Introduction
Endometrial carcinoma is one of the most common
malignancies of the female genital tract in developed counties,
with its life time risk estimated at 2–3% (1,2).
Endometrial carcinomas are sporadic in 95% of cases, while ≤5% of
cases is associated with a hereditary predisposition, especially
when presenting in younger patients (3). Predisposing factors of the sporadic
cases include hypertension, diabetes mellitus, late menopause,
oestrogen replacement treatment and obesity. The most common
symptom that leads to its diagnosis is postmenopausal vaginal
bleeding. There are various histological subtypes of endometrial
adenocarcinomas, including endometrioid, papillary serous and clear
cell adenocarcinomas.
Endometrial carcinomas have been categorized into
two major groups: Type I and type II carcinomas. The most common
form of type I endometrial carcinoma is endometrioid
adenocarcinoma, an oestrogen-dependent neoplasm that usually arises
soon after menopause. Endometrioid adenocarcinomas also develop
from endometrial hyperplasia in up to 80% of cases. The most common
type II endometrial carcinoma is papillary serous adenocarcinoma,
which is an oestrogen-independent neoplasm that develops in the
atrophic endometrium, presents in elderly postmenopausal women and
is associated with an aggressive behaviour and poor prognosis
(3–8).
Endometrial cancer is successfully treated with
hysterectomy and bilateral oophorectomy. Risk factors in dilation
and curettage histological specimens include high-grade tumors or
serous papillary or clear cell adenocarcinomas. Suggestive risk
factors in MRI include tumor invasion greater than one-half of the
myometrial thickness and/or involvement of pelvic lymph nodes.
Therefore, when risk factors are present, then extensive surgery
and postoperative radiotherapy are required. These patients might
also benefit from new targeted therapies (9). Early-stage endometrial carcinomas
result in favourable prognosis and excellent survival rates, with a
5-year overall survival of 75–90%. However, women with more
advanced or recurrent disease tend to have an extremely poor
clinical outcome, with a 5-year survival rate of only 10–30%
(3–8).
Understanding the pathogenesis of endometrial
carcinomas, particular at the molecular and cellular levels via the
identification of genetic alterations, such as mutations, gene
expression or dysregulation of apoptotic cell death, is essential
for recognizing useful biomarkers for early tumor diagnosis and new
therapies using target genes. An example of the involvement of
apoptosis in carcinogenetic development is the permanent expression
of survivin in various human malignancies by blocking the action of
caspase-3 and caspase-7, which are proteases and effectors of cell
death (10–13). Survivin is a unique member of the
inhibitor of the apoptosis protein family, which regulates the
anti-apoptotic activity of the v-Rel and nuclear factor-κB
transcription factor families (13).
It plays essential roles in mitosis, cellular stress response and
inhibition of programmed cell death induced by both extrinsic and
intrinsic apoptotic stimuli (14).
The survivin protein is present in large amounts of fetal tissues
and absent in most normal adult differentiated tissues (15). Survivin overexpression is linked to
tumor progression, metastasis and increased rates of recurrence,
treatment resistance and unfavorable prognosis in different types
of cancer (14,16–20). The
survivin protein comprises 142 amino acids and has a molecular
weight of ~16.5 kD (21). The gene
encoding the survivin protein is BIRC5 which, in humans is located
on chromosome 17q25 and encodes an mRNA that is divided into 4
exons and 3 introns (22). There are
5 different transcript isoforms of the human gene encoding
survivin: survivin, survivin 2A, survivin 2B, survivin DEx2 and
survivin 3B (23). Survivin is
located in the nucleus, cytosol and mitochondria. In the nucleus,
survivin shows its mitotic role. In response to apoptotic stimulus,
survivin is transported from the mitochondria to the cytosol, where
it exerts its anti-apoptotic potential (24). Survivin is regulated by a variety of
oncogenes and tumor suppressor genes, including the
phosphatidylinositol 3-kinase (PI3K)/mTOR pathway genes (14). The survivin and phosphatase and
tensin homolog (PTEN) proteins are both thought to be inverse
factors of apoptosis (25). Guha
et al (26), found that the
expression of tumor suppressor gene PTEN silences the expression of
survivin in tumor cells, independently of the p53 pathway. In
addition, Kim et al (27)
reported that the inhibition of the PI3K/AKT pathway down-regulates
survivin in neuroblastoma. Moreover, Sui et al (28) found that PTEN expression is
negatively associated with survivin expression in the development
and progression of ovarian cancer. In addition to all these
findings, there has been evidence that the transcription of
survivin is partially under the control of the p53 protein. Indeed,
it has been found that wild-type p53 actively suppresses survivin
expression (29–31). The findings of Kannangai et al
(32), supported the role of p53 in
regulating survivin expression by demonstrating an association
between the abnormal nuclear accumulation of p53 and the
overexpression of survivin in hepatocellular carcinomas, as
determined by immunohistochemical analysis. Cohen et al
(15), found that there was a
significant positive correlation between nuclear survivin
expression and mutant p53 in ovarian carcinoma. In a study
published by the Polish Ovarian Cancer Group, the high nuclear
expression of survivin was found to be a favorable predictor of
progression-free survival and increased platinum sensitivity in
patients with a positive p53 expression (33). In addition, Gąsowska-Bodnar et
al (34) found that a high
survivin expression could be a favorable prognostic factor in
ovarian carcinomas, especially in the group of patients with a
positive p53 expression. On the other hand, Gonzalez et al
(35) found that high expression
levels of survivin in combination with high expression levels of
p53 were associated with an increased risk of local tumor
progression in urothelial carcinomas of the bladder. These findings
raise the question of whether there is a strong correlation between
the concomitant protein expression of survivin, PTEN and p53 in
endometrial carcinomas with remarkable clinical significance.
However, the findings regarding endometrial carcinomas are not
consistent so far in the international literature. Pallares et
al (36), found that survivin
expression was statistically significantly correlated with a
decreased PTEN expression, while Erkanli et al (37) did not identify any such
correlation.
Since the findings in the literature are conflicting
with respect to the prognostic value of survivin expression in
endometrial carcinomas, in the present study, immunohistochemical
staining was performed to investigate the distribution of survivin
expression in primary endometrial carcinomas from Greek patients
who underwent surgery and the survivin protein expression levels
were analyzed in association with well-established
clinicopathological prognostic factors (16,36–42).
Furthermore, in order to gain better insight into the synergistic
function of survivin with tumor suppressor genes, the effects of
PTEN and p53 expression, which had been previously evaluated by
immunohistochemical analysis (43),
were investigated on the expression of survivin in endometrial
carcinomas. The main goal was to analyze the co-expression of
survivin, PTEN and p53 using traditional clinicopathological
prognostic factors and assess their prognostic value, as such
evidence has still not been fully elucidated in the literature.
Finally, in the current study, the frequencies of p53, PTEN and
survivin were compared in women with normal, hyperplastic and
carcinomatous endometria. An attempt was also made to determine the
survivin expression differences between normal, hyperplastic and
carcinomatous endometria when survivin was concomitantly expressed
with PTEN or p53 in order to investigate the mechanisms of
endometrial carcinogenesis.
Materials and methods
Case selection
Tissue analysis was randomly performed on
paraffin-embedded blocks of surgically resected tissues from 99
patients with primary endometrial carcinomas, who underwent surgery
between 2006 and 2015, and had previously undergone evaluation for
PTEN and p53 expression using immunohistochemical analysis
(43). Samples were also obtained
from 15 patients with normal endometria and 15 patients with
endometrial hyperplasia. All patients included in this study at the
time of data collection provided informed consent for the use of
their data, as well as surgical specimens that remained following
pathological diagnosis, in this study. The study was approved by
The Ethical Committee of the Medical School of the Kapodistrian
University of Athens, (Athens, Greece). The following
histopathological parameters were determined: Histological type and
grade, clinical stage, depth of myometrial invasion, lymph-vascular
space invasion, fallopian tube and/or ovarian invasion, and
presence of tumor necrosis.
Histological analysis and
immunohistochemistry
For histological examination, endometrial carcinomas
were routinely fixed with formalin and embedded in paraffin.
Sections cut at a thickness of ~4 µm, which included sufficient
quantities of endometrial carcinoma mass, were mounted on
silane-coated glass slides. Hematoxylin and eosin staining was
performed.
Unstained slides containing endometrial carcinoma
sections from each patient were used at the same time to
investigate the immunohistochemical expression of p53, PTEN and
survivin. For immunohistochemical analysis, sections of the
formalin-fixed 5-µm thick sections were cut from the
paraffin-embedded endometrial carcinoma specimens. The following
primary antibodies were used: Monoclonal rabbit anti-survivin
antibody (Cell Signaling Techology Inc.), mouse monoclonal anti-p53
antibody (clone DO-7; Thermo Fisher Scientific, Inc.) and
monoclonal PTEN (clone MMAC; Novocastra). Deparaffinization,
rehydration and epitope retrieval was performed using the PT link
system (Agilent Technologies Inc.). The pretreatment Module buffer
(100X citrate at pH 9.0) was used for 1 h. The slides were rinsed
in gently running followed by distilled water. The slides were
immersed into 3.3% H2O2 for 10 min in the
dark, washed with tap water followed by distilled water and buffer,
and incubated with the appropriate monoclonal antibody. Slides were
washed extensively in buffer. Horseradish peroxidase-polymer
secondary antibodies (Agilent Technologies, Inc.) were used for a
30-min incubation. 3′,3′-Diaminobenzidine was used as a chromogen
and the slides were incubated for 5 min. The sections were
counterstained in Meyer's hematoxylin, washed again and dehydrated
with ethanol and xylene prior to mounting. The dilutions used for
the primary antibodies were 1:200 for p53, 1:100 for PTEN and 1:400
for survivin.
No significant differences in immunoreactivity were
identified when comparing sections from older paraffin blocks with
others from recent blocks, suggesting that activated survivin
immunoreactivity is well presented in paraffin-embedded tissues.
The fraction of the positively stained tumor cells was scored
following the examination of 20 high-power fields (magnification,
×400) of endometrial adenocarcinomas. The scores for the
immunohistochemical expression of survivin, p53 and PTEN were
classified into the following 4 categories, according to the
percentage of the positive staining area in relation to the whole
carcinoma area: 0, <5% Immunopositive cells; 1, 5–25%
immunopositive cells (low scores); 2, 25–75% immunopositive cells
(moderate scores); 3, ≥75% immunopositive cells (high scores).
Cases with ≥5% positive endometrial carcinoma cells were defined as
positive, and all other cases as negative.
The intensity of survivin staining in every stained
slide was assessed as red-brown staining and classified into the
following 4 categories: 0, Absent; 1, weak (faintly perceptible
staining); 2, moderate staining; 3, strong staining (corresponded
to staining intensity similar to that seen in positive control
tissue sections).
In every stained slide, the scores of
immunohistochemical expression of survivin, p53 and PTEN, and their
staining intensity was then combined to produce the final sum of
immunohistochemical expression, which was classified into the
following 4 categories: –, (0); +, (1–2); ++,
(3–4); +++, (5–6).
Since endometrial adenocarcinomas showed
heterogeneous staining, the dominant pattern was used for scoring.
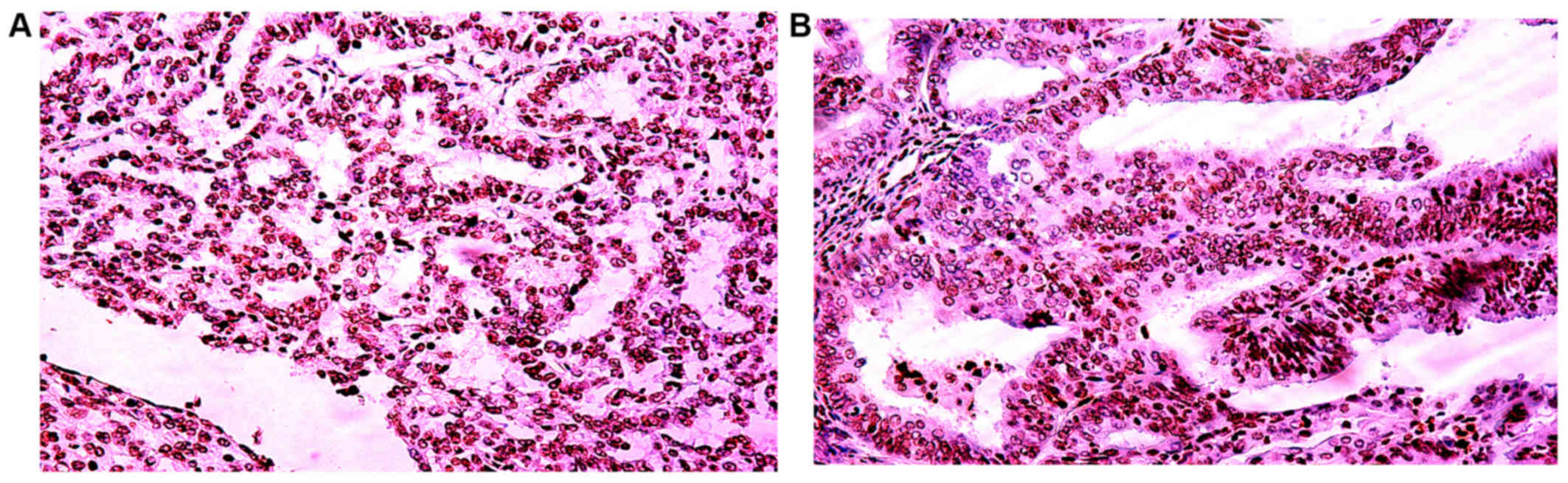
Overall the exclusively nuclear localization of survivin protein
was identified in all survivin-positive cases. The
immunohistochemical staining for survivin is shown in Figs. 1 and 2. The slides were scored independently by 2
pathologists who were unaware of the clinicopathological data.
Statistical analysis
Categorical variables are presented as absolute (n)
and relative (%) frequencies, while continuous variables are
presented as medians (min, max). Associations between categorical
variables were assessed by exact Pearson's χ2 test. To
investigate whether the median levels of a quantitative variable
differed between 2 groups, the Mann-Whitney test was used, while
for ≥3 groups the Kruskal-Wallis was used (with Dunn test for post
hoc analysis). Finally, to investigate correlations between 2
parameters, Spearman's correlation coefficient was used.
Statistical significance was set at a two-tailed P<0.05. Data
were analyzed using IBM SPSS Statistics, v25.0 (IBM Corp.).
Results
Clinicopathological and
immunohistochemical study of endometrioid, papillary serous and
clear cell endometrial adenocarcinomas
The clinicopathological data of our study was
previously described and reported (43). The specimens included 20 endometrioid
endometrial carcinomas of histological grade 1 (G1), 47 of G2 and
19 of G3, as well as 13 papillary serous carcinomas or clear cell
carcinomas. A total of 68 tumors were clinical stage I, 15 stage II
and 5 stage III. The correlation between survivin
immunohistological expression (score, intensity and sum of score
and intensity) and clinicopathological parameters, such as tumor
grade, clinical stage and depth of myometrial invasion was
evaluated.
The survivin immunohistochemical expression scores
were not statistically significant, as compared to the mean age of
patients (P=0.121), depth of myometrial invasion (P=0.255),
fallopian tube and/or ovarian invasion (P=0.073) and histological
types (P=0.241; Table I).
 | Table I.Associations between
clinicopathological characteristics and scores of
immunohistochemical survivin expression. |
Table I.
Associations between
clinicopathological characteristics and scores of
immunohistochemical survivin expression.
|
| Survivin staining
pattern-Scores (%) |
|
|---|
|
|
|
|
|---|
|
| 5-25%
positivity | 25-75%
positivity | >75%
positivity |
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
parameters | No. (%) | Median (IQR) | No. (%) | Median (IQR) | No. (%) | Median (IQR) | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
|
<60 | 13 (37.1) | 10.0 (8.0,
17.5) | 10 (20.4) | 27.5 (25.0,
35.0) | 0 (0.0) | – | 0.121 |
|
≥60 | 22 (62.9) | 15.0 (7.3,
20.0) | 39 (79.6) | 50.0 (30.0,
60.0) | 3 (100.0) | 80.0 (80.0,
80.0) |
|
| Histological
type |
|
|
|
|
|
|
|
|
Endometrioid | 33 (94.3) | 10.0 (8.0,
17.5) | 41 (83.7) | 40.0 (30.0,
50.0) | 3 (100.0) | 80.0 (80.0,
80.0) | 0.241 |
| Clear
cell and papillary serous | 2 (5.7) | 17.5 (15.0, -) | 8 (16.3) | 60.0 (35.0,
67.5) | 0 (0.0) | – |
|
| Clinical stage |
|
|
|
|
|
|
|
| I | 29 (93.5) | 10.0 (8.0,
15.0) | 32 (76.2) | 37.5 (25.0,
50.0) | 0 (0.0) | – | <0.001 |
| II | 2 (6.5) | 17.5 (15.0, -) | 9 (21.4) | 60.0 (40.0,
60.0) | 1 (33.3) | 80.0 (80.0,
80.0) |
|
|
III | 0 (0.0) | – | 1 (2.0) | 70.0 (70.0,
70.0) | 2 (66.7) | 80.0 (80.0,
80.0) |
|
| IV | 0 (0.0) | – | 0 (0.0) | – | 0 (0.0) | – |
|
| Histological
differentiation |
|
|
|
|
|
|
|
| G1 | 11 (31.4) | 15.0 (5.0,
5.0) | 7 (14.3) | 30.0 (25.0,
50.0) | 0 (0.0) | – | <0.001 |
| G2 | 22 (62.9) | 10.0 (8.0,
20.0) | 23 (46.9) | 30.0 (25.0,
50.0) | 0 (0.0) | – |
|
| G3 | 2 (5.7) | 17.5 (15.0,
15.0) | 19 (38.8) | 60.0 (50.0,
70.0) | 3 (100.0) | 80.0 (80.0,
80.0) |
|
| Myometrial
invasion |
|
|
|
|
|
|
|
|
<1/2 | 14 (40.0) | 12.5 (10.0,
16.3) | 14 (28.6) | 30.0 (25.0,
38.8) | 0 (0.0) | – | 0.255 |
|
≥1/2 | 21 (60.0) | 15.0 (5.0,
20.0) | 35 (71.4) | 50.0 (30.0,
60.0) | 3 (100.0) | 80.0 (80.0,
80.0) |
|
| Lymph-vascular
space invasion |
|
|
|
|
|
|
|
|
Positive | 3 (15.8) | 15.0 (8.0, -) | 6 (17.1) | 45.0 (37.5,
60.0) | 2 (100.0) | 80.0 (80.0,
80.0) | 0.040 |
|
Negative | 16 (84.2) | 15.0 (8.0,
15.0) | 29 (82.9) | 40.0 (27.5,
60.0) | 0 (0.0) | – |
|
| Fallopian tube
and/or ovarian invasion |
|
|
|
|
|
|
|
|
Positive | 3 (20.0) | 20.0 (15.0, -) | 10 (41.7) | 60.0 (45.0,
60.0) | 2 (100.0) | 80.0 (80.0,
80.0) | 0.073 |
|
Negative | 12 (80.0) | 10.0 (8.0,
15.0) | 14 (58.3) | 45.0 (25.0,
62.5) | 0 (0.0) | – |
|
| Tumoral
necrosis |
|
|
|
|
|
|
|
|
Yes | 2 (11.1) | 17.5 (15.0, -) | 3 (9.7) | 60.0 (60.0, -) | 2 (100.0) | 80.0 (80.0,
80.0) | 0.017 |
| No | 16 (88.9) | 12.5 (8.0,
15.0) | 28 (90.3) | 35.0 (26.3,
57.5) | 0 (0.0) | – |
|
There was, however, a statistically significant
correlation between clinical stage and immunohistochemical survivin
expression scores (P<0.001; Table
I). Among those with a 5–25% survivin staining pattern, 29 were
classified as clinical stage I (93.5%), and 2 as clinical stage II
(6.5%). Among those that exhibited a 25–75% survivin staining
pattern, 32 were classified as clinical stage I (76.2%), 9 as
clinical stage II (21.4%) and 1 as clinical stage III. Finally,
among those with a survivin staining pattern of >75%, 1 was
classified as clinical stage II (33.3%) and 2 as clinical stage III
(66.7%). Earlier clinical stages were associated with lower
survivin-positive immunostaining counts, while higher clinical
stages were associated with medium/higher survivin-positive
immunostaining counts. Fig. 3 shows
the survivin immunohistochemical expression in different clinical
stages of endometrial carcinomas, while Fig. 4 shows the moderate and high scores of
survivin immunohistochemical expression.
There was a statistically significant correlation
between histological grades and scores of immunohistochemical
survivin expression (P<0.001; Table
I). Among those with a 5–25% survivin staining pattern, 11 were
classified as G1 (31.4%), 22 as G2 (6.5%) and 2 as G3 (5.7%). Among
those with a 25–75% survivin staining pattern, 7 were classified as
G1 (14.3%), 23 as G2 (46.9%) and 19 as G3 (38.8%). Finally, all 3
patients with a survivin staining pattern >75% were classified
as G3 (100.0%). Lower grade levels were associated with lower
survivin-positive immunostaining counts than expected, while higher
grade levels were associated with medium/higher survivin-positive
immunostaining counts than expected.
Furthermore, there was a statistically significant
correlation between lymph-vascular space invasion and scores of
immunohistochemical survivin expression (P=0.040; Table I). Among those with a 5–25% survivin
staining pattern, 3 had a lymph-vascular space invasion (15.8%),
while there was an absence of lymph-vascular space invasion in 16
patients (84.2%). Among those with a 25–75% survivin staining
pattern, 6 exhibited lymph-vascular space invasion (17.1%) and 29
did not (82.9%). Finally, 2 cases with a >75% surviving staining
pattern exhibited lymph-vascular space invasion (100.0%). The
presence of lymph-vascular space invasion was associated with
higher survivin positive immunostaining counts, while the absence
of lymph-vascular space invasion was associated with medium/lower
survivin-positive immunostaining counts than expected.
A statistically significant association was observed
between the presence of tumor necrosis and immunohistochemical
survivin expression scores (P=0.017; Table I). Among those with a 5–25% survivin
staining pattern, 2 had tumor necrosis (11.1%) and 16 did not
(88.9%). Among those with a 25–75% survivin staining pattern, tumor
necrosis was present in 3 cases (9.7%) and absent in 28 cases
(90.3%). Finally, tumor necrosis was absent in 2 cases with a
>75% survivin staining pattern (100.0%). The presence of tumor
necrosis was associated with higher survivin-positive
immunostaining counts, while its absence was associated with
medium/lower survivin-positive immunostaining counts.
Out of 99 cases, 43 (43.4%) exhibited a strong
survivin expression intensity and 44 (44.4%) a moderate one. The
survivin expression intensity was marginally associated with the
patient's age (P=0.051; Table II).
Specifically, the patients with a strong survivin expression
intensity had a median age of 65 years (range, 53–90), while those
with a moderate survivin expression had a median age of 62 years
(range 42–85).
 | Table II.Associations between
clinicopathological characteristics and intensity of
immunohistochemical survivin expression. |
Table II.
Associations between
clinicopathological characteristics and intensity of
immunohistochemical survivin expression.
|
| Survivin staining
pattern-intensity (%) |
|
|---|
|
|
|
|
|---|
| Clinicopathological
parameters | Moderate, n
(%) | Strong, n (%) | P-value |
|---|
| Age, years |
|
|
|
|
<60 | 16 (36.4) | 7 (16.3) | 0.051 |
|
≥60 | 28 (63.6) | 36 (83.7) |
|
| Histological
type |
|
|
|
|
Endometrioid | 42 (95.5) | 35 (81.4) | 0.049 |
| Clear
cell and papillary serous | 2 (4.5) | 8 (18.6) |
|
| Clinical stage |
|
|
|
| I | 36 (92.3) | 25 (67.6) | 0.012 |
| II | 3 (7.7) | 9 (24.3) |
|
|
III | 0 (0.0) | 3 (8.1) |
|
| IV | 0 (0.0) | 0 (0.0) |
|
| Histological
differentiation |
|
|
|
| G1 | 14 (31.8) | 4 (9.3) | 0.001 |
| G2 | 25 (56.8) | 20 (46.5) |
|
| G3 | 5 (11.4) | 19 (44.2) |
|
| Myometrial
invasion |
|
|
|
|
<1/2 | 21 (47.7) | 7 (16.3) | 0.003 |
|
≥1/2 | 23 (52.3) | 36 (83.7) |
|
| Lymph-vascular
space invasion |
|
|
|
|
Positive | 0 (0.0) | 11 (33.3) | 0.002 |
|
Negative | 23 (100.0) | 22 (66.7) |
|
| Fallopian tube
and/or ovarian invasion |
|
|
|
|
Positive | 3 (18.7) | 12 (48.0) | 0.097 |
|
Negative | 13 (81.3) | 13 (52.0) |
|
| Tumoral
necrosis |
|
|
|
|
Yes | 1 (4.3) | 6 (21.4) | 0.112 |
| No | 22 (95.7) | 22 (78.6) |
|
The intensity of survivin expression was
statistically significantly associated with the histological type
of the endometrial carcinomas (P=0.049; Table II). A strong positive expression was
observed in 35 (81.4%) cases of endometrioid carcinomas and in 8
(18.6%) cases of clear cell or papillary serous adenocarcinomas.
The corresponding frequencies for moderate expression were 42
(95.5%) and 2 (4.5%) respectively.
In addition, survivin expression intensity was
statistically significantly associated with histological grade
(P=0.001; Table II). Among cases
with a strong positive expression, 4 (9.3%) were histological G1,
20 (46.5%) G2, and 19 (44.2%) G3. The corresponding frequencies for
moderate survivin expression were 14 (31.8%), 25 (56.8%) and 5
(11.4%) respectively. Lower grade levels were associated with a
moderate survivin expression, while higher grade levels with a
stronger survivin expression.
Moreover, the histological types of endometrial
carcinomas and the intensity of survivin staining were
statistically correlated (P=0.049; Table II). In cases with a strong positive
survivin expression, 35 cases had endometrioid carcinomas (81.4%)
and 8 had clear cell and papillary serous carcinomas (18.6%). On
the contrary, in cases with a moderate positive survivin
expression, 42 had endometrioid carcinomas (95.5%) and 2 had clear
cell and papillary serous carcinomas (4.5%). Endometrioid
carcinomas were associated with a more moderate survivin
expression, while clear cell and papillary serous endometrial
carcinomas were associated with a stronger survivin expression.
In addition, the depth of myometrial invasion and
survivin staining intensity were statistically correlated
(P=0.003). Among the 43 cases with a strong survivin expression, 21
(47.7%) had a depth of myometrial invasion of <50% of the
thickness of the myometrium, while 23 (52.3%) had a depth of ≥50%
of the myometrial thickness. Among the 44 cases with moderate
staining, 7 (16.3%) had a depth of myometrial invasion of <50%
of the myometrial thickness, while the other 36 (83.7%) had a depth
of myometrial invasion of ≥50% of the myometrial thickness.
Moderate survivin expression intensity was associated with a lower
myometrial invasion, while strong survivin intensity with a deeper
myometrial invasion.
Moreover, clinical stage and survivin staining
intensity were statistically significantly correlated (P=0.012;
Table II). Among the cases with a
strong survivin expression, 25 were clinical stage I (67.6%), 9 in
stage II (24.3%) and 3 in clinical stage III (8.1%). The
corresponding frequencies of moderate intensity were 36 (92.3%), 3
(7.7%) and 0 (0.0%), respectively. Moderate survivin expression was
associated with earlier clinical stages, while strong survivin
expression with advanced clinical stages.
The lymph-vascular space invasion and the intensity
of survivin staining were also statistically significantly
correlated (P=0.002; Table II). The
23 cases (100.0%) with a moderate survivin staining intensity did
not exhibit lymph-vascular space invasion. Among the cases with a
strong survivin staining intensity, 22 (66.7%) did not exhibit
lymph-vascular space invasion, while the other 11 (33.3%) did.
Lymph-vascular space invasion was associated with strong survivin
staining, while the absence of lymph-vascular space invasion with
moderate survivin staining.
No statistically significant association was
identified between survivin staining intensity and the presence of
tumor necrosis (P=0.112) or fallopian tube and/or ovary invasion
(P=0.097), although the results were suggestive of fallopian tube
and/or ovary invasion (Table
II).
Table III shows the
sum of staining intensity and scores of survivin immunopositive
cells in association with the clinicopathological characteristics.
A significant association was observed between the sum of survivin
positive cell scores and staining intensity, and patient age
(P=0.028), histological grade (P<0.001), clinical stage
(P=0.018) and presence of fallopian tube and/or ovarian invasion
(P=0.039). No significant correlation was identified with
histological type (P=0.141), depth of myometrial invasion
(P=0.083), presence of lymph-vascular space invasion (P=0.107) or
tumor necrosis (P=0.240).
 | Table III.Associations between
clinicopathological characteristics and sum of stain intensity and
scores of survivin expression. |
Table III.
Associations between
clinicopathological characteristics and sum of stain intensity and
scores of survivin expression.
|
|
| IHC results for
survivin, n (%) |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | Cases, n (%) | + | ++ | +++ | P-value |
|---|
| Age, years |
|
<60 | 23 (26.4) | 6 (33.3) | 13 (38.2) | 4 (11.4) | 0.028 |
|
≥60 | 64 (73.6) | 12 (66.7) | 21 (61.8) | 31 (88.6) |
|
| Histological
type |
|
Endometrioid | 77 (88.5) | 17 (94.4) | 32 (94.1) | 28 (80.0) | 0.141 |
| Clear
cell and papillary serous | 10 (11.5) | 1 (5.6) | 2 (5.9) | 7 (20.0) |
|
| Clinical stage |
| I | 61 (80.3) | 15 (100.0) | 27 (87.1) | 19 (63.3) | 0.018 |
| II | 12 (15.8) | 0 (0.0) | 4 (12.9) | 8 (26.7) |
|
|
III | 3 (3.9) | 0 (0.0) | 0 (0.0) | 3 (10.0) |
|
| Histological
differentiation |
| G1 | 18 (20.7) | 8 (44.4) | 6 (17.6) | 4 (11.4) | <0.001 |
| G2 | 45 (51.7) | 10 (55.6) | 22 (64.7) | 13 (37.1) |
|
| G3 | 24 (27.6) | 0 (0.0) | 6 (17.6) | 18 (51.4) |
|
| Myometrial
invasion |
|
<1/2 | 28 (32.2) | 9 (50.0) | 12 (35.3) | 7 (20.0) | 0.083 |
|
≥1/2 | 59 (67.8) | 9 (50.0) | 22 (64.7) | 28 (80.0) |
|
| Lymph-vascular
space invasion |
|
Positive | 11 (19.6) | 0 (0.0) | 3 (14.3) | 8 (30.8) | 0.107 |
|
Negative | 45 (80.4) | 9 (100.0) | 18 (85.7) | 18 (69.2) |
|
| Fallopian tube
and/or ovarian invasion |
|
Positive | 15 (36.6) | 0 (0.0) | 5 (33.3) | 10 (52.6) | 0.039 |
|
Negative | 26 (63.4) | 7 (100.0) | 10 (66.7) | 9 (47.4) |
|
| Tumoral
necrosis |
|
Yes | 7 (13.7) | 0 (0.0) | 2 (10.0) | 5 (22.7) | 0.240 |
| No | 44 (86.3) | 9 (100.0) | 18 (90.0) | 17 (77.3) |
|
Correlation between survivin
expression and concomitant p53 or PTEN expression and
clinicopathological parameters
This experiment utilized data from all the
endometrial carcinomas of endometrioid, papillary serous and clear
cell histological type. Immunopositivity for survivin was
identified in 88% (87/99) of cases, according to the scores of
immunopositive endometrial carcinoma cells, immunostaining
intensity or the sum of positive cell scores and staining
intensity; the same frequency was identified in all 3 staining
categories. Our previous study described PTEN and p53 expression in
endometrial adenocarcinomas from Greek patients (43). The overall rate of PTEN and p53
positivity was 77 and 89%, respectively, according to the sum of
staining intensity and positive cell scores. A survivin(−)/PTEN(+),
survivin(−)/p53(+) or survivin (−)/PTEN(+)/p53(+) expression was
identified in 6.1, 6.1 and 3.0% of patients respectively. A
survivin(+)/PTEN(−), or survivin(+)/p53(−) or survivin
(+)/PTEN(−)/p53(−) expression was identified in 25.3, 10.1 and
28.3% of patients, respectively. Concurrent survivin, PTEN and p53
expression (scores and intensity) was found in 59 (60%) endometrial
adenocarcinomas, 56 which were endometrioid endometrial
adenocarcinomas (95%). Figs. 5 and
6 show the immunohistochemical
staining patterns of PTEN and p53 in endometrial carcinomas,
respectively.
The correlation between survivin and PTEN expression
was investigated using Spearman's correlation coefficient.
According to the proportion of immunopositive cell scores, there
was a co-expression of survivin and PTEN in 25.8% of cases (16/62),
as compared to 74.2% without such co-expression. The findings were
not statistically significant (P=0.062, ρ=−0.238), but they were
suggestive for a correlation between the survivin and PTEN positive
cell scores. The correlation between the positive cell scores of
survivin and PTEN is presented in the scatterplot (Fig. 7A). Cases with high immunopositive
survivin scores tended to have low scores of PTEN
immunostaining.
According to the intensity of staining there was a
coexistence of survivin and PTEN expression in 23.8% of cases
(15/63), as compared to 76.2% without such co-expression. The
findings were not statistically significant (P=0.119, ρ=−0.198).
The correlation between the intensity of survivin and PTEN
immunopositivity is presented in the scatterplot (Fig. 7B).
According to the sum of staining and positive cell
scores, there was a coexistence of survivin and PTEN expression in
32.3% of cases (20/62), as compared to 67.7% without such
co-expression. The findings were not statistically significant
(P=0.399, ρ=−0.109). The correlation between the sum of survivin
and PTEN immunopositivity is presented in the scatterplot (Fig. 7C).
In addition, the correlation between survivin and
p53 expression was investigated using Spearman's rank correlation.
According to the proportion of immunopositive cell scores there was
a coexistence of survivin and p53 expression in 46.8% of cases
(36/77), as compared to 53.2% without such co-expression. The
findings were not statistically significant (P=0.442, ρ=0.089). The
correlation between the scores of survivin and p53 immunopositivity
is presented in the scatterplot (Fig.
7D).
According to the intensity of staining there was a
co-expression of survivin and p53 in 42.3% of cases (33/78), as
compared to 57.7% without such co-expression. The findings were
statistically significant (P=0.001, ρ=0.372) and the correlation
between survivin and p53 staining intensity is presented in the
scatterplot (Fig. 7E). According to
the sum of staining intensity and positive cell scores, there was a
co-expression of survivin and p53 in 46.8% of cases (36/77), as
compared to 53.2% without such co-expression. The findings were
statistically significant (P=0.008, ρ=0.300) and the correlation
between survivin and p53 sum immunopositivity is presented in the
scatterplot (Fig. 7F). Therefore,
the increase in the intensity or sum (intensity and scores) of
survivin expression was associated with an increase in the
intensity or sum (intensity and scores) of the p53 expression.
Tables IV–IX present the co-expression of survivin
with PTEN or p53 immunopositivity in relation to
clinicopathological factors in endometrial carcinomas. There was a
statistically significant correlation between the co-expression of
survivin and PTEN in endometrial carcinomas, according to staining
intensity and histologic grade (P=0.023), lymph-vascular space
involvement (P=0.007) and presence of fallopian tube and/or ovarian
invasion (P=0.031; Table V).
Furthermore, there was a statistically significant correlation
between the sum of staining intensity and immunopositive cell
scores, and the histological type (P=0.020; Table VI).
 | Table IV.Co-expression of survivin and PTEN in
endometrial carcinoma according to scores of immunopositive cells
in relation to clinopathological parameters. |
Table IV.
Co-expression of survivin and PTEN in
endometrial carcinoma according to scores of immunopositive cells
in relation to clinopathological parameters.
|
Characteristics | Patients with
survivin and PTEN low scores positive expression, n (%) | Patients with
either survivin or PTEN moderate scores positive expression, n
(%) | P-value |
|---|
| Age, years |
|
|
|
|
<60 | 4 (57.1) | 17 (25.4) | 0.095 |
|
≥60 | 3 (42.9) | 50 (74.6) |
|
| Histological
type |
|
|
|
|
Endometrioid | 6 (85.7) | 58 (86.6) | >0.999 |
| Clear
cell and papillary serous | 1 (14.3) | 9 (13.4) |
|
| Clinical stage |
|
|
|
| I | 7 (100.0) | 46 (79.3) | 0.444 |
| II | 0 (0.0) | 9 (15.5) |
|
|
III | 0 (0.0) | 3 (5.2) |
|
| Histological
differentiation |
|
|
|
| G1 | 0 (0.0) | 13 (19.4) | 0.143 |
| G2 | 6 (85.7) | 32 (47.8) |
|
| G3 | 1 (14.3) | 22 (32.8) |
|
| Myometrial
invasion |
|
|
|
|
<1/2 | 1 (14.3) | 22 (32.8) | 0.424 |
|
≥1/2 | 6 (85.7) | 45 (67.2) |
|
| Lymph-vascular
space invasion |
|
|
|
|
Yes | 3 (50.0) | 8 (18.6) | 0.117 |
| No | 3 (50.0) | 35 (81.4) |
|
| Fallopian tube
and/or ovarian invasion |
|
|
|
|
Yes | 0 (0.0) | 11 (35.5) | 0.285 |
| No | 4 (100.0) | 20 (64.5) |
|
| Tumoral
necrosis |
|
|
|
|
Yes | 1 (20.0) | 4 (10.5) | >0.999 |
| No | 4 (80.0) | 34 (89.5) |
|
 | Table IX.Co-expression of survivin and p53 in
endometrial carcinoma according to sum of stain intensity and
scores of immunopositive cells in relation to clinicopathological
parameters. |
Table IX.
Co-expression of survivin and p53 in
endometrial carcinoma according to sum of stain intensity and
scores of immunopositive cells in relation to clinicopathological
parameters.
|
Characteristics | Patients with
survivin and p53 + expression, n (%) | Patients with
either survivin or p53 ++ expression, n (%) | Patients with
survivin and p53 +++ expression, n (%) | P-value |
|---|
| Age, years |
|
|
|
|
|
<60 | 2 (50.0) | 21 (35.6) | 0 (0.0) | 0.001 |
|
≥60 | 2 (50.0) | 38 (64.4) | 8 (100.0) |
|
| Histological
type |
|
|
|
|
|
Endometrioid | 4 (100.0) | 56 (94.9) | 6 (75.0) | 0.020 |
| Clear
cell and papillary serous | 0 (0.0) | 3 (5.1) | 2 (25.0) |
|
| Clinical stage |
|
|
|
|
| I | 3 (100.0) | 47 (88.7) | 3 (42.9) | 0.037 |
| II | 0 (0.0) | 5 (9.4) | 3 (42.9) |
|
|
III | 0 (0.0) | 1 (1.9) | 1 (14.3) |
|
| Histological
differentiation |
|
|
|
|
| G1 | 3 (75.0) | 11 (18.6) | 1 (12.5) | 0.001 |
| G2 | 1 (25.0) | 37 (62.7) | 2 (25.0) |
|
| G3 | 0 (0.0) | 11 (18.6) | 5 (62.5) |
|
| Myometrial
invasion |
|
|
|
|
|
<1/2 | 2 (50.0) | 24 (40.7) | 0 (0.0) | 0.100 |
|
≥1/2 | 2 (50.0) | 35 (59.3) | 8 (100.0) |
|
| Lymph-vascular
space invasion |
|
|
|
|
|
Yes | 0 (0.0) | 7 (18.9) | 2 (28.6) | 0.842 |
| No | 2 (100.0) | 30 (81.1) | 5 (71.4) |
|
| Fallopian tube
and/or ovarian invasion |
|
|
|
|
|
Yes | 0 (0.0) | 7 (26.9) | 3 (60.0) | 0.026 |
| No | 2 (100.0) | 19 (73.1) | 2 (40.0) |
|
| Tumoral
necrosis |
|
|
|
|
|
Yes | 0 (0.0) | 4 (12.1) | 1 (16.7) | >0.999 |
| No | 2 (100.0) | 29 (87.9) | 5 (83.3) |
|
 | Table V.Co-expression of survivin and PTEN in
endometrial carcinomas according to stain intensity of
immunopositive cells in relation to clinopathological
parameters. |
Table V.
Co-expression of survivin and PTEN in
endometrial carcinomas according to stain intensity of
immunopositive cells in relation to clinopathological
parameters.
|
Characteristics | Patients with
survivin and PTEN weak positive expression, n (%) | Patients with
either survivin or PTEN moderate positive expression, n (%) | Patients with
survivin and PTEN strong positive expression, n (%) | P-value |
|---|
| Age, years |
|
|
|
|
|
<60 | 1 (9.1) | 15 (32.6) | 1 (33.3) | 0.145 |
|
≥60 | 10 (90.9) | 31 (67.4) | 2 (66.7) |
|
| Histological
type |
|
|
|
|
|
Endometrioid | 9 (81.8) | 44 (95.7) | 2 (66.7) | 0.098 |
| Clear
cell and papillary serous | 2 (18.2) | 2 (4.3) | 1 (33.3) |
|
| Clinical stage |
|
|
|
|
| I | 8 (88.9) | 34 (82.9) | 3 (100.0) | 0.366 |
| II | 1 (11.1) | 4 (9.8) | 0 (0.0) |
|
|
III | 0 (0.0) | 3 (7.3) | 0 (0.0) |
|
| Histological
differentiation |
|
|
|
|
| G1 | 6 (54.5) | 11 (23.9) | 0 (0.0) | 0.023 |
| G2 | 3 (27.3) | 24 (52.2) | 2 (66.7) |
|
| G3 | 2 (18.2) | 11 (23.9) | 1 (33.3) |
|
| Myometrial
invasion |
|
|
|
|
|
<1/2 | 5 (45.5) | 17 (37.0) | 0 (0.0) | 0.479 |
|
≥1/2 | 6 (54.5) | 29 (63.0) | 3 (100.0) |
|
| Lymph-vascular
space invasion |
|
|
|
|
|
Yes | 0 (0.0) | 4 (14.3) | 2 (100.0) | 0.007 |
| No | 9 (100.0) | 24 (85.7) | 0 (0.0) |
|
| Fallopian tube
and/or ovarian invasion |
|
|
|
|
|
Yes | 1 (16.7) | 6 (28.6) | 0 (0.0) | 0.031 |
| No | 5 (83.3) | 15 (71.4) | 0 (0.0) |
|
| Tumoral
necrosis |
|
|
|
|
|
Yes | 0 (0.0) | 4 (15.4) | 0 (0.0) | 0.516 |
| No | 9 (100.0) | 22 (84.6) | 0 (0.0) |
|
 | Table VI.Co-expression of survivin and PTEN in
endometrial carcinomas according to sum of stain intensity and
scores of immunopositive cells in relation to clinopathological
parameters. |
Table VI.
Co-expression of survivin and PTEN in
endometrial carcinomas according to sum of stain intensity and
scores of immunopositive cells in relation to clinopathological
parameters.
|
Characteristics | Patients with
either survivin or PTEN ++ expression, n (%) | Patients with
survivin and PTEN +++ expression, n (%) | P-value |
|---|
| Age, years |
|
|
|
|
<60 | 17 (29.8) | 1 (50.0) | 0.101 |
|
≥60 | 40 (70.2) | 1 (50.0) |
|
| Histological
type |
|
|
|
|
Endometrioid | 54
(94.7) | 1 (50.0) | 0.020 |
| Clear
cell and papillary serous | 3 (5.3) | 1 (50.0) |
|
| Clinical stage |
|
|
|
| I | 40 (80.0) | 2 (100.0) | 0.837 |
| II | 7 (14.0) | 0 (0.0) |
|
|
III | 3 (6.0) | 0 (0.0) |
|
| Histological
differentiation |
|
|
|
| G1 | 12 (21.1) | 0 (0.0) | 0.363 |
| G2 | 32 (56.1) | 1 (50.0) |
|
| G3 | 13 (22.8) | 1 (50.0) |
|
| Myometrial
invasion |
|
|
|
|
<1/2 | 18 (31.6) | 0 (0.0) | 0.425 |
|
≥1/2 | 39 (68.4) | 2 (100.0) |
|
| Lymph-vascular
space invasion |
|
|
|
|
Yes | 8 (21.6) | 1 (50.0) | 0.272 |
| No | 29 (78.4) | 1 (50.0) |
|
| Fallopian tube
and/or ovarian invasion |
|
|
|
|
Yes | 10 (34.5) | 0 (0.0) | 0.352 |
| No | 19 (65.5) | 0 (0.0) |
|
| Tumoral
necrosis |
|
|
|
|
Yes | 5 (14.7) | 0 (0.0) | 0.687 |
| No | 29 (85.3) | 0 (0.0) |
|
In the case of concomitant survivin and p53
expression, there was a statistically significant correlation
between the positive cell scores and the patient's age (P=0.001),
and the presence of fallopian tube and/or ovarian invasion
(P=0.027; Table VII). The staining
intensity was correlated with patient age (P=0.014), histological
type (P=0.001), clinical stage (P=0.032) and histological
differentiation (P<0.001; Table
VIII). The sum of immunopositive cell-scores and staining
-intensity was correlated with patient age (P=0.001), histological
type (P=0.020), clinical stage (P=0.037), histological
differentiation (P=0.001) and presence of fallopian tube and/or
ovarian invasion (P=0.026; Table
IX).
 | Table VII.Co-expression of survivin and p53 in
endometrial carcinoma according to scores of immunopositive cells
in relation to clinicopathological parameters. |
Table VII.
Co-expression of survivin and p53 in
endometrial carcinoma according to scores of immunopositive cells
in relation to clinicopathological parameters.
|
Characteristics | Patients with
survivin and p53 low scores positive expression, n (%) | Patients with
either survivin or p53 moderate scores positive expression, n
(%) | P-value |
|---|
| Age, years |
|
|
|
|
<60 | 5 (71.4) | 18 (23.7) | 0.001 |
|
≥60 | 2 (28.6) | 58 (76.3) |
|
| Histological
type |
|
|
|
|
Endometrioid | 7 (100.0) | 65 (85.5) | 0.676 |
| Clear
cell and papillary serous | 0 (0.0) | 11 (14.5) |
|
| Clinical stage |
|
|
|
| I | 6 (100.0) | 52 (78.8) | 0.320 |
| II | 0 (0.0) | 10 (15.2) |
|
|
III | 0 (0.0) | 4 (6.1) |
|
| Histological
differentiation |
|
|
|
| G1 | 3 (42.9) | 13 (17.1) | 0.315 |
| G2 | 4 (57.1) | 38 (50.0) |
|
| G3 | 0 (0.0) | 25 (32.9) |
|
| Myometrial
invasion |
|
|
|
|
<1/2 | 2 (28.6) | 27 (35.5) | 0.933 |
|
≥1/2 | 5 (71.4) | 49 (64.5) |
|
| Lymph-vascular
space invasion |
|
|
|
|
Yes | 0 (0.0) | 11 (22.0) | 0.667 |
| No | 4 (100.0) | 39 (51.3) |
|
| Fallopian tube
and/or ovarian invasion |
|
|
|
|
Yes | 0 (0.0) | 14 (37.8) | 0.027 |
| No | 3 (100.0) | 23 (62.2) |
|
| Tumoral
necrosis |
|
|
|
|
Yes | 0 (0.0) | 5 (11.1) | 0.618 |
| No | 4 (100.0) | 40 (88.9) |
|
 | Table VIII.Co-expression of survivin and p53 in
endometrial carcinoma according to stain intensity of
immunopositive cells in relation to clinicopathological
parameters. |
Table VIII.
Co-expression of survivin and p53 in
endometrial carcinoma according to stain intensity of
immunopositive cells in relation to clinicopathological
parameters.
|
Characteristics | Patients with
survivin and p53 weak positive expression, n (%) | Patients with
either survivin or p53 moderate positive expression, n (%) | Patients with
survivin and p53 strong positive expression, n (%) | P-value |
|---|
| Age, years |
|
|
|
|
|
<60 | 3 (23.1) | 18 (35.3) | 0 (0.0) | 0.014 |
|
≥60 | 10 (76.9) | 33 (64.7) | 8 (100.0) |
|
| Histological
type |
|
|
|
|
|
Endometrioid | 12 (92.3) | 50 (98.0) | 6 (75.0) | 0.001 |
| Clear
cell and papillary serous | 1 (7.7) | 1 (2.0) | 2 (25.0) |
|
| Clinical stage |
|
|
|
|
| I | 10 (90.9) | 41 (87.2) | 2 (33.3) | 0.032 |
| II | 1 (9.1) | 5 (10.6) | 3 (50.0) |
|
|
III | 0 (0.0) | 1 (2.1) | 1 (16.7) |
|
| Histological
differentiation |
|
|
|
|
| G1 | 6 (46.2) | 10 (19.6) | 1 (12.5) | <0.001 |
| G2 | 7 (53.8) | 31 (60.8) | 2 (25.0) |
|
| G3 | 0 (0.0) | 10 (19.6) | 5 (62.5) |
|
| Myometrial
invasion |
|
|
|
|
|
<1/2 | 6 (46.2) | 20 (39.2) | 1 (12.5) | 0.274 |
|
≥1/2 | 7 (53.8) | 31 (60.8) | 7 (87.5) |
|
| Lymph-vascular
space invasion |
|
Yes | 1 (14.3) | 6 (18.2) | 2 (25.0) | 0.799 |
| No | 6 (85.7) | 27 (81.8) | 6 (75.0) |
|
| Fallopian tube
and/or ovarian invasion |
|
|
|
|
|
Yes | 1 (20.0) | 6 (27.3) | 4 (66.7) | 0.084 |
| No | 4 (80.0) | 16 (72.7) | 2 (33.3) |
|
| Tumoral
necrosis |
|
|
|
|
|
Yes | 0 (0.0) | 3 (10.3) | 1 (14.3) | 0.733 |
| No | 7 (100.0) | 26 (89.7) | 6 (85.7) |
|
Comparison of the immunohistochemical
expression of p53, PTEN and survivin among normal, hyperplastic and
carcinomatous endometria
Fig. 8 shows the p53
immunohistochemical expression in normal and hyperplastic
endometria (Fig. 8A and B) and
absence of p53 expression in a normal endometrium (Fig. 8C). Therefore, in the case of p53
expression, the staining scores were statistically significantly
correlated between normal and hyperplastic endometria (P=0.035);
patients with endometrial hyperplasia showed an increased count of
high p53 expression scores, while patients with normal endometria
showed an increased count of low or moderate p53 scores (Fig. 9A). In addition, the scores of p53
expression were statistically significantly correlated between
normal endometria and endometrial carcinomas (P=0.016); patients
with endometrial carcinomas exhibited more p53 positive cell scores
than those with normal endometria (Fig.
9B).
Fig. 10 shows the
PTEN immunohistochemical expression in normal and hyperplastic
endometria (Fig. 10A and B) and
absence of PTEN expression in a normal endometrium (Fig. 10C). Therefore, in the case of PTEN
expression, there was a statistical significance in the staining
intensity of immunopositive cells between normal endometria and
endometrial carcinomas (P<0.001); more patients with endometrial
carcinomas exhibited weak or moderate or high PTEN expression
intensity, as compared to those with normal endometria (Fig. 11A). In addition, there was a
statistically significant association between the sum of staining
intensity and PTEN immunopositive cell scores in normal endometria
and endometrial carcinomas (P=0.002); patients with normal
endometria showed a lower count of (++) sum of PTEN positive
expression, as compared to those with endometrial carcinomas
(Fig. 11B). The intensity of PTEN
expression was statistically significantly correlated between
endometrial hyperplasias and carcinomas (P=0.002); patients with
endometrial hyperplasia exhibited lower counts of moderate and
strong PTEN expression than those with endometrial carcinomas
(Fig. 11C). Finally, there was a
statistically significant correlation in the sum of staining
intensity and positive PTEN cell scores between endometrial
hyperplasias and endometrial carcinomas (P=0.006); patients with
endometrial hyperplasia exhibited lower counts of (+) and (++)
expression, as compared to those with endometrial carcinomas
(Fig. 11D).
Fig. 12 shows the
immunohistochemical expression of survivin in normal and
hyperplastic endometria (Fig.
12A-C) and absence of survivin expression in a normal
endometrium (Fig. 12D). In the case
of concomitant expression of survivin and p53, according to the
proportion of immunopositive cell scores, there was a statistically
significant expression of survivin between normal endometria and
endometrial carcinomas (P=0.048); patients with endometrial
carcinomas demonstrated increased concurrent positive survivin and
p53 cell scores as compared to those with a normal endometrium
(Fig. 13A). In the case of
concomitant survivin and PTEN expression, according to the
intensity of immunopositivity, there was a statistically
significant expression of survivin between endometrial hyperplasias
and endometrial carcinomas (P=0.008); patients with endometrial
carcinomas showed more weak or moderate intensity for concurrent
positive survivin and PTEN immunostaining, as compared to those
with endometrial hyperplasias (Fig.
13B). In addition, there was a statistically significant
correlation in the sum of staining scores and intensity in the case
of survivin and PTEN coexistence between endometrial hyperplasias
and endometrial carcinomas (P=0.016); patients with endometrial
carcinomas had an increased count of (+) or (++) sum expression for
concurrent survivin and PTEN expression, as compared to patients
with endometrial hyperplasias.
Discussion
In the present study, the immunohistochemical
expression of survivin was examined in 99 endometrial
adenocarcinomas from Greek patients. Survivin expression was
observed in 88% (87/99) of cases, taking into account the overall
histological types and confirming that survivin is a very common
genetic alteration in endometrial carcinomas. In the international
literature the distribution of survivin expression in endometrial
carcinomas was 43–100% (36,38,40,44,45).
Lambropoulou et at (38),
also studied the expression of survivin in Greek patients with
endometrial carcinomas and found a frequency of only 43%. Brunner
et al (40), identified the
expression of survivin in 45% of cases, Pallares et al
(36) in 76%, Chuwa et al
(45) in 86% and Lehner et al
(44) in 100%. These wide variations
in the frequency of survivin expression in endometrial carcinomas
could be due to a number of reasons, including geographic location,
antibodies used, antibody dilutions, interpretation of staining,
heterogeneity of endometrial carcinomas and differences in the
immunohistochemichal protocols.
In the endometrium, survivin is not exclusively
expressed in carcinomas and is therefore not a specific marker for
endometrial malignancies. Lehner et al identified the mRNA
expression of survivin in both normal and malignant endometria
(44). In addition, Saitoh et
al (46), Konno et al
(47) and Lehner et al
(44) identified the mRNA expression
of survivin in normal endometria. However, Saitoh et al
(46) demonstrated that the mRNA
expression of survivin in endometrial cancer specimens was
significantly higher than in the normal endometria. Ai et al
(48), had the same results as
Saitoh et al (46). The
staining of survivin in the present study showed exclusively
nuclear localization. A combination of nuclear and cytoplasmic
staining for survivin has been described in the international
literature. However, Yilmaz et al (39) did not determine any statistically
significant difference between cytoplasmic and nuclear
expression.
In the present study, a significant correlation was
observed between the sum of staining intensity and scores of
survivin immunopositive cells, and patient age (P=0.028),
histological grade (P<0.001), clinical stage (P=0.018) and
fallopian tube and/or ovarian invasion (P=0.039). The findings of
the present study suggested that survivin may be an indicator of
unfavorable outcome in older patients with endometrial carcinomas.
In particular, it was found that higher levels of survivin were
associated with a higher number of older patients, more cases with
higher histological G3 and less cases with G2. In addition, the
higher levels of survivin were associated with a larger number of
stage II and III patients and a smaller number of stage I patients.
However, in previous reports investigating the role of survivin in
endometrial cancer, controversial results were obtained.
Lambropoulou et al (38),
suggested a significant correlation between survivin expression and
histological grade, stage, myometrial invasion and survival rates.
Lehner et al (44), found a
correlation between survivin mRNA expression and the histological
grade of tumors. Takai et al (16), reported that survivin expression was
significantly associated with histological grade, surgical stage,
myometrial invasion and survival rate. On the other hand, Ai et
al (48) did not observe any
association with patient age, histological grade or stage of
endometrial carcinomas. Pallares et al (36), had similar results with Ai et
al (48). In addition, Erkanli
et al (41) found no
correlation between survivin and the classic prognostic factors for
endometrial carcinomas, such as histological grade, stage and
myometrial invasion or survival in patients with endometrial
carcinomas. Their results were supported by Yilmaz et al
(39) as they did not find any
association between survivin expression and the clinical prognostic
factors including lymphovascular space involvement and extrauterine
spread of disease or survival. Aksoy et al (42), found no statistical association
between survival and prognostic factors such as histological grade,
stage, or cytoplasmic and nuclear expression of survivin in
endometrial carcinomas. All these differences with regard to the
prognostic role of survivin in endometrial carcinoma may be due to
different concomitant genetic alterations and different molecular
pathways taking place during endometrial carcinogenesis and
metastatic expansion. It is possible that the plethora of different
concomitant genetic interactions results in a different biological
behavior of endometrial carcinomas. In fact, it has been indicated
that poor prognosis was associated with concomitant PI3K/AKT and
p53 alterations in endometrial carcinomas (49). Nout et al (50), reported that the concomitant
activation of p53 with microsatellite instability was a strong
genetic prognostic factor for disease-free survival in endometrial
carcinomas. It has also been found that the PTEN-positive and
phosphorylated-AKT-negative expression is a good predictor of
survival in patients with advanced endometrial carcinomas (51). In addition to these findings,
survivin appears to demonstrate multi-functional action that
promotes proliferation, angiogenesis and metastatic spread on top
of inhibiting apoptosis (52–54). In
order to determine the exact prognostic significance of the
expression of survivin in endometrial carcinoma, a deeper
understanding of the interaction of survivin with other genetic
alterations that take place during tumor development, progression
and metastatic process was required. It appears that molecular
interaction between survivin and X-linked inhibitor of apoptosis
protein stimulates tumor cell invasion and promotes metastasis
(55). An association between the
co-expression of survivin and the vascular endothelial growth
factor-C (VEGF-C) in lymph node metastasis has been identified in
breast (56), gastric (57) and papillary thyroid carcinomas
(58). All these findings indicated
that the regulated expression of VEGF-C by survivin is essential
for the invasion and lymphatic metastasis of these tumors (58). With regards to the relationship
between survivin and p53 at the molecular level, the following are
known so far: Functional p53-binding sites have been identified
withing the BIRC5 gene promoter, suggesting the possible
involvement of p53 in the direct repression of BIRC5 gene promoter
activity (59,60). There have been indications that
wild-type, but not mutated p53 represses BIRC5 expression upon DNA
damage at the transcription level, and regulates normal cell cycle
and apoptosis (60–62). In addition, p53 represses BIRC5 gene
promoter activity by counteracting the BIRC5 promoter through the
binding of Sp1 factor and hypoxia inducing factor (63,64). It
has been found that, in normal endometria, the BIRC5 gene promoter
is completely unmethylated, while in endometrial cancers the
hypermethylation of the BIRC5 gene promoter blocks the binding of
p53 to its promoter region and causes the elevated expression of
the survivin protein (65). On the
other hand, survivin overexpression mediates the p53-dependent
apoptotic pathway through the mouse double minute 2 homolog (MDM2)
oncoprotein. In fact, it has been shown that the overexpression of
survivin causes the promotion of p53 degradation through the
inhibition of MDM2 cleavage (66).
With regards to the association between survivin and PTEN at the
molecular level, the survivin gene is negatively regulated by PTEN
(67). It has been reported that the
acute silencing of the survivin gene is essential for endogenous
PTEN-mediated tumor suppression through the binding of Forkhead Box
O1 (FOXO1) and FOXO2 factors to the proximal survivin promoter in
several types of cancers (26,68,69).
In the present study, we investigated the effects of
immunohistochemical PTEN and p53 expression on the expression of
survivin in endometrial carcinomas. In our previous study we
investigated the distribution of tumor suppressor genes p53 and
PTEN in primary endometrial carcinoma specimens acquired from Greek
patients and analyzed the clinical significance of the combination
of p53 and PTEN expression (43). We
believe that the present findings are interesting, since the
clinical significance of the interplay between the expression of
the tumor suppressor genes PTEN and p53, and survivin in
endometrial carcinomas remained unknown so far. In our previous
study the levels of p53 and PTEN co-expression were found to be
correlated with patient age (P=0.08) and histological
differentiation (P=0.028), according to the scores of
immunopositive cells. We therefore suggested that the concomitant
expression of p53 and PTEN may play a role in the development of
high-grade endometrial carcinomas in older patients (43). In addition, these findings suggested
the involvement of different molecular pathways in the development
of low- and high-grade endometrial carcinomas (43). In the present study, a negative
tendency was identified between the scores of survivin and PTEN
expression (P=0.062, ρ=−0.238). It appears that cases with high
scores of survivin immunoexpression tended to have decreased scores
of PTEN immunostaining, and vice versa. This finding is very
interesting, as PTEN and survivin are two inverse factors of
apoptosis (28). This finding was
strengthened by the study of Pallares et al (36), in which they identified a
statistically significant negative correlation between survivin and
PTEN expression. PTEN and survivin appear to act in opposite ways
from each other in endometrial carcinoma, and PTEN modulates the
survivin level via the PI3K/AKT pathway (28,36).
However, Erkanli et al (37)
did not report such correlation probably due to i) the low number
of studied patients with endometrial carcinoma and ii) the fact
that no patients with papillary serous or clear cell
adenocarcinomas were included in the study. Therefore, further
research that includes a larger number of patients is required in
order to clarify the exact role of the interaction and relationship
between the expression of survivin and that of PTEN in endometrial
carcinoma. This could lead to the development of novel target gene
therapies for the effective treatment of endometrial carcinoma. In
the present study, a statistically significant correlation was
identified between the sum of staining intensity and scores of
survivin and PTEN immunopositivity and histological type in
endometrial adenocarcinomas (P=0.020). In fact, it was found that
moderate levels of survivin and PTEN co-expression were associated
with a higher frequency of endometrioid histological types, while
the high levels of concomitant survivin and PTEN expression were
associated with a lower frequency of endometrioid histological
types. In cases of concomitant survivin and PTEN expression, a
statistically significant correlation was observed according to the
staining intensity and histologic grade (P=0.023), presence of
lymph-vascular space invasion (P=0.007) and fallopian tube and/or
ovarian invasion (P=0.031). These findings suggested that the
co-expression of and interaction between survivin and PTEN may play
a role in the development of more aggressive endometrial
carcinomas. In the present study, in cases with concomitant PTEN
and survivin, and according to the intensity of immunochemical
positivity, a statistically significant difference in the
expression of survivin was identified between endometrial
hyperplasias and endometrial carcinomas (P=0.008). In addition, the
sum of survivin staining scores and intensity in cases with
concurrent PTEN positive staining showed a statistically
significance in the expression of survivin between endometrial
hyperplasias and endometrial carcinomas (P=0.016). Therefore, the
present findings clearly indicated the interaction between survivin
and PTEN during endometrial carcinogenesis.
Survivin and p53 co-expression and their association
with the clinical behavior and histological types of endometrial
carcinomas were investigated in the present study, due to the
limited related data in the literature so far. A statistically
significantly positive correlation between survivin and p53
concurrent expression was identified based on staining intensity
(P=0.001, ρ=0.372) or the sum of staining intensity and scores
(P=0.008, ρ=0.300). These findings suggested that these proteins
are strongly positively correlated and may share a common molecular
pathway through which they regulate each other's action and promote
endometrial carcinogenesis. Herein, a significant correlation was
found between the sum staining intensity and scores of survivin and
p53 immunopositivity and age of patients (P=0.001), histological
type (P=0.020), clinical stage (P=0.037), histological
differentiation (P=0.001) and presence of fallopian tube and/or
ovarian invasion (P=0.026). These results showed the synergic
action of survivin and p53 and indicated their prognostic value for
endometrial cancer. A concomitant expression of survivin and p53
seems to favor the growth of more aggressive endometrial carcinomas
in older patients. The concomitant expression of these markers
could have a prognostic significance. Survivin and p53 could be
important targets for therapeutic interventions in new target gene
therapies for endometrial malignancies. A statistically significant
difference in the scores of immunohistochemical survivin staining
was observed between normal endometria and endometrial carcinomas
(P=0.048). The present findings suggested the importance of
concurrent survivin and p53 expression for the development of
endometrial carcinomas.
In conclusion, the expression of survivin in
specific circumstances appears to depend on different concomitant
genetic alterations and the different combinations of molecular
pathways in endometrial carcinogenesis. This study showed a
negative tendency for correlation between the concurrent
immunopositive survivin and PTEN cell scores, and suggested their
pivotal role in the development of more aggressive tumors in such
circumstances. Moreover, the survivin and p53 proteins may share a
common molecular pathway, and their combined evaluation may
implicate them in the prediction of tumor behavior and
prognosis.
Acknowledgements
The present study was part of a thesis for a Doctor
of Philosophy (Ph.D.) in Obstetrics and Gynecology, Medical School,
Kapodistrian University of Athens, Greece for AS.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors were responsible for the conception and
design of the present study. TV and AT were responsible for the
provision of the study materials. TV, AT, VKV and FNV were
responsible for the collection and assembly of the data. AS, MV,
TV, VKV, FNV, AT, AN, NK and ACL performed the data analysis and
interpretation. AS, MV, TV, VKV, FNV, AT, AN, NK and ACL
contributed to writing of the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Medical School of Kapodistrian University of Athens,
Greece. Written informed consent was obtained from all patients for
the use of their tumor specimens and their data in analysis.
Patient consent for publication
All patients included in the present study provided
consent for their data to be used in this publication at the time
of data collection.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sadlecki P, Bodnar M, Grabiec M, Marszalek
A, Walentowicz P, Sokup A, Zegarska J and Walentowicz-Sadlecka M:
The role of Hypoxia-inducible factor 1α, glucose transporter-1
(GLUT-1) and carbon anhydrase IX in endometrial cancer patients.
Biomed Res Int. 2014:6168502014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Daniilidou K, Frangou-Plemenou M,
Grammatikakis J, Grigoriou O, Vitoratos N and Kondi-Pafiti A:
Prognostic significance and diagnostic value of PTEN and p53
expression in endometrial carcinoma. A retrospective
clinicopathological and immunohistochemical study. J BUON.
18:195–201. 2013.PubMed/NCBI
|
|
4
|
Talhouk A and McAlpine JN: New
classification of endometrial cancers: The development and
potential applications of genomic-based classification in research
and clinical care. Gynecol Oncol Res Pract. 3:142016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taoussi N, Alghamdi A, Futyma K and
Rechberger T: Biological markers with potential clinical value in
endometrial cancer-review of the literature. Ginekol Pol.
88:331–336. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mancebo G, Sole-Sedeno JM, Pino O,
Miralpeix E, Mojal S, Garrigos L, Lloveras B, Navarro P, Gibert J,
Lorenzo M, et al: Prognostic impact of CD133 expression in
endometrial cancer patients. Sci Rep. 7:76872017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang HM, Fan TT, Li W and Li XX:
Expressions and significances of TTF-1 and PTEN in early
endometrial cancer. Eur Rev Med Pharmacol. 21 (3 Suppl):S20–S26.
2017.
|
|
8
|
Hashmi AA, Hussain ZF, Qadri A, Irfan M,
Ramzan S, Faridi N, Khan A and Edhi MM: Androgen receptor
expression in endometrial carcinoma and its correlation with
clinicopathologic features. BMC Res Notes. 11:2892018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bakkum-Gamez JN, Gonzalez-Bosquet J, Laack
NN, Mariani A and Dowdy SC: Current issues in the management of
endometrial cancer. Mayo Clin Proc. 83:97–112. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rudin CM and Thompson CB: Apoptosis and
disease: Regulation and clinical relevance of programmed cell
death. Annu Rev Med. 48:267–281. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
LaCasse EC, Baird S, Korneluk RG and
MacKenzie AE: The inhibitors of apoptosis (IAPs) and their emerging
role in cancer. Oncology. 17:3247–3259. 1998.
|
|
13
|
Tamm I, Wang Y, Sausville E, Scudiero DA,
Vigna N, Oltersdorf T and Reed JC: IAP-family protein survivin
inhibits caspase activity and apoptosis induced by Fas (CD95), Bax,
caspases, and anticancer drugs. Cancer Res. 58:5315–5320.
1998.PubMed/NCBI
|
|
14
|
Altieri DC: Survivin, cancer networks and
path-directed drug discovery. Nat Rev Cancer. 8:61–70. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cohen C, Lohmann CM, Cotsonis G, Lawson D
and Santoianni R: Survivin expression in ovarian carcinoma:
Correlation with apoptotic markers and prognosis. Mod Pathol.
16:574–583. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takai N, Miyazaki T, Nishida M, Nasu K and
Miyakawa I: Survivin expression correlates with clinical stage,
histological grade, invasive behavior and survival rate in
endometrial carcinoma. Cancer Lett. 184:105–116. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tran J, Master Z, Yu JL, Rak J, Dumont DJ
and Kerbel RS: A role for survivin in chemoresistance of
endothelial cells mediated by VEGF. Proc Natl Acad Sci USA.
99:4349–4354. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Altieri DC: Survivin, versatile modulation
of cell division and apoptosis in cancer. Oncogene. 22:8581–8589.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Altieri DC: Survivin and apoptosis
control. Adv Cancer Res. 88:31–52. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mita AC, Mita MM, Nawrocki ST and Giles
FJ: Survivin: Key regular of mitosis and apoptosis and novel target
for cancer therapeutics. Clin Cancer Res. 14:5000–5005. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li F, Ambrosini G, Chu EY, Plescia J,
Tognin S, Marchisio PC and Altieri DC: Control of apoptosis and
mitotic spindle checkpoing by survivin. Nature. 396:580–584. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mahotka C, Wenzel M, Springer E, Gabbert
HE and Gerharz CD: Survivin-deltaEx3 and survivin-2B: Two novel
splice variants of the apoptosis inhibitor survivin with different
antiapoptotic properties. Cancer Res. 59:6097–6102. 1999.PubMed/NCBI
|
|
23
|
Zhang M, Yang J and Li F: Transcriptional
and post-transcriptional controls of survivin in cancer cells:
Novel approaches for cancer treatment. J Exp Clin Cancer Res.
25:391–402. 2006.PubMed/NCBI
|
|
24
|
Dohi T, Beltrami E, Wall NR, Plescia J and
Alteiri DC: Mitochondrial survivin inhibits apoptosis and promotes
tumorigenesis. J Clin Invest. 114:1117–1127. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sui L, Dong Y, Ohno M, Watanabe Y,
Sugimoto K and Tokuda M: Survivin expression and its correlation
with cell proliferation and prognosis in epithelial ovarian tumors.
Int J Oncol. 21:315–120. 2002.PubMed/NCBI
|
|
26
|
Guha M, Plescia J, Leav I, Li J, Languino
LR and Altieri DC: Endogenous tumor suppression mediated by PTEN
involves survivin gene silencing. Cancer Res. 69:4954–4958. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim S, Kang J, Qiao J, Thomas RP, Evers BM
and Chung DH: Phosphatidylinositol 3-kinase inhibition
down-regulates survivin and facilitates TRAIL-mediated apoptosis in
neuroblastomas. J Padiatr Surg. 39:516–521. 2004. View Article : Google Scholar
|
|
28
|
Sui L, Dong, Watanabe Y, Yamaguchi F,
Sugimoto K and Tokuda M: Alteration and clinical relevance of PTEN
expression and its correlation with survivin expression in
epithelial ovarian tumors. Oncol Rep. 15:773–778. 2006.PubMed/NCBI
|
|
29
|
Grossman D, Kim PJ, Blanc-Brude OP, Brash
DE, Tognin S, Marchisio PC and Altieri DC: Transgenic expression of
survivin in keratinocytes counteracts UVB-induced apoptosis and
cooperates with loss of p53. J Clin Invest. 108:991–999. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hoffman WH, Biade S, Zilfou JT, Chen J and
Murphy M: Trascriptional repression of the anti-apoptotic survivin
gene by wild type p53. J Biol Chem. 277:3247–3257. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mirza A, McGuirk M, Hockenberry TN, Wu Q,
Ashar H, Black S, Wen SF, Wang L, Kirschmeier P, Bishop WR, et al:
Human survivin is negatively regulated by wild-type p53 and
participates in p53-dependent apoptotic pathway. Oncogene.
21:2613–2622. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kannangai R, Wang J, Liu QZ, Sahin F and
Torbenson M: Survivin overexpression in hepatocellular carcinoma is
associated with p53 dysregulation. Int J Gastrointest Cancer.
35:53–60. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Felisiak-Golabek A, Rembiszewska A,
Rzepecka IK Szafron L, Madry R, Murawska M, Napiorkowski T,
Sobiczewski P, Osuch B and Kupryjanczyk J; PolishOvarian Cancer
Study Group (POCSG), : Nuclear survivin expression is a positive
prognostic factor in taxane-platinum-treated ovarian cancer
patients. J Ovarian Res. 4:202011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gąsowska-Bodnar A, Bodnar L, Dąbek A,
Cichowicz M, Jerzak M, Cierniak S, Kozłowski W and Baranowski W:
Survivin expression as a prognostic factor in patients with
epithelial ovarian cancer or primary peritoneal cancer treated with
neoadjuvant chemotherapy. Int J Gynecol Cancer. 24:687–696. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gonzalez S, Aubert S, Kerdraon O, Haddad
O, Fantoni JC, Bisere J and Lorey X: Prognostic value of combined
p53 and survivin in pT1G3 urothelial carcinoma of the bladder. Am J
Clin Pathol. 129:232–237. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pallares J, Martínez-Guitarte JL, Dolcet
X, Llobet D, Rue M, Palacios J, Prat J and Matias-Guiu X: Survivin
expression in endometrial carcinoma: A tissue microarray study with
correlation with PTEN and STAT-3. Int J Gynecol Pathol. 24:247–253.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Erkanli S, Kayaselcuk F, Kuscu E, Bagis T,
Bolat F, Haberal A and Demirhan B: Expression of survivin, PTEN and
p27 in normal, hyperplastic, and carcinomatous endometrium. Int J
Gynecol Cancer. 16:1412–1418. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lambropoulou M, Papadopoulos N, Tripsianis
G, Alexiadis G, Pagonopoulou O, Kiziridou A, Liberis V, Kakolyris S
and Chatzaki E: Co-expression of survivin, c-erbB2, and
cyclooxygenase-2 (COX-2): Prognostic value and survival of
endometrial cancer patients. J Cancer Res Clin Oncol. 136:427–435.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yilmaz E, Koyuncuoglu M, Görken IB, Okyay
E, Saatli B, Ulukus EC and Saygili U: Expression of matrix
metalloproteinase-2 and survivin in endometrioid and
nonendometrioid endometrial cancers and clinicopathologic
significance. J Gynecol Oncol. 22:89–96. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Brunner A, Riss P, Heinze G and Brustmann
H: pHH3 and survivin are co-expressed in high-risk endometrial
cancer and are prognostic relevant. Br J Cancer. 107:84–90. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Erkanli S, Bolat F, Kayaselcuk F, Demirhan
B and Kuscu E: COX-2 and survivin are overexpressed and positively
correlated in endometrial carcinoma. Gynecol Oncol. 104:320–325.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Aksoy RT, Turan AT, Boran N, Tokmak A,
Isikdogan BZ, Tulunay HG and Dogan M: Lack of relation of survivin
gene expression with survival and surgical prognostic factors in
endometrial carcinoma patients. Asian Pac J Cancer Prev.
15:6905–6910. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Stavropoulos A, Varras M, Vasilakaki T,
Varra VK, Tsavari A, Varra FN, Nonni A, Kavantzas N and Lazaris AC:
Expression of p53 and PTEN in human primary endometrial carcinomas:
Clinicopathological and immunohistochemical analysis and study of
their concomitant expression. Oncol Lett. 17:4575–4589.
2019.PubMed/NCBI
|
|
44
|
Lehner R, Enomoto T, McGregor JA, Shroyer
L, Haugen BR, Pugazhenthi U and Shroyer KR: Correlation of survivin
mRNA detection with histologic diagnosis in normal endometrium and
endometrial carcinoma. Acta Obstet Gynecol Scand. 81:162–167. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chuwa AH, Sone K, Oda K, Ikeda Y, Fukuda
T, Wada-Hiraike O, Inaba K, Makii C, Takeuchi M, Oki S, et al:
Significance of survivin as a prognostic factor and a therapeutic
target in endometrial cancer. Gynecol Oncol. 141:564–569. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Saitoh Y, Yaginuma Y and Ishikawa M:
Analysis of Bcl-2, Bax and survivin genes in uterine cancer. Int J
Oncol. 15:137–141. 1999.PubMed/NCBI
|
|
47
|
Konno R, Yamakawa H, Utsunomiya H, Ito K,
Sato S and Yajima A: Expression of survivin and Bcl-2 in normal
human endometrium. Mol Hum Reprod. 6:529–534. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ai Z, Yin L, Zhou X, Zhu Y, Zhu D, Yu Y
and Feng Y: Inhibition of survivin reduces cell proliferation and
induces apoptosis in human endometrial cancer. Cancer. 107:746–756.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Catasus L, Gallardo A, Cuatrecasas M and
Prat J: Concomitant PI3K-AKT and p53 alterations in endometrial
carcinomas are associated with poor prognosis. Mod Pathol.
22:522–529. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nout RA, Bosse T, Creutzberg CL,
Jürgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, van der Steen-Banasik
EM, van Eijk R, Ter Haar NT and Smit VT: Improved risk assessment
of endometrial cancer by combined analysis of MSI, PI3K-AKT,
Wnt/β-caterin and P53 pathway activation. Gynecol Oncol.
126:466–473. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Uegaki K, Kanamori Y, Kigawa J, Kawaguchi
W, Kaneko R, Naniwa J, Takahashi M, Shimada M, Oishi T, Itamochi H
and Terakawa N: PTEN-positive and phosphorylated-Akt-negative
expression is a predictor of survival for patients with advanced
endometrial carcinoma. Oncol Rep. 14:389–392. 2005.PubMed/NCBI
|
|
52
|
Deveraux QL and Reed JC: IAP family
proteins-suppressors of apoptosis. Genes Dev. 13:239–252. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sanhueza C, Wehinger S, Castillo Bennett
J, Valenzuela M, Owen GI and Quest AF: The twisted survivin
connection to angiogenesis. Mol Cancer. 14:1982015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yan YL and Li XM: The IAP family:
Endogenous caspase inhibitors with multiple biological activities.
Cell Res. 10:169–177. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mehrotra S, Languino LR, Raskett CM,
Mercurio AM, Dohi T and Altieri DC: IAP regulation of metastasis.
Cancer Cell. 17:53–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cai X, Ma S, Gu M, Zu C, Qu W and Zheng X:
Survivin regulates the expression of VEGF-C in lymphatic metastasis
of breast cancer. Diagn Pathol. 7:522012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang J, Zhu Z, Sun Z, Sun X, Wang Z and
Xu H: Survivin gene expression increases gastric cancer cell
hymphatic metastasis by upregulating vascular endothelial growth
factor-C expression levels. Mol Med Red. 9:600–606. 2014.
View Article : Google Scholar
|
|
58
|
Selemtjev S, Savin S, Paunovic I, Tatic S
and Cvejic D: Concomitant high expression of survivin and vascular
endothelial growth factor-C is strong associated with metastatic
status of lymph nodes in papillary thyroid carcinoma. J Cancer Res
Ther. 14 (Suppl):S114–S119. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Nakano J, Huang CL, Liu D, Ueno M,
Sumitomo S and Yokomise H: Survivin gene expression is negatively
regulated by the p53 tumor suppressor gene in non-small cell lung
cancer. Int J Oncol. 27:1215–1221. 2005.PubMed/NCBI
|
|
60
|
Lyu H, Huang J, He Z and Liu B: Epigenetic
mechanist of survivin dysregulation in human cancer. Sci China Life
Sci. 61:808–814. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chen X, Duan N, Zhang C and Zhang W:
Survivin and tumorigenesis: Molecular mechanisms and therapeutic
strategies. J Cancer. 7:314–323. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Pei SG, Wang JX, Wang XL, Zhang QJ and
Zhang H: Correlation of survivin, p53 and Ki-67 in laryngeal cancer
Hep-2 cell proliferation and invasion. Asian Pac J Trop Med.
8:636–642. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Estève PO, Chin HG and Pradhan S:
Molecular mechanisms of transactivation and doxorubicin-mediated
repression of survivin gene in cancer cells. J Biol Chem.
282:2615–2625. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Smallwood A, Estève PO, Pradhan S and
Carey M: Functional cooperation between HP1 and DNMT1 mediates gene
silencing. Genes Dev. 21:1169–1178. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Nabilsi NH, Broaddus RR and Loose DS: DNA
methylation inhibits p53-mediated survivin repression. Oncogene.
28:2046–2050. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang Z, Fukuda S and Pelus LM: Survivin
regulates the p53 tumor suppressor gene family. Oncogene.
23:8146–8153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Guha M and Altieri DC: Survivin as a
global target of intrinsic tumor suppression networks. Cell Cycle.
8:2708–2710. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Calnan DR and Brunet A: The FoxO code.
Oncogene. 27:2276–2288. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Accili D and Arden KC: FoxOs at the
crossroads of cellular metabolism, differentiation, and
transformation. Cell. 117:421–426. 2004. View Article : Google Scholar : PubMed/NCBI
|