Introduction
Gliomas are malignant tumors derived from
neuroepithelial tissue and are the most common malignant tumors of
the central nervous system, accounting for 40–50% of intracranial
tumors (1). According to the WHO
classification, gliomas are divided into I–IV grades. Among them,
astrocytoma and polar glioblastoma are common, followed by
oligodendymal glioma and medulloblastoma (2). Malignant glioma generally refers to
gliomas of grades III and IV, with a high recurrence rate and poor
prognosis (3). Moreover, the
recurrence of glioma is also a major threat to the health of
patients. The recurrence time of glioma depends on the degree of
differentiation and treatment. Glioma patients receiving
conventional treatments usually relapse within 1 to 5 years
(4). Because the tumor has not been
completely resected or regenerated, a large proportion of gliomas
will recur after surgery. Therefore, it is necessary to actively
search for effective diagnosis and treatment.
In recent years, microRNAs (miRNAs/miRs) have been
reported to regulate multiple biological activities of human
cancers by blocking the expression of their target genes (5). In particular, the function of many
miRNAs has been identified in glioma. For example, miR-124-3p
inhibited cell proliferation and motility by targeting EphA2 in
gliomas (6). In addition, miR-499a
was found to inhibit cell proliferation and promote apoptosis in
glioma through inactivating Notch1 and the MAPK signaling pathway
(7). In contrast, miR-423-3p was
demonstrated to promote tumor growth of glioma by targeting PANX2
(8). Recently, the specific role of
miR-505-3p in human diseases caught our attention. For example,
miR-505 was identified as a potential biomarker for the imatinib
response in patients with chronic myeloid leukemia (9). It has been reported that miR-505
identified endothelial cell migration and tube formation in
patients with essential hypertension (10). Moreover, miR-505 was found to inhibit
growth factor-induced epithelial mesenchymal transition (EMT)
(11). However, the regulatory
mechanism of miR-505-3p remains unclear in glioma, and needs to be
further elucidated.
As a member of the HMGB superfamily, high-mobility
group box 1 (HMGB1) has been found to regulate DNA organization and
gene transcription (12).
Furthermore, HMGB1-mediated expression of matrix
metalloproteinase-9 was found to promote tumor cell invasiveness in
non-small cell lung cancer (13). In
addition, it was found that HMGB1 silencing inhibited cell
proliferation and invasion of MGC-803 gastric cancer cells
(14). Overexpression of HMGB1 was
correlated with tumor progression and poor prognosis in human
colorectal carcinoma (15).
Knockdown of HMGB1 has been found to inhibit cell proliferation and
to induce apoptosis in hemangioma by blocking AKT pathway (16). It was also reported that miR-1231
exerted tumor suppressive effects by regulating the EGFR/PI3K/AKT
axis in glioma (17). However, the
molecular mechanism of miR-505-3p/HMGB1/AKT axis remains unclear in
glioma.
In this study, the alteration of miR-505-3p
expression was first identified in glioma tissues and cell lines.
In addition, the relationship between miR-505-3p and HMGB1 was
confirmed. The regulatory mechanism of miR-505-3p/HMGB1/AKT was
also investigated in glioma cells. These results will help improve
the diagnosis and treatment of gliomas.
Patients and methods
Glioma specimens
In total, 44 human glioma tissues were acquired from
the Department of Neurosurgery, Zhangye People's Hospital
Affiliated to Hexi University (Zhangye, China). Glioma tissue
samples were classified according to the World Health Organization
(WHO) standards. Gliomas (11 of the 44) were classified as
low-grade (5 WHO I and 6 WHO II, diffuse astrocytoma) and 33 were
classified as high-grade (19 WHO III and 14 WHO IV, anaplastic
astrocytoma). Clinicopathological information of the patients with
glioma included in the present study is summarized in Table I. Eight samples of normal brain
tissues were obtained from internal decompression patients
undergoing surgical operation. The patients with glioma did not
receive any treatment prior to surgery. The tissues were frozen in
liquid nitrogen and then stored at −80°C in a refrigerator.
Informed consents were obtained from the patients. The study was
approved by the Institutional Ethics Committee of Department of
Neurosurgery, Zhangye People's Hospital Affiliated to Hexi
University.
 | Table I.Relationship between miR-505-3p
expression and their clinicopathological characteristics of glioma
patients. |
Table I.
Relationship between miR-505-3p
expression and their clinicopathological characteristics of glioma
patients.
|
|
| miR-505-3p |
|
|---|
|
|
|
|
|
|---|
| Characteristics | Cases | High | Low | P-value |
|---|
| Age (years) |
|
|
| 0.205 |
| ≥55 | 24 | 10 | 14 |
|
|
<55 | 20 | 7 | 13 |
|
| Sex |
|
|
| 0.361 |
| Male | 26 | 11 | 15 |
|
|
Female | 18 | 6 | 12 |
|
| Tumor size (mm) |
|
|
| 0.284 |
| ≤5.0 | 16 | 6 | 10 |
|
|
>5.0 | 28 | 11 | 17 |
|
| Necrosis |
|
|
|
0.032a |
|
Yes | 14 | 5 | 9 |
|
| No | 30 | 12 | 18 |
|
| WHO grade |
|
|
|
0.019a |
|
I–II | 11 | 6 | 5 |
|
|
III–IV | 33 | 11 | 22 |
|
Cell culture
The U251 (BNCC337874), A172 (BNCC341782), LN229
(BNCC341218) glioma cell lines and normal human astrocyte NHA cells
(BNCC341796) were acquired from Cell Bank of the Chinese Academy of
Sciences. Glioma cells were cultured in Dulbecco's modified Eagle's
medium (DMEM; Thermo Fisher Scientific, Inc.) supplemented with 10%
fetal bovine serum (FBS) (Thermo Fisher Scientific, Inc.) at 37°C
in a humidified incubator containing 5% CO2. NHAs were
cultured using AGM Astrocyte Growth Medium Bullet kit (Lonza)
containing astrocyte growth media and supplements.
Cell transfection
The miR-505-3p mimics (5′-GGGAGCCAGGAAGUAUUGAUGU-3′)
or inhibitor (5′-ACUACUGAGUGACAGUAGA-3′) and negative control (NC,
5′-UUCUCCGAACGUGUCACGUTT-3′) were obtained from GenePharma. Then
they were transferred into U251 cells, respectively, with
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
based on the protocols of the manufacturers. After 48 h of
transfection, the effcacy was determined by RT-qPCR analysis.
RT-qPCR analysis
Total RNA was extracted from the tissues and cells
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
Complementary DNA was synthesized by RevertAid RT Reverse
Transcription kit (Thermo Fisher Scientific, Inc.). PCR
amplifcation was performed by SYBR® Green qPCR Assay kit
(Thermo Fisher Scientific, Inc.) on ABI 7500 Fast system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) with primers. U6 or
GAPDH was used as control for miR-505-3p or HMGB1. Relative miRNA
or mRNA expression was calculated using the 2−ΔΔCq
method, normalized against U6 or GAPDH and then compared with the
control group (18). The primer
sequences used in qPCR were as follows: miR-505-3p forward,
5′-CTACGTGGGTCACCCCCTC-3′ and reverse, 5′-CCAAAGGAGACCTCGTAGT-3′;
and U6 forward, 5′-GCTTCGGCAGCACATATACTAAA-3′ and reverse,
5′-GCTTCACGAATTTGCGTGTCAT-3′. HMGB1 forward,
5′-TATGGCAAAAGCGGACAAGG-3′ and reverse,
5′-CTTCGCAACATCACCAATGGA-3′; GAPDH forward,
5′-ACAACTTTGGTATCGTGGAAGG-3′ and reverse,
5′-GCCATCACGCCACAGTTTC-3′.
Western blot analysis
The protein samples were obtained using RIPA lysis
buffer. The protein concentration was detected using a BCA protein
kit (Beyotime). A total of 50 µg of protein was separated by 10%
SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF)
membrane (Bio-Rad Laboratories, Inc.). The membrane was then
blocked using 5% skim milk and incubated with E-cadherin (Rabbit
monoclonal; dilution, 1:1,000; cat. no. ab1416; Abcam), N-cadherin
(Rabbit polyclonal; dilution, 1:1,000; cat. no. ab18203; Abcam),
Vimentin (Rabbit polyclonal; dilution, 1:1,000; cat. no. ab137321;
Abcam), MMP-2 (Rabbit polyclonal, dilution, 1:300; cat. no.
10373-2-AP; Proteintech), MMP-9 (Rabbit polyclonal, dilution,
1:600; cat. no. 10375-2-AP; Proteintech), AKT (Rabbit polyclonal;
dilution, 1:1,000; cat. no. ab8805; Abcam), p-AKT (phospho S473,
Rabbit monoclonal; dilution, 1:1,000; cat. no. ab81283; Abcam),
HMGB1 (Rabbit monoclonal; dilution, 1:1,000; cat. no. ab227168;
Abcam) and GAPDH (Rabbit monoclonal; dilution, 1:1,000; cat. no.
ab181602; Abcam) antibodies overnight at 4°C. After washing, the
membrane was washed and incubated with horseradish
peroxidase-conjugated secondary antibodies (dilution, 1:5,000; cat
no. ab190492; Abcam) for 2 h at 37°C. The protein bands were
visualized using ECL (Pierce; Thermo Fisher Scientific, Inc.).
MTT assay
To analyze cellular proliferation, 5×103
U251 cells with miR-505-3p mimics or inhibitor were cultured in a
96-well plate with 100 µl of DMEM containing 0.5 g/l MTT (Thermo
Fisher Scientific, Inc.). Then, U251 cells were cultured at 37°C
for 12, 24, 48 or 72 h, after which the medium was removed. Next,
50 µl dimethyl sulfoxide (Thermo Fisher Scientific, Inc.) was
added. Following incubation at 37°C for 10 min, the absorbance at
490 nm of each sample was determined using a plate reader (Bio-Rad
Laboratories, Inc.). All experiments were run in triplicate.
Transwell assay
Transwell chambers (8-µm pore size membranes) were
employed to perform cell migration and invasion assays. For
detection of cell migration, 5×103 cells transfected
with miR-505-3p mimics or miR-505-3p inhibitor were resuspended in
500 µl of medium without FBS and placed in the upper chambers. The
lower chamber was filled with medium containing 10% FBS as a
chemoattractant. For cell invasion, the upper chambers were coated
with Matrigel (BD Biosciences). After 24 h at 37°C, the
non-migrating or non-invading cells on the top well were gently
removed. The cells on the lower surface of the membrane were fixed
with 70% ethanol and stained with 0.1% violet (Sigma-Aldrich; Merck
KGaA). Finally, the number of removed cells were counted using a
microscope (Olympus Corporation). All experiments were run in
triplicate.
Bioinformatics prediction
TargetScan version 7.1 online software (www.targetscan.org) was used to predict the potential
targets of miR-505-3p, according to the manufacturer's
instructions. Briefly, ‘human’ was selected as the target species,
and miR-505 was inserted as the investigated miRNA. miR-505-3p was
predicted to be able to directly bind to the seeding sequences of
the 3′-UTR of HMGB1.
Dual luciferase assay
The 3′-UTR of wild-type or mutant HMGB1 was inserted
into pcDNA3.1 plasmid vector (Promega Corporation) to perform
luciferase reporter experiments. Then, the luciferase vector and
miR-505-3p mimics were transfected into U251 cells using
Lipofectamine 2000 and incubated for 48 h. Finally, the luciferase
activity was measured through dual luciferase assay system (Promega
Corporation). All experiments were run in triplicate. The U251
cells with NC and luciferase vector were used as the control.
Statistical analysis
Data are shown as mean±SD and were analyzed using
SPSS 19.0 (SPSS, Inc.) or Graphpad Prism 6 (GraphPad Software,
Inc.). The association between miR-505-3p and clinicopathological
features in glioma patients was calculated by Chi-square test.
Differences between multiple groups were calculated by one-way
ANOVA followed by Tukey's post hoc test. Pearson's correlation
analysis was performed to examine the correlation between the
miR-505-3p and HMGB1 expression in glioma tissues. Survival curves
were plotted by Kaplan-Meier analysis, and log-rank test was used
to compare survival differences. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of miR-505-3p is reduced in
glioma
The expression of miR-505-3p was detected in glioma
tissues by RT-qPCR assay. The expression of miR-505-3p was reduced
in the 44 cases of glioma tissues compared to normal tissues
(Fig. 1A). The median relative
expression level of miR-505-3p in glioma tissues was taken as a
cut-off point to separate low and high expression of miR-505-3p.
Moreover, miR-505-3p was also found to be associated with WHO grade
(P=0.019) and necrosis (P=0.032; Table
I). In addition, we found that low miR-505-3p expression was
associated with shorter overall survival in glioma patients
(P=0.0306; Fig. 1B). These findings
suggest that miR-505-3p may be involved in the pathogenesis of
glioma.
miR-505-3p restrains proliferation,
migration and invasion of glioma cells
Expression levels of miR-505-3p were also measured
in U251, A172, LN229 and NHA cell lines. Downregulation of
miR-505-3p was also detected in U251, A172 and LN229 cell lines
compared to NHA cells (Fig. 2A).
miR-505-3p mimics or inhibitor was then transfected into U251 cells
to investigate its function in glioma cells. RT-qPCR assay showed
that miR-505-3p expression was increased by miR-505-3p mimics and
decreased by miR-505-3p inhibitor (Fig.
2B). Functionally, upregulation of miR-505-3p inhibited
proliferation of U251 cells (Fig.
2C). In contrast, downregulation of miR-505-3p was found to
promote cell proliferation in U251 cells (Fig. 2D). As with the above results,
overexpression of miR-505-3p inhibited cell migration in U251
cells, whereas downregulation of miR-505-3p promoted migration of
U251 cells (Fig. 2E). Moreover,
similar effect of miR-505-3p on cell invasion was also identified
in U251 cells (Fig. 2F). Based on
these results, miR-505-3p might have inhibitory effect on the
development of glioma.
HMGB1 is a direct target of miR-505-3p
in glioma
In addition, TargetScan database (http://www.targetscan.org/) shows that HMGB1 has a
binding site with miR-505-3p (Fig.
3A). Next, luciferase reporter assay was performed to confirm
the prediction. We found that miR-505-3p mimics significantly
reduced the luciferase activity of Wt-HMGB1. However, the
luciferase activity of Mut-HMGB1 was not affected by miR-505-3p
mimics (Fig. 3B). Moreover, HMGB1
expression was found to be negatively correlated with miR-505-3p in
glioma tissues (P=0.0105, R2=0.1825; Fig. 3C). HMGB1 expression was examined in
U251 cells with miR-505-3p mimics or inhibitor. The results showed
that miR-505-3p mimics reduced the expression level of HMGB1
(Fig. 3D), and miR-505-3p inhibitor
enhanced the expression of HMGB1 in U251 cells (Fig. 3E). Taken together, HMGB1 is a direct
target of miR-505-3p, which is inversely related to miR-505-3p
expression in glioma.
HMGB1 is upregulated in glioma
Next, alteration of HMGB1 expression was identified
in glioma tissues and cell lines. First, HMGB1 was found to be
upregulated in the 44 cases of glioma tissues compared to adjacent
normal tissues (Fig. 4A). Similarly,
upregulation of HMGB1 was also identified in U251, A172 and LN229
cell lines compared to NHA cells (Fig.
4B). HMGB1 expression was associated with a prognosis in glioma
patients, and high HMGB1 expression predicted a poor prognosis in
glioma patients (P=0.0407; Fig. 4C).
Therefore, we consider that HMGB1 may be involved in the
development of glioma.
miR-505-3p inhibits the development of
glioma through targeting HMGB1
To further verify the relationship between
miR-505-3p and HMGB1, HMGB1 vector was transfected into U251 cells
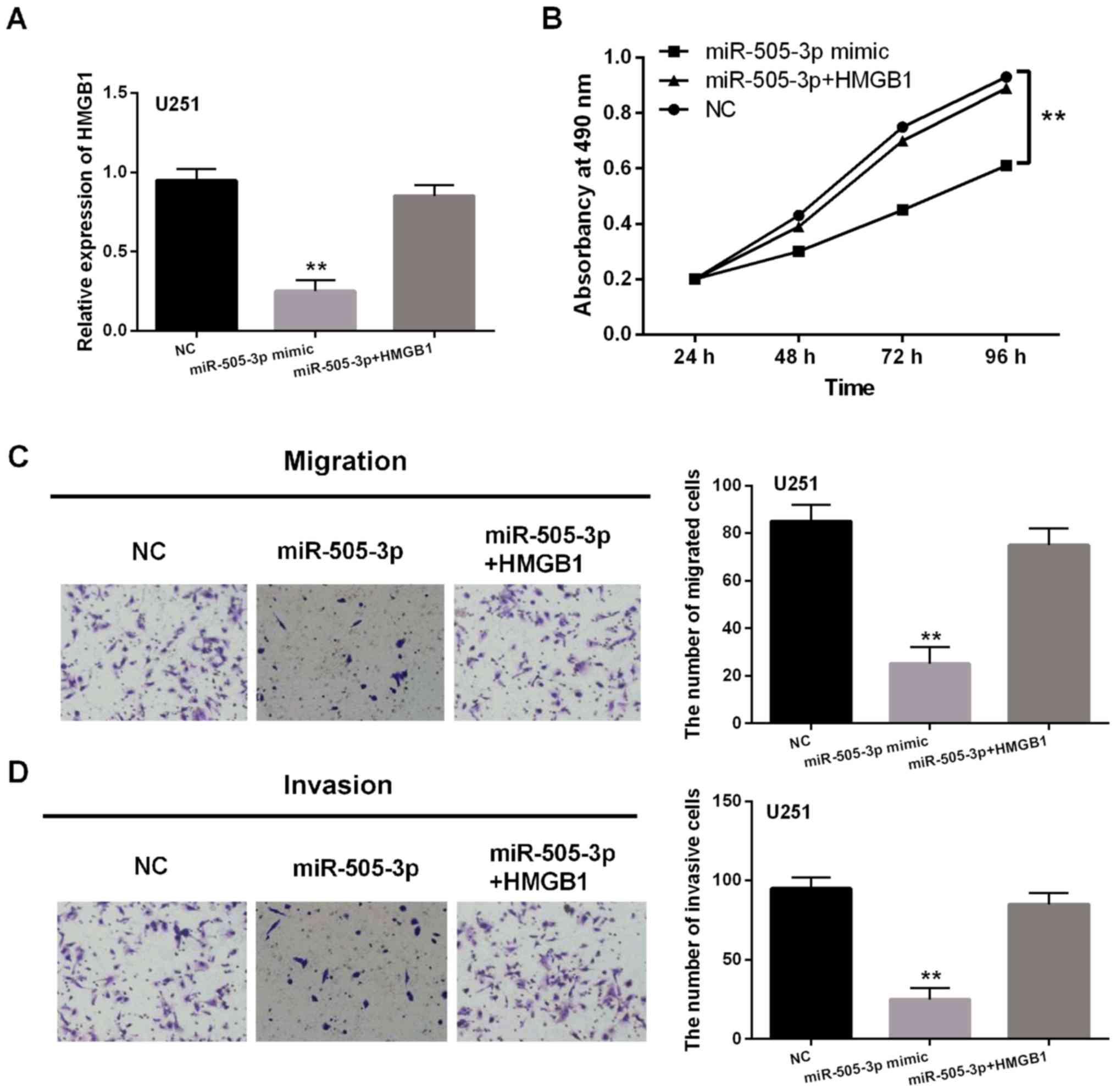
with miR-505-3p mimics. Reduction of HMGB1 expression induced by
miR-505-3p mimics was restored by HMGB1 vector in U251 cells
(Fig. 5A). Then, it was found that
the inhibitory effect of miR-505-3p on cell proliferation was
hindered by HMGB1 vector in U251 cells (Fig. 5B). Similar results for cell migration
and invasion were also identified in U251 cells (Fig. 5C and D). Collectively, miR-505-3p
inhibited the development of glioma by targeting HMGB1.
miR-505-3p suppresses EMT regulating
AKT expression in glioma
The effect of miR-505-3p on EMT and AKT expression
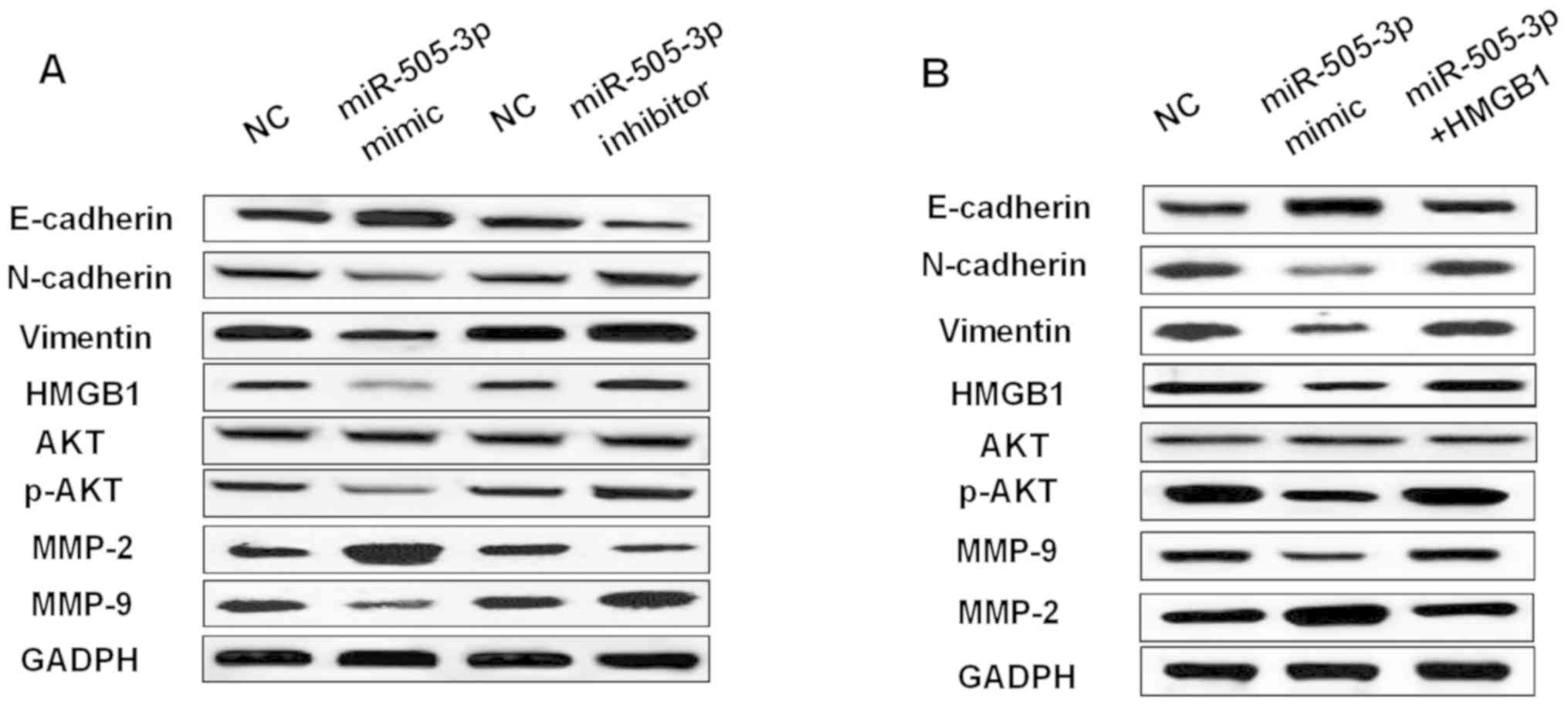
was investigated in glioma cells. We found that overexpression of
miR-505-3p promoted E-cadherin expression and inhibited N-cadherin
and Vimentin expression in U251 cells (Fig. 6A). In contrast, knockdown of
miR-505-3p had the opposite effect on these markers (Fig. 6A). Moreover, miR-505-3p mimics also
promoted MMP-2 expression and suppressed MMP-9 expression.
miR-505-3p inhibitor decreased MMP-2 expression and increased MMP-9
expression (Fig. 6A). Furthermore,
overexpression of miR-505-3p was found to inhibit p-AKT expression
(Fig. 6), while downregulation of
miR-505-3p promoted p-AKT expression (Fig. 6A). However, the expression of AKT was
not affected by miR-505-3p mimics or inhibitor in glioma cells. As
shown in Fig. 6B, upregulation of
HMGB1 weakened the effect of miR-505-3p on these makers. Briefly,
miR-505-3p blocked EMT and suppressed p-AKT expression in glioma
cells.
Discussion
Recently, many studies have shown that miRNAs play
important roles in the pathogenesis of glioma (19,20). It
has been reported that miR-505-3p regulated tumorigenesis of human
diseases and cancers. Especially, the combination of TMZ and
miR-505 were proposed to inhibit the development of glioblastoma by
modulating the WNT7B/Wnt/β-catenin signaling pathway (21). In the current study, downregulation
of miR-505-3p was detected in the 44 cases of glioma tissues
(low-grade and high-grade glioma). Moreover, miR-505-3p inhibited
the development of glioma by targeting HMGB1. In conclusion,
miR-505-3p was identified as an inhibitory miRNA in glioma.
Consistent with our results, miR-505 was found to
predict prognosis and act as tumor inhibitor in cervical carcinoma
(22). Moreover, miR-505 was found
to be downregulated in human osteosarcoma and suppress cell
proliferation, migration and invasion (23). In this study, miR-505-3p expression
was also reduced in glioma tissues, and overexpression of
miR-505-3p inhibited proliferation, migration and invasion of
glioma cells. Moreover, miR-505-3p also blocked EMT in glioma cells
by promoting E-cadherin expression and inhibiting N-cadherin and
Vimentin expression, which has not been reported in previous
studies. Furthermore, miR-505 was found to suppress proliferation
and invasion of liver cancer cells by directly targeting HMGB1
(24). Our study also found that
miR-505-3p inhibited the development of glioma through targeting
HMGB1.
We confirmed that HMGB1 is a direct target of
miR-505-3p, which is inversely related to miR-505-3p expression in
glioma. HMGB1, as an extracellular signal molecule, has been
reported to regulate tumor differentiation and metastasis (25). As an oncogene, HMGB1 was identified
to promote cell proliferation and invasion in osteosarcoma
(26). It was reported that
knockdown of HMGB1 improved apoptosis and suppressed proliferation
and invasion of glioma cells (27).
Here, HMGB1 was also upregulated and functioned as an oncogene in
glioma. High HMGB1 expression was identified to predict poor
prognosis in glioma patients. Similarly, previous study also showed
the clinical and prognostic significance of HMGB1 in human gliomas
(28). Besides, upregulation of
HMGB1 was found to restore the inhibitory effect of miR-505-3p in
glioma.
Furthermore, we found that miR-505-3p was involved
in AKT pathway by downregulating p-AKT expression in glioma.
Similarly, it has been reported that miR-505 can inactivate the AKT
pathway, and the AKT pathway partially was partially restored by
HMGB1 in hepatocellular carcinoma (29). The AKT pathway has been proposed to
be involved in the progression of many human cancers, including
glioma (30). For example, tumor
suppressor miRNA-34a suppressed glioma cell proliferation and tumor
growth by modulating the AKT signaling pathway (31). This study showed that miR-505-3p
could inactivate the AKT signaling pathway by suppressing p-AKT
expression. Therefore, the inhibitory effect of miR-505-3p on
proliferation, migration and invasion of glioma cells can be
regulated by the HMGB1/AKT axis. However, we only explained
initially the regulatory mechanism of miR-505-3p in glioma. Thus,
further study is planned to address the small sample size and lack
of animal experiments.
In conclusion, downregulation of miR-505-3p was
identified in glioma, which was associated with shorter overall
survival in glioma patients. Importantly, miR-505-3p inhibited cell
proliferation, migration and invasion in glioma by targeting HMGB1.
Furthermore, miR-505-3p blocked EMT and inhibited p-AKT expression
in glioma cells.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZC contributed to the study design, data analysis
and drafted the manuscript; BW was involved in data acquisition and
revision of the manuscript; CZ contributed to the study design,
data analysis, and revised the manuscript. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Institutional Ethics
Committee of Department of Neurosurgery, Zhangye People's Hospital
Affiliated to Hexi University (Zhangye, China). Informed consents
were obtained from the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Omuro A and DeAngelis LM: Glioblastoma and
other malignant gliomas: A clinical review. JAMA. 310:1842–1850.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zeng T, Cui D and Gao L: Glioma: An
overview of current classifications, characteristics, molecular
biology and target therapies. Front Biosci. 20:1104–1115. 2015.
View Article : Google Scholar
|
|
3
|
Ostrom QT, Gittleman H, Stetson L, Virk S
and Barnholtz-Sloan JS: Epidemiology of intracranial gliomas. Prog
Neurol Surg. 30:1–11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qaddoumi I, Sultan I and Gajjar A: Outcome
and prognostic features in pediatric gliomas: A review of 6212
cases from the Surveillance, Epidemiology, and End Results
database. Cancer. 115:5761–5770. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu Q, Xu L, Wang C, Fan W, Yan H and Li Q:
MicroRNA-124-3p represses cell growth and cell motility by
targeting EphA2 in glioma. Biochem Biophys Res Commun.
503:2436–2442. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang BQ, Yang B, Yang HC, Wang JY, Hu S,
Gao YS and Bu XY: MicroRNA-499a decelerates glioma cell
proliferation while accelerating apoptosis through the suppression
of Notch1 and the MAPK signaling pathway. Brain Res Bull.
142:96–106. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu J, He J, Huang H, Peng R and Xi J:
MicroRNA-423-3p promotes glioma growth by targeting PANX2. Oncol
Lett. 16:179–188. 2018.PubMed/NCBI
|
|
9
|
Ramachandran SS, Muiwo P, Ahmad HM, Pandey
RM, Singh S, Bakhshi S, Kumar L, Bhattacharya A and Gupta YK:
miR-505-5p and miR-193b-3p: Potential biomarkers of imatinib
response in patients with chronic myeloid leukemia. Leuk Lymphoma.
58:1981–1984. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang Q, Jia C, Wang P, Xiong M, Cui J, Li
L, Wang W, Wu Q, Chen Y and Zhang T: MicroRNA-505 identified from
patients with essential hypertension impairs endothelial cell
migration and tube formation. Int J Cardiol. 177:925–934. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qin Z, He W, Tang J, Ye Q, Dang W, Lu Y,
Wang J, Li G, Yan Q and Ma J: MicroRNAs orovide feedback regulation
of epithelial-mesenchymal transition induced by growth factors. J
Cell Physiol. 231:120–129. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ding J, Cui X and Liu Q: Emerging role of
HMGB1 in lung diseases: Friend or foe. J Cell Mol Med.
21:1046–1057. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu PL, Tsai JR, Hwang JJ, Chou SH, Cheng
YJ, Lin FY, Chen YL, Hung CY, Chen WC, Chen YH, et al:
High-mobility group box 1-mediated matrix metalloproteinase-9
expression in non-small cell lung cancer contributes to tumor cell
invasiveness. Am J Respir Cell Mol Biol. 43:530–538. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song B, Song WG, Li ZJ, Xu ZF, Wang XW,
Wang CX and Liu J: Effect of HMGB1 silencing on cell proliferation,
invasion and apoptosis of MGC-803 gastric cancer cells. Cell
Biochem Funct. 30:11–17. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yao X, Zhao G, Yang H, Hong X, Bie L and
Liu G: Overexpression of high-mobility group box 1 correlates with
tumor progression and poor prognosis in human colorectal carcinoma.
J Cancer Res Clin Oncol. 136:677–684. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pan C, Wang Y, Qiu MK, Wang SQ, Liu YB,
Quan ZW and Ou JM: Knockdown of HMGB1 inhibits cell proliferation
and induces apoptosis in hemangioma via downregulation of AKT
pathway. J Biol Regul Homeost Agents. 31:41–49. 2017.PubMed/NCBI
|
|
17
|
Zhang J, Zhang J, Qiu W, Zhang J, Li Y,
Kong E, Lu A, Xu J and Lu X: MicroRNA-1231 exerts a tumor
suppressor role through regulating the EGFR/PI3K/AKT axis in
glioma. J Neurooncol. 139:547–562. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma C, Wei F, Xia H, Liu H, Dong X, Zhang
Y, Luo Q, Liu Y and Li Y: MicroRNA-10b mediates TGF-β1-regulated
glioblastoma proliferation, migration and epithelial-mesenchymal
transition. Int J Oncol. 50:1739–1748. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang Y, Wang X, Zhang J and Lai R:
MicroRNA-599 suppresses glioma progression by targeting RAB27B.
Oncol Lett. 16:1243–1252. 2018.PubMed/NCBI
|
|
21
|
Zhang C, Yang X, Fu C and Liu X:
Combination with TMZ and miR-505 inhibits the development of
glioblastoma by regulating the WNT7B/Wnt/β-catenin signaling
pathway. Gene. 672:172–179. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma C, Xu B, Husaiyin S, Wang L,
Wusainahong K, Ma J, Zhu K and Niyazi M: MicroRNA-505 predicts
prognosis and acts as tumor inhibitor in cervical carcinoma with
inverse association with FZD4. Biomed Pharmacother. 92:586–594.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu YJ, Li W, Chang F, Liu JN, Lin JX and
Chen DX: MicroRNA-505 is downregulated in human osteosarcoma and
regulates cell proliferation, migration and invasion. Oncol Rep.
39:491–500. 2018.PubMed/NCBI
|
|
24
|
Lu L, Qiu C, Li D, Bai G, Liang J and Yang
Q: MicroRNA-505 suppresses proliferation and invasion in hepatoma
cells by directly targeting high-mobility group box 1. Life Sci.
157:12–18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stoetzer OJ, Fersching DM, Salat C,
Steinkohl O, Gabka CJ, Hamann U, Braun M, Feller AM, Heinemann V,
Siegele B, et al: Circulating immunogenic cell death biomarkers
HMGB1 and RAGE in breast cancer patients during neoadjuvant
chemotherapy. Tumour Biol. 34:81–90. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meng Q, Zhao J, Liu H, Zhou G, Zhang W, Xu
X and Zheng M: HMGB1 promotes cellular proliferation and invasion,
suppresses cellular apoptosis in osteosarcoma. Tumour Biol.
35:12265–12274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang J, Liu C and Hou R: Knockdown of
HMGB1 improves apoptosis and suppresses proliferation and invasion
of glioma cells. Chin J Cancer Res. 26:658–668. 2014.PubMed/NCBI
|
|
28
|
Wang XJ, Zhou SL, Fu XD, Zhang YY, Liang
B, Shou JX, Wang JY and Ma J: Clinical and prognostic significance
of high-mobility group box-1 in human gliomas. Exp Ther Med.
9:513–518. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu L, Zhang D, Xu Y, Bai G, Lv Y and Liang
J: miR-505 enhances doxorubicin-induced cytotoxicity in
hepatocellular carcinoma through repressing the Akt pathway by
directly targeting HMGB1. Biomed Pharmacother. 104:613–621. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar
|
|
31
|
Rathod SS, Rani SB, Khan M, Muzumdar D and
Shiras A: Tumor suppressive miRNA-34a suppresses cell proliferation
and tumor growth of glioma stem cells by targeting Akt and Wnt
signaling pathways. FEBS Open Bio. 4:485–495. 2014. View Article : Google Scholar : PubMed/NCBI
|