Introduction
Cervical cancer is the fourth most common type of
malignant cancer in women, with one of the highest incidences among
all types of female genital tract malignant tumors (1). Cervical cancer is caused by a number of
factors, including premature sexual activity, genetics, infection,
long-term use of oral contraceptives, oral immunosuppressive drugs
and poor health conditions (2,3).
However, it is generally accepted that the major contributing
factor towards the development of cervical cancer is human
papillomavirus (HPV) infection, especially high-risk (HR) HPV. HPV
16 and 18 are the most common forms of HPV (4). HPV detection has been implemented as
one of the screening methods for the prevention of cervical cancer
(5). With the application of HPV
detection technology, the resection rate of cervical
intraepithelial neoplasia and early cervical cancer has increased,
but a large number of patients present with advanced cervical
cancer at diagnosis (6). Therefore,
to detect precancerous lesions as early as possible and reduce the
incidence of cervical cancer, it is necessary to identify a
biomarker for the early diagnosis of cervical cancer.
Squamous cell carcinoma antigen (SCCA) belongs to
the serine/cysteine protease inhibitor family (7). As a tumor marker of squamous cell
carcinomas, SCCA exhibits low expression levels in most normal
tissues, but is highly expressed in malignant tumors or squamous
intraepithelial lesions (8). SCCA
has been extensively studied in various squamous cell carcinomas,
including vulvar, esophageal and liver cancer (7–9), but
also non-neoplastic diseases, such as psoriasis (10). A number of studies have demonstrated
that SCCA is not only an important reference index for the
diagnosis of cervical cancer types and cervical intraepithelial
neoplasia, but also can provide information for the effective
treatment for patients with cervical cancer (11–13).
SCCA has been extensively studied in cervical lesions; however,
there is no study on whether the overexpression of SCCA is
associated with HPV infection.
Metastasis-associated gene 1 (MTA1) is a biomarker
whose expression profile is associated with the invasive and
migratory capabilities of tumor cells (14). MTA1 is expressed at low levels in
normal tissues with the exception of the testis, but upregulated in
the majority of tumor types, such as ovarian (15), breast (16), and cervical cancer (17). In the process of tumorigenesis and
metastasis, MTA1 can promote the expression of tumor invasion and
metastasis related factors, and inhibit the expression of tumor
suppressor genes (18). MTA1 is
expressed at high levels in the cervical cancer cell line SiHa,
which contains the HPV16 genome (19). The HPV E6 protein can also bind to
P53 (19–21). Therefore, it is hypothesized that
MTA1 upregulation in cervical lesions may be associated with HR-HPV
infections. However, there is no study investigating the
relationship between HR-HPV infection and MTA1 in cervical tissue,
to the best of our knowledge.
The multiple tumor suppressor gene P16 directly
participates in the regulation of the cell cycle (22). Upregulation of P16 in cervical
lesions is associated with the expression of HPV16 and 18 genes,
suggesting that the upregulation of P16 is secondary to the HPV
infection (23). Therefore, P16 may
be used as a marker for the differential diagnosis of benign and
neoplastic hyperplasia of cervical epithelium. The nuclear
associated antigen Ki-67 is expressed in the nucleus of
proliferating cells and can be used to judge the proliferative
activity of cells (24). Ki-67 is
localized at the basal and parabasal layers of cervical
intraepithelial neoplasia (24). The
expression of Ki-67 is strongly positive in cells with a typical
nucleus, and the expression profile of Ki-67 widens and becomes
more intense with the increase in the degree of cervical
intraepithelial neoplasia (25,26).
Additionally, HR-HPV can promote Ki-67 expression in the cervical
epithelium (27). Therefore, Ki-67
can be used as a sensitive biomarker to reflect the degree and
grade of lesions. Recently, P16/Ki-67 dual staining has been used
for cervical cancer screening (28,29).
In the present study, the expression levels of SCCA,
MTA1, P16 and Ki-67 proteins and the HR-HPV were detected in
various cervical tissues. The relationship between the four
proteins and the HR-HPV infection was further evaluated. The aims
of the present study were to evaluate whether the trend in the
expression of SCCA and MTA1 in various types of cervical lesions
increased with the lesion grade and to investigate whether the
expression profiles of SCCA and MTA1 were related to HR-HPV
infections.
Materials and methods
Patients
The present study was approved by the Ethics
Committee of the North China University of Science and Technology
Affiliated Hospital, and carried out in accordance with the ethical
guidelines of the World Medical Association (Declaration of
Helsinki) for experiments involving humans. All patients signed
informed consent for the use of their cervical tissue, secretions
and clinical information.
From March 2015 to November 2016, in the North China
University of Science and Technology Affiliated Hospital, 123
patients aged from 21 to 65 were enrolled in the present study,
none of whom had received prior radiotherapy, chemotherapy or
hormonotherapy. Of these patients, there were 31 cases with
low-grade squamous intraepithelial lesions (LSILs), 31 cases with
high-grade squamous intraepithelial lesions (HSILs) and 29 cases
with cervical squamous cell carcinoma (CSCC). A total of 32 cases
with chronic cervicitis (CCE) were recruited as the control group.
Cervical tissue was obtained from surgeries or colposcopic biopsies
and immediately frozen at 80°C for further study. Secretions were
collected using a cervical sampling brush and stored in cell
preservation solution (BestBio Co., Ltd.).
Immunohistochemistry analysis
The 4% paraformaldehyde-fixed (24 h at 4°C) and
paraffin-embedded tissue sections (4 µm) were deparaffinized and
dehydrated. For antigen retrieval, the sections were boiled in EDTA
antigen retrieval solution (1:50; OriGene Technologies, Inc.) for
2.5 min. Endogenous peroxidases and non-specific reactions were
blocked by incubating the sections with 3%
H2O2 for 15 min at 37°C and with 10% normal
goat serum (cat. no. ZLI-9022; ZSGB-BIO Co., Ltd.) for 20 min at
37°C, respectively. All sections were incubated separately with the
following primary antibodies: Rabbit anti-human SCCA (1:600; cat.
no. A6960ABclonal Biotech Co., Ltd.), rabbit anti-human MTA1
(1:600; cat. no. A16085; ABclonal Biotech Co., Ltd.), rabbit
anti-human Ki-67 (1:600; cat. no. bs-23103R; Bioss Inc.) or rabbit
anti-human P16 (1:600; cat. no. bs-1856R; Bioss Inc.) at 4°C
overnight, followed by incubation with goat anti-rabbit horseradish
peroxidase labelled immunoglobulin (IgG-HRP) secondary antibodies
(1:2,000, cat. no. sc2030; Santa Cruz Biotechnology Inc.) at 37°C
for 40 min. The staining of tissue sections was performed using a
DAB Staining kit (Origene Technologies, Inc.) according to the
manufacturer's instructions. Images (magnification, ×200) were
captured using a Micro Publisher 5.0 Confocal Microscope (Roper
Technologies, Inc.) equipped with a CMOS camera (Olympus
Corporation). The SCCA-, MTA1-, Ki-67- and P16-positive tissues
were analyzed using CellSens Dimension software (version 1.6,
Olympus Corporation).
The criteria for interpretation of the SCCA, MTA1
and P16 staining as positive were as follows: i) The presence of
brown and yellow granules; ii) SCCA was located in the cytoplasm;
MTA1 was in the cytoplasm, membrane and nucleus; P16 was in the
cytoplasm and nucleus; iii) in each sample, the intensity of
immunoreactivity (ICH) was scored as 0 (no color), 1 (light
yellow), 2 (yellow) and 3 (brown), and at least 500 cells from five
randomly selected staining regions were counted. The incidence of
ICH was scored as 0 (<5% of cells stained), 1 (between 5–25%), 2
(between 25–50%), 3 (between 50–75%) and 4 (≥75%), respectively.
Finally, the product of intensity and incidence was used as the
criteria for the expression of protein: 0 (negative, -), 1–3 (weak
positive, +), 4–7 (moderate positive, ++) and 8–12 (strong
positive, +++). For Ki-67, positive expression was considered as
the presence of brown-yellow granules in the nucleus. The
percentage of positive cells for Ki-67 was graded as previously
described (30): Negative (−),
<5%; weak positive (+), 5–25%; moderate positive (++), 26–50%;
and strong positive (+++), >50%.
Western blot analysis
Total protein was obtained from cervical tissue
using RIPA lysis buffer (BestBio Co., Ltd.) and quantified using a
bicinchoninic acid Protein Assay kit (MultiSciences Biotech Co.,
Ltd.). Equal amounts of proteins (4 µl per lane) were separated
using 10% SDS-PAGE (Beyotime Institute of Biotechnology) and
transferred to PVDF membrane using a TRANS-BLOT SD Semidry Transfer
Cell (Bio-Rad Laboratories, Inc.). The PVDF membrane was incubated
in 5% skim milk for 1 h at 37°C and then probed with primary rabbit
anti-human SCCA (1:1,000; cat. no. A6960; ABclonal Biotech Co.,
Ltd.) or rabbit anti-human MTA1 antibodies (1:1,000; cat. no.
A16085; ABclonal Biotech Co., Ltd.) antibodies at 4°C overnight.
After washing in 1X TBST buffer (Tween-20 0.05%, v/v) for 5 min on
a shaker three times, the membrane was incubated with IgG-HRP
secondary antibodies (1:2,000; cat. no. sc2030; Santa Cruz
Biotechnology Inc.) for 1 h at 37°C and washed in 1X TBST buffer
(Tween-20, 0.05% v/v; cat. no. T1085; Beijing Solarbio Science
& Technology Co., Ltd.) three times. Finally, the results were
visualized using the ECL Chemiluminescence Detection kit (BestBio
Co., Ltd.) and signals were observed using Image Lab 5.0 (Bio-Rad
Laboratories, Inc.). For quantification, SCCA and MTA1 signals were
normalized against GAPDH (1:1,000; cat. no. TA346930; ZSGB-BIO Co.,
Ltd.) and β-actin (1:1,000; cat. no. TA09; ZSGB-BIO Co., Ltd.)
signals to obtain the relative expression levels of SCCA and MTA1,
respectively.
HPV detection
HPV detection of cervical secretion was carried out
by Cobas 4800 HPV (Roche Diagnostics), which consisted of Cobas
4800 DNA extractor (Roche Diagnostics) and Cobas z 4800 PCR-cycler
(Roche Diagnostics). Relevant reagents, including sample
preparation kit, HPV detection kit (PCR fluorescence method), HPV
quality control kit, liquid-based cell preparation kit and PCR 96
pore plate, were purchased from Roche Diagnostics. Using a single
signal, the Cobas 4800 HPV test can not only detect HR-HPV-16 and
HPV-18 specifically, but also for 12 HR-HPV types, namely 31, 33,
35, 39, 45, 51, 52, 56, 59, 66 and 68 (31). The β-globulin gene was selected as an
internal control, and a Cobas 4800 real-time PCR system was applied
for the PCR. Data processing was as previously described (32). The experimental conditions followed
the guidelines of the manufacturer's instructions.
Statistical analysis
Statistical analyses were performed using SPSS 22.0
statistical software (IBM Corp.). The one-way ANOVA with Tukey's
test was used for continuous data. Kruskal-Wallis with Dunn's post
hoc test were used for comparing the results of
immunohistochemistry in two different cervical lesion tissues. The
relative expression quantities of SCCA and MTA1 are presented as
the mean ± SD. Dichotomous variables are presented as ratios, and
comparisons between groups were performed using the χ2
and Fisher's exact tests. P≤0.05 was considered to indicate a
statistically significant difference.
Results
Expression of SCCA during the
development of cervical cancer
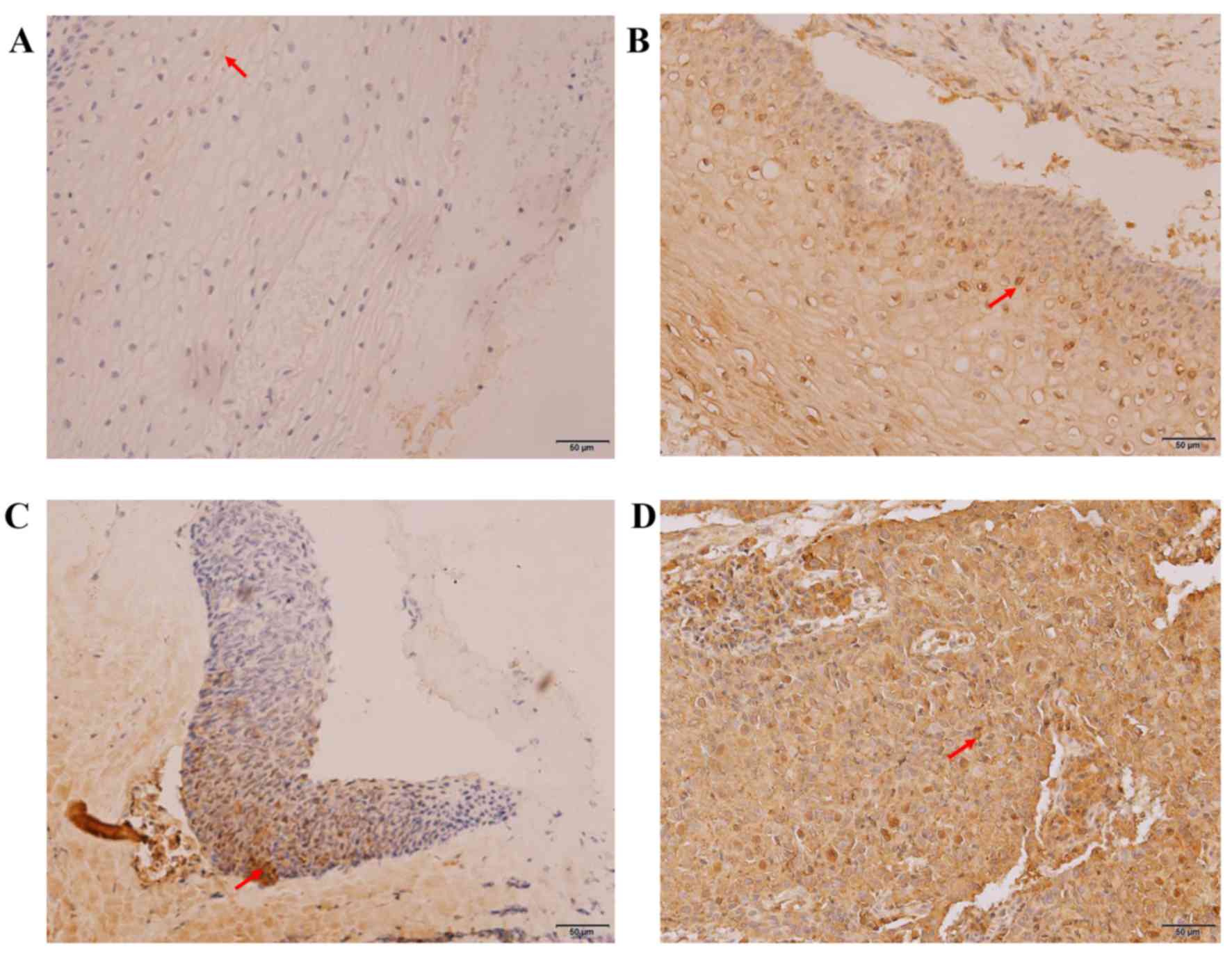
Immunohistochemistry was used to test the expression
levels of SCCA in various cervical lesion tissues. As presented in
Fig. 1, positive expression of SCCA
was characterized by brown and yellow granules in cytoplasm. The
count and rate of positive expression of SCCA in each sample group
is listed in Table I. The expression
levels and staining range of SCCA increased gradually with the
degree of severity of the cervical lesion and the positive rates
were 12.50, 45.16, 54.84 and 89.66% in the CCE, LSIL, HSIL and CSCC
groups, respectively. Fisher's exact test demonstrated that the
expression profile of SCCA was closely related to the development
of cervical carcinogenesis (P<0.05). In addition, the number of
SCCA-positive cells was lower in the CCE samples compared to the
LSIL samples (P<0.008), as well as being lower between the HSIL
samples compared to CSCC samples (P<0.008). However, the
difference in the number of positive SCCA cells between LSIL and
HSIL samples was not significant (P=0.197).
 | Table I.Immunohistochemical examination for
SCCA protein in cervical tissues from the CCE, LSIL, HSIL and CSCC
groups. |
Table I.
Immunohistochemical examination for
SCCA protein in cervical tissues from the CCE, LSIL, HSIL and CSCC
groups.
|
|
| No. of specimens
with IHC score |
|
|
|---|
|
|
|
|
|
|
|---|
| Groups | n | − | + | ++ | +++ | Positive rate,
% | P-value |
|---|
| CCE | 32 | 28 | 4 | 0 | 0 | 12.50 |
|
| LSIL | 31 | 17 | 9 | 4 | 1 | 45.16 |
<0.008a,b |
|
|
|
|
|
|
|
| 0.197b,c |
| HSIL | 31 | 14 | 5 | 11 | 1 | 54.84 |
<0.008a,c |
|
|
|
|
|
|
|
|
<0.008c,d |
| CSCC | 29 | 3 | 2 | 8 | 16 | 89.66 |
<0.008a,d |
|
|
|
|
|
|
|
|
<0.008b,d |
Western blotting was also used to examine the
relative protein expression levels of SCCA in various cervical
lesion tissues (Fig. 2A). The
relative expression level of SCCA was strongest in the CSCC samples
(1.26±0.07), followed by the HSIL (0.59±0.08), LSIL (0.49±0.04) and
CCE samples (0.19±0.05) (Fig. 2B).
With the degree of cervical lesions, the expression levels of SCCA
gradually increased, demonstrating a significant upward trend
(P<0.05). However, there was no significant difference in the
relative expression levels of SCCA between the LSIL and HSIL
samples (P=0.13). This was consistent with the immunohistochemistry
results.
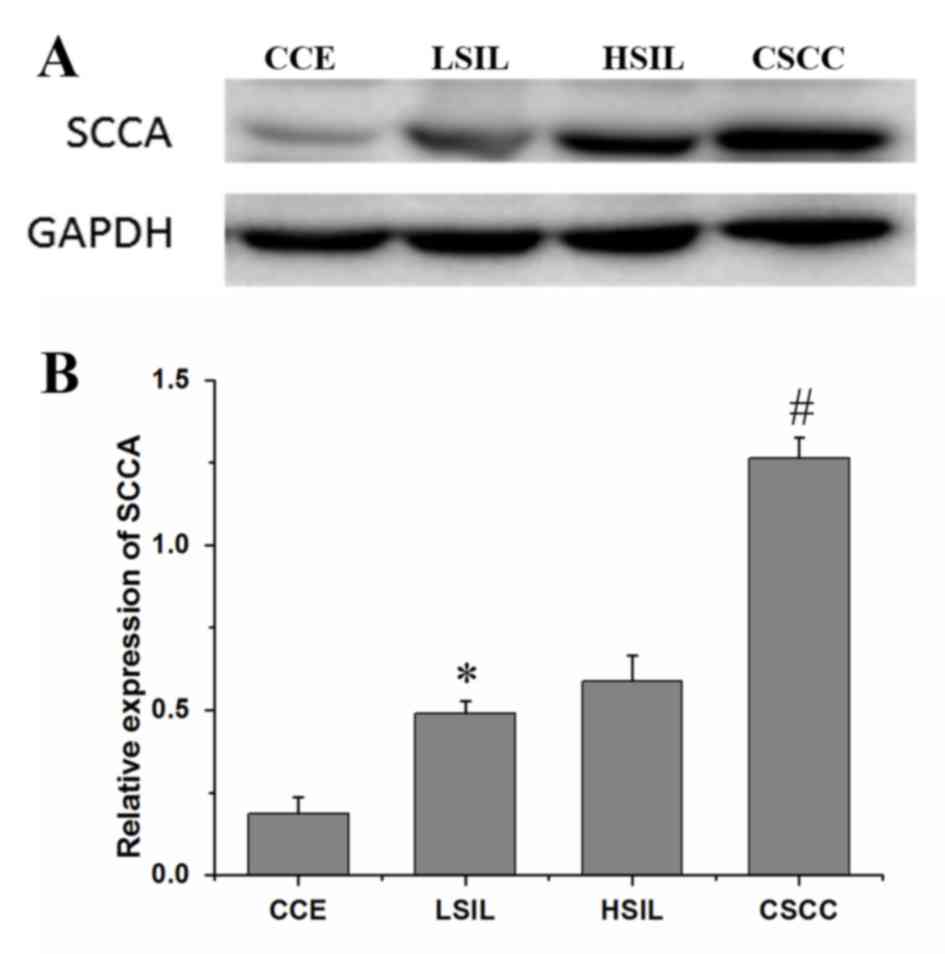 | Figure 2.(A) Western blot analysis of SCCA
expression levels in CCE, LSIL, HSIL and CSCC lysates. (B) The
relative expression levels of SCCA (ratio of optical densities of
SCCA and GAPDH) derived from western blot assays on the CCE, LSIL,
HSIL and the CSCC groups. *P<0.05 vs. CCE, #P<0.05
vs. HSIL. CCE, chronic cervicitis; CSCC, cervical squamous cell
carcinoma; HSIL, high-grade squamous intraepithelial lesion; LSIL,
low-grade squamous intraepithelial lesion; SCCA, squamous cell
carcinoma antigen. |
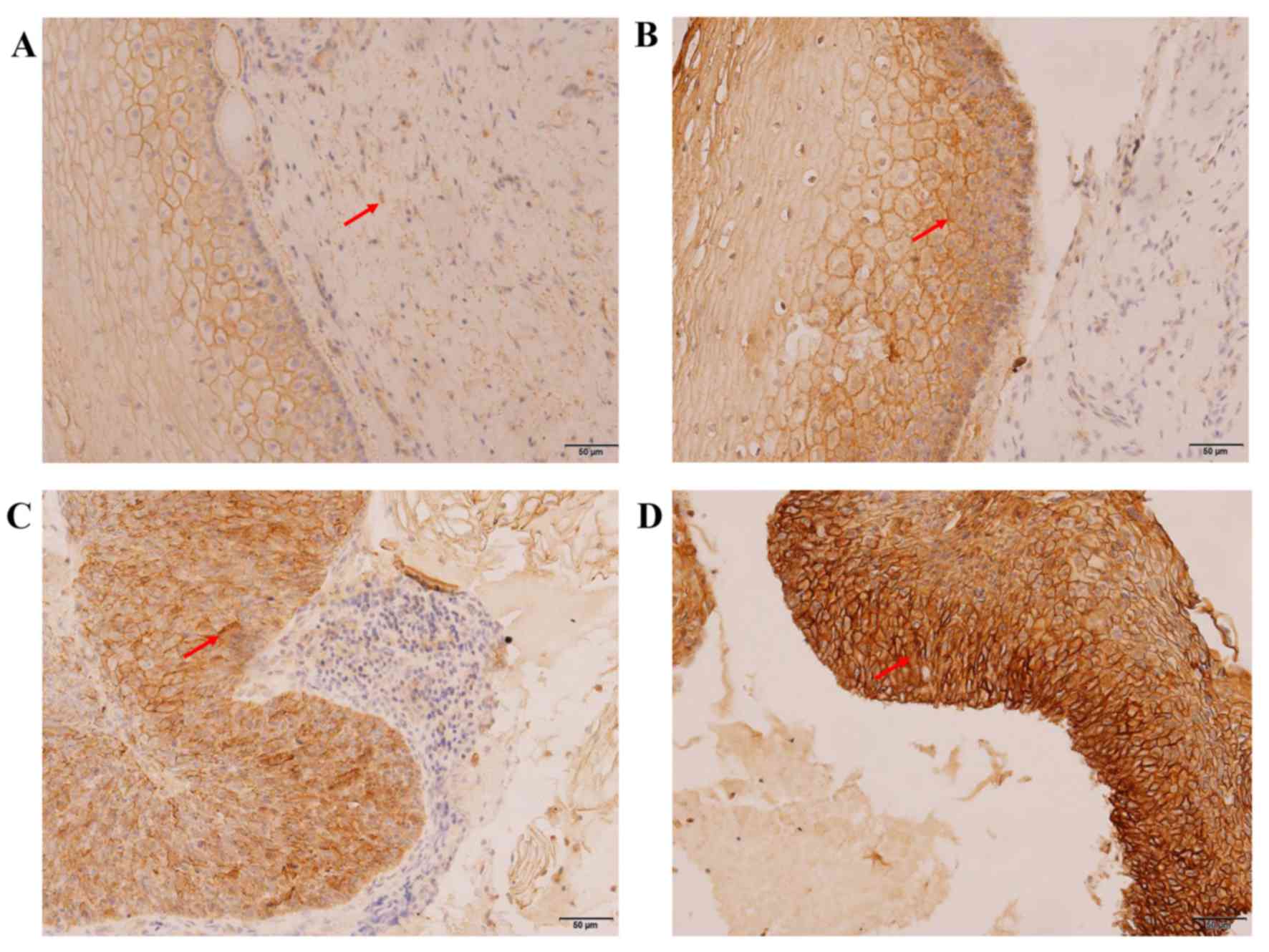
Expression of MTA1 in the development
of cervical carcinogenesis
The expression levels of MTA1 in the various
cervical lesion tissues were characterized using
immunohistochemistry (Fig. 3) and
western blotting (Fig. 4). As
presented in Fig. 3, positive
expression of MTA1 was observed as brown and yellow granules in the
cytoplasm, membrane and nucleus, and the expression level and
staining range of MTA1 increased gradually between the CCE, LSIL,
HSIL and CSCC groups. The positive rates were 9.38, 19.35, 58.06
and 82.76% in the CCE, LSIL, HSIL and CSCC groups, respectively
(Table II). Fisher's exact test
revealed that the expression of MTA1 was associated to the
development stage of cervical cancer (P<0.05) and that MTA1
expression presented a similar expression profile with that of
SCCA. The only difference in the expression profile was that there
was no significant difference in the number of positive MTA1 cells
between the CCE and LSIL groups (P=0.258).
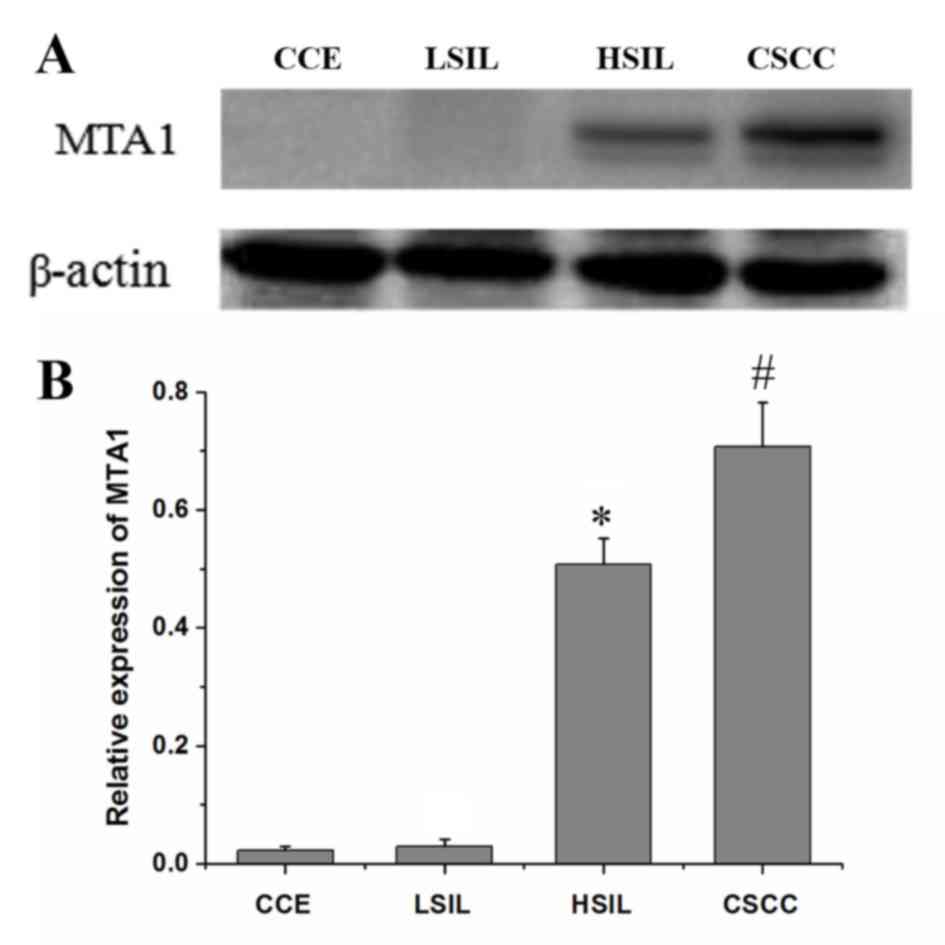 | Figure 4.(A) Western blot analysis of MTA1
expression in CCE, LSIL, HSIL and CSCC. (B) The relative expression
of MTA1 (ratio of optical density of SCCA to β-actin) derived from
Western blot analysis of the CCE, LSIL, HSIL and CSCC groups.
ANOVA, P≤0.05; Tukey's tests, *P<0.05 vs. LSIL,
#P<0.05 vs. HSIL. CCE, chronic cervicitis; CSCC,
cervical squamous cell carcinoma; HSIL, high-grade squamous
intraepithelial lesion; LSIL, low-grade squamous intraepithelial
lesion; MTA1, metastasis-associated gene 1; SCCA, squamous cell
carcinoma antigen. |
 | Table II.Immunohistochemical examination for
metastasis-associated gene 1 protein in cervical tissue from the
CCE, LSIL, HSIL and CSCC groups. |
Table II.
Immunohistochemical examination for
metastasis-associated gene 1 protein in cervical tissue from the
CCE, LSIL, HSIL and CSCC groups.
|
|
| Number of specimens
with different IHC scores |
|
|
|---|
|
|
|
|
|
|
|---|
| Groups | n | − | + | ++ | +++ | Positive rate
(%) | P-value |
|---|
| CCE | 32 | 29 | 2 | 1 | 0 | 9.38 |
|
| LSIL | 31 | 25 | 4 | 1 | 1 | 19.35 | 0.258a,b |
|
|
|
|
|
|
|
|
<0.008b,c |
| HSIL | 31 | 13 | 3 | 11 | 4 | 58.06 |
<0.008a,c |
|
|
|
|
|
|
|
|
<0.008c,d |
| CSCC | 29 | 5 | 2 | 6 | 16 | 82.76 |
<0.008a,d |
|
|
|
|
|
|
|
|
<0.008b,d |
According to the western blotting data, the relative
expression level of MTA1 was the lowest in the CCE group
(0.023±0.007), followed by the LSIL (0.030±0.011), HSIL
(0.508±0.044) and CSCC (0.707±0.076) groups (Fig. 4B). The expression levels of MTA1 in
the late stage of cervical carcinogenesis (HSIL and CSCC) were
higher compared with those in the early stage (CCE and LSIL). No
significant difference was observed for the relative expression
levels of MTA1 between the CCE and LSIL groups (P=0.42).
Experimental results of the western blotting and
immunohistochemical results for MTA1 were consistent.
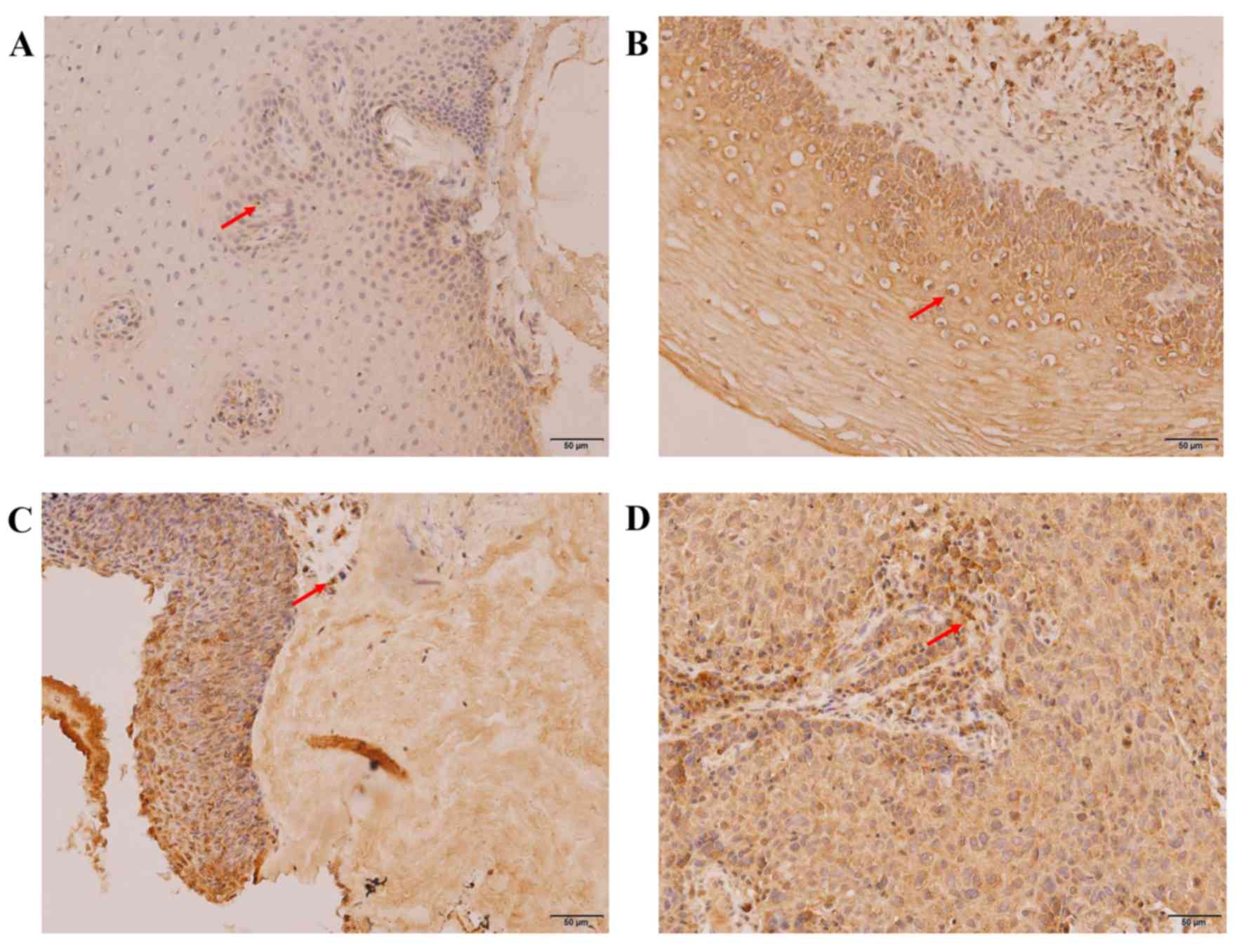
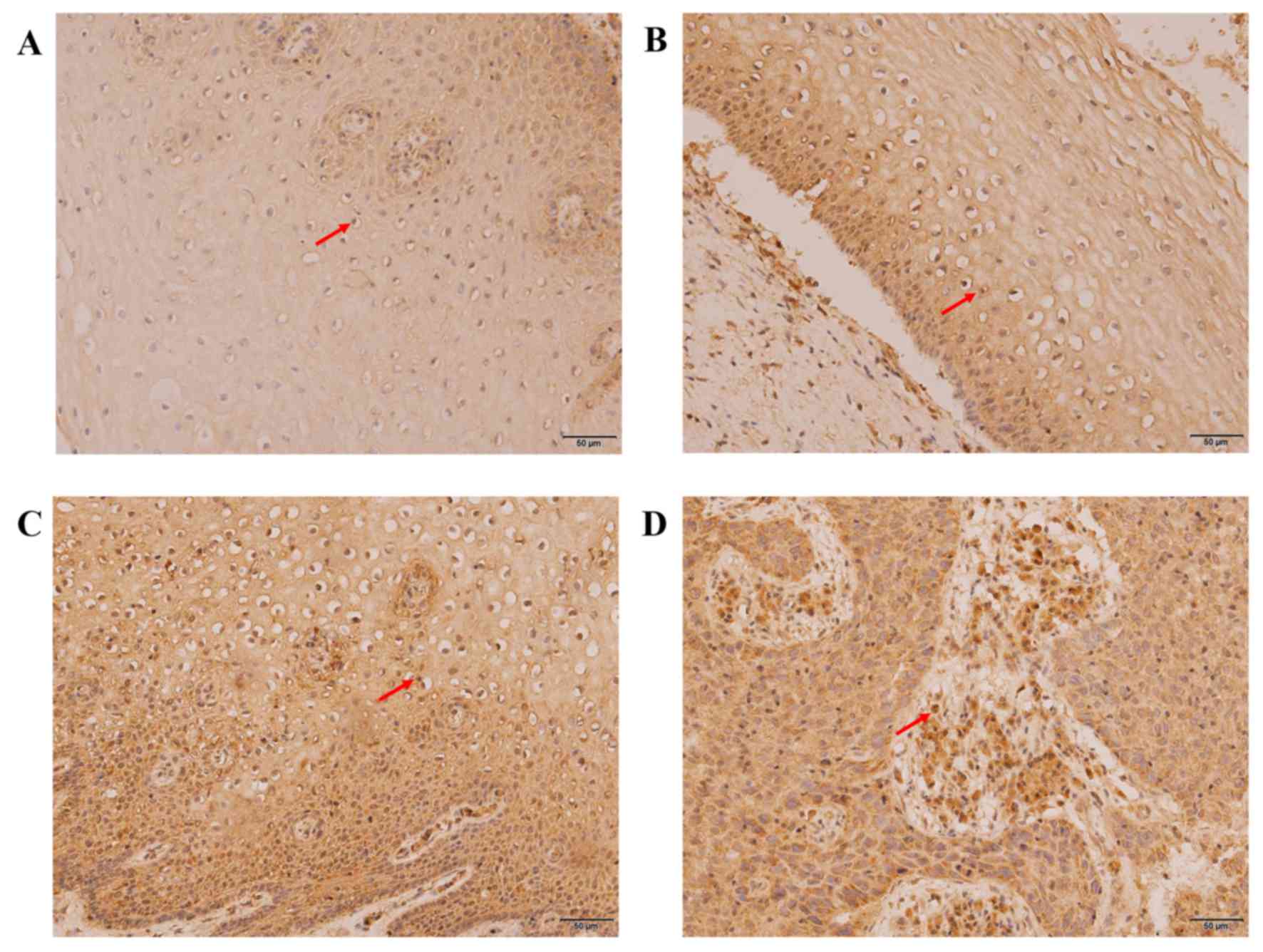
Expression of P16 and Ki-67 in the
development of cervical cancer
Using immunohistochemistry, positive expression of
Ki-67 (Fig. 5) and P16 (Fig. 6) were characterized by the
distribution of brown-yellow granules in the nucleus or the
cytoplasm/nucleus, respectively. Fisher's exact test revealed that
the staining range and expression levels of P16 and Ki-67 were
closely associated with the degree of cervical lesion, which
increased significantly with the development of cervical lesions
(P16, P<0.05; Ki-67, P<0.05). The positive rates were 15.63,
45.16, 87.10 and 100.00% for P16 (Table III) and 21.86, 83.87, 93.55 and
100.00% for Ki-67 (Table IV) in the
CCE, LSIL, HSIL and CSCC groups, respectively.
 | Table III.Immunohistochemical examination for
P16 protein in cervical tissues from the CCE, LSIL, HSIL and CSCC
groups. |
Table III.
Immunohistochemical examination for
P16 protein in cervical tissues from the CCE, LSIL, HSIL and CSCC
groups.
|
|
| Number of specimens
with different IHC score |
|
|
|---|
|
|
|
|
|
|
|---|
| Groups | n | − | + | ++ | +++ | Positive rate
(%) | P-value |
|---|
| CCE | 32 | 27 | 4 | 1 | 0 | 15.63 |
|
| LSIL | 31 | 17 | 9 | 3 | 2 | 45.16 | 0.009a,b |
|
|
|
|
|
|
|
|
<0.008b,c |
| HSIL | 31 | 4 | 5 | 7 | 15 | 87.10 |
<0.008a,c |
|
|
|
|
|
|
|
|
<0.008c,d |
| CSCC | 29 | 0 | 1 | 4 | 24 | 100.0 |
<0.008a,d |
|
|
|
|
|
|
|
|
<0.008b,d |
 | Table IV.Immunohistochemical examination for
Ki-67 protein in cervical tissues from the CCE, LSIL, HSIL and CSCC
groups. |
Table IV.
Immunohistochemical examination for
Ki-67 protein in cervical tissues from the CCE, LSIL, HSIL and CSCC
groups.
|
|
| Number of specimens
with different IHC score |
|
|
|---|
|
|
|
|
|
|
|---|
| Groups | n | − | + | ++ | +++ | Positive rate
(%) | P-value |
|---|
| CCE | 32 | 25 | 6 | 1 | 0 | 21.86 |
|
| LSIL | 31 | 5 | 19 | 6 | 1 | 83.87 |
<0.008a,b |
|
|
|
|
|
|
|
|
<0.008b,c |
| HSIL | 31 | 2 | 6 | 9 | 14 | 93.55 |
<0.008a,c |
|
|
|
|
|
|
|
|
<0.008c,d |
| CSCC | 29 | 0 | 0 | 2 | 27 | 100.0 |
<0.008a,d |
|
|
|
|
|
|
|
|
<0.008b,d |
Expression of the four analyzed
proteins and HR-HPV infection
As presented in Table
V, positive rates of HR-HPV in the CCE, LSIL, HSIL and CSCC
groups were 53.13, 70.97, 93.55 and 100.00%, respectively. With the
increases in the cervical lesion grade, the infection rate of
HR-HPV increased significantly (P<0.05). However, the positive
rates of HR-HPV positive samples between the CCE and LSIL groups
(P=0.145) and between the HSIL and CSCC groups (P=0.164) were not
statistically significant.
 | Table V.Infection rates of HR-HPV in cervical
tissues from the CCE, LSIL, HSIL and CSCC groups. |
Table V.
Infection rates of HR-HPV in cervical
tissues from the CCE, LSIL, HSIL and CSCC groups.
|
|
| HR-HPV (+) | HR-HPV (−) |
|
|---|
|
|
|
|
|
|
|---|
| Groups | n | n1 | Positive % | n2 | Negative % | P-value |
|---|
| CCE | 32 | 17 | 53.13 | 15 | 46.87 |
|
| LSIL | 31 | 22 | 70.97 | 9 | 29.03 | 0.145a,b |
|
|
|
|
|
|
|
<0.008b,c |
| HSIL | 31 | 29 | 93.55 | 2 | 6.45 | 0.020a,c |
|
|
|
|
|
|
| 0.164c,d |
| CSCC | 29 | 29 | 100.0 | 0 | 0.00 |
<0.008a,d |
|
|
|
|
|
|
|
<0.008b,d |
Fisher's exact test analysis was used to analyze the
significant between the expression levels of the four proteins and
the HR-HPV infection rate in cervical tissues. As demonstrated in
Table VI, the expression ratio of
SCCA between HR-HPV infection and non-infection groups was no
statistically significant (P=0.38), but the expression ratios of
MTA1, P16 and Ki-67 between HR-HPV infection and non-infection
groups were statistically significant (MTA1, P<0.05; P16,
P<0.05; Ki-67, P<0.05).
 | Table VI.The expression of the four analyzed
proteins and infection of HR-HPV in cervical tissues. |
Table VI.
The expression of the four analyzed
proteins and infection of HR-HPV in cervical tissues.
|
|
| Number of specimens
with IHC score |
|
|---|
|
|
|
|
|
|---|
| Variable | HR-HPV | − | + | ++ | +++ | P-value |
|---|
| SCCA | – | 15 | 6 | 3 | 2 | 0.38 |
|
| + | 47 | 14 | 20 | 16 |
|
| MTA1 | – | 19 | 4 | 2 | 1 | <0.05 |
|
| + | 53 | 7 | 17 | 20 |
|
| P16 | – | 15 | 7 | 3 | 1 | <0.05 |
|
| + | 33 | 12 | 12 | 40 |
|
| Ki-67 | – | 14 | 7 | 3 | 2 | <0.05 |
|
| + | 18 | 24 | 15 | 40 |
Discussion
Cervical cancer is one of the most common
gynecological tumor types that affects women (33). The pathology behind cervical cancer
is caused by a variety of factors, among which HPV infection,
especially HR-HPV, is currently recognized as a major pathogenic
factor (4). When cervical squamous
epithelial cells are infected with HPV, morphological changes
occur, followed by the appearance of pre-cancerous lesions; after
5–10 years, some of these lesions develop into cervical cancer
(34). The results of the present
study demonstrated that the expression levels of SCCA, MTA1, P16
and Ki-67 increased with the development of cervical lesions and
were associated with the HR-HPV infection status. Among the
patients included in the present study, the infection rate of
HR-HPV in CCE was low, but the infection rates of HR-HPV in
cervical pre-cancerous lesions (LSIL and HSIL) and cervical cancer
(CSCC) were high, which was similar to a previous report (35). Therefore, HPV infection is associated
with the occurrence of cervical cancer.
The expression levels of P16 and Ki-67 gradually
increase with the development of cervical intraepithelial lesions
and cervical cancer (23,25,26).
Consequently, P16/Ki-67 dual staining has been used for cervical
cancer screening (28,29). In this present study, the positive
rates of P6 and Ki-67 in the CCE, LSIL, HSIL and CSCC samples
exhibited a significant increasing trend: Their expression levels
in the LSIL, HSIL and CSCC samples were significantly higher
compared with those in CCE; the expression levels in the CSCC
samples were significantly higher compared with those in the HSIL
and LSIL samples; and the expression levels in the HSIL samples
were significantly higher compared with those in the LSIL samples.
The results were consistent with previous reports (23,25,26).
With the increase of P16 and Ki67 expression levels, the number of
HR-HPV copies increases (34). The
results of the present study revealed that the expression of P16
and Ki-67 were positively associated with the HR-HPV infection
status. Therefore, upregulation of P16 and Ki-67 indicated
infection with HR-HPV. This observation may provide a basis for the
early diagnosis of cervical lesions and was consistent with a
previous report (34,36).
As a specific antigen produced by squamous cell
carcinomas, SCCA has been studied extensively in cervical cancer
(8–13). Although it has been confirmed that
SCCA expression is highly associated with the occurrence and
development of CVCC (8,12,13),
there are limited reports investigating the expression of SCCA in
cervical pre-cancerous lesions. In addition, HPV, especially
high-risk HPV, is the main cause of cervical lesions (4). However, to the best of our knowledge,
there are no studies investigating the relationship between SCCA
and HPV infections. In this present study, the expression levels of
SCCA in the LSIL, HSIL and CSCC samples were significantly higher
compared with those in the CCE samples. The expression of SCCA in
the CSCC samples was significantly higher compared with that in the
HSIL and LSIL samples, and the expression of SCCA in the HSIL
samples was higher compared with that in the LSIL samples; however,
there was no significant difference between the HSIL and LSIL
samples. The expression levels of SCCA increased with the
development of cervical cancer, which indicated that SCCA may have
a clinical role in the diagnosis of cervical intraepithelial
neoplasia and cervical cancer. Combining SCCA and P16/Ki-67 dual
staining may be used to improve the accuracy of disease diagnosis,
especially for distinguishing HSIL and CSCC; therefore, it may help
to ensure that appropriate treatment is provided for patients with
cervical cancer. No association was observed between SCCA
expression levels and HR-HPV infection in various cervical lesions
(P=0.20) in the present study, which may be due to the small sample
size.
MTA1 is a gene associated with tumor metastasis and
has been extensively studied in the field of gynecological cancer
(14,15,17).
Abnormal expression of MTA1 is not only related to the occurrence
and metastasis of ovarian cancer, but is also associated with the
clinical stage of ovarian cancer (15). The expression levels of MTA1 in
cervical cancer have been demonstrated to be higher compared with
those in the normal cervical tissue, and upregulation of MTA1 is
positively correlated with the clinical stage and lymph node
metastasis in patients with cervical cancer (17). However, the expression of MTA1 in
cervical lesions has been not reported. In this present study, the
expression intensity of MTA1 was associated with the degree of the
cervical lesions. The expression of MTA1 in the CSCC samples was
significantly higher compared with that in the LSIL, HSIL and CCE
samples; the expression of MTA1 in the HSIL samples also appeared
higher compared with that in the CCE samples, but no significant
difference was observed. In addition, the expression of MTA1 in
cervical tissue was associated with the HR-HPV infection status.
Previous studies have demonstrated that MTA1 can interact with P53
(19–21). It is assumed that the HPV E6 protein
may interact with P53 after infection with HR-HPV and that the
decrease of P53 may provide a feedback stimulation leading to
raised protein expression levels of MTA1 (20,21). In
addition, the expression of MTA1 in various cervical lesions was
consistent with that of P16 and Ki-67. Upregulation of MTA1 was
associated with infection with HR-HPV; therefore, MTA1 may be a
useful biomarker for the early diagnosis of cervical lesions.
The main limitation to this study was that the
relationship between the development of cervical lesions and the
blood concentration of the four proteins was not determined.
However, SCCA, MTA1, P16 and Ki-67 may indicate the occurrence and
development of cervical lesions. The mechanism of action behind
their specific roles needs to be further studied, which will lay a
theoretical foundation for the early diagnosis and effective
treatment of cervical lesions in the clinic.
In conclusion, SCCA, MTA1, P16 and Ki-67 were
upregulated in cervical lesions and their positive rates increased
with the development of cervical lesions. Furthermore, the
expression levels of MTA1, P16 and Ki-67 were associated with the
infection rate of HR-HPV. SCCA and MTA1 may be used for cervical
cancer screening in a similar manner to P16/Ki-67 double staining.
In particular, SCCA may be used to distinguish between patients
with HSIL and CSCC, and MTA1 can be used to distinguish between
those with LSIL and HSIL.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CH and YC conceived and designed the study. CH, FZ
and CW performed the experiments. CH and YH analyzed the data. CH
and YC wrote the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethnic
committee of the North China University of Science and Technology
Affiliated Hospital. Informed consent was obtained from all
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Arbyn M, Weiderpass E, Bruni L, de Sanjosé
S, Saraiya M, Ferlay J and Bray F: Estimates of incidence and
mortality of cervical cancer in 2018: A worldwide analysis. Lancet
Glob Health. 8:e191–e203. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Plummer M, Peto J and Franceschi S;
International Collaboration of Epidemiological Studies of Cervical
Cancer, : Time since first sexual intercourse and the risk of
cervical cancer. Int J Cancer. 130:2638–2644. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shrestha AD, Neupane D, Vedsted P and
Kallestrup P: Cervical cancer prevalence, incidence and mortality
in low and middle income countries: A systematic review. Asian Pac
J Cancer Prev. 19:319–324. 2018.PubMed/NCBI
|
|
4
|
Arbyn M, Snijders PJ, Meijer CJ, Berkhof
J, Cuschieri K, Kocjan BJ and Poljak M: Which high-risk HPV assays
fulfil criteria for use in primary cervical cancer screening? Clin
Microbiol Infect. 21:817–826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wright TC, Stoler MH, Behrens CM, Sharma
A, Zhang G and Wright TL: Primary cervical cancer screening with
human papillomavirus: End of study results from the ATHENA study
using HPV as the first-line screening test. Gynecol Oncol.
136:189–197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dasari S, Wudayagiri R and Valluru L:
Cervical cancer: Biomarkers for diagnosis and treatment. Clin Chim
Acta. 445:7–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pozzan C, Cardin R, Piciocchi M, Cazzagon
N, Maddalo G, Vanin V, Giacomin A, Pontisso P, Cillo U and Farinati
F: Diagnostic and prognostic role of SCCA-IgM serum levels in
hepatocellular carcinoma (HCC). J Gastroenterol Hepatol.
29:1637–1644. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maddalo G, Fassan M, Cardin R, Piciocchi
M, Marafatto F, Rugge M, Zaninotto G, Pozzan C, Castoro C, Ruol A,
et al: Squamous cellular carcinoma antigen serum determination as a
biomarker of barrett esophagus and esophageal cancer. J Clin
Gastroenterol. 52:401–406. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chechlinska M, Kowalewska M,
Brzoska-Wojtowicz E, Radziszewski J, Ptaszynski K, Rys J, Kaminska
J and Nowak R: Squamous cell carcinoma antigen 1 and 2 expression
in cultured normal peripheral blood mononuclear cells and in vulvar
squamous cell carcinoma. Tumor Biol. 31:559–567. 2010. View Article : Google Scholar
|
|
10
|
El-Rachkidy RG, Young HS, Griffiths CE and
Camp RD: Humoral autoimmune responses to the squamous cell
carcinoma antigen protein family in psoriasis. J Invest Dermatol.
128:2219–2224. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jeong BK, Choi DH, Huh SJ, Park W, Bae DS
and Kim BG: The role of squamous cell carcinoma antigen as a
prognostic and predictive factor in carcinoma of uterine cervix.
Radiat Oncol J. 29:191–198. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shimura K. Mabuchi S, Yokoi T, Sasano T,
Sawada K, Hamasaki T and Kimura T: Utility of serum squamous cell
carcinoma antigen levels at the time of recurrent cervical cancer
diagnosis in determining the optimal treatment choice. J Gynecol
Oncol. 24:321–329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim BG: Squamous cell carcinoma antigen in
cervical cancer and beyond. J Gynecol Oncol. 24:291–292. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sen N, Gui B and Kumar R: Role of MTA1 in
cancer progression and metastasis. Cancer Metastasis Rev.
33:879–889. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yi S, Guangqi H and Guoli H: The
association of the expression of MTA1, NM23H1 with the invasion,
metastasis of ovarian carcinoma. Chin Med Sci J. 18:87–92.
2003.PubMed/NCBI
|
|
16
|
Guddeti RK, Bali P, Karyala P and Pakala
SB: MTA1 coregulator regulates LDHA expression and function in
breast cancer. Biochem Biophys Res Commun. 520:54–59. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bilikezi·Aikemu F, Min L, Xiumei Z,
Junling G, Danjin G and Qi: Expression and significance of MTA1,
MMP-2, MMP-7 in cervical cancer. Chin J Mod Med. 23:51–54.
2013.
|
|
18
|
Dannenmann C, Shabani N, Friese K, Jeschke
U, Mylonas I and Brüning A: The metastasis-associated gene MTA1 is
upregulated in advanced ovarian cancer, represses ERβ, and enhances
expression of oncogenic cytokine GRO. Cancer Biol Ther.
7:1460–1467. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rao Y, Wang H, Fan L and Chen G: Silencing
MTA1 by RNAi reverses adhesion, migration and invasiveness of
cervical cancer cells (SiHa) via altered expression of p53, and
E-cadherin/β-catenin complex. J Huazhong Univ Sci Technolog Med
Sci. 31:1–9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Scheffner M, Werness BA, Huibregtse JM,
Levine AJ and Howley PM: The E6 oncoprotein encoded by human
papillomavirus types 16 and 18 promotes the degradation of p53.
Cell. 63:1129–1136. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moody CA and Laimins LA: Human
papillomavirus oncoproteins: Pathways to transformation. Nat Rev
Cancer. 10:550–560. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pan FP, Zhou HK, Bu HQ, Chen ZQ, Zhang H,
Xu LP, Tang J, Yu QY, Chu YQ, Pan J, et al: Emodin enhances the
demethylation by 5-Aza-CdR of pancreatic cancer cell
tumor-suppressor genes P16, RASSF1A and ppENK. Oncol Rep.
35:1941–1949. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bleotu C, Botezatu A, Goia CD, Socolov D,
Corniţescu F, Teleman S, Huică I, Iancu I and Anton G: P16INK4A-A
possible marker in HPV persistence screening. Roum Arch Microbiol
Immunol. 68:183–189. 2009.PubMed/NCBI
|
|
24
|
Sun X and Kaufman PD: Ki-67: More than a
proliferation marker. Chromosoma. 127:175–186. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kimura M, Matsumoto T, Morizane T, Sonoue
H, Ogishima D and Kinoshita K: Histopathological study of the
spreading neoplastic cells in cervical glands and surface epithelia
in cervical intra-epithelial neoplasia and microinvasive squamous
cell carcinoma: Ki-67 immunostaining is a useful marker for
pathological diagnosis from the gland involvement site. Pathol Int.
56:428–433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carreras R, Alameda F, Mancebo G,
García-Moreno P, Mariñoso ML, Costa C, Fusté P, Baró T and Serrano
S: A study of Ki-67, c-erbB2 and cyclin D-1 expression in CIN-I,
CIN-III and squamous cell carcinoma of the cervix. Histol
Histopathol. 22:587–592. 2007.PubMed/NCBI
|
|
27
|
Yang D, Xu Q and Mao Q: Expression of P16,
Ki67 and HPV16/18 in cervical lesions and their clinical
implications. Int J Pathol Clin Med. 32:211–215. 2012.
|
|
28
|
Clarke MA, Cheung LC, Castle PE, Schiffman
M, Tokugawa D, Poitras N, Lorey T, Kinney W and Wentzensen N:
Five-year risk of cervical precancer following p16/ki-67 dual-stain
triage of HPV-positive women. JAMA Oncol. 5:181–186. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wentzensen N, Clarke MA, Bremer R, Poitras
N, Tokugawa D, Goldhoff PE, Castle PE, Schiffman M, Kingery JD,
Grewal KK, et al: Clinical evaluation of human papillomavirus
screening with p16/ki-67 dual stain triage in a large organized
cervical cancer screening program. JAMA Intern Med. 179:881–888.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhong P, Li J, Gu Y, Liu Y, Wang A, Sun Y
and Lu L: P16 and Ki-67 expression improves the diagnostic accuracy
of cervical lesions but not predict persistent high risk human
papillomavirus infection with CIN1. Int J Clin Exp Pathol.
8:2979–2986. 2015.PubMed/NCBI
|
|
31
|
Pesic A, Krings A, Hempel M, Preyer R,
Chatzistamatiou K, Agorastos T and Kaufmann AM: CIN2+ detection of
the HPV DNA array genotyping assay in comparison with the cobas
4800 HPV test and cytology. Virol J. 16:922019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang Z, Xu Y, Song N, Zou D, Liao Y, Li Q
and Pan C: A comparison of the MeltPro® HPV test with
the Cobas® HPV test for detecting and genotyping 14
high-risk human papillomavirus types. Arch Virol. 163:725–730.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brusselaers N, Shrestha S, van de Wijgert
J and Verstraelen H: Vaginal dysbiosis and the risk of human
papillomavirus and cervical cancer: Systematic review and
meta-analysis. Am J Obstet Gynecol. 221:9–18. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Melnikow J, Henderson JT, Burda BU, Senger
CA, Durbin SS and Weyrich MS: Screening for cervical cancer with
high-risk human papillomavirus testing. JAMA. 320:687–705. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kjær SK, Munk C, Junge J and Iftner T:
Carcinogenic HPV prevalence and age-specific type distribution in
40,382 women with normal cervical cytology, ASCUS/LSIL, HSIL, or
cervical cancer: What is the potential for prevention? Cancer
Causes Control. 25:179–189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Song SH, Park HM, Eom DW, Lee JK, Lee NW,
Kim AR, Hur JY, Lee KW, Park YK and Saw HS: The expression of p16
(INK4a) and Ki-67 in relation to high-risk human papilloma viral
load and residual disease after conization with positive margins.
Int J Gynecol Cancer. 17:858–867. 2007. View Article : Google Scholar : PubMed/NCBI
|