Introduction
Gastric cancer (GC) is the fifth most common
malignancy in the world with 951,600 new cases (6.8% of total) and
723,100 deaths (8.8% of total) in 2012 (1,2). Due to
the non-specific symptoms of early-stage GC, and patients with
advanced GC exhibit poor prognosis. Therefore, it is necessary to
identify novel specific and sensitive biomarkers for GC that can
assist in early diagnosis and improve its prognosis (3,4).
Adenosine diphosphate ribosylation factor guanylate
kinase 1 (ASAP1) influences tumor cell migration and invasion, and
therefore promotes the metastasis of tumor cells in the body. ASAP1
expression is significantly upregulated in various tumors and is
closely associated with the malignant biological behavior and
prognosis of these tumors (5–9).
However, to the best of our knowledge, ASAP1 expression in GC and
its association with GC prognosis have not been previously
reported. Focal adhesion kinase (FAK) is an important non-receptor
tyrosine kinase that influences cell proliferation, adhesion,
invasion and migration, and is associated with tumor growth,
anti-apoptotic mechanisms and tumor recurrence (10–12).
Previous studies have demonstrated that FAK expression is
upregulated in thyroid, esophageal, breast, gastric and intestinal
cancer, and its expression is associated with a poor tumor
prognosis (13,14). The present study used
immunohistochemistry to detect the expression levels of ASAP1 and
FAK in GC tissues, and analyzed their associations with multiple
clinicopathological factors and with GC prognosis.
Materials and methods
Patients and tissue samples
All clinical GC tissue samples were isolated from 32
patients who had received a subtotal gastrectomy or radical total
gastrectomy between December 2011 and February 2012 at the Union
Hospital of Fujian Medical University (Fuzhou, China). The patients
enrolled in the present study had not received radiotherapy or
chemotherapy before surgery. In addition, all patients had complete
follow-up data and available paraffin-embedded normal gastric
mucosal tissues. The follow-up rate was 100%.
The clinical staging was evaluated based on the
International Union Against Cancer (UICC) Tumor-Node-Metastasis
(TNM) Classification of Malignant Tumors (15). Ethical approval for the present study
was obtained from the Union Hospital of Fujian Medical University
Ethics Committee. All patients or their guardians provided written
informed consent.
Clinical information of the 32 patients is presented
in Table I. There were 23 males and
9 females. The median age of the patients was 63.9 years (range,
40–87 years; patients ≥60 years, n=23; patients <60 years, n=9).
Pathological findings indicated that 53.1% (n=17) of the tumors
were classified as being moderately or highly differentiated, and
46.9% (n=15) as undifferentiated or poorly differentiated. A total
of 22 patients (68.8%) presented with lymph node metastasis (N1-4).
The depth of invasion was pT1+2 in 15 patients (46.9%) and pT3+4 in
17 patients (53.1%). A total of 14 patients (43.8%) were allocated
to TNM stage I+II and 18 patients (56.3%) to stage III+IV.
 | Table I.Clinicopathological characteristics of
patients with gastric cancer. |
Table I.
Clinicopathological characteristics of
patients with gastric cancer.
| Characteristic | n | % |
|---|
| Age, years |
|
|
|
<60 | 9 | 28.13 |
| ≥60 | 23 | 71.88 |
| Sex |
|
|
|
Female | 9 | 28.13 |
|
Male | 23 | 71.88 |
| Invasion depth |
|
|
|
pT1+2 | 14 | 43.75 |
|
pT3+4 | 18 | 56.25 |
| Lymph node
metastasis |
|
|
| No | 10 | 31.25 |
|
Yes | 22 | 68.75 |
| TNM stage |
|
|
|
I+II | 15 | 46.88 |
|
III+IV | 17 | 53.12 |
| Degree of
differentiation |
|
|
|
Moderate/high | 17 | 53.12 |
|
Undifferentiated/low | 15 | 46.88 |
Disease-free survival (DFS) time was defined as the
time from surgery to recurrence or mortality from any cause during
the 5 years of follow-up. Overall survival (OS) time was defined as
the time between initial surgery and the day of the last follow-up
or mortality from any cause during the 5 years of follow-up. The
mean 5-year DFS time was 77.37±35.08 months and the mean 5-year OS
time was 77.57±34.02 months.
Immunohistochemistry
Serial 3-µm thick sections were cut from tissue
samples, mounted onto glass slides, dewaxed in xylene and
rehydrated in different concentrations (100, 95, 90, 85 and 70%) of
ethanol. The sections were autoclaved in in citrate buffer (pH 6.0)
at 121°C for 10 min for antigen retrieval. Samples were blocked
with 5% normal goat serum (cat. no. KL-D1418; Kalang Biologicals)
at 37°C for 30 min and then incubated with primary anti-ASAP1
(1:250; cat. no. 125729; Abcam) and anti-FAK (1:250; cat. no.
40794; Abcam) overnight at 4°C. Subsequently, samples were rinsed
in PBS three times and incubated with a horseradish
peroxidase-labeled secondary antibody (1:1,000; cat. no. K4003;
Dako; Agilent Technologies, Inc.) at 37°C for 30 min. The slides
were stained with 3,3′-diaminobenzidine (Dako; Agilent
Technologies, Inc.) at room temperature for 2 min. Slides were
washed in PBS three times. For color development: DAB color kit
(cat. no. C520017; Sangon Biotech), reaction solution (10 ml)
reagents A (200 µl) and reagents C (20 µl) were used to form the
DAB color development solution. After rinsing with 50 µl extra
water, the color development solution was added to each section in
a dark place at 37°C for ~10 min. The sections were rinsed with
purified water three times to terminate the color development.
Hematoxylin (cat. no. E607318; Sangon Biotech) staining was
performed at 37°C for 5 min and then counterstained with eosin
(cat. no. E607318; Sangon Biotech) at 37°C for 30 sec. Slides were
then dehydrated in 95% ethanol twice for 5 min each, and washed in
xylene twice for 5 min each. Finally, sections were sealed using
neutral balsam and left to dry naturally.
Immunohistochemistry scoring
Immunohistochemistry staining of ASAP1 and FAK was
performed and scored by two experienced pathologists using a light
microscope at ×200 magnification, according to a previous study
published by Hou et al (5).
The staining intensity was graded as follows: 0, unstained; 1, low
signal (light yellow); 2, moderate signal (yellow brown); and 3,
strong signal (brown). In addition, the score associated with the
percentage of positive cells was assigned as follows: 0, <5%
positive cells; 1, 5–10% positive cells; 2, 11–50% positive cells;
3, 51–80% positive cells; and 4, >80% positive cells. The final
score was calculated by multiplying the scores associated with the
percentage of positive cells by the score associated with the
intensity. The scores were divided into the negative expression
(final score, 0–4) and positive expression (final score, 6–12)
groups.
Statistical analysis
All data were analyzed using SPSS v11.5 (SPSS Inc.).
Quantitative data are presented as the mean ± SD, and qualitative
data are presented as the rate or ratio. The survival time is
presented as the median and quartiles. The χ2 test or
Fisher's exact test were used to analyze the differences among
groups. The κ value was used to investigate the association between
variables. Kaplan-Meier curves were used to evaluate the 5-year DFS
rate or 5-year OS rate, and the log-rank test was used to analyze
differences in survival rates. P<0.05 was considered to indicate
a statistically significant difference.
Results
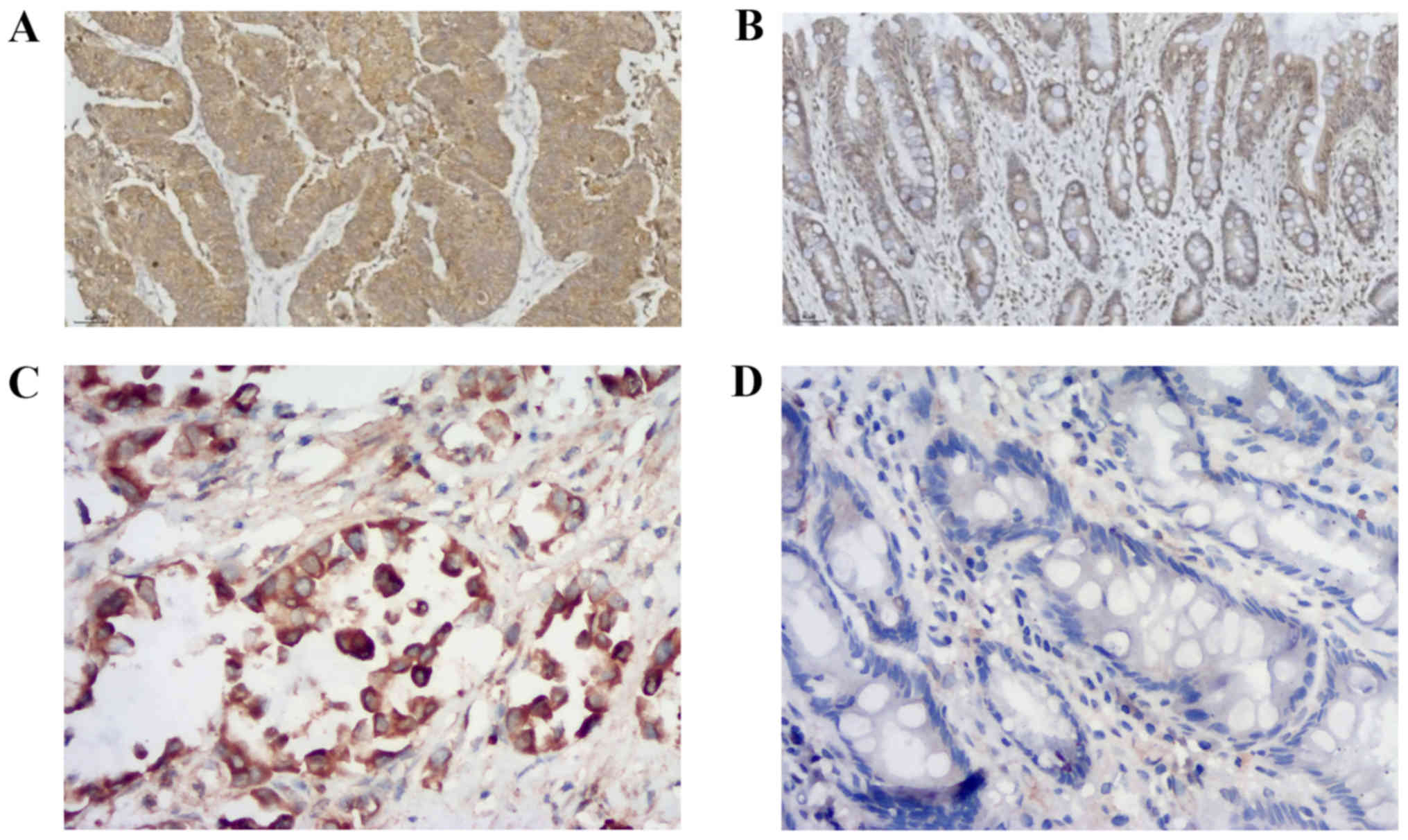
Protein expression of ASAP1 and FAK in
GC tissues
The expression levels of ASAP1 and FAK were detected
in GC tissues, and a brown/yellow signal was identified in the
cytoplasm of positive cells by microscopy. Most of the positive
cells for ASAP1 (Fig. 1A) and FAK
(Fig. 1C) were found in tumor
tissues. however, a limited number of cells presenting a low signal
for ASAP1 (Fig. 1B) and FAK
(Fig. 1D) were found in normal
tissues adjacent to cancerous tissues. The positive expression
rates of ASAP1 in 32 GC and normal gastric mucosal tissues were
59.4% (19/32) and 28.1% (9/32), respectively, and the difference
was statistically significant (χ2=6.349; P=0.012). The
positive expression rate of FAK was 68.8% (22/32) and 40.6% (13/32)
in GC and normal gastric mucosal tissues, respectively, and the
difference was statistically significant (χ2=5.107;
P=0.024). As presented in Table II,
the expression levels of ASAP1 and FAK in GC tissues were
significantly associated with depth of invasion, lymph node
metastasis, TNM stage and differentiation (P<0.05).
 | Table II.Association between ASAP1 and FAK
expression and clinicopathological features of patients with
gastric cancer (n=32). |
Table II.
Association between ASAP1 and FAK
expression and clinicopathological features of patients with
gastric cancer (n=32).
|
| ASAP1 | FAK |
|---|
|
|
|
|
|---|
| Characteristic | −, n | +, n | P-value | −, n | +, n | P-value |
| Age, years |
|
|
|
|
|
|
|
<60 | 6 | 3 | 0.109 | 4 | 5 | 0.407 |
|
≥60 | 7 | 16 |
| 6 | 17 |
|
| Sex |
|
|
|
|
|
|
|
Female | 5 | 4 | 0.427 | 4 | 5 | 0.407 |
|
Male | 8 | 15 |
| 6 | 17 |
|
| Invasion depth |
|
|
|
|
|
|
|
pT1+2 | 11 | 3 | <0.001 | 9 | 5 | 0.001 |
|
pT3+4 | 2 | 16 |
| 1 | 17 |
|
| Lymph node
metastasis |
|
|
|
|
|
|
| No | 8 | 2 | 0.005 | 7 | 3 | 0.003 |
|
Yes | 5 | 17 |
| 3 | 19 |
|
| TNM stage |
|
|
|
|
|
|
|
I+II | 11 | 4 | 0.001 | 9 | 6 | 0.002 |
|
III+IV | 2 | 15 |
| 1 | 16 |
|
| Degree of
differentiation |
|
|
|
|
|
|
|
Moderate/high | 13 | 4 | <0.001 | 10 | 7 | <0.001 |
|
Undifferentiated/low | 0 | 15 |
| 0 | 15 |
|
Association between ASAP1 and FAK
expression in GC tissues
Among the 19 GC tissues with positive ASAP1
expression, 19 were positive for FAK (100.0%). Among the 13 GC
tissues with negative ASAP1 expression, 3 were positive for FAK
(23.1%). κ analysis indicated that the expression levels of ASAP1
were associated with FAK expression in GC tissues (κ=0.798;
P<0.001; Table III).
 | Table III.Association between ASAP1 and FAK
expression in gastric cancer tissues. |
Table III.
Association between ASAP1 and FAK
expression in gastric cancer tissues.
|
| FAK expression |
|
|
|
|---|
|
|
|
|
|
|
|---|
| ASAP1
expression | positive | negative | Total | κ | P-value |
|---|
| Positive | 19 | 0 | 19 | 0.798 | <0.001 |
| Negative | 3 | 10 | 13 |
|
|
| Total | 22 | 10 | 32 |
|
|
Association between ASAP1 expression,
FAK expression and GC prognosis
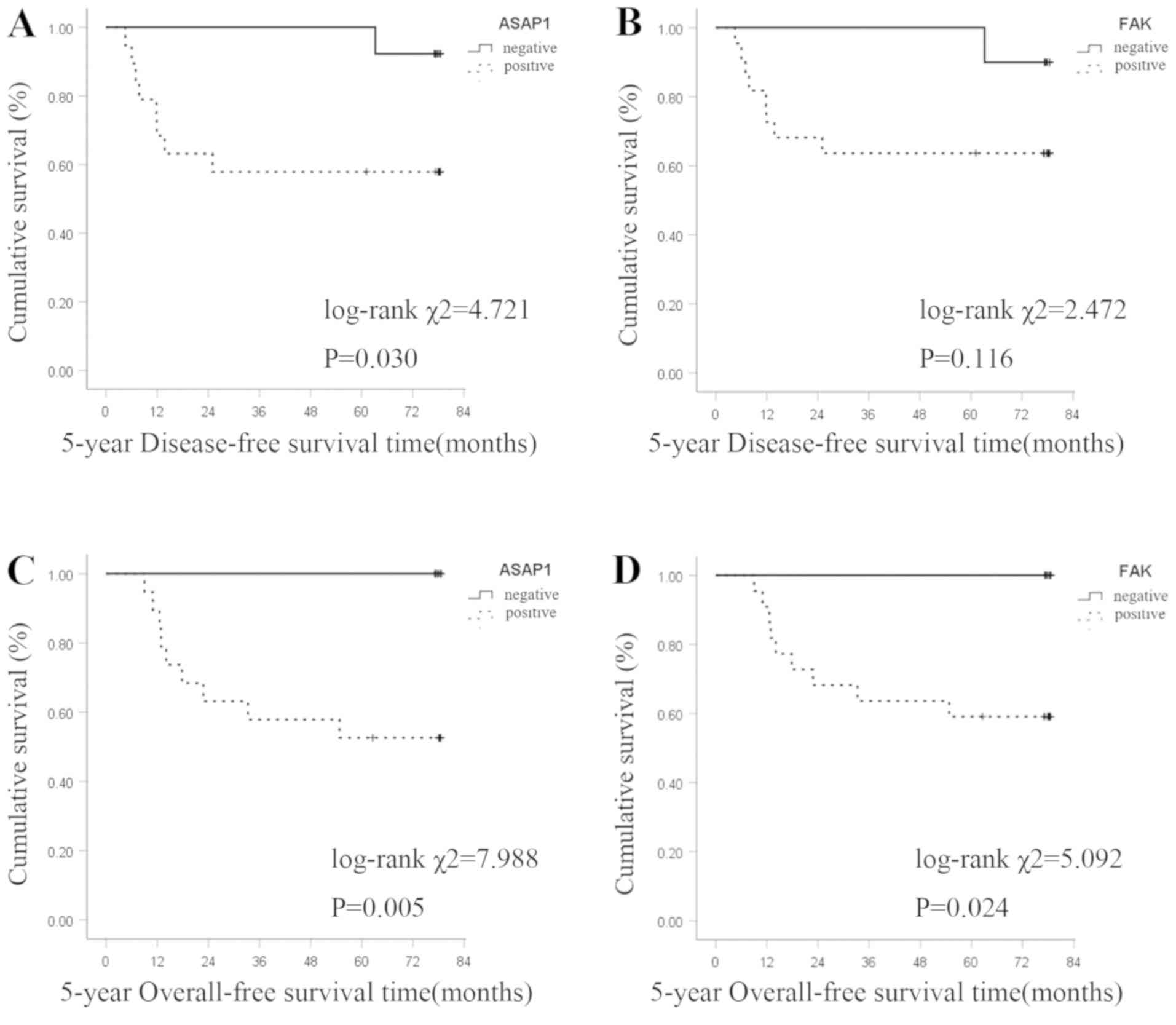
The Kaplan-Meier survival curve analysis revealed
that ASAP1 and FAK expression levels were negatively associated
with 5-year DFS time in patients with GC. The 5-year DFS rate was
57.9% (11/19) in the ASAP1-positive group and 92.3% (12/13) in the
negative group. The difference was statistically significant
(log-rank χ2=4.721; P=0.030; Fig. 2A). The 5-year DFS rate was 63.6%
(14/22) in the FAK-positive group and 90.0% (9/10) in the negative
group. The difference was not statistically significant (log-rank
χ2=2.472; P=0.116; Fig.
2B).
The 5-year OS rate of patients with positive and
negative ASAP1 expression was 52.8% (10/19) and 100.0% (13/13),
respectively, and the difference was statistically significant
(log-rank χ2=7.988; P=0.005; Fig. 2C). The 5-year OS rate of patients
with positive and negative FAK expression was 59.1% (13/22) and
100.0% (10/10), respectively, and the difference was statistically
significant (log-rank χ2=5.092; P=0.024; Fig. 2D).
Discussion
GC is one of the most common malignant tumors that
poses a severe threat to human health. Genome-driven targeted
cancer therapies may provide novel and promising strategies for
cancer prevention and control (16).
In 2009, the American Society of Clinical Oncology reported the
first targeted therapy for GC in the ‘trastuzumab for gastric
cancer trial’, demonstrating that trastuzumab combined with
chemotherapy as first-line treatment may improve the survival of
human epidermal growth factor receptor 2 (HER2)-positive patients
with advanced GC (17). However, the
global positive rate of HER2 in advanced GC is only 25%, and half
of these patients exhibit a poor response to trastuzumab due to
unknown causes (18). To the best of
our knowledge, the only first-line targeted drug for GC is
trastuzumab, the only second-line drug is ramucirumab and the only
third-line drug is apatinib (19).
Therefore, the available targeted treatments for GC are limited
compared with those for other types of cancer, and further studies
are required to develop novel therapeutic strategies to improve GC
treatment.
ASAP1 is a phospholipid-dependent GTPase-activating
protein (GAP) located on the long arm of chromosome 8, at
24.1–24.2. As a member of the ARF GAP family, ASAP1 hydrolyzes GTP
to regulate actin re-organization and actin cytoskeletal dynamics,
in this way controlling motility, regulating the formation of focal
adhesions and invasive pseudopods, and contributing to the folding
of the plasma membrane (20–24). In addition, ASAP1 binds to the SH3
domain-containing kinase-binding protein 1, the CD2-associated
protein, cortactin, the CRK-like proto-oncogene adaptor protein and
the SRC proto-oncogene non-receptor tyrosine kinase through its
structural domains to exert its biological activities and to
regulate cell invasion (25–27). Recent studies have described the
association between ASAP1 and the biological features of malignant
tumors. Müller et al (20)
demonstrated that ASAP1 promotes the invasion of colorectal cancer
cells in vitro and stimulates the metastasis of colorectal
cancer cells in vivo. Hou et al (5) revealed that ASAP1 expression in ovarian
cancer tissues is significantly higher than that in normal ovarian
tissues. In-depth analysis has demonstrated that positive ASAP1
expression is an indicator of poor prognosis in ovarian cancer and
is an independent prognostic factor for the OS rate of patients
with ovarian cancer. In addition, Liu et al (28) demonstrated that downregulation of
ASAP1 expression by RNA interference inhibits cell proliferation
and migration. However, to the best of our knowledge, no previous
studies have investigated ASAP1 expression in GC tissues. In the
present study, the expression levels of ASAP1 in GC tissues were
significantly higher than those in tumor-adjacent normal gastric
mucosal tissues (P=0.012). ASAP1 expression in GC tissues from
patients with a T3+T4 infiltration depth was higher compared with
that in patients with a T1+T2 infiltration depth (P<0.001).
Furthermore, ASAP1 expression in GC tissues from patients with
lymph node metastasis was higher than that of patients without
lymph node metastasis (P=0.005). ASAP1 expression in GC tissues
from patients at stages III and IV was significantly higher than
that of patients at stages I and II (P=0.001). Compared with
moderate or higher degree of differentiation, patients with
undifferentiated or lower degree of differentiation had a higher
ASAP1 expression levels (P<0.001). The DFS rates of patients
with positive and negative ASAP1 expression were 57.9% (11/19) and
92.3% (12/13), respectively, with a significant difference between
the two groups (P=0.030). The OS rates of patients with positive
and negative ASAP1 expression were 52.8% (10/19) and 100.0%
(13/13), respectively, with a significant difference between the
two groups (P=0.005). Therefore, the present results suggested that
ASAP1 may serve important roles in the growth, invasion and
metastasis of malignant gastric tumors, and may represent a novel
molecular marker for evaluating the biological behavior and
prognosis of GC.
FAK was first identified and cloned by Schaller
et al (29) from
v-src-transfected chicken embryo fibroblasts in the 1990s. The
human FAK gene is located on the long arm of chromosome 8
(8q24). The FAK protein is a non-receptor tyrosine protein kinase
with six tyrosine sites that can be phosphorylated: Tyr397 and
Tyr407 are located at the amino terminus, Tyr576 and Tyr577 are
located in the activation loop of the kinase domain, and Tyr861 and
Tyr925 are located at the carboxyl terminus. Activated FAK
participates in tumor proliferation, growth, invasion and
metastasis via multiple signaling pathways, such as the FAK-Ras-PK,
FAK-PI3K and FAK-STAT pathways (12). A study by Weiner et al
(30) published in 1993 revealed
that FAK expression is increased in invasive tumors and that
positive FAK expression is present in all metastatic tumors,
suggesting that it may promote tumor cell invasion and metastasis.
A study by Miyazaki et al (31) published in 2003 demonstrated that FAK
upregulation is associated with infiltration depth, lymph node
metastasis and the number of metastatic lymph nodes in esophageal
cancer. In 2010, Park et al (32) used immunohistochemistry to detect FAK
expression in 444 surgically resected GC tissues. Additionally, a
study by Lai et al (33)
revealed that FAK can be autophosphorylated at the 397-tyrosine
residue and that after this activation it initiates processes such
as the proliferation, invasion and migration of GC cells. A study
by Fan et al (11) in 2013
demonstrated that FAK activation increases endogenous A2 protein
phosphorylation and leads to changes in epithelial-mesenchymal
transition markers, including matrix metalloproteases, thereby
inducing tumorigenesis and tumor progression. A study by Wang et
al (34) in 2019 revealed that
FAK binds directly to microRNA-1224 to inhibit the activation of
the STAT3 and NF-κB pathways, thereby suppressing the metastasis of
GC. The present results suggested that the expression levels of FAK
in GC tissues were significantly upregulated compared with those in
tumor-adjacent normal gastric mucosal tissues (P=0.024).
Additionally, FAK expression was associated with depth of
infiltration, lymph node metastasis, increased tumor stage and
decreased differentiation degree. The OS rates of patients with
positive and negative FAK expression were 59.1% (13/22) and 100.0%
(10/10), respectively, with a significant difference between the
two groups (P=0.024). The present results suggested that FAK may
serve a role in the tumorigenesis and progression of GC. Abnormal
FAK expression may lead to more malignant GC cell proliferation,
growth, invasion and metastasis. Therefore, FAK may be useful as a
novel molecular marker to evaluate the biological behavior and
prognosis of GC.
ASAP1 is a multi-domain protein with rich in proline
structural regions which binds to various structural proteins to
exert its biological function. ASAP1 interacts with non-muscle
myosin II-A to regulate actin cytoskeleton remodeling and the
transport of integrin, thereby affecting cell invasion and
metastasis (35,36). FAK, as a key molecule in
integrin-dependent signal transduction pathways, serves an
important role in the binding between integrin and ligands, thus
promoting the formation of focal adhesions (37). A study by Liu et al (28) used yeast two-hybrid screening and a
co-immunoprecipitation assay to demonstrate a direct interaction
between ASAP1 and FAK. In the present study, 29 samples were found
to co-express ASAP1 and FAK in GC tissues, which resulted in a κ
value of 0.798 (P<0.001), indicating that ASAP1 expression was
associated with FAK expression and that ASAP1 and FAK promoted the
pathophysiology of malignant GC. The present results suggested an
association between ASAP1 and FAK, which affected the
pathophysiology of malignant GC. Therefore, ASAP1 may promote the
malignant phenotype of GC cells through FAK.
In conclusion, ASAP1 and FAK were highly expressed
in GC tissues and were associated with degree of invasion, lymph
node metastasis, pathological staging and degree of differentiation
in the present study. In addition, positive expression of ASAP1 and
FAK may be risk factors for the prognosis of patients with GC. The
present findings suggested that ASAP1 and FAK may synergistically
promote the tumorigenesis, tumor progression, invasion and
metastasis of GC, and are closely associated with the survival of
patients with GC. Although the sample size was too small, which was
one of the limitations of the present study, it provided a basis
for a follow-up study on the association between ASAP1, FAK and GC.
ASAP1 and FAK may represent novel molecular markers for the
pathophysiology and prognosis of GC. In addition, further in
vitro and in vivo studies investigating the molecular
mechanisms of ASAP1 and FAK in the tumorigenesis and tumor
progression of GC may help to elucidate the pathophysiology of
malignant GC. Furthermore, additional studies may provide important
information for the early diagnosis, treatment and prognostic
assessment of GC, as well as novel molecular targets and
therapeutic strategies to develop novel drugs to treat GC.
Acknowledgements
Not applicable.
Funding
The present study was partially supported by the
Health- Education Joint Research Project of Fujian Province (grant
no. WKJ2016-223), the Science Technology Innovation Joint Project
Foundation of Fujian Province (grant no. 2017Y9003), the Startup
Fund for Scientific Research, Fujian Medical University (grant no.
2017XQ2037), the Science Technology Innovation Joint Project
Foundation of Fujian Province (grant no. 2018Y9038) and the Program
for Innovative Research Team in Science and Technology in Fujian
Province University.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SY and XC conceived and supervised the project. QL,
SZ, DZ and FY performed the experiments and analyzed the data. QL,
SZ and DZ wrote the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Ethical approval for the present study was obtained
from the Union Hospital of Fujian Medical University Ethics
Committee (Fuzhou, China). All patients or their guardians provided
written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li J, Meng Q, Sun Y and Qing H: Inhibition
of focal adhesion kinase induces apoptosis in human gastric
carcinoma cells (SGC-7901). Mol Biol Rep. 40:401–406. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Z, Lei H, Luo M, Wang Y, Dong L, Ma Y,
Liu C, Song W, Wang F, Zhang J, et al: DNA methylation
downregulated mir-10b acts as a tumor suppressor in gastric cancer.
Gastric Cancer. 18:43–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kanda M and Kodera Y: Recent advances in
the molecular diagnostics of gastric cancer. World J Gastroenterol.
21:98382015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hou T, Yang C, Tong C, Zhang H, Xiao J and
Li J: Overexpression of ASAP1 is associated with poor prognosis in
epithelial ovarian cancer. Int J Clin Exp Pathol.
7:2802014.PubMed/NCBI
|
|
6
|
Zhang L, Shi SB, Zhu Y, Qian TT and Wang
HL: Long non-coding RNA ASAP1-IT1 promotes cell proliferation,
invasion and metastasis through the PTEN/AKT signaling axis in
non-small cell lung cancer. Eur Rev Med Pharmacol Sci. 22:142–149.
2018.PubMed/NCBI
|
|
7
|
Randazzo PA, Inoue H and Bharti S: Arf
GAPs as regulators of the actin cytoskeleton. Biol Cell.
99:583–600. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang T, Zhao G, Yang C, Dong P, Watari H,
Zeng L, Pfeffer LM and Yue J: Lentiviral vector mediated-ASAP1
expression promotes epithelial to mesenchymal transition in ovarian
cancer cells. Oncol Lett. 15:4432–4438. 2018.PubMed/NCBI
|
|
9
|
Yang L, Xue Y, Liu J, Zhuang J, Shen L,
Shen B, Yan J and Guo H: Long noncoding RNA ASAP1-IT1 promotes
cancer stemness and predicts a poor prognosis in patients with
bladder cancer. Neoplasma. 64:847–855. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sulzmaier FJ, Jean C and Schlaepfer DD:
FAK in cancer: Mechanistic findings and clinical applications. Nat
Rev Cancer. 14:5982014. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fan H, Zhao X, Sun S, Luo M and Guan JL:
Function of focal adhesion kinase scaffolding to mediate endophilin
A2 phosphorylation promotes epithelial-mesenchymal transition and
mammary cancer stem cell activities in vivo. J Biol Chem.
288:3322–3333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fu W, Hall JE and Schaller MD: Focal
adhesion kinase-regulated signaling events in human cancer. Biomol
Concepts. 3:225–240. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thanapprapasr D, Previs RA, Hu W, Ivan C,
Armaiz-Pena GN, Dorniak PL, Hansen JM, Rupaimoole R, Huang J,
Dalton HJ, et al: PTEN expression as a predictor of response to
focal adhesion kinase inhibition in uterine cancer. Mol Cancer
Ther. 14:1466–1475. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zeng XQ, Li N, Ma LL, Tseng YJ, Zhao NQ
and Chen SY: Prognostic value of focal adhesion kinase (FAK) in
human solid carcinomas: A meta-analysis. PLoS One. 11:e01626662016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sobin L: International Union Against
Cancer (UICC) TNM classification of malignant tumours. Oesophagus
including Oesophagogastric Junction. 66–72. 2009.
|
|
16
|
Buqué A, Bloy N, Aranda F, Castoldi F,
Eggermont A, Cremer I, Fridman WH, Fucikova J, Galon J, Marabelle
A, et al: Trial watch: Immunomodulatory monoclonal antibodies for
oncological indications. Oncoimmunology. 4:e10088142015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Petrelli NJ, Winer EP, Brahmer J, Dubey S,
Smith S, Thomas C, Vahdat LT, Obel J, Vogelzang N, Markman M, et
al: Clinical Cancer Advances 2009: Major research advances in
cancer treatment, prevention, and screening-a report from the
American Society of Clinical Oncology. J Clin Oncol. 27:6052–6069.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yazici O, Sendur MA, Ozdemir N and Aksoy
S: Targeted therapies in gastric cancer and future perspectives.
World J Gastroenterol. 22:471–489. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feng R, Zhang X and Yang S: Research
status quo and progression in targeted therapy for advanced gastric
cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 19:1191–1196. 2016.(In
Chinese). PubMed/NCBI
|
|
20
|
Müller T, Stein U, Poletti A, Garzia L,
Rothley M, Plaumann D, Thiele W, Bauer M, Galasso A, Schlag P, et
al: ASAP1 promotes tumor cell motility and invasiveness, stimulates
metastasis formation in vivo, and correlates with poor survival in
colorectal cancer patients. Oncogene. 29:2393–2403. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Randazzo PA, Andrade J, Miura K, Brown MT,
Long YQ, Stauffer S, Roller P and Cooper JA: The Arf
GTPase-activating protein ASAP1 regulates the actin cytoskeleton.
Proc Natl Acad Sci USA. 97:4011–4016. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Yerushalmi GM, Grigera PR and
Parsons JT: Mislocalization or reduced expression of Arf
GTPase-activating protein ASAP1 inhibits cell spreading and
migration by influencing Arf1 GTPase cycling. J Biol Chem.
280:8884–8892. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Inoue H and Randazzo PA: Arf GAPs and
their interacting proteins. Traffic. 8:1465–1475. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nie Z and Randazzo PA: Arf GAPs and
membrane traffic. J Cell Sci. 119:1203–1211. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brown MT, Andrade J, Radhakrishna H,
Donaldson JG, Cooper JA and Randazzo PA: ASAP1, a
phospholipid-dependent arf GTPase-activating protein that
associates with and is phosphorylated by Src. Mol Cell Biol.
18:7038–7051. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kowanetz K, Husnjak K, Höller D, Kowanetz
M, Soubeyran P, Hirsch D, Schmidt MHH, Pavelic K, De Camilli P,
Randazzo PA and Dikic I: CIN85 associates with multiple effectors
controlling intracellular trafficking of epidermal growth factor
receptors. Mol Biol Cell. 15:3155–3166. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oda A, Wada I, Miura K, Okawa K, Kadoya T,
Kato T, Nishihara H, Maeda M, Tanaka S, Nagashima K, et al: CrkL
directs ASAP1 to peripheral focal adhesions. J Biol Chem.
278:6456–6460. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu Y, Loijens JC, Martin KH, Karginov AV
and Parsons JT: The association of ASAP1, an ADP ribosylation
factor-GTPase activating protein, with focal adhesion kinase
contributes to the process of focal adhesion assembly. Mol Biol
Cell. 13:2147–2156. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schaller MD, Hildebrand JD and Parsons JT:
Complex formation with focal adhesion kinase: A mechanism to
regulate activity and subcellular localization of Src kinases. Mol
Biol Cell. 10:3489–3505. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Weiner TM, Craven RJ, Craven RJ and Cance
WG: Expression of focal adhesion kinase gene and invasive cancer.
Lancet. 342:1024–1025. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Miyazaki T, Kato H, Nakajima M, Sohda M,
Fukai Y, Masuda N, Manda R, Fukuchi M, Tsukada K and Kuwano H: FAK
overexpression is correlated with tumour invasiveness and lymph
node metastasis in oesophageal squamous cell carcinoma. Br J
Cancer. 89:1402003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Park JH, Lee BL, Yoon J, Kim J, Kim MA,
Yang HK and Kim WH: Focal adhesion kinase (FAK) gene amplification
and its clinical implications in gastric cancer. Hum Pathol.
41:1664–1673. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lai HC, Zhuang LF, Liu X, Wieland M and
Zhang ZY and Zhang ZY: The influence of surface energy on early
adherent events of osteoblast on titanium substrates. J Biomed
Mater Res A. 93:289–296. 2010.PubMed/NCBI
|
|
34
|
Wang J, Wen T, Li Z, Che X, Gong L, Yang
X, Zhang J, Tang H, He L, Qu X and Liu Y: MicroRNA-1224 inhibits
tumor metastasis in intestinal-type gastric cancer by directly
targeting FAK. Front Oncol. 9:2222019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yoon HY, Jacques K, Nealon B, Stauffer S,
Premont RT and Randazzo P: Differences between AGAP1, ASAP1 and Arf
GAP1 in substrate recognition: Interaction with the N-terminus of
Arf1. Cell Signal. 16:1033–1044. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vitali T, Girald-Berlingeri S, Randazzo PA
and Chen PW: Arf GAPs: A family of proteins with disparate
functions that converge on a common structure, the integrin
adhesion complex. Small GTPases. 10:280–288. 2019.PubMed/NCBI
|
|
37
|
Brami-Cherrier K, Gervasi N, Arsenieva D,
Walkiewicz K, Boutterin MC, Ortega A, Leonard PG, Seantier B, Gasmi
L, Bouceba T, et al: FAK dimerization controls its kinase-dependent
functions at focal adhesions. EMBO J. 33:356–370. 2014. View Article : Google Scholar : PubMed/NCBI
|