Introduction
As one of the most common malignant tumors in the
world, stomach cancer (SC) is the third leading cause of
cancer-related deaths although its incidence has been decreasing in
the past few decades (1,2). According to the latest statistical
report, more than 1 million people newly developed the disease
worldwide in 2018, and the number of patients who succumbed to this
disease was close to 800,000, thus the disease poses a serious
threat to human life and health (3).
At present, surgery is the only potential radical treatment for SC,
however the disease is mostly in its advanced stage when diagnosed
and as a result, most patients are ineligible for radical surgery
(4). Currently, chemotherapy is the
first choice for SC patients who cannot undergo surgery, but there
is no standard chemotherapeutic scheme with a satisfactory
prognosis for them (5). Therefore, a
current research hotspot is the discovery of a therapeutic scheme
with better efficacy and higher safety to improve the prognosis of
SC.
As an anticancer drug composed of tegafur (FT),
gimeracil (CDHP), and oteracil (Oxo), S-1 is well tolerated in
clinical practice. This drug was approved for the treatment of
advanced non-small cell lung cancer (NSCLC) in Japan in 2004, and
then for the treatment of various malignant tumors including SC
(6,7). Palliative care (PC) is a
multidisciplinary therapeutic method, and aims to improve the
quality of life (QOL) of patients with serious diseases and their
families (8). Its core components
include the assessment and treatment of physical and psychological
symptoms, the identification and support of mental pain, and the
expert communication to set nursing goals and to assist in complex
medical decisions and nursing coordination (9).
The application of S-1 combined with PC to patients
with advanced stomach cancer (ASC) has been rarely explored.
Therefore, the effects of the combination on the efficacy (ORR),
survival rate, safety, negative emotions, QOL, and immune function
of patients were observed in the present study, to provide more
effective data for the clinical application of the therapeutic
method.
Materials and methods
General information
This prospective study was approved by the Medical
Ethics Committee of Tianjin Fifth Central Hospital (Tianjin,
China). The research subjects included 168 patients (106 males and
62 females) with ASC treated at Tianjin Fifth Central Hospital from
September 2016 to March 2018. Inclusion criteria for the patients
were as follows: Patients aged >18 years; patients confirmed
with SC by pathological examination; patients who had not taken
drugs for the digestive tract system and anticancer drugs in the
past month; patients in stages III and IV according to the TNM
staging diagnostic criteria issued by American Joint Committee on
Cancer (AJCC) in 2017 (10);
patients who signed the informed consent form. Exclusion criteria
for the patients were as follows: Patients with other malignant
tumors except SC; patients with poor compliance; patients with
expected survival time >3 months; patients who did not complete
follow-up; patients with contraindications to the drugs used in
this study; patients with incomplete clinical data.
Therapeutic methods
Patients in the single drug group (SDG; n=77) were
orally administrated with S-1 (Taiho Pharmaceutical Co., Ltd.)
twice a day, 40–60 mg each time according to their body surface
area. Medication for 4 consecutive weeks was considered as one
course of treatment, and the patients were treated for 2 courses.
The second course started at 2 weeks after the first course. Those
in the combined drug group (CDG; n=91) were treated as in the SDG
group but were additionally treated with PC, which mainly included
humanistic care, nutritional support, the prevention of toxic and
side effects, and pain treatment. Following the treatment, the
patients in both groups were followed-up by telephone and
out-patient re-examinations to record their 1-year overall survival
rate (OSR). The follow-up was conducted once a month and for a
total of 12 months.
Outcome measures
The following day after the end of treatment, the
clinical efficacy of the treatment on patients was assessed based
on version 1.1 Response Evaluation Criteria in Solid Tumors
(RECIST1.1) (11). The efficacy was
classified into complete remission (CR), partial remission (PR),
stable disease (SD), and progressive disease (PD). The overall
response rate (ORR) was calculated as follows: ORR=(CR + PR
cases)/total number of cases ×100%.
The occurrence of major adverse reactions during the
treatment was observed, including loss of appetite, nausea and
vomiting, leukopenia, diarrhea, insomnia, and bone marrow
depression (BMD).
The Self-Rating Anxiety Scale (SAS) (12) and the Self-Rating Depression Scale
(SDS) (13) were respectively used
to score the anxiety and depression of the patients one day before
and after treatment. Each scale had a total score of 100 points. A
high score indicated serious anxiety and depression.
Serum samples were collected from the patients one
day before and after treatment, in which levels of nutritional
indices [albumin (ALB), prealbumin (PA), and transferrin (TF)] were
measured using a Cobas C312 fully automatic biochemical analyzer
(Roche Diagnostics).
At 15 days after treatment, the QOL of patients was
evaluated according to Karnofsky Performance Scale (KPS) score
(14). Improved indicated that the
score was increased by >10 points. Stable indicated that the
score was reduced or increased by ≤10 points. Worsened indicated
that the score was reduced by >10 points. The total improvement
rate was calculated as follows: Total improvement rate=(improved +
stable cases)/total number of cases ×100%.
Serum samples were collected from the patients one
day before and after treatment, in which peripheral blood T
lymphocyte subsets were assessed using a FACSCalibur flow cytometer
(BD Biosciences). Anticoagulated whole blood (100 µl) was
respectively added with CD4-PE and CD8-PE antibodies (cat. nos.
FAB3791P and FAB1509P, respectively; R&D Systems, Inc.) (20 µl
each), mixed well, and then allowed to stand at room temperature
for 15 min. Next, hemolysin (R&D Systems, Inc.) (370 µl) was
added to the mixture, mixed well, and then allowed to stand at room
temperature for 15 min. Peripheral blood T lymphocyte subsets were
detected on the flow cytometer.
Statistical analysis
In the present study, SPSS 21.0 (IBM Corp.) was used
for statistical analysis. GraphPad Prism 7 (GraphPad Software,
Inc.) was used to plot figures. Count data such as general
information were expressed by [n(%)], and the comparison of rate
between groups was conducted by chi-square test. Measurement data
such as SAS and SDS scores were expressed by (mean ± SD), and their
comparison between groups was conducted by independent samples
t-test, while the comparison within groups before and after
treatment was conducted by paired t-test. Kaplan-Meier method was
used to plot curves of the 1-year OSR after the follow-up. Log-rank
test was used to analyze the difference in the survival between the
two groups. P<0.05 indicated a statistically significant
difference.
Results
Comparison of general information
There were no significant differences between the
SDG and the CDG in characteristics including sex, age, body weight,
educational level, food preference, place of residence, exercise
habits, marital status, history of smoking, history of drinking,
TNM staging, and pathological types (P>0.05; Table I).
 | Table I.Comparison of general information
(mean ± SD). |
Table I.
Comparison of general information
(mean ± SD).
| Groups | SDG (N=77) n (%) | CDG (N=91) n (%) |
χ2/F-value | P-value |
|---|
| Sex |
|
| 0.601 | 0.438 |
| Male | 51 (66.23) | 55 (60.44) |
|
|
|
Female | 26 (33.77) | 36 (39.56) |
|
|
| Age (years) | 56.24±8.67 | 58.45±10.11 | 1.506 | 0.134 |
| BMI
(kg/m2) | 23.13±1.89 | 23.45±2.12 | 1.024 | 0.307 |
| Educational
level |
|
| 0.881 | 0.348 |
|
<Senior high school | 36 (46.75) | 36 (39.56) |
|
|
| ≥Senior
high school | 41 (53.25) | 55 (60.44) |
|
|
| Food preference |
|
| 1.062 | 0.303 |
|
Bland | 50 (64.94) | 52 (57.14) |
|
|
|
Greasy | 27 (35.06) | 39 (42.86) |
|
|
| Place of
residence |
|
| 2.907 | 0.088 |
| City | 49 (63.64) | 46 (50.55) |
|
|
|
Countryside | 28 (36.36) | 45 (49.45) |
|
|
| Exercise
habits |
|
| 0.362 | 0.547 |
|
Yes | 39 (50.65) | 33 (36.26) |
|
|
| No | 38 (49.35) | 58 (63.74) |
|
|
| Marital status |
|
| 2.382 | 0.304 |
|
Married | 62 (80.52) | 64 (70.33) |
|
|
|
Unmarried | 6 (7.79) | 12 (13.19) |
|
|
|
Divorced | 9 (11.69) | 15 (16.48) |
|
|
| History of
smoking |
|
| 1.338 | 0.247 |
|
Yes | 55 (71.43) | 72 (79.12) |
|
|
| No | 22 (28.57) | 19 (20.88) |
|
|
| History of
drinking |
|
| 0.887 | 0.346 |
|
Yes | 35 (45.45) | 46 (50.55) |
|
|
| No | 42 (54.55) | 45 (49.45) |
|
|
| TNM staging |
|
| 0.794 | 0.373 |
|
III | 37 (48.05) | 50 (54.95) |
|
|
| IV | 40 (51.95) | 41 (45.05) |
|
|
| Pathological
types |
|
| 0.869 | 0.648 |
|
Adenocarcinoma | 65 (84.42) | 72 (79.12) |
|
|
|
Squamous cell carcinoma | 7 (9.09) | 10 (10.99) |
|
|
|
Signet-ring cell
carcinoma | 5 (6.49) | 9 (9.89) |
|
|
Comparison of clinical efficacy
No patient died during the treatment. After
treatment, there were 22 cases (28.57%) of CR, 35 cases (45.46%) of
PR, 12 cases (15.58%) of SD, and 8 cases (10.39%) of PD in the SDG,
with an ORR of 74.03%. There were 32 cases (35.16%) of CR, 48 cases
(52.75%) of PR, 6 cases (6.59%) of SD, and 5 cases (5.50%) of PD in
the CDG, with an ORR of 87.91%. After treatment, the ORR in the CDG
was significantly higher than that in the SDG (P<0.05; Table II).
 | Table II.Comparison of clinical efficacy. |
Table II.
Comparison of clinical efficacy.
| Groups | n | CR n (%) | PR n (%) | SD n (%) | PD n (%) | ORR n (%) |
|---|
| SDG | 77 | 22 (28.57) | 35 (45.46) | 12 (15.58) | 8 (10.39) | 57 (74.03) |
| CDG | 91 | 32 (35.16) | 48 (52.75) | 6 (6.59) | 5 (5.50) | 80 (87.91) |
| χ2
test | – | – | – | – | – | 5.345 |
| P-value | – | – | – | – | – | 0.021 |
Comparison of occurrence of adverse
reactions
During the treatment, patients in the CDG and SDG
had no drug allergy but good tolerance. Their major adverse
reactions could be cured by clinical symptomatic treatment. There
was no significant difference between the two groups in the
occurrence of loss of appetite, insomnia, and BMD (P>0.05). The
occurrence of nausea and vomiting, leukopenia, and diarrhea in the
CDG was significantly lower than that in the SDG, and the total
number of adverse reactions was also significantly lower in the CDG
(P<0.05; Table III).
 | Table III.Comparison of occurrence of adverse
reactions. |
Table III.
Comparison of occurrence of adverse
reactions.
| Groups | SDG (N=77) n
(%) | CDG (N=91) n
(%) | χ2
test | P-value |
|---|
| Loss of
appetite | 14 (18.18) | 8 (8.79) | 3.232 | 0.072 |
| Nausea and
vomiting | 22 (28.57) | 12 (13.79) | 6.115 | 0.013 |
| Leukopenia | 12 (15.58) | 5 (5.49) | 4.669 | 0.031 |
| Diarrhea | 16 (20.78) | 8 (8.79) | 4.895 | 0.027 |
| Insomnia | 11 (14.29) | 7 (7.69) | 1.895 | 0.169 |
| BMD | 7 (9.09) | 5 (5.49) | 0.813 | 0.367 |
| Total number of
adverse reactions | 39 (50.65) | 29 (31.87) | 6.106 | 0.014 |
Comparison of SAS and SDS scores
Before treatment, there were no significant
differences between the SDG and CDG in SAS and SDS scores
(P>0.05). After treatment, the two scores significantly
decreased in the two groups, and the scores in the CDG were
significantly lower than those in the SDG (P<0.05; Table IV).
 | Table IV.Comparison of SAS and SDS scores
(mean ± SD, points). |
Table IV.
Comparison of SAS and SDS scores
(mean ± SD, points).
|
| SAS | SDS |
|---|
|
|
|
|
|---|
| Groups | Before
treatment | After
treatment | Before
treatment | After
treatment |
|---|
| SDG (n=77) | 86.56±10.54 |
78.56±11.54a | 85.86±10.12 |
76.51±12.52a |
| CDG (n=91) | 88.56±9.38 |
69.56±8.54a | 87.56±9.68 |
70.23±9.56a |
| t-value | 1.301 | 5.798 | 1.111 | 3.682 |
| P-value | 0.195 | <0.001 | 0.268 | <0.001 |
Comparison of changes in nutritional
indices
Before treatment, there were no significant
differences between the SDG and CDG in levels of ALB, PA, and TF
(P>0.05). After treatment, the levels in the two groups
significantly increased, and the levels in the CDG were
significantly higher than those in the SDG (P<0.05; Table V).
 | Table V.Comparison of changes in nutritional
indices (mean ± SD). |
Table V.
Comparison of changes in nutritional
indices (mean ± SD).
|
| ALB (g/l) | PA (mg/l) | TF (g/l) |
|---|
|
|
|
|
|
|---|
| Groups | Before
treatment | After
treatment | Before
treatment | After
treatment | Before
treatment | After
treatment |
|---|
| SDG (n=77) | 28.15±3.78 |
33.34±4.21a | 125.14±18.61 |
192.24±25.34a | 1.38±0.34 |
1.72±0.33a |
| CDG (n=91) | 28.89±4.13 |
36.67±4.45a | 127.34±20.43 |
257.15±27.67a | 1.43±0.36 |
2.27±0.41a |
| t-value | 1.203 | 4.953 | 0.724 | 15.743 | 0.920 | 9.460 |
| P-value | 0.231 | <0.001 | 0.470 | <0.001 | 0.359 | <0.001 |
Comparison of QOL improvement
In the SDG, the QOL was improved in 27 cases
(35.06%), stable in 32 cases (41.56%), and worsened in 18 cases
(23.38%), with an improvement rate of 76.62%. In the CDG, the QOL
was improved in 39 cases (42.86%), stable in 43 cases (47.25%), and
worsened in 9 cases (9.89%), with an improvement rate of 90.11%.
The total improvement rate of the QOL in the CDG was significantly
higher than that in the SDG (P<0.05; Table VI).
 | Table VI.Comparison of QOL improvement. |
Table VI.
Comparison of QOL improvement.
| Groups | N | Improved n (%) | Stable n (%) | Worsened n (%) | Total improvement
rate n (%) |
|---|
| SDG | 77 | 27 (35.06) | 32 (41.56) | 18 (23.38) | 59 (76.62) |
| CDG | 91 | 39 (42.86) | 43 (47.25) | 9 (9.89) | 82 (90.11) |
| χ2 | – | – | – | – | 5.624 |
| P-value | – | – | – | – | 0.018 |
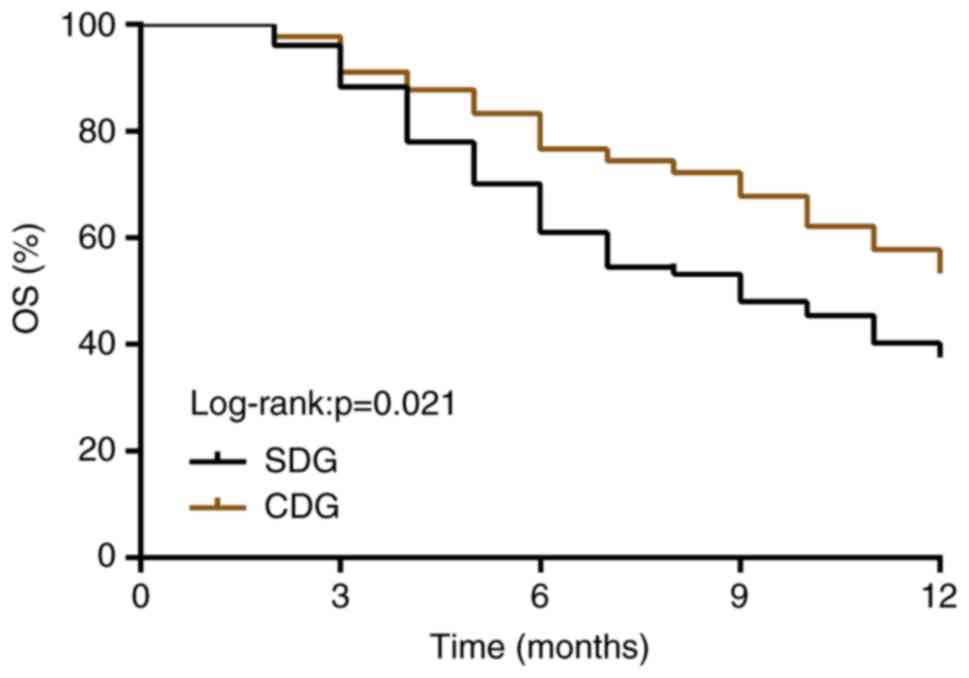
Comparison of 1-year OSR
After the follow-up, the 1-year OSR in the CDG
(57.14%) was significantly higher than that in the SDG (38.96%)
(P<0.05, log-rank test; Fig.
1).
Comparison of immune function indices
before and after treatment
Before treatment, there were no significant
differences between the SDG and CDG in levels of serum
CD4+, CD8+, and
CD4+/CD8+ (P>0.05). After treatment, the
levels of CD4+ and CD4+/CD8+ in
the two groups significantly increased, and the levels in the CDG
were significantly higher than those in the SDG (P<0.05).
However, the CD8+ level in the two groups significantly
decreased, and the level in the CDG was significantly lower than
that in the SDG (P<0.05; Fig.
2).
Discussion
Changes in the life and dietary habits of people
leads to a gradual increase in the incidence of digestive system
diseases (15). In addition to a
common malignant tumor of the digestive system worldwide and a
major factor of cancer-related deaths (16), SC is also a genetically heterogeneous
tumor with multiple causes, closely related to heredity,
helicobacter pylori infection, diet, and lifestyle (17). ASC is characterized by high
metastasis and high recurrence, which result in the worst clinical
results of the disease among all solid organ tumors (18). Therefore, the therapeutic effect on
ASC urgently requires improvement in clinical practice.
FT is a prodrug converted into 5-fluorouracil
(5-Fu), whose catabolism can be delayed by CDHP and whose
phosphorylation can be inhibited by Oxo (19). S-1 that is composed of the three
drugs can prolong and maintain 5-Fu concentration and reduce its
toxicity (19). This drug is common
for the treatment of various tumor diseases including SC (20–22). PC,
an interdisciplinary specialty, is dedicated to improving the QOL
of critically ill patients and their families through symptom
management, communication, and patient autonomy (23). Its combination with chemotherapy is
commonly used to treat tumors, but there are currently few studies
on S-1 combined with PC to treat ASC. In the study, the ORR and
1-year OSR in the CDG were significantly higher than those in the
SDG, which demonstrated that S-1 combined with PC has a better
efficacy and higher safety than S-1 alone in the treatment of
patients with ASC. Improving the QOL is one of the basic goals of
treatment, however cancer patients, especially those with advanced
cancers, have a markedly decreased QOL owing to disease symptoms,
pain, and negative emotions (24,25). In
addition, patients with ASC suffer from less food intake and poor
absorption caused by the disease itself or the toxic and side
effects of chemotherapeutic drugs, eventually resulting in the
patients experiencing malnutrition which directly affects their QOL
(26,27). Therefore, apart from the treatment of
the disease itself, all efforts should be made to appease the
emotions of the patients and provide adequate nutritional support,
thus improving the QOL more effectively. In the present study,
compared with those in the SDG, patients in the CDG had a decreased
number of adverse reactions, SAS score, and SDS score, but an
increased QOL improvement rate and levels of nutritional indices
(ALB, PA, and TF). This indicated that S-1 combined with PC had
higher safety, and could improve the QOL of patients with ASC more
significantly, because PC aims to improve the QOL of patients with
serious diseases and their families through humanistic care,
symptomatic treatment, as well as other means.
The immune system has a great effect on controlling
and curing cancers, and the immune function of cancer patients is
generally inhibited compared with that of healthy people (28). Accordingly, immunotherapy for tumors
has been widely valued in the medical field, and thus it has a
broad application prospect (29).
Studies have revealed that nutritional support has a great effect
on improving the immune function of the cancer patients (30,31). T
lymphocyte-mediated cellular immunity plays a pivotal role in the
antitumor immune mechanism of the body, thus changes in T
lymphocyte subsets (CD4+ and CD8+) are
important markers reflecting immune dysfunction (32,33). In
the present study, post-treatment levels of CD4+ and
CD4+/CD8+ in the CDG were higher than those
in the SDG, while the post-treatment CD8+ level was
lower than that in the SDG, indicating that S-1 combined with PC
has a better effect on relieving immunosuppression in patients with
ASC. This may be due to the fact that nutritional support provided
to the patients in the CDG provides various nutrients, and thereby
improves their immune function.
In summary, S-1 combined with PC is more effective
than S-1 alone in the treatment of patients with ASC. The patients
treated with the combination had improved efficacy (higher ORR),
higher QOL, and better immune function, and thus this treatment can
be clinically popularized. However, there are some shortcomings in
the present study. Firstly, the number of the research subjects was
small and the follow-up time was short, which may lead to bias in
the K-M survival analysis. Secondly, the QOL of the family members
of the patients and the disease-free survival time were not
investigated. Additionally, the cost for these treatments was not
recorded. Therefore, our aim is to improve these deficiencies in
future studies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW wrote the manuscript, interpreted and analyzed
the data. GL designed the study and performed the experiment. YW
was responsible for the analysis and discussion of the data. Both
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Medical Ethics
Committee of Tianjin Fifth Central Hospital (Tianjin, China).
Patients who participated in this research, provided signed
informed consent and had complete clinical data. Signed written
informed consents were obtained from the patients and/or
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang Y, Liu H, Li W, Yu J, Li J, Shen Z,
Ye G, Qi X and Li G: CircRNA_100269 is downregulated in gastric
cancer and suppresses tumor cell growth by targeting miR-630. Aging
(Albany NY). 9:1585–1594. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sitarz R, Skierucha M, Mielko J, Offerhaus
GJA, Maciejewski R and Polkowski WP: Gastric cancer: Epidemiology,
prevention, classification, and treatment. Cancer Manag Res.
10:239–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wagner AD, Syn NL, Moehler M, Grothe W,
Yong WP, Tai BC, Ho J and Unverzagt S: Chemotherapy for advanced
gastric cancer. Cochrane Database Syst Rev.
8:CD0040642017.PubMed/NCBI
|
|
5
|
Xu W, Yang Z and Lu N: Molecular targeted
therapy for the treatment of gastric cancer. J Exp Clin Cancer Res.
35:12016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li F, Ju Y, Guan Y and Zhao H: Tegafur
gimeracil oteracil potassium capsule induced acute interstitial
lung disease: A case report. Zhongguo Fei Ai Za Zhi. 17:53–56.
2014.(In Chinese). PubMed/NCBI
|
|
7
|
Kobayakawa M and Kojima Y:
Tegafur/gimeracil/oteracil (S-1) approved for the treatment of
advanced gastric cancer in adults when given in combination with
cisplatin: A review comparing it with other fluoropyrimidine-based
therapies. Onco Targets Ther. 4:193–201. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zimmermann C, Swami N, Krzyzanowska M,
Leighl N, Rydall A, Rodin G, Tannock I and Hannon B: Perceptions of
palliative care among patients with advanced cancer and their
caregivers. CMAJ. 188:E217–E227. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kelley AS and Morrison RS: Palliative care
for the seriously ill. N Engl J Med. 373:747–755. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
In H, Solsky I, Palis B, Langdon-Embry M,
Ajani J and Sano T: Validation of the 8th edition of the AJCC TNM
staging system for gastric cancer using the national cancer
database. Ann Surg Oncol. 24:3683–3691. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eisenhauer A and Verweij J: 11 New
response evaluation criteria in solid tumors: RECIST GUIDELINE
VERSION 1.1. Eur J Cancer Suppl. 7:52009. View Article : Google Scholar
|
|
12
|
Chen ML, Liu J, Zhang J and Wu TJ:
Investigation for the incidences of cognitive emotion regulation,
quality of life, anxiety and depression in patients with chronic
heart failure. Chin Circ J. 32:956–959. 2017.
|
|
13
|
Liu N, Liu S, Yu N, Peng Y, Wen Y, Tang J
and Kong L: Correlations among psychological resilience,
self-efficacy, and negative emotion in acute myocardial infarction
patients after percutaneous coronary intervention. Front
Psychiatry. 9:12018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang JY, Verma V, Li M, Zhang W, Komaki
R, Lu C, Allen PK, Liao Z, Welsh J, Lin SH, et al: Proton beam
radiotherapy and concurrent chemotherapy for unresectable stage III
non-small cell lung cancer: Final results of a phase 2 study. JAMA
Oncol. 3:e1720322017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Van Cutsem E, Sagaert X, Topal B,
Haustermans K and Prenen H: Gastric cancer. Lancet. 388:2654–2664.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Plummer M, Franceschi S, Vignat J, Forman
D and de Martel C: Global burden of gastric cancer attributable to
helicobacter pylori. Int J Cancer. 136:487–490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng XJ, Lin JC and Tu SP: Etiology and
prevention of gastric cancer. Gastrointest Tumors. 3:25–36. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Y, Feng Y, Gao Y and Hou R: Clinical
benefits of combined chemotherapy with S-1, oxaliplatin, and
docetaxel in advanced gastric cancer patients with palliative
surgery. Onco Targets Ther. 9:1269–1273. 2016.PubMed/NCBI
|
|
19
|
Lv X, Zhang L, Huang R and Song W: A
clinical exploration of neoadjuvant chemotherapy with tegafur,
gimeracil, and oteracil potassium capsules combined with
oxaliplatin for advanced gastric cancer. Int J Clin Exp Med.
8:19030–19036. 2015.PubMed/NCBI
|
|
20
|
Yeo W, Lam KO, Law LY, Lee CC, Chiang CL,
Au KH, Mo FK, So TH, Lam KC, Ng WT and Li L: Adjuvant S-1
chemotherapy after curative resection of gastric cancer in Chinese
patients: Assessment of treatment tolerability and associated risk
factors. Hong Kong Med J. 23:54–62. 2017.PubMed/NCBI
|
|
21
|
Otsuka H, Fujii T, Toh U, Iwakuma N,
Takahashi R, Mishima M, Takenaka M, Kakuma T, Tanaka M and Shirouzu
K: Phase II clinical trial of metronomic chemotherapy with combined
irinotecan and tegafur-gimeracil-oteracil potassium in metastatic
and recurrent breast cancer. Breast Cancer. 22:335–342. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang T, Zhang SF, Qiu MQ and Li QL:
Efficacy and safety of S-1 (tegafur, gimeracil, and oteracil
potassium) concurrent with 3-dimensional conformal radiotherapy for
newly diagnosed squamous cell carcinoma of the lung in elderly
patients. Cancer Radiother. 20:181–186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
May P, Normand C, Cassel JB, Del Fabbro E,
Fine RL, Menz R, Morrison CA, Penrod JD, Robinson C and Morrison
RS: Economics of palliative care for hospitalized adults with
serious illness: A meta-analysis. JAMA Intern Med. 178:820–829.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Polanski J, Jankowska-Polanska B,
Rosinczuk J, Chabowski M and Szymanska-Chabowska A: Quality of life
of patients with lung cancer. Onco Targets Ther. 9:1023–1028.
2016.PubMed/NCBI
|
|
25
|
Park SA, Chung SH and Lee Y: Factors
influencing the quality of life of patients with advanced cancer.
Appl Nurs Res. 33:108–112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bicakli DH, Ozveren A, Uslu R, Dalak RM,
Cehreli R, Uyar M, Karabulut B and Akcicek F: The effect of
chemotherapy on nutritional status and weakness in geriatric
gastrointestinal system cancer patients. Nutrition. 47:39–42. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cotogni P, De Carli L, Passera R, Amerio
ML, Agnello E, Fadda M, Ossola M, Monge T, De Francesco A and
Bozzetti F: Longitudinal study of quality of life in advanced
cancer patients on home parenteral nutrition. Cancer Med.
6:1799–1806. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Garcia-Anguita A, Kakourou A and Tsilidis
KK: Biomarkers of inflammation and immune function and risk of
colorectal cancer. Curr Colorectal Cancer Rep. 11:250–258. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li K, Zhang Q, Zhang Y, Yang J and Zheng
J: T-cell-associated cellular immunotherapy for lung cancer. J
Cancer Res Clin Oncol. 141:1249–1258. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang F, Hou MX, Wu XL, Bao LD and Dong PD:
Impact of enteral nutrition on postoperative immune function and
nutritional status. Genet Mol Res. 14:6065–6072. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ding D, Feng Y, Song B, Gao S and Zhao J:
Effects of preoperative and postoperative enteral nutrition on
postoperative nutritional status and immune function of gastric
cancer patients. Turk J Gastroenterol. 26:181–185. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun H, Song Y and Wang XY: Effects of
different anesthetic methods on cellular immune and neuroendocrine
functions in patients with hepatocellular carcinoma before and
after surgery. J Clin Lab Anal. 30:1175–1182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ivanova EA and Orekhov AN: T helper
lymphocyte subsets and plasticity in autoimmunity and cancer: An
overview. Biomed Res Int. 2015:3274702015. View Article : Google Scholar : PubMed/NCBI
|