Introduction
Colorectal cancer is a malignant tumor originating
from colon or rectal mucosal epithelium due to various factors
(1), with incidence that ranks third
among all malignant tumors and mortality that ranks fourth
(2). There are no obvious clinical
symptoms in the early stage of the disease and the diagnosis is
difficult. Therefore, most patients with clinical symptoms are in
the advanced stage at the time of diagnosis and metastasis of
cancer cells has occurred (3,4). The
pathogenesis of colorectal cancer still remains unknown as there
are numerous pathogenic factors, which makes the treatment of
colorectal cancer more difficult (5–7).
Surgical resection has been the radical treatment for colorectal
cancer; however, the probability of infection after surgery is
high, resulting in poor survival rate of patients (8,9).
Therefore, the relevant mechanisms of colorectal cancer need to be
clarified and new potential therapeutic targets should be
investigated to improve the prognosis of patients with colorectal
cancer.
MicroRNA, a small RNA with length of ~20–24
nucleotides, regulates cell endogenous functions (10). MicroRNA mainly inhibits the protein
synthesis of target genes, through incomplete and complete
complementation or degradation of the target genes, to regulate the
biological growth and development, and to play an important
regulatory role in cell proliferation, apoptosis and migration
(11). It has been revealed that
microRNA is a key regulator in tumor metastasis and targeted
therapy (12). Upregulation of
miR-452-5p inhibits stem-like traits and tumorigenesis of gliomas
by inhibiting the regulatory factors of various stem cells
(13). It has been reported that
miR-452-5p is a tumor factor for breast malignancies, gliomas, and
prostate tumors; the expression of miR-452-5p in cancerous tissues
is significantly lower than that in adjacent normal tissues, and is
negatively correlated with lymph node metastasis and tumor staging
(14). Furthermore, miR-215-5p is
significantly downregulated in cancerous tissues. The
overexpression of miR-215-5p reduces cell proliferation,
differentiation and migration, as well as the formation of cell
clones. In addition, upregulation of miR-215-5p in gliomas dually
inhibits tumors, effectively inhibits target genes, and reduces
tumor proliferation and migration (15). However, the relationship of
miR-452-5p and miR-215-5p with colorectal cancer remains
unclear.
In the present study, the expression levels of
miR-452-5p and miR-215-5p in colorectal cancer tissues, and their
association with the clinicopathological features of colorectal
cancer patients were explored, in order to provide potential
targets for the treatment and prognosis of colorectal cancer.
Patients and methods
General data
A total of 50 specimens of cancerous and adjacent
normal tissues were collected from colorectal cancer patients who
underwent surgical resection at the Xingtai People's Hospital
(Xingtai, China) from March 2012 to February 2014. The patients'
clinical data were collected, including name, sex, age, tumor
location, tumor diameter, tumor-node-metastasis (TNM) staging,
lymph node metastasis, differentiation degree, and infiltration
degree. Inclusion criteria: Patients confirmed with colorectal
cancer by pathological examinations; patients who had not received
any antitumor therapy (e.g., radiotherapy, chemotherapy, targeted
therapy or drug therapy) before surgery; patients with complete
clinical data. Exclusion criteria: Patients with familial
adenomatous polyposis; patients with previous gastrointestinal
diseases; patients with hypertension, diabetes, severe liver and
kidney diseases, infectious diseases or other malignant tumors; and
patients with mental or communication disorders. The study was
approved by the Ethics Committee of Xingtai People's Hospital. All
patients and their families were informed of the study and signed
informed consents were obtained from the patients.
Main instruments and reagents
Roche LightCycler® 480 II fluorescence
PCR instrument (cat. no. 480II; Beijia Genetool Biotechnology Co.,
Ltd.); Ultraviolet-visible (UV–Vis) spectrophotometer Pharo 300
(cat. no. ZMK-1.00707.0001; Shanghai ZZBIO Co., Ltd.); 3% agarose
gel electrophoresis (cat. no. JKL2116; Shanghai Jingke Scientific
Instrument Co., Ltd.); TRIzol® kit (cat. no. 15596-018;
Beijing Solarbio Science & Technology Co., Ltd.);
TRIzol® reagent (cat. no. 15596018; Yanke Biotechnology
Co., Ltd.); 5X TransScript All-in-One No-RT Control SuperMix for
qPCR (cat. no. GL141-01; Beijing Transgen Biotech Co., Ltd.); 2X
TransTaq HiFi PCR SuperMix II (cat. no. AS131-21; Beijing Transgen
Biotech Co., Ltd.); TaqMan miRNA kit (cat. no. D1802; HaiGene).
miR-452-5p, miR-215-5p and U6 internal reference primers were
designed and synthesized by Beijing Dingguo Changsheng
Biotechnology Co., Ltd. The required primer sequences are shown in
Table I.
 | Table I.Primer sequences of miR-452-5p,
miR-215-5p and U6. |
Table I.
Primer sequences of miR-452-5p,
miR-215-5p and U6.
| Gene | Forward | Reverse |
|---|
| miR-452-5p |
5′-GCGCAACTGTTTGCAGAG-3′ |
5′-GTGCAGGGTCCGAGGT-3′ |
| miR-215-5p |
5′-CTCGAGATGTCATCCTCAG-3′ |
5′-GAATTCGTGAGTTCTTCTG-3′ |
| U6 |
5′-CTCGCTTCGGCAGCACA-3′ |
5′-AACGCTTCACGAATTTGCGT-3′ |
Experimental methods
Colorectal cancer and adjacent normal tissues were
stored at −80°C after being resected, and then they were cut into
pieces and ground with liquid nitrogen in order to obtain the
tissue suspension. Total RNA was extracted using TRIzol®
reagent in strict accordance with the manufacturer's instructions.
The concentration and purity of the extracted RNA were analyzed by
UV–Vis spectrophotometer Pharo 300. The integrity of RNA was
analyzed by 3% agarose gel electrophoresis. An A260/A280 value
between 1.8 and 2.1 was considered to meet the experimental
requirements. After the RNA extraction was completed, reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) was
carried out. The reaction system was 4 µl of 5X TransScript
All-in-One No-RT Control SuperMix for PCR, 2 µg of total RNA, and
ribonuclease distilled water for a final volume of 20 µl. The
reaction conditions were 25°C for 10 min, 42°C for 30 min, 85°C for
5 sec; and that was the end of the inactivation of the reverse
transcriptase.
After the reverse transcription reaction was
completed, PCR amplification was carried out. The PCR amplification
system was 2 µl of cDNA, 25 µl of 2X TransTaq HiFi PCR SuperMix II,
1 µl of upstream primers, 1 µl of downstream primers, and
double-distilled water for a final volume of 50 µl. The PCR
thermocycling conditions were as follows: Pre-denaturation at 95°C
for 3 min, at 94°C for 2 min, at 94°C for 30 sec, at 55°C for 30
sec, at 72°C 1–2 kb/min, 40 cycles, and extension at 72°C for 5 min
after completion of the cycle. The amplification data were analyzed
by the Roche LightCycler® 480 II fluorescence
quantitative PCR instrument. TaqMan miRNA kit was used to detect
the microRNA expression levels. U6 was used as the internal
reference and mRNA levels were quantified using the
2−ΔΔCq method (16).
Follow up
Patients were followed up for 5 years by telephone,
letters and visits. The patients were followed up every 2 months on
average until February 2019. The overall survival (OS) was
calculated from the first day after surgery to the last day of
follow up or the patient's date of death.
Statistical analysis
Statistical software SPSS 22.0 (IBM Corp.) was used
to analyze the experimental data. Counting data were expressed as
the number of cases and percentage [n (%)]. Measurement data were
expressed as the mean ± standard deviation (SD). The comparison of
measurement data between groups was conducted using paired t-test.
Kaplan-Meier survival analysis was used for the generation of the
survival curves of miR-452-5p and miR-215-5p high- and
low-expression groups, and the difference of the curves between
groups was evaluated by log-rank test. Cox regression model was
used to carry out univariate and multivariate analysis in order to
analyze the independent factors affecting prognosis. P<0.05 was
considered to indicate a statistically significant difference.
Results
General data
Of the 50 colorectal cancer patients with complete
clinical data, 32 were males and 18 were females. The patients were
27–78 years of age, including 27 patients <53 years of age and
23 patients ≥53 years of age. The tumor diameter was 1.2–8.6 cm and
the tumor location was in the colon (27 cases) and rectum (23
cases). There were 31 cases of good and moderate differentiation
and 19 cases of poor differentiation. A total of 24 cases were in
stage I+II, whereas 26 cases were in stage III+IV. There were 19
cases with lymph node metastasis and 31 cases without lymph node
metastasis. As for infiltration depth, 21 cases were in T1+T2
stage, and 29 cases were in T3+T4 stage. Details are shown in
Table II.
 | Table II.General clinical data of patients with
colorectal cancer [n (%)]. |
Table II.
General clinical data of patients with
colorectal cancer [n (%)].
| Clinicopathological
feature | Cases |
|---|
| Sex |
|
| Male | 32 (64.00) |
|
Female | 18 (36.00) |
| Age, years |
|
|
<53 | 27 (54.00) |
| ≥53 | 23 (46.00) |
| Tumor diameter,
cm |
|
|
<5 | 30 (60.00) |
| ≥5 | 20 (40.00) |
| Tumor location |
|
|
Colon | 27 (54.00) |
|
Rectum | 23 (46.00) |
| Differentiation
degree |
|
| Well and
moderately differentiated | 31 (62.00) |
| Poorly
differentiated | 19 (38.00) |
| TNM staging |
|
| I+II | 24 (48.00) |
|
III+IV | 26 (52.00) |
| Lymph node
metastasis |
|
| Yes | 19 (38.00) |
| No | 31 (62.00) |
| Infiltration
depth |
|
|
T1+T2 | 21 (42.00) |
|
T3+T4 | 29 (58.00) |
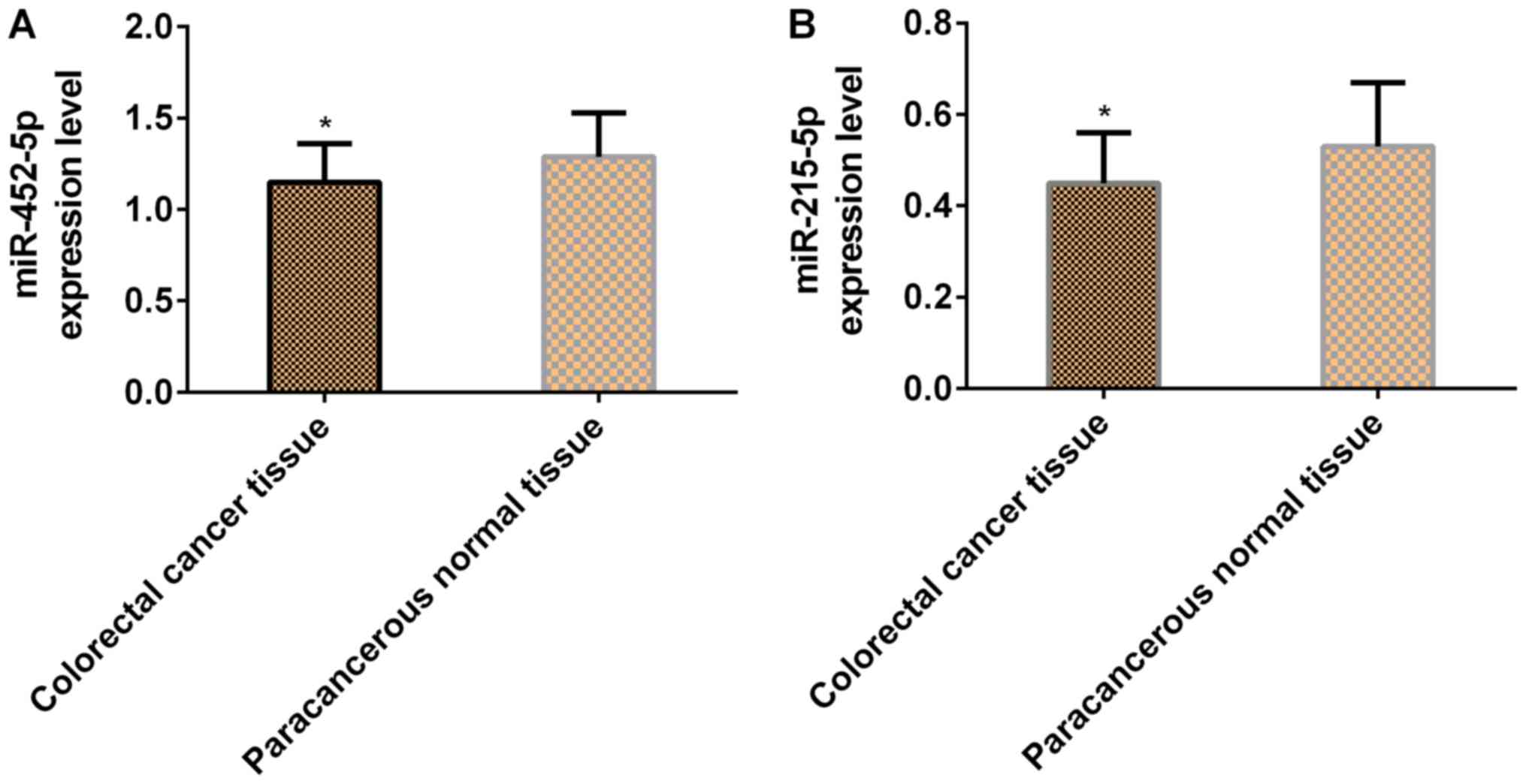
Expression levels of miR-452-5p and
miR-215-5p in cancerous and adjacent normal tissues
The expression levels of both miR-452-5p and
miR-215-5p in colorectal cancer tissues were significantly lower
than those in adjacent normal tissues (P<0.05; Table III and Fig. 1).
 | Table III.Expression levels of miR-452-5p and
miR-215-5p in cancerous and adjacent normal tissues (mean ±
SD). |
Table III.
Expression levels of miR-452-5p and
miR-215-5p in cancerous and adjacent normal tissues (mean ±
SD).
| Group | n | miR-452-5p | miR-215-5p |
|---|
| Colorectal cancer
tissues | 50 | 1.15±0.21 | 0.45±0.11 |
| Adjacent normal
tissues | 50 | 1.29±0.24 | 0.53±0.14 |
| t | – | 3.104 | 3.177 |
| P-value | – | 0.003 | 0.002 |
Relationship between miR-452-5p
expression and clinicopathological features
There was no significant difference in the
expression level of miR-452-5p in colorectal cancer patients in
terms of sex, age, tumor diameter, tumor location, lymph node
metastasis and infiltration depth (P≥0.05). The expression level of
miR-452-5p in colorectal cancer tissues was associated with TNM
staging and differentiation degree (P<0.05; Table IV).
 | Table IV.Relationship between miR-452-5p
expression and clinicopathological features of colorectal cancer
patients (mean ± SD). |
Table IV.
Relationship between miR-452-5p
expression and clinicopathological features of colorectal cancer
patients (mean ± SD).
| Clinicopathological
feature | n | miR-452-5p
expression | t | P-value |
|---|
| Sex |
|
| 0.314 | 0.755 |
|
Male | 32 | 1.14±0.22 |
|
|
|
Female | 18 | 1.16±0.21 |
|
|
| Age, years |
|
| 0.488 | 0.628 |
|
<53 | 27 | 1.16±0.23 |
|
|
|
≥53 | 23 | 1.13±0.20 |
|
|
| Tumor diameter,
cm |
|
| 0.433 | 0.667 |
|
<5 | 30 | 1.16±0.24 |
|
|
| ≥5 | 20 | 1.13±0.24 |
|
|
| Tumor location |
|
| 0.354 | 0.725 |
|
Colon | 27 | 1.16±0.19 |
|
|
|
Rectum | 23 | 1.14±0.21 |
|
|
| Differentiation
degree |
|
| 2.408 | 0.020 |
| Well
and moderately differentiated | 31 | 1.19±0.21 |
|
|
| Poorly
differentiated | 19 | 1.04±0.22 |
|
|
| TNM staging |
|
| 2.132 | 0.038 |
|
I+II | 24 | 1.21±0.24 |
|
|
|
III+IV | 26 | 1.08±0.19 |
|
|
| Lymph node
metastasis |
|
| 0.477 | 0.636 |
|
Yes | 19 | 1.13±0.24 |
|
|
| No | 31 | 1.16±0.20 |
|
|
| Infiltration
depth |
|
| 0.326 | 0.736 |
|
T1+T2 | 21 | 1.16±0.22 |
|
|
|
T3+T4 | 29 | 1.14±0.21 |
|
|
Relationship between miR-215-5p
expression and clinicopathological features
There was no significant difference in the
expression level of miR-215-5p in colorectal cancer patients in
terms of sex, age, tumor diameter, tumor location and
differentiation degree (P≥0.05). The expression level of miR-215-5p
in colorectal cancer tissues was associated with TNM staging, lymph
node metastasis and infiltration depth (P<0.05; Table V).
 | Table V.Relationship between miR-215-5p
expression and clinicopathological features of colorectal cancer
patients (mean ± SD). |
Table V.
Relationship between miR-215-5p
expression and clinicopathological features of colorectal cancer
patients (mean ± SD).
| Clinicopathological
feature | n | miR-215-5p
expression | t | P-value |
|---|
| Sex |
|
| 0.947 | 0.348 |
|
Male | 32 | 0.44±0.10 |
|
|
|
Female | 18 | 0.47±0.12 |
|
|
| Age, years |
|
| 0.581 | 0.564 |
|
<53 | 27 | 0.46±0.13 |
|
|
|
≥53 | 23 | 0.44±0.11 |
|
|
| Tumor diameter,
cm |
|
| 0.673 | 0.504 |
|
<5 | 30 | 0.46±0.09 |
|
|
| ≥5 | 20 | 0.44±0.12 |
|
|
| Tumor location |
|
| 1.525 | 0.134 |
|
Colon | 27 | 0.48±0.12 |
|
|
|
Rectum | 23 | 0.43±0.11 |
|
|
| Differentiation
degree |
|
| 1.611 | 0.114 |
| Well
and moderately differentiated | 31 | 0.47±0.12 |
|
|
| Poorly
differentiated | 19 | 0.41±0.14 |
|
|
| TNM staging |
|
| 3.196 | 0.003 |
|
I+II | 24 | 0.49±0.08 |
|
|
|
III+IV | 26 | 0.38±0.15 |
|
|
| Lymph node
metastasis |
|
| 2.324 | 0.024 |
|
Yes | 19 | 0.47±0.10 |
|
|
| No | 31 | 0.37±0.17 |
|
|
| Infiltration
depth |
|
| 2.057 | 0.045 |
|
T1+T2 | 21 | 0.46±0.13 |
|
|
|
T3+T4 | 29 | 0.39±0.11 |
|
|
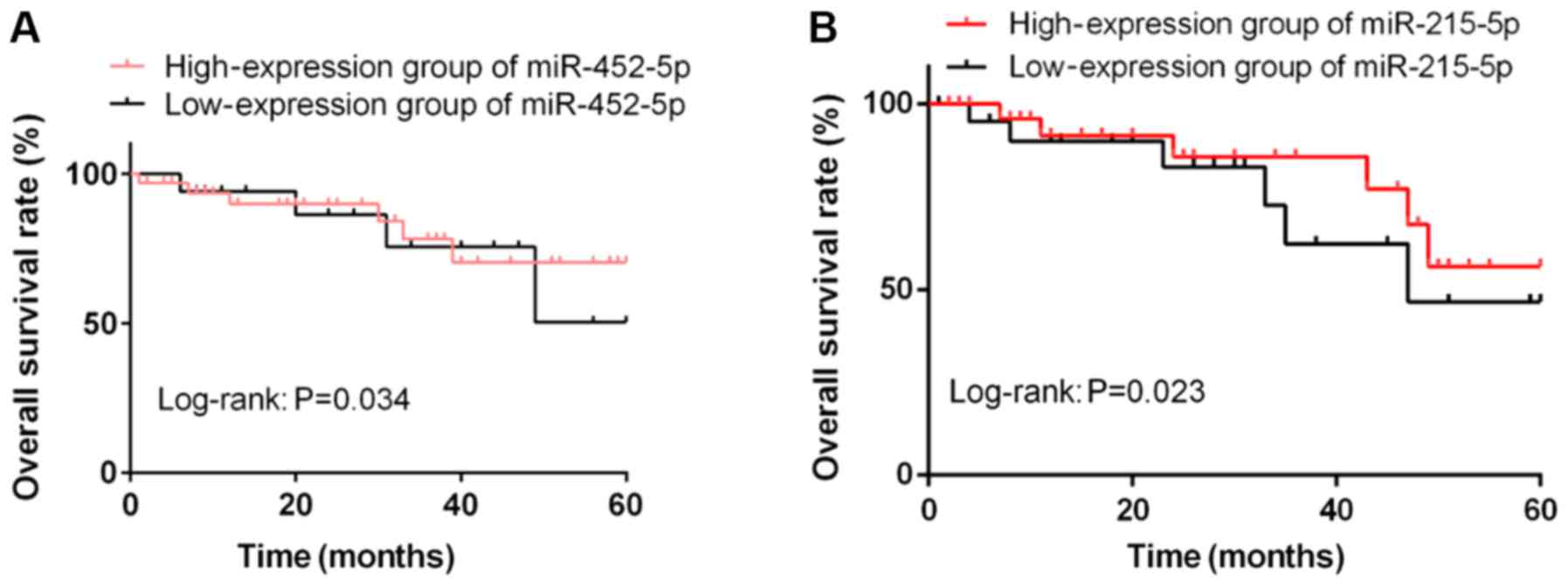
Survival of colorectal cancer
patients
According to the average expression levels of
miR-452-5p and miR-215-5p, the patients were classified into
miR-452-5p high-expression group (≥1.15, n=33), miR-452-5p
low-expression group (<1.15, n=17), miR-215-5p high-expression
group (≥0.45, n=28) and miR-215-5p low-expression group (<0.45,
n=22). The patients were followed up for 5 years by telephone,
letters and visits. The 5-year OS rate was 78.79% (26/33) in the
miR-452-5p high-expression group and 52.94% (9/17) in the
miR-452-5p low-expression group. In other words, the 5-year OS rate
in the miR-452-5p high-expression group was significantly higher
than that in the low-expression group (P<0.05). The 5-year OS
rates in miR-215-5p high- and low-expression groups were 53.57%
(15/28) and 40.91% (9/22), respectively, indicating that the 5-year
OS rate in the miR-452-5p high-expression group was significantly
higher than that in the low-expression group (P<0.05; Fig. 2).
Univariate and multivariate analyses
on prognosis of colorectal cancer
Multivariate Cox proportional hazards regression
model was used to analyze the variables found in univariate
analysis. The results showed that TNM staging, lymph node
metastasis, miR-452-5p and miR-215-5p expression levels were
independent risk factors affecting colorectal cancer prognosis
(P<0.05), whereas differentiation degree and infiltration depth
were not (P>0.05). The details of the analyses are presented in
Table VI.
 | Table VI.Univariate and multivariate analysis
on prognosis of colorectal cancer. |
Table VI.
Univariate and multivariate analysis
on prognosis of colorectal cancer.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI |
|---|
| TNM staging (I+II
vs. III+IV) | 18.26 | 5.43–61.38 | <0.001 | 6.56 | 1.55–27.8 |
| Lymph node
metastasis (yes vs. no) |
3.98 | 1.71–9.30 |
0.015 | 3.24 | 1.32–7.96 |
| miR-452-5p (high
vs. low expression) |
3.61 | 1.85–7.09 |
0.001 | 4.81 | 1.98–11.7 |
| miR-215-5p (high
vs. low expression) |
3.01 | 1.08–8.39 |
0.024 | 4.51 | 1.56–13.01 |
| Infiltration depth
(T1+T2 vs. T3+T4) |
4.59 | 2.13–9.89 |
0.111 | – | – |
| Differentiation
degree (well and moderately vs. poorly) |
6.46 | 2.61–15.96 |
0.204 | – | – |
Discussion
Colorectal cancer is the second leading cause of
cancer death in adults (17). In
most cases, metastasis and diffusion of tumor cells are the
ultimate causes of death (18). In a
gradual way, normal colonic epithelial cells transform and grow
benignly forming polyps, and then develop into benign adenomas,
which can eventually develop into invasive cancers and lesions
(19). Therefore, discovering
biological indicators that affect the prognosis of colorectal
cancer is of great significance for improving the prognosis of
patients and increasing the survival rate.
MicroRNA is an evolutionarily endogenous,
conservative and non-coding small RNA, which achieves negative
regulation of gene expression through binding with specific target
mRNA after transcription (20).
MicroRNA also plays a role in tumor inhibition, cell growth
induction, and inhibition of metastasis and invasion of colorectal
cancer cells, and is a key regulator in cancer progression
(21). miR-452-5p is related to
tumor progression and has different expression in different
cancers. miR-452-5p targets multiple genes and plays an important
role in cancer development and occurrence through various
mechanisms (22,23). Furthermore, the expression of
miR-215-5p in tumor tissue is significantly downregulated, which
may lead to enhanced cell proliferation. A study has reported that
miR-215-5p is an effective inhibitor for tumor and primary colon
tumor initial cells (24). Its tumor
suppression also leads to reduced proliferation, increased
apoptosis and formation of new colonies (25). In the present study, the expression
levels of miR-452-5p and miR-215-5p in cancerous tissues were
significantly lower than those in adjacent normal tissues. In
addition, the investigation of the association between the
clinicopathological characteristics of colorectal cancer patients
and the expression levels of miR-452-5p and miR-215-5p revealed
that miR-452-5p expression was related to TNM staging and
differentiation degree, whereas miR-215-5p expression was
associated with TNM staging, lymph node metastasis and infiltration
depth. These results indicate that miR-452-5p and miR-215-5p may be
involved in the occurrence and progression of colorectal cancer. In
the study by Gao et al (26),
the expression of miR-452-5p was shown to be associated with TNM
staging and lymph node metastasis in colorectal cancer, and
miR-452-5p could target CDKNIB to stimulate tumor growth and
inhibit cell apoptosis. Vychytilova-Faltejskova et al
(27) reported that miR-215-5p
affects specific cells; higher levels of miR-215-5p were shown to
significantly reduce the metabolism and proliferation of colorectal
cancer cells, as well as to inhibit cell migration, indicating the
potential use of miR-215-5p in the diagnosis and prognosis of
colorectal cancer. miR-452-5p and miR-215-5p may play a key role as
tumor suppressor genes in colorectal cancer, and therefore, might
be involved to a certain extent in the progression of colorectal
cancer. Thus, miR-452-5p and miR-215-5p are both expected to be
therapeutic targets and biomarkers for colorectal cancer.
In the study by He et al (28), the expression level of miR-452-5p was
shown to be related to the survival rate of non-small cell lung
cancer patients, and the highly expressed miR-452-5p was reported
to be associated with a better OS. In addition, according to the
study by Halvorsen et al (29), the low expression of miR-215-5p was
related to a poorer OS of patients with non-small cell lung cancer.
According to the results of the present study, the 5-year OS rates
in the miR-452-5p and miR-215-5p high-expression groups were
significantly higher than those in the miR-452-5p and miR-215-5p
low-expression groups. Cox proportional hazards model showed that
miR-452-5p, miR-215-5p, TNM staging and lymph node metastasis were
independent prognostic factors that may affect the survival time of
colorectal cancer patients, whereas differentiation degree and
infiltration depth were not. Therefore, it is believed that the
degree of differentiation and the depth of invasion are prognostic
factors that affect patients with colorectal cancer, although they
are not independent prognostic factors. When the degree of tumor
invasion increases, then the condition of the patient becomes more
serious resulting in a poorer prognosis. However, the expression
levels of miR-452-5p and miR-215-5p in colorectal cancer and
adjacent normal tissues were not associated with sex, age, tumor
diameter or tumor location, indicating that the expression levels
of miR-452-5p and miR-215-5p are less susceptible to individual
factors or other factors, and therefore could be used as prognostic
indicators for clinical evaluation of colorectal cancer patients.
miR-452-5p and miR-215-5p may play an important role in the
occurrence, development and prognosis of colorectal cancer, and
could be considered new biological target indices.
Although the present study confirmed the role of
miR-452-5p and miR-215-5p in the occurrence, development and
prognosis of colorectal cancer, there are still some limitations.
Firstly, the expression levels of miR-452-5p and miR-215-5p in
serum were not detected. Secondly, their effects on proliferation,
apoptosis, migration and invasion of colorectal cancer cells were
not further studied. These will be the aim of our future research.
In addition, a greater sample size and the expression of the
proteins regulated by these microRNAs will be investigated in the
future.
In conclusion, the expression levels of miR-452-5p
and miR-215-5p were significantly downregulated in colorectal
cancer tissues, suggesting that they might promote the occurrence,
progression, invasion and metastasis of colorectal cancer. Thus,
miR-452-5p and miR-215-5p could be used as prognostic indicators
for patients with colorectal cancer.
Acknowledgements
Not applicable.
Funding
The study was supported by the Self-financing
Project of Xingtai Municipal Science and Technology Plan (no.
2017ZC119).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JY analyzed and interpreted the patient general
data. HL performed PCR. YD and JW were responsible for the
statistical analysis of the data. RW was responsible for the
follow-up of the patients. JY wrote the manuscript. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Xingtai People's Hospital. Patients who participated in this
research had complete clinical data and provided a signed informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Al Dahhan SA and Al Lami FH: Epidemiology
of Colorectal Cancer in Iraq, 2002–2014. Gulf J Oncolog. 1:23–26.
2018.PubMed/NCBI
|
|
2
|
Abdulla MH, Valli-Mohammed MA, Al-Khayal
K, Al Shkieh A, Zubaidi A, Ahmad R, Al-Saleh K, Al-Obeed O and
McKerrow J: Cathepsin B expression in colorectal cancer in a Middle
East population: Potential value as a tumor biomarker for late
disease stages. Oncol Rep. 37:3175–3180. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vatandoust S, Price TJ and Karapetis CS:
Colorectal cancer: Metastases to a single organ. World J
Gastroenterol. 21:11767–11776. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li CX, An XX, Zhao B, Wu SJ, Xie GH and
Fang XM: Impact of operation timing on post-operative infections
following colorectal cancer surgery. ANZ J Surg. 86:294–298. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Katsidzira L, Gangaidzo I, Thomson S,
Rusakaniko S, Matenga J and Ramesar R: The shifting epidemiology of
colorectal cancer in sub-Saharan Africa. Lancet Gastroenterol
Hepatol. 2:377–383. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Legrand N, Dixon DA and Sobolewski C:
AU-rich element-binding proteins in colorectal cancer. World J
Gastrointest Oncol. 11:71–90. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Govaert JA, van Dijk WA, Fiocco M,
Scheffer AC, Gietelink L, Wouters MW and Tollenaar RA: Nationwide
outcomes measurement in colorectal cancer surgery: Improving
quality and reducing costs. J Am Coll Surg. 222:19–29.e2. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Curtis NJ, Taylor M, Fraser L, Salib E,
Noble E, Hipkiss R, Allison AS, Dalton R, Ockrim JB and Francis NK:
Can the combination of laparoscopy and enhanced recovery improve
long-term survival after elective colorectal cancer surgery? Int J
Colorectal Dis. 33:231–234. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Obad S, dos Santos CO, Petri A, Heidenblad
M, Broom O, Ruse C, Fu C, Lindow M, Stenvang J, Straarup EM, et al:
Silencing of microRNA families by seed-targeting tiny LNAs. Nat
Genet. 43:371–378. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bhome R, Del Vecchio F, Lee G-H, Bullock
MD, Primrose JN, Sayan AE and Mirnezami AH: Exosomal microRNAs
(exomiRs): Small molecules with a big role in cancer. Cancer Lett.
420:228–235. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhai W, Li S, Zhang J, Chen Y, Ma J, Kong
W, Gong D, Zheng J, Xue W and Xu Y: Sunitinib-suppressed miR-452-5p
facilitates renal cancer cell invasion and metastasis through
modulating SMAD4/SMAD7 signals. Mol Cancer. 17:1572018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu L, Chen K, Wu J, Shi L, Hu B, Cheng S,
Li M and Song L: Downregulation of miR-452 promotes stem-like
traits and tumorigenicity of gliomas. Clin Cancer Res.
19:3429–3438. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He Z, Xia Y, Pan C, Ma T, Liu B, Wang J,
Chen L and Chen Y: Up-regulation of MiR-452 inhibits metastasis of
non-small cell lung cancer by regulating BMI1. Cell Physiol
Biochem. 37:387–398. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang C, Chen Q, Li S, Li S, Zhao Z, Gao H,
Wang X, Li B, Zhang W, Yuan Y, et al: Dual inhibition of PCDH9
expression by miR-215-5p up-regulation in gliomas. Oncotarget.
8:10287–10297. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Simon K: Colorectal cancer development and
advances in screening. Clin Interv Aging. 11:967–976. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ling H, Pickard K, Ivan C, Isella C, Ikuo
M, Mitter R, Spizzo R, Bullock M, Braicu C, Pileczki V, et al: The
clinical and biological significance of MIR-224 expression in
colorectal cancer metastasis. Gut. 65:977–989. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang S, Sun Z, Zhou Q, Wang W, Wang G,
Song J, Li Z, Zhang Z, Chang Y, Xia K, et al: MicroRNAs, long
noncoding RNAs, and circular RNAs: Potential tumor biomarkers and
targets for colorectal cancer. Cancer Manag Res. 10:2249–2257.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wolter JM, Le HH, Linse A, Godlove VA,
Nguyen TD, Kotagama K, Lynch A, Rawls A and Mangone M: Evolutionary
patterns of metazoan microRNAs reveal targeting principles in the
let-7 and miR-10 families. Genome Res. 27:53–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wei W, Yang Y, Cai J, Cui K, Li RX, Wang
H, Shang X and Wei D: miR-30a-5p suppresses tumor metastasis of
human colorectal cancer by targeting ITGB3. Cell Physiol Biochem.
39:1165–1176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gan XN, Gan TQ, He RQ, Luo J, Tang RX,
Wang HL, Zhou H, Qing H, Ma J, Hu XH, et al: Clinical significance
of high expression of miR-452-5p in lung squamous cell carcinoma.
Oncol Lett. 15:6418–6430. 2018.PubMed/NCBI
|
|
23
|
Kolligs FT: Diagnostics and Epidemiology
of Colorectal Cancer. Visc Med. 32:158–164. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ullmann P, Nurmik M, Schmitz M, Rodriguez
F, Weiler J, Qureshi-Baig K, Felten P, Nazarov PV, Nicot N, Zuegel
N, et al: Tumor suppressor miR-215 counteracts hypoxia-induced
colon cancer stem cell activity. Cancer Lett. 450:32–41. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bouvier AM and Launoy G: Epidemiology of
colorectal cancer. Rev Prat. 65:767–773. 2015.(In French).
PubMed/NCBI
|
|
26
|
Gao L, Gan XN, Ye ZH, et al: MiR-452-5p
may serve as an oncogene in colorectal cancer through targeting
CDKN1B: A study based on bioinformatics analysis and
dual-luciferase reporter assay. Int J Clin Exp Med. 12:2151–2166.
2019.
|
|
27
|
Vychytilova-Faltejskova P, Merhautova J,
Machackova T, Gutierrez-Garcia I, Garcia-Solano J, Radova L,
Brchnelova D, Slaba K, Svoboda M, Halamkova J, et al: MiR-215-5p is
a tumor suppressor in colorectal cancer targeting EGFR ligand
epiregulin and its transcriptional inducer HOXB9. Oncogenesis.
6:3992017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He Z, Xia Y, Liu B, Qi X, Li Z, Wang J,
Chen L and Chen Y: Down-regulation of miR-452 is associated with
poor prognosis in the non-small-cell lung cancer. J Thorac Dis.
8:894–900. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Halvorsen AR, Sandhu V, Sprauten M, Flote
VG, Kure EH, Brustugun OT and Helland Å: Circulating microRNAs
associated with prolonged overall survival in lung cancer patients
treated with nivolumab. Acta Oncol. 57:1225–1231. 2018. View Article : Google Scholar : PubMed/NCBI
|