Introduction
Osteosarcoma is a malignant bone cancer with high
invasiveness and poor prognosis that commonly occurs in children
and adolescents (1,2). Osteosarcoma develops from mesenchymal
cell lines and is most likely to develop at the ends of long bones,
such as proximal tibia and distal femur, because of their abundant
blood supply (3). Currently, the
most popular treatment for osteosarcoma is neoadjuvant chemotherapy
combined with surgical resection; however, the 5-year survival rate
with modern protocols has only seen a mild improvement over the
past 40 years (from 65 to 70%) (4,5). The
main reason for the poor prognosis is the development of
chemoresistance, side effects from chemotherapy and early
metastases (6). Therefore, there is
an urgent need to identify new, more effective antineoplastic drugs
to inhibit the growth and metastasis of osteosarcoma.
Enoxacin is a broad-spectrum, third-generation
fluoroquinolone antibiotic with strong bactericidal activity
(7). It inhibits the growth of
numerous cancer cells types (8–11),
including gastric (SNU-638 and SNU-1), colorectal (RKO and
HCT-116), liver (HepG2), lung (H23, H1299, and A549), lymphoma
(RAJI), and leukaemia (KG1a), and is relatively selective for
cancer cells, resulting in low toxicity to noncancerous cells
(11,12). However, its efficacy and the
mechanisms of action in osteosarcoma have not been reported.
This study examined the antitumour effects of
enoxacin on osteosarcoma 143B cells in vitro and in a murine
xenograft model, and explored the underlying molecular
mechanisms.
Materials and methods
Cell culture and treatment
The human osteosarcoma cell line 143B and human
osteoblast hFOB1.19 cell line were purchased from the American Type
Culture Collection. The 143B cells were cultured in DMEM (Hyclone
GE healthcare) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and 100 U/ml streptomycin and penicillin. The
hFOB1.19 cells were maintained in DMEM/F-12 (Hyclone; GE
healthcare) containing 15% FBS. All cells were cultured in a
humidified atmosphere containing 5% CO2 at 37°C. All
cells used in the present study were subjected to >20 passages
and were in exponential cell growth.
Cell proliferation assay
Cells were seeded in 96-well plates
(3×103 cells/well) and incubated overnight. The
following day, enoxacin (Sigma Aldrich; Merck KGaA) diluted in DMEM
supplemented with 10% FBS was added to the wells at 0, 3.125, 6.25,
12.5, 25, 50 or 100 mg/l. Viability of 143B cells was measured at
24, 36 and 48 h, and that of hFOB1.19 cells was assessed at 24 h,
using the Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc.), according to the manufacturer's protocol. The
absorbance was measured at 450 nm using an ELX800 absorbance
microboard reader (Bio-Tek Corporation).
Tumour-cell clonogenic assay
Osteosarcoma 143B cells were seeded in a 6-well
culture plate (103 cells/well). Enoxacin diluted in DMEM
supplemented with 10% FBS was added to the wells at 0, 1.25, 2.5,
5, 10 or 20 mg/l and the plates were incubated for 7 days.
Following incubation, the culture medium was removed, the cells
were fixed in 4% paraformaldehyde for 15 min at 4°C, then stained
with 0.1% crystal violet solution (Beijing Solarbio Science &
Technology Co., Ltd.) for 20 min at room temperature. The number of
colonies (clusters of >50 cells) were counted under a light
microscope (magnification, ×10; Olympus Corporation).
Transwell assays
For the cell invasion assay, 143B cells were
suspended in serum-free medium with enoxacin, then 200 µl of cell
suspension (1×104 cells) was added on top of
Matrigel-coated Transwell chambers (8-µm pore size; Corning, Inc.).
The chambers were incubated in 600 µl of 10% serum medium for 18 h.
Following incubation, the culture medium in the lower chamber was
removed and the cells were washed twice with PBS, then fixed with
4% paraformaldehyde for 15 min at 4°C, and stained with 0.05%
crystal violet solution for 20 min at room temperature. A cotton
swab was used to remove the cells that had not passed through the
membrane, while the transmembrane cells were imaged (magnification,
×10) and counted in 5 microscopic fields using a light microscope
(Olympus Corporation).
The cell migration assay was performed under similar
experimental conditions as the invasion assay but using
non-Matrigel coated cell culture inserts.
Annexin V/propidium iodide (PI)
apoptosis assay
Osteosarcoma 143B cells were seeded in 6-well plates
(105 cells/well). The next day, enoxacin diluted in DMEM
containing 10% FBS was added to the wells at 0, 5, 10 or 20 mg/l
and the plates were incubated for 24 h. Following incubation, the
supernatants and adherent cells were collected and Annexin V and PI
staining was performed using the YF® 488 Annexin V and
PI Apoptosis kit (US Everbright® Inc.), according to the
manufacturer's protocol. Briefly, the cells were resuspended in
Annexin V binding buffer (100 µl) before Annexin V (4–5 µl) and PI
(1–2 µl) working solutions were added to each tube. The tubes were
set on ice and incubated in the dark for 15 min. Apoptotic cells
were quantified using a flow cytometer (BD FACSCanto II; Becton,
Dickinson and Company) and the apoptosis rates were calculated
using FlowJo 7.6 software (Tree Star, Inc.).
Western blot analysis
Osteosarcoma 143B cells were seeded in 6-well plates
(3×105 cells/well) and treated with enoxacin (0, 10 or
20 mg/l) for 24 h, then washed twice with PBS and lysed with RIPA
lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 5 mM EDTA, 1% Triton
X-100, 1 mM sodium fluoride, 1 mM sodium vanadate, 1% deoxycholate,
and protease inhibitor cocktail; Beyotime Institute of
Biotechnology). The cells were collected into centrifuge tubes
using a cell scraper and lysed on ice for 30 min, with frequent
shaking to ensure full lysis. The cells were centrifuged at 12,000
× g at 4°C for 15 min before the supernatant was collected and the
total protein concentration was determined using the bicinchoninic
acid method (Beyotime Institute of Biotechnology).
Following the instructions for the use of the
electrophoresis instrument (Bio-Rad Laboratories, Inc.), a total of
20 µl protein (1.5 µg/µl) from each sample were separated by 10%
SDS-PAGE and then transferred to PVDF membranes (EMD Millipore).
The membranes were blocked with 5% skimmed milk (Becton, Dickinson
and Company) for 1 h at room temperature before they were probed
with primary antibodies [rabbit anti-human matrix metalloproteinase
(MMP)2 monoclonal antibody; Cell signalling Technology, Inc. (CST);
cat. no. 87809S; 1:1,000; rabbit anti-human MMP9 monoclonal
antibody; CST; cat. no. 13667S; 1:1,000; rabbit anti-human Bcl-xL
monoclonal antibody; CST; cat. no. 2764S; 1:1,000; rabbit
anti-human Bcl-2 monoclonal antibody; CST; cat. no. 4223S, 1:1,000;
rabbit anti-human GAPDH; CST; cat. no. 5174S; 1;1,000] at 4°C
overnight. The following day, the membranes were washed three times
with TBS-T (0.05% Tween-20; Boster Biological Technology) and then
the second antibody (goat anti-rabbit IgG; HRP-linked antibody;
CST; 7074S; 1:2,000) was incubated for 2 h at room temperature.
Immunoreactive protein bands were detected by enhanced
chemiluminescence (ECL kit, TransGen Biotech Co., Ltd.) according
to the manufacturer's protocol.
Tumour xenograft model
A total of 20 4-week-old female BALB/c-nu/nu nude
mice (~19.0 g), were obtained from Shrek Jingda Experimental Animal
Centre (Hunan, China) and raised in a laminar mouse house, with 50
± 5% humidity, 24 ± 2°C and a 12-h light/dark cycle. The mice were
fed standard rodent food and mineral water. Osteosarcoma 143B cells
were diluted with PBS (up to a density of 1×107
cells/ml) and 100 µl of cell suspension was injected into the
tibial plateau of anesthetized nude mice. Subsequently, the mice
were randomly divided into 4 groups: Blank control, saline (NaCl),
low enoxacin concentration (enoxacin diluted with NaCl, 4 mg/kg/d),
and high enoxacin concentration (enoxacin diluted with NaCl, 8
mg/kg/d), with 5 mice in each group. One week later, the mice were
injected intraperitoneally with NaCl or enoxacin every day for 3
weeks. Tumour volume (measured with the Vernier calliper, V=length
× width2 ×0.5) and body weight (measured electronically)
of the mice were recorded every week. At the end of the study
period, the mice were euthanised and the tumours were dissected to
measure their volume and weight. Cardiac blood (200 µl) was
collected to evaluate liver and kidney function.
Statistical analyses
The data were analysed by GraphPad Prism 5.0
software. Statistical significance between groups was analysed
using student's t-tests or ANOVA followed by the Tukey test. The
results are presented as means ± SD. All the experiments were
performed at least three times. P<0.05 was considered to
indicate statistically significant differences between groups.
Results
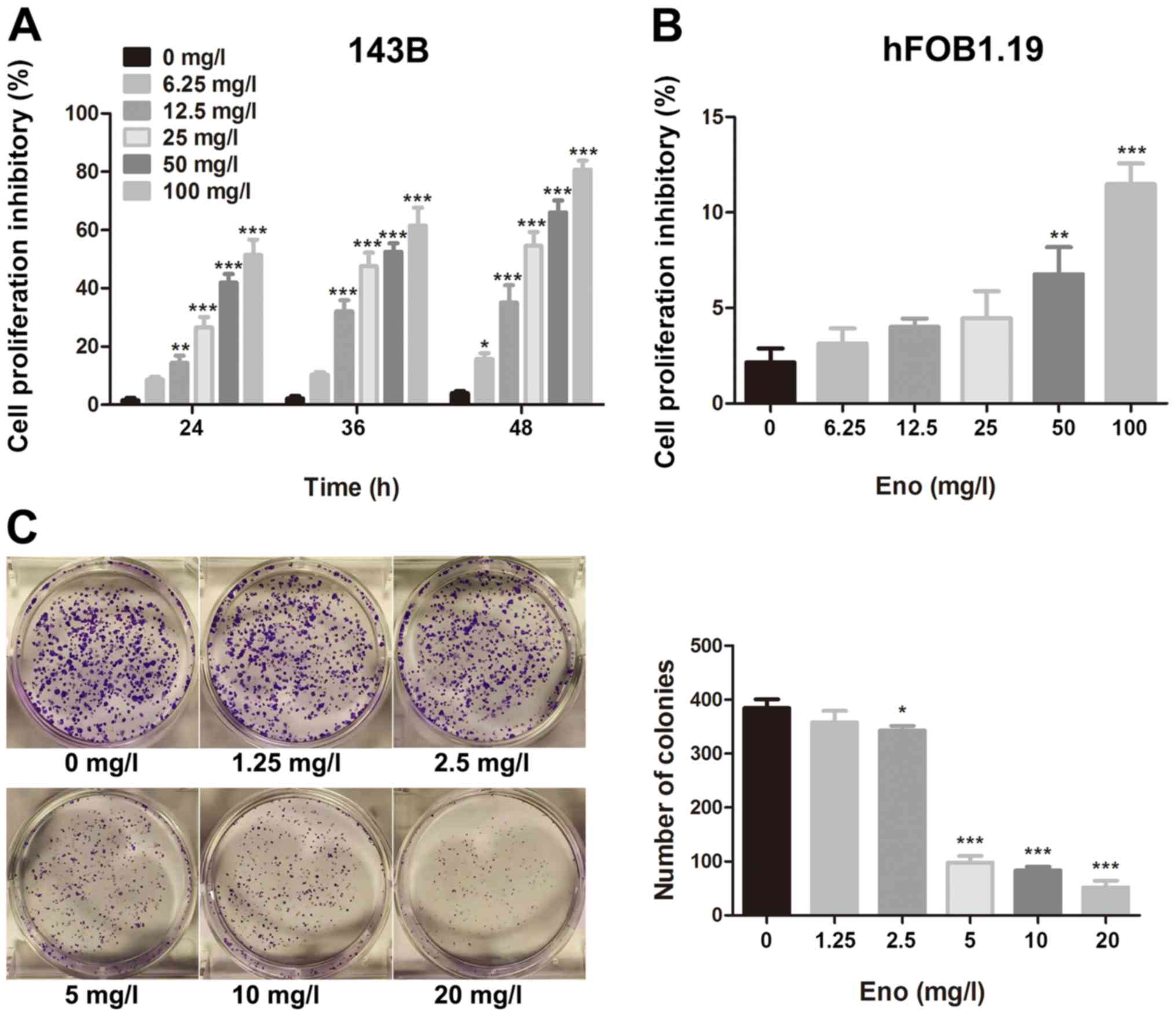
Enoxacin inhibits the proliferation of
143B cells but not that of hFOB1.19 cells
A CCK-8 assay was used to assess the
antiproliferative effect of enoxacin on human osteosarcoma and
osteoblast cell lines. Enoxacin inhibited the proliferation of 143B
cells in a time- and concentration-dependent manner (Fig. 1A), without a significant effect on
the proliferation of hFOB1.19 cells at up to 25 mg/l (Fig. 1B). The present results indicate that
enoxacin is effective and selective towards human osteosarcoma
cells and exhibits no obvious toxicity towards normal osteoblasts
at up to 25 mg/l (within the concentration range used in this
study).
Enoxacin impairs the colony-forming
ability of 143B cells
The effect of enoxacin on the colony-forming ability
of single osteosarcoma 143B cells was assessed with a clonogenic
assay. In the control group (0 mg/l) and low enoxacin concentration
group (1.25 mg/l), a large number of colonies formed within one
week of culture (colony formation rate ≥80.34%). Increasing
enoxacin concentration was associated with decreasing size and
number of colonies, indicating that the drug inhibits 143B cell
proliferation and colony formation in a dose-dependent manner
(Fig. 1C).
Enoxacin inhibits the migration and
invasion of 143B cells
The effect of enoxacin on the migration and invasion
of osteosarcoma 143B cells was evaluated using Transwell assays.
The drug significantly impaired the migratory and invasive
abilities of 143B cells in a dose-dependent manner (Fig. 2), suggesting that it could inhibit
the metastasis of osteosarcoma.
Enoxacin stimulates apoptosis of 143B
cells
Flow cytometric analysis with Annexin V/PI staining
was performed to investigate whether enoxacin inhibited the growth
of osteosarcoma 143B cells by stimulating apoptosis.
Enoxacin-treated cells exhibited a significantly increased rate of
apoptosis compared with that of the control cells (Fig. 3).
Enoxacin downregulates the expression
of MMP2, MMP9, Bcl-xL and Bcl-2 in 143B cells
To explore the molecular mechanisms underlying the
antitumour effects of enoxacin, the expression of MMPs (MMP2 and
MMP9) and Bcl-2 family proteins (Bcl-xL, Bcl-2) in enoxacin-treated
osteosarcoma 143B cells were assessed using western blot analysis.
Expression levels of MMP2, MMP9, Bcl-xL and Bcl-2 were
significantly decreased in enoxacin-treated cells compared with
that in control cells (Fig. 4).
These observations suggest that the pro-apoptotic and anti-invasion
effects of enoxacin are mediated by downregulation of Bcl-2 family
proteins and MMP expression.
Enoxacin impairs the growth of
osteosarcoma in vivo
A xenograft tumour model with 143B cells was
established in nude mice to assess the effect of enoxacin on tumour
growth. The tumour volume and weight were significantly decreased
in enoxacin-treated mice compared with in untreated mice. In
contrast, there were no significant differences in tumour volume or
weight between the NaCl group and the control group (Fig. 5A-C). Body weight of the mice in the
treatment groups did not differ significantly from that of control
mice during the study period (Fig.
5D). These data show that enoxacin hinders the development of
osteosarcoma and has no obvious drug toxicity in nude mice.
Side effects of enoxacin in vivo
Circulating levels of liver enzymes (alanine
transaminase and aspartate aminotransferase) and renal function
markers [blood urea nitrogen (BUN) and creatinine (Cr)] were
measured in nude mice heart blood to evaluate hepatotoxicity and
nephrotoxicity following enoxacin treatment. The functions of the
liver and kidney were normal in all groups (Table I). Erythrocyte, leukocyte and
platelet counts of the mice were within normal limits, indicating
that enoxacin has no obvious haematological side effects within the
dosage range tested (Table II).
 | Table I.Effect of enoxacin on liver and
kidney function in nude mice. |
Table I.
Effect of enoxacin on liver and
kidney function in nude mice.
| Enoxacin | n | ALT (u/l) | AST (u/l) | BUN (mmol/l) | Cr (mmol/l) |
|---|
| Control | 5 | 32.05±3.03 | 98.96±13.10 | 5.79±1.00 | 21.23±2.30 |
| NaCl | 5 | 29.24±3.37 | 106.04±17.55 | 6.51±0.90 | 21.63±3.00 |
| 4 mg/kg/day | 5 | 29.65±3.55 | 98.93±12.72 | 5.56±0.59 | 22.82±3.06 |
| 8 mg/kg/day | 5 | 31.43±3.00 | 100.04±21.50 | 5.97±1.96 | 22.62±3.82 |
 | Table II.Effect of enoxacin on blood cell
count of nude mice. |
Table II.
Effect of enoxacin on blood cell
count of nude mice.
| Enoxacin | n | Erythrocyte
(×1012/l) | Leukocyte
(×109/l) | Platelet
(×109/l) |
|---|
| Control | 5 | 8.18±0.44 | 4.98±1.03 | 803.18±85.72 |
| Nacl | 5 | 8.12±0.83 | 4.32±0.82 | 743.52±108.70 |
| 4 mg/kg/day | 5 | 7.96±0.69 | 4.56±0.64 | 789.76±70.71 |
| 8 mg/kg/day | 5 | 8.46±0.54 | 4.66±0.70 | 793.46±94.11 |
Discussion
Osteosarcoma is a bone cancer that originates from
mesenchymal tissue and exhibits high degree of invasiveness,
malignancy, and poor prognosis; its incidence is reported to be ~5
cases per million persons per year. The disease is particularly
prevalent among children and adolescents (13,14). At
present, the mainstream treatment protocol for patients with
osteosarcoma is a combination of neoadjuvant chemotherapy and
surgery, and although the prognosis has improved (14–17), the
5-year overall survival rate is still unsatisfactory (18). The early detection rate of
osteosarcoma is low and pulmonary metastases are often discovered
at diagnosis, which complicates treatment (19,20).
Thus, it is necessary to explore the molecular mechanisms of
metastasis and invasion of osteosarcoma to identify new avenues for
the treatment of this lethal disease.
Enoxacin is a highly effective broad-spectrum
fluoroquinolone antibiotic with low toxicity (12). Several studies have confirmed that,
in addition to its antibacterial properties, enoxacin also displays
antitumour activity (8–11). Thus, its effect on the proliferation,
migration and invasion of human osteosarcoma 143B cells was
investigated. The present results indicate that enoxacin could
prevent metastasis and promote apoptosis in osteosarcoma, and
improve patient survival.
Metastasis, a biological characteristic of malignant
tumours, is a dynamic process that involves the spread of tumour
cells from the primary site to the surrounding or distant tissue.
Early lung metastasis by hematogenous dissemination is the cause of
high mortality in osteosarcoma (21). In this study, it was found that
enoxacin blocked the migration and invasion of 143B cells,
highlighting its potential in the prevention of osteosarcoma
metastasis.
The formation, progression, invasion and metastasis
of malignant tumours are often accompanied by changes in the
expression of extracellular matrix (ECM) components and their cell
surface receptors. The degradation of ECM by MMPs is a key step in
the invasion and metastasis processes in human cancers, and
increased secretion and activity of MMPs are implicated in numerous
types of malignant tumours (22,23).
MMP2 and MMP9 are important members of the MMP family and
inhibition of their expression is a strategy for reducing the
invasion and metastasis of osteosarcoma (24,25). The
current western blot analysis showed that enoxacin treatment
downregulated the expression of MMP2 and MMP9 in 143B cells, which
could be an effective way to inhibit osteosarcoma metastasis.
Apoptosis is a physiological phenomenon that is
necessary for normal growth, development and maintenance of
cellular homeostasis, which also plays a central role in tumour
development (26). The present
results demonstrated that enoxacin stimulated apoptosis in 143B
cells and decreased tumour volume and weight in the xenograft model
of osteosarcoma. Therefore, enoxacin might block tumour growth in
osteosarcoma.
Apoptosis is a complex process that is tightly
regulated by multiple genes (27).
Bcl-2 family proteins can prevent the release of cytochrome C from
mitochondria to the cytoplasm, thus inhibiting apoptosis (28,29).
Bcl-2 and Bcl-xL are the main anti-apoptotic molecules among the
members of the Bcl-2 family; reducing their protein levels can
promote apoptosis of tumour cells. In the current study, enoxacin
downregulated protein expression of both Bcl-2 and Bcl-xL to
trigger the mitochondrial apoptosis pathway in 143B cells.
In the mouse study, the tumour volume and weight
were decreased in the enoxacin-treated group compared with in the
untreated group. However, mean body weights did not differ between
enoxacin-treated and untreated mice. Furthermore, there was no
evidence of disturbed liver or kidney function, or altered blood
cell count in enoxacin-treated mice, which confirms that the drug
has good antitumour efficacy without obvious toxic effects in
vivo.
The current study describes the pharmacological
activities of enoxacin against osteosarcoma and explores its
potential as a novel approach to the treatment of this disease. The
use of fluoroquinolones, such as enoxacin, is limited in children
because of their potential to cause cartilage dysplasia.
Nevertheless, it is estimated that in the Unites States, 520,000
prescriptions were issued for children under the age of 18 in 2002
alone. Of those, 13,800 were for infants and children aged between
2 and 6, and 2,750 were for infants under the age of 2 (30). Accumulating evidence suggests that
the incidence and severity of articular cartilage injury in
children treated with fluoroquinolones is markedly lower than that
in animals (31,32), suggesting that the side effects of
fluoroquinolones are not be as serious as initially thought. Thus,
the use of enoxacin to treat osteosarcoma in children may increase.
Although osteosarcoma is common in people under the age of 20, as
the global population grows and ages, the prevalence of
osteosarcoma and other types of bone cancer will rise in the
middle-aged and elderly (33).
Enoxacin might become valuable in the treatment of these patient
groups in the future.
The limitation of this study is that it only used a
single cell line for experiments and further research is necessary
in the future to verify the current findings.
Collectively, the present data demonstrated that
enoxacin promotes apoptosis and inhibits proliferation, migration,
and invasion of osteosarcoma 143B cells in vitro, and
effectively impairs the growth of osteosarcoma in vivo.
Based on these findings, enoxacin might represent a novel strategy
for the treatment of osteosarcoma. However, its exact mechanism of
action and clinical effects warrant further evaluation.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Key Research
Plan of Jiangxi Province (grant no. 20171ACG70006) and National
Natural Science Foundation of China (grant nos. 81860404, 81860405,
81601912 and 81660365).
Availability of data and materials
All data generated or analysed during the present
study are included in the published article.
Authors' contributions
XWL, XQL, XLY, MD and TN designed the study. XWL,
HXF, BZ and QYT performed in vivo experiments, while XWL,
FQW, ZPG, CY and FLL performed in vitro experiments and
collected the data. XWL, XQL, CY, and TN analyzed the data. XWL and
CY drafted the initial manuscript. XWL, HXF, BZ and MD instructed
the experimental technology and critically reviewed the
intellectual content of the article. All authors have read and
approved the final manuscript.
Ethics approval
All animal studies were approved by the Ethics
Committee of The First Affiliated Hospital of Nanchang University
(Nanchang, China). All procedures were performed in accordance with
the ethical standards of the institution or practice.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BUN
|
blood urea nitrogen
|
|
Cr
|
creatinine
|
|
DMEM
|
Dulbecco's Modified Eagle Medium
|
|
PBS
|
phosphate-buffered saline
|
|
FBS
|
foetal bovine serum
|
|
PI
|
propidium iodide
|
|
CST
|
Cell Signalling Technology, Inc.
|
References
|
1
|
Delebinski CI, Georgi S, Kleinsimon S,
Twardziok M, Kopp B, Melzig MF and Seifert G: Analysis of
proliferation and apoptotic induction by 20 steroid glycosides in
143B osteosarcoma cells in vitro. Cell Prolif. 48:600–610. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu SY, Deng SY, He YB and Ni GX: miR-451
inhibits cell growth, migration and angiogenesis in human
osteosarcoma via down-regulating IL 6R. Biochem Biophys Res Commun.
482:987–993. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang J, Hou W, Chai M, Zhao H, Jia J, Sun
X, Zhao B and Wang R: MicroRNA-127-3p inhibits proliferation and
invasion by targeting SETD8 in human osteosarcoma cells. Biochem
Biophys Res Commun. 469:1006–1011. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Isakoff MS, Bielack SS, Meltzer P and
Gorlick R: Osteosarcoma: Current treatment and a collaborative
pathway to success. J Clin Oncol. 33:3029–3035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao JZ, Chen FH, Wang L, Wei H and Meng
SL: YM155 inhibits tumor growth and enhances chemosensitivity to
cisplatin in osteosarcoma. Eur Rev Med Pharmacol Sci. 19:2062–2069.
2015.PubMed/NCBI
|
|
6
|
Brown HK, Tellez-Gabriel M and Heymann D:
Cancer stem cells in osteosarcoma. Cancer Lett. 386:189–195. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wise R, Andrews JM and Danks G: In-vitro
activity of enoxacin (CL-919), a new quinoline derivative, compared
with that of other antimicrobial agents. J Antimicrob Chemother.
13:237–244. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao S, Sun R, Wang W, Meng X, Zhang Y,
Zhang N and Yang S: RNA helicase DHX9 may be a therapeutic target
in lung cancer and inhibited by enoxacin. Am J Transl Res.
9:674–682. 2017.PubMed/NCBI
|
|
9
|
Mondal ER, Das SK and Mukherjee P:
Comparative evaluation of antiproliferative activity and induction
of apoptosis by some fluoroquinolones with a human non-small cell
lung cancer cell line in culture. Asian Pac J Cancer Prev.
5:196–204. 2004.PubMed/NCBI
|
|
10
|
Sousa E, Graça I, Baptista T, Vieira FQ,
Palmeira C, Henrique R and Jerónimo C: Enoxacin inhibits growth of
prostate cancer cells and effectively restores microRNA processing.
Epigenetics. 8:548–558. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Melo S, Villanueva A, Moutinho C, Davalos
V, Spizzo R, Ivan C, Rossi S, Setien F, Casanovas O, Simo-Riudalbas
L, et al: Small molecule enoxacin is a cancer-specific growth
inhibitor that acts by enhancing TAR RNA-binding protein 2-mediated
microRNA processing. Proc Natl Acad Sci USA. 108:4394–4399. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bhanot SK, Singh M and Chatterjee NR: The
chemical and biological aspects of fluoroquinolones: Reality and
dreams. Curr Pharm Des. 7:311–335. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sampo M, Koivikko M, Taskinen M, Kallio P,
Kivioja A, Tarkkanen M and Böhling T: Incidence, epidemiology and
treatment results of osteosarcoma in Finland-a nationwide
population-based study. Acta Oncol. 50:1206–1214. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mirabello L, Troisi RJ and Savage SA:
International osteosarcoma incidence patterns in children and
adolescents, middle ages and elderly persons. Int J Cancer.
125:229–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dai X, Ma W, He X and Jha RK: Review of
therapeutic strategies for osteosarcoma, chondrosarcoma, and
Ewing's sarcoma. Med Sci Monit. 17:RA177–RA190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ando K, Heymann MF, Stresing V, Mori K,
Rèdini F and Heymann D: Current therapeutic strategies and novel
approaches in osteosarcoma. Cancers (Basel). 5:591–616. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lamoureux F, Trichet V, Chipoy C,
Blanchard F, Gouin F and Redini F: Recent advances in the
management of osteosarcoma and forthcoming therapeutic strategies.
Expert Rev Anticancer Ther. 7:169–181. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Picci P: Osteosarcoma (osteogenic
sarcoma). Orphanet J Rare Dis. 2:62007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Liao Q, Li K, Zhong D, Weng X and Mi
M: Knockdown of endothelin A receptor expression inhibits
osteosarcoma pulmonary metastasis in an orthotopic xenograft mouse
model. Mol Med Rep. 5:1391–1395. 2012.PubMed/NCBI
|
|
20
|
Kato H, Wakabayashi H, Naito Y, Kato S,
Nakagawa T, Matsumine A and Sudo A: Anti-tumor necrosis factor
therapy inhibits lung metastasis in an osteosarcoma cell line.
Oncology. 88:139–146. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meazza C and Scanagatta P: Metastatic
osteosarcoma: A challenging multidisciplinary treatment. Expert Rev
Anticancer Ther. 16:543–556. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shay G, Lynch CC and Fingleton B: Moving
targets: Emerging roles for MMPs in cancer progression and
metastasis. Matrix Biol. 44-46:200–206. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jabłońska-Trypuć A, Matejczyk M and
Rosochacki S: Matrix metalloproteinases (MMPs), the main
extracellular matrix (ECM) enzymes in collagen degradation, as a
target for anticancer drugs. J Enzyme Inhib Med Chem. 31 (Suppl
1):S177–S183. 2016. View Article : Google Scholar
|
|
24
|
Zhu KP, Ma XL and Zhang CL: LncRNA ODRUL
contributes to osteosarcoma progression through the miR-3182/MMP2
Axis. Mol Ther. 25:2383–2393. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qiu R, Li X, Qin K, Chen X, Wang R, Dai Y,
Deng L and Ye Y: Antimetastatic effects of calycosin on
osteosarcoma and the underlying mechanism. Biofactors. 45:975–982.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li J, Yang Z, Li Y, Xia J, Li D, Li H, Ren
M, Liao Y, Yu S, Chen Y, et al: Cell apoptosis, autophagy and
necroptosis in osteosarcoma treatment. Oncotarget. 7:44763–44778.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Silvestris F, Ribatti D, Nico B,
Silvestris N, Romito A and Dammacco F: Apoptosis or programmed cell
death: Regulatory and pathophysiological mechanisms. Ann Ital Med
Int. 10:7–13. 1995.(In Italian). PubMed/NCBI
|
|
28
|
Cardenas C, Montagna MK, Pitruzzello M,
Lima E, Mor G and Alvero AB: Adipocyte microenvironment promotes
Bclxl expression and confers chemoresistance in ovarian
cancer cells. Apoptosis. 22:558–569. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park SS, Lee DM, Lim JH, Lee D, Park SJ,
Kim HM, Sohn S, Yoon G, Eom YW, Jeong SY, et al: Pyrrolidine
dithiocarbamate reverses Bcl-xL-mediated apoptotic resistance to
doxorubicin by inducing paraptosis. Carcinogenesis. 39:458–470.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Committee on Infectious Diseases: The use
of systemic fluoroquinolones. Pediatrics. 118:1287–1292. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hampel B, Hullmann R and Schmidt H:
Ciprofloxacin in pediatrics: Worldwide clinical experience based on
compassionate use-safety report. Pediatr Infect Dis J. 16:127–129,
160-162. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bacci C, Galli L, de Martino M and
Chiappini E: Fluoroquinolones in children: Update of the
literature. J Chemother. 27:257–265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huvos AG: Osteogenic sarcoma of bones and
soft tissues in older persons. A clinicopathologic analysis of 117
patients older than 60 years. Cancer. 57:1442–1449. 1986.
View Article : Google Scholar : PubMed/NCBI
|