Introduction
According to the World Health Organization report
2016, meningiomas are the most common primary tumors of the central
nervous system in adults in the world (1). Glioma grows in an expansive and
invasive manner, and tends to progress to a higher grade (2). Despite aggressive treatment (such as
surgery followed by radiotherapy or chemotherapy), the median
survival time for patients with GBM is only 14.6 months and most
patients die within 2 years (3). The
complexity of the genesis of malignant gliomas involves different
genetic and molecular pathways (4).
Epidermal growth factor receptor gene amplification and phosphatase
and tensin homolog mutations are more common in primary GBM than
secondary GBM. In secondary GBM, mutations occur more commonly in
the isocitrate dehydrogenase 1 or 2 and TP53 genes (5,6). In ~80%
of GBMs, there are also changes in tyrosine kinase activity
transmembrane receptor signaling pathways, the p53 pathway
(TP53/mouse double minute 2 homolog/p14ARF), the phosphorylated
retinoblastoma (RB) pathway [RB1/cyclin-dependant kinase (CDK)
inhibitor 2A/CDK4] and the telomerase reverse transcriptase
promoter region (pTERT) (7,8). The high variation in the genes involved
in GBM is an important reason for the poor efficacy of chemotherapy
drugs. Therefore, treatment of these highly aggressive tumors is
quite challenging. The understanding of the various important genes
involved in glioma and the underlying signaling pathways activated
during the process of carcinogenesis will reveal the nature of
glioma development and provide new insight into the treatment of
glioma.
Human pituitary tumor-transforming gene 1 (PTTG11)
is a multifunctional proto-oncogene that is upregulated in various
tumors, including glioma and hepatocellular carcinoma (9). The upregulation of PTTG11 is associated
with tumor invasion, progression and angiogenesis, suggesting that
PTTG1 may play a crucial role in tumorigenesis (10). PTTG1 has been identified as a key
‘signature gene’, with high levels of expression predicting
metastasis in multiple tumor types, such as breast, prostate and
ovarian cancer (11). Our previous
study demonstrated that the downregulation of PTTG11 gene
expression significantly inhibited the proliferation, migration and
invasion ability, and increased the apoptosis of SHG44 glioma cells
(12). These studies suggest that
PTTG1 is a potential oncogene involved in tumor development,
invasion and angiogenesis. However, the molecular mechanisms
involved in the regulation of PTTG1 and its actions remain
elusive.
Signal transducer and activator of transcription 3
(STAT3) is an important regulatory factor that modulates tumor cell
proliferation, apoptosis, invasion and metastasis (13). Several previous studies have
demonstrated that STAT3 signaling plays an important role in the
growth of gliomas, and increased STAT3 activation has been
associated with the progression of pathological stages and worse
overall survival (14–16). S3I-201 is a novel and selective STAT3
inhibitor of the Stat3/Stat3 complex, STAT3 tyrosine
phosphorylation and DNA binding, exerting antitumor properties.
Furthermore, the interleukin (IL)-6/JAK/STAT3 pathway is involved
in the pathogenesis of numerous human malignancies (17,18). In
cancer, increased IL-6 levels result in hyperactivation of
JAK/STAT3 signaling, which is typically associated with a poorer
prognosis (19). In the process of
tumorigenesis and development, PTTG11 and STAT3 can affect the
regulation of the cell cycle and participate in biological
processes, such as cell apoptosis and proliferation. PTTG11 and
STAT3 regulate some mutual downstream target genes, including c-Myc
and Bax/Bcl-2 (20,21). Overall, the PTTG11 pathway may be
involved in STAT3 modulated tumor cell proliferation and apoptosis,
although additional studies are required to confirm this
hypothesis.
Our previous study demonstrated that the
downregulation of PTTG11 gene expression significantly inhibited
the proliferation, migration and invasion ability, and increased
the apoptosis of SHG44 glioma cells. However, the molecular
mechanisms that regulate PTTG11 and its actions remain elusive. In
the present study, CCK-8 and flow cytometry assays were used to
assess the proliferation/viability and apoptosis, respectively, of
the human glioma U251 cell line. The purpose of this study is to
explore the effect of PTTG1 on the proliferation and apoptosis of
glioma cell U251 and explore its mechanism.
Materials and methods
Materials
siRNA-PTTG11, scrambled negative control siRNA
(siRNA-NC) and riboFECT CP Transfection kit were purchased from
Guangzhou RiboBio Co., Ltd (https://www.ribobio.com/). Cell Counting Kit-8 (CCK-8)
was purchased from Dalian Meilun Biotechnology Co., Ltd. An
inhibitor (S3I-201) and agonist (IL-6) of STAT3 were purchased from
Sigma-Aldrich; Merck KGaA. Based on previous reports, a dose of 200
µm S3I-201 was used in the present study (21–23). The
annexin V-FITC/propidium iodide (PI) apoptosis kit was purchased
from Nanjing KeyGen Biotech Co., Ltd. The primary antibodies
against PTTG11 (cat. no. ab79546) and Bax (cat. no. ab32503) were
purchased from Abcam. The antibodies against c-myc (cat. no.
D84C12), p-STAT3 (cat. no. D3A7) and STAT3 (cat. no. 124H6) were
purchased from Cell Signaling Technology, Inc. The primary antibody
against β-actin (cat. no. 20536-1-AP) was purchased from the
Proteintech Group, Inc. The secondary antibodies, horse radish
peroxidase (HRP)-labeled goat anti-rabbit IgG (cat. no. GAR007) and
HRP-labeled goat anti-mouse IgG (cat. no. GAM007) were purchased
from Hangzhou Multi Sciences (Lianke) Biotech. Co., Ltd.
Cell culture
U251 cells were cultured in Dulbecco's Modified
Eagle's Medium (DMEM) (Hyclone; GE Healthcare Life Sciences)
containing 10% fetal bovine serum (Thermo Fisher Scientific Inc.)
at 37°C in 5% CO2. The medium was replaced at 48-h
intervals. After the cells had reached 80–90% confluency, the cells
were passaged.
Cell transfection
U251 cells in the logarithmic growth phase were
selected for transfection. The cells were divided into 3 groups: A
blank group (non-transfected cells), a negative control group
(cells transfected with 50 nM siRNA-negative control) and an
siRNA-PTTG11 group (cells transfected with siRNA-PTTG11). According
to our previous study (12), the
sequence of the siRNA-PTTG11 was 5′-GGGAGATCTCAGTTTCAA-3′ and it
was used at a concentration of 50 nM. The cells were placed in
6-well plates at a density of 1×106 cells/well. When
their confluence reached 70%, cell transfection was performed
according to the manufacturer's instructions from the transfection
kit. After 24 h, the medium was replaced with fresh medium in which
siRNA-NC and siRNA-PTTG11 were added along with 80 ng/ml IL-6 for
24 h.
CCK-8 assay
To assess the proliferation of the U251 cells after
different treatments, 5,000 cells/well were inoculated into 96-well
plates and cultured for 24 h. The cells were treated with different
concentrations of S3I-201 (0, 50, 100 or 200 µM) or IL-6 (20, 40 or
80 ng/ml) for 0, 6, 12, 24, 48 or 72 h. The 0 µM was used as the
control. Next, 10 µl of a CCK-8 solution was added to each well and
the cells were incubated at 37°C for 1 h, according to the
manufacturer's protocols. Subsequently, the absorbance at 450 nm of
each group was measured. The cell viability was calculated using
the formula given in the manufacturer's instructions from the
kit.
Flow cytometry
Transfected U251 cells were digested with 0.25%
trypsinase, washed twice with 300 ml prechilled PBS and then
collected by centrifugation 800 × g for 5 min at 4°C. The cells
were resuspended at 1–5×105 cells/ml in 100 µl binding
buffer (eBioscience; Thermo Fisher Scientific, Inc.) followed by
the addition of 5 µl Annexin V labeled with FITC and 10 µl PI in
the dark at room temperature. After 15 min, 400 µl additional
binding buffer was added to the reaction system. Cell apoptosis was
analyzed using a flow cytometer (Attune NxT, Thermo Fisher
Scientific, Inc.) at an excitation wavelength of 488 nm. FlowJo
version 10.5 (BD Biosciences) was used for analysis.
Western blotting
Human glioma U251 cells were lysed with RIPA buffer
(Beijing Solarbio Science & Technology Co., Ltd.) that
contained protease inhibitors. After quantification with a BCA
protein assay kit (Pierce; Thermo Fisher Scientific, Inc.) using a
Multifunctional microplate reader (Molecular Devices). Total
protein (20 µg/lane) was separated on a 12% gel using sodium
dodecyl sulfate polyacrylamide gel electrophoresis. Next, the
proteins were transferred to polyvinylidene difluoride membranes.
The 5% Nonfat-Dried milk (Solarbio, Beijing, China) in TBST was
used to block PVDF membranes at room temperature for 1 h followed
by incubation with primary antibodies against PTTG11 (1:5,000), Bax
(1:1,000), c-Myc (1:1,000), p-STAT3 (1:2,000), STAT3 (1:1,000), or
β-actin (1:5,000) overnight at 4°C. The membranes were then
incubated with the the secondary antibodies (both 1:10,000) at room
temperature for 1 h. ECL solution (EMD Millipore) was used to
detect the proteins on the membrane. The relative protein levels
were normalized to β-actin.
Statistical analysis
Experimental result data are expressed as the mean ±
SEM of three separate experiments. Data for cell viability were
compared using two-way ANOVA followed by Bonferroni's post hoc
test, and one-way ANOVA with Tukey's post hoc test for the
remainder of the multiple comparisons performed. The analyses were
performed using Prism 7 for Windows (GraphPad Software Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of S3I-201 and IL-6 on
viability and apoptosis of U251 cells
It has been reported that S3I-201 and IL-6 are an
inhibitor and an agonist of STAT3, respectively (22,23). To
investigate the effects of IL-6 and S3I-201 on glioma cells, the
CCK-8 assay was used at 0, 6, 12, 24, 48 and 72 h after treatment
with different doses of S3I-201 (0, 50, 100 or 200 µM) or IL-6 (0,
20, 40 or 80 ng/ml) to measure U251 cell viability and
proliferation, respectively. The results demonstrated that the
viability of U251 cells treated with S3I-201 at dosage of 200 µM
was significantly decreased compared with that of the control group
(Fig. 1A). When U251 cells were
treated with IL-6 at dosage of 80 ng/ml, the proliferation of the
U251 cells was significantly increased compared with that in the
control group (Fig. 1B).
In addition, flow cytometry was performed to confirm
that IL-6 (80 ng/ml) and S3I-201 (200 µM) affected apoptosis of the
U251 cells. The results revealed that the percentage of apoptotic
cells was significantly increased in the S3I-201 group compared
with that in the control group (Fig.
1C). However, the percentage of apoptotic cells was decreased
in the IL-6 group compared with the control group, although this
did not reach statistical significance (Fig. 1C). In conclusion, these data implied
that S3I-201 could decrease U251 cell viability and promote
apoptosis, while IL-6 increased proliferation and had no effect on
apoptosis. Thus, it was hypothesized that STAT3 could promote the
viability of and inhibit the apoptosis of U251 cells.
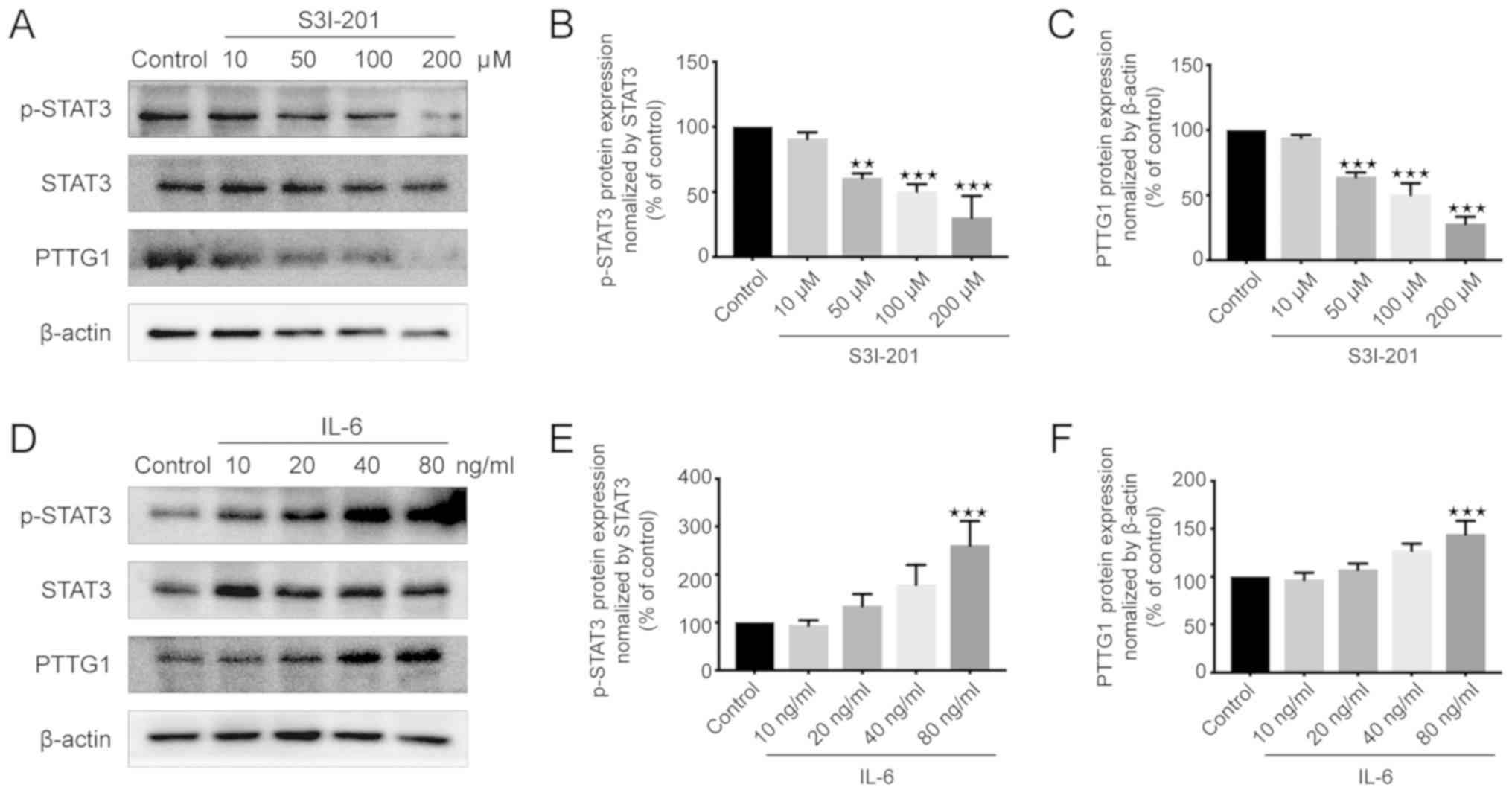
Effects of S3I-201 and IL-6 on the
protein expression of p-STAT3 and PTTG11 in U251 cells
PTTG11 is known to promote the proliferation of
glioma cells (24). To elucidate the
association between STAT3 and PTTG11, western blotting was used to
measure the protein expression levels of p-STAT3 and PTTG11. The
results demonstrated that the expression levels of p-STAT3 and
PTTG11 were significantly decreased in the 50, 100 and 200 µM
S3I-201 groups compared with those in the control group (Fig. 2A-C). However, there was no
statistically significant difference between the 10 µM S3I-201 and
control groups (Fig. 2A-C). By
contrast, the p-STAT3 and PTTG11 levels were increased in the IL-6
group compared with those in the control group, with this effect
reaching significance when cells were treated with 80 ng/ml IL-6
(Fig. 2D-F). The changes in PTTG11
and p-STAT3 demonstrated similar trends to each other when treated
with S3I-201 and IL-6. Therefore, it was concluded that the protein
expression of the p-STAT3 protein was positively associated with
PTTG11 protein expression.
IL-6 promotes the proliferation of
U251 cells by affecting the expression of PTTG11
The aforementioned results demonstrated that IL-6
promoted the proliferation of U251 cells and increased the
expression of PTTG11 in U251 cells. To verify whether IL-6 promotes
the proliferation of U251 by upregulating the expression of PTTG11,
U251 cells were transfected with siRNA-PTTG11 and treated with
IL-6, and the cell viability and apoptotic rates of U251 cells were
investigated. The results of the CCK-8 assays revealed that the
difference in U251 cell viability between the control and siRNA-NC
group was not statistically significant (Fig. 3A). Compared with the siRNA-NC group,
the viability of the siRNA-PTTG11 and siRNA-PTTG11+IL-6 groups was
significantly decreased, while for the siRNA-NC+IL-6 group it was
significantly increased between 24 and 72 h (Fig. 3A). There was no statistically
significant difference between the siRNA-PTTG11 and
siRNA-PTTG11+IL-6 groups (Fig.
3A).
 | Figure 3.Effect of siRNA-PTTG11 on the
viability and apoptosis of U251 cells. The control group was
untreated, and negative control group cells were treated with NC
siRNA. (A) Viability of U251 cells in each group after cell
transfection. The viability of the U 251 cells decreased after the
cells were transfected with siRNA-PTTG11, and there was no
statistically significant difference between the siRNA-PTTG11 and
siRNA-PTTG11+IL-6 (80 ng/ml) groups after 24 h, while the viability
of the siRNA-NC+IL-6 group was significantly increased from 24 to
72 h. (B and C) Apoptosis rate of U251 cells in each group after
transfection. Apoptosis was significantly increased in the
siRNA-PTTG11 and siRNA-PTTG11+IL-6 groups, but decreased in the
siRNA-NC+IL-6 group compared with siRNA-NC group. *P<0.05 and
***P<0.001 compared with the control group (set to 100%). IL,
interleukin; SS, side scatter; FS, forward scatter; PI, propidium
iodide; PTTG11, pituitary tumor transforming gene 1; NC, negative
control; si, small interfering. |
Flow cytometry experiments were performed to further
verify these results. The results demonstrated that compared with
that in the siRNA-NC group, the percentage of apoptotic cells was
significantly increased in the siRNA-PTTG11 and siRNA-PTTG11+IL-6
groups; however, it was decreased in the siRNA-NC+IL-6 group
(Fig. 3B and C). There was no
statistically significant difference in the apoptosis rate between
the siRNA-PTTG11+IL-6 group and the siRNA-PTTG11 group (Fig. 3B and C). These results suggest that
silencing PPTG1 could affect cell proliferation and apoptosis, but
that IL-6 could not effectively weaken this effect. In conclusion,
IL-6 regulates the proliferation and apoptosis of U251 cells by
affecting the expression of PTTG11.
IL-6 affects U251 apoptosis and cell
viability by altering the expression of STAT3-PTTG11-related
proteins
The aforementioned results demonstrated that the
expression levels of p-STAT3 and PTTG11 were positively associated
in U251 cells, while IL-6, as an agonist of STAT3, could upregulate
the expression of PTTG11 to promote cell proliferation. It was
therefore hypothesized that STAT3 might act upstream of PTTG11 and
regulate the apoptosis and proliferation of U251 cells by altering
the expression of PTTG11. To prove this hypothesis, U251 cells were
transfected with siRNA-PTTG11. Following confirmation of
transfection efficiency, western blotting was performed to observe
the changes in protein levels (Fig. 4A
and C). The results indicated that silencing PTTG11 had no
significant effect on p-STAT3 (Fig. 4A
and B). However, IL-6 could upregulate the expression of
p-STAT3 in U251 cells (Fig. 4B). The
expression of PTTG11 was increased in the siRNA-NC+IL-6 group;
however, IL-6 did not obviously increase the level of PTTG11 after
silencing of PTTG11 (Fig. 4C). In
addition, proliferation-associated proteins (c-Myc and Bcl-2)
(25) were decreased in the
siRNA-PTTG11 and siRNA-PTTG11+IL-6 groups compared with the levels
in the siRNA-NC group (Fig. 4D and
E). By contrast, the expression of c-Myc and Bcl-2 was enhanced
in the siRNA-NC-IL-6 group compared with the siRNA-NC group
(Fig. 4D and E). In addition, the
levels of the apoptosis-associated protein (Bax) (26) in the siRNA-PTTG11 and
siRNA-PTTG11+IL-6 groups were significantly higher compared with
the level in the siRNA-NC group (Fig.
4F). In the siRNA-NC+IL-6 group, there was a decrease in the
level of Bax protein compared with the siRNA-NC group (Fig. 4F). In conclusion, IL-6 could
upregulate the expression of PTTG11, c-Myc and Bcl-2, and decrease
the expression of BAX by inducing the expression of p-STAT3, which
could be achieved by regulating the target gene PTTG11.
 | Figure 4.IL-6 affects apoptosis and
proliferation by altering the expression of related proteins in
U251 cells. The control group was untreated, and negative control
group cells were treated with NC siRNA. (A) Expression of
proliferation and apoptosis-related proteins in U251 cells of each
group as shown by western blotting. The expression levels of
p-STAT3, PTTG11, c-Myc, Bcl-2 and Bax in U251 cells after the
indicated treatment. (B) Whether PTTG11 was silenced or not, IL-6
could upregulate the expression of p-STAT3. (C) IL-6 at dosage of
80 ng/ml could upregulate the expression of PTTG11. This effect was
not obvious when PTTG11 was silenced. (D) c-Myc and (E) Bcl-2 were
decreased in the siRNA-PTTG11 and siRNA-PTTG11+IL-6 groups, but
enhanced in the siRNA-NC+IL-6 group compared with siRNA-NC group.
(F) Bax levels in the siRNA-PTTG11 and siRNA-PTTG11+IL-6 groups
were notably higher but were at their lowest in the siRNA-NC+IL-6
group compared with siRNA-NC group. *P<0.05, **P<0.01 and
***P<0.001 compared with the control group (set to 100%). IL,
interleukin; PTTG11, pituitary tumor transforming gene 1; NC,
negative control; si, small interfering. |
Discussion
Due to the failure of conventional therapeutic
strategies (surgery combined with radiotherapy and chemotherapy)
(27,28), an understanding of the molecular
mechanisms of glioma will be helpful for treating the disease and
for delivering tumoricidal agents. The oncogenic transcription
factor STAT3 is a key signaling hub, regulating a number of
tumor-related processes, including proliferation, migration,
apoptosis-resistance, angiogenesis and immune evasion (29). A previous study demonstrated that
PTTG11 plays an important role in gene modulation, angiogenesis,
mitoses, cell cycle control, cell transformation, DNA repair and
cell apoptosis (30). A high
expression level of PTTG11 has been observed in U251 glioma cells.
Both STAT3 and PTTG11 have been implicated in multiple steps of
glioma development and progression (19,24), but
the mechanisms that regulate their coordinated functions were not
known.
The findings of the present study used an activator
and inhibitor of STAT3 to reveal that a positive association exists
between the extent of STAT3 and PTTG11 activation. The present
study demonstrated that both p-STAT3 and PTTG11 protein levels were
significantly decreased in the S3I-201 group compared with those in
the NC group (Fig. 2A-C). However,
p-STAT3 and PTTG11 protein levels increased significantly in the
IL-6 group compared with those in the NC group (Fig. 2D-F). When PTTG1-siRNA was used, the
effects of STAT3 on proliferation were significantly suppressed and
PTTG1 silencing increased the apoptosis of U251 cells. These
results demonstrated that PTTG1 may be one of the genes targeted by
the STAT3 signaling pathway that acts to promote glioma
proliferation and apoptosis.
In addition, in the present study, the protein
expression of genes downstream of the STAT3-PTTG1 signaling pathway
was assessed. Therefore, the effect of PTTG1-siRNA transfection on
IL-6-induced proliferation and apoptosis-related protein expression
in U251 cells was investigated. Following PTTG1-siRNA transfection,
the protein expression levels of PTTG11, c-Myc and Bcl-2 were
decreased, while that of Bax was increased. IL-6-induced STAT3
activation upregulated PTTG11, c-Myc and Bcl-2 protein expression,
and downregulated Bax protein expression, which was achieved by
regulating the target gene PTTG11. The findings of the present
study demonstrated that c-Myc, which is responsible for the
inhibition of cell proliferation, was downregulated after
PTTG11-siRNA transfection. It was also demonstrated that Bcl-2 was
downregulated and BAX was upregulated following PTTG11-siRNA
transfection, showing that together, these processes are
responsible for increased levels of cell apoptosis. These results
are similar to previous reports (24,25).
Of course, this study also has limitations. We used
the U251 cell line to study the role of PTTG1 in glioma
proliferation and apoptosis, and explored its mechanism. Although
U251 is a widely used and recognized cell for gliomas study, but
the use of single cell line still makes this research slightly
underweight. In conclusion, the findings of the present study
demonstrated that PTTG11 is a downstream gene of STAT3. STAT3
induces PTTG11 expression, which then induces c-Myc and Bcl-2
expression, and inhibits BAX expression, thereby promoting cell
proliferation and inhibiting apoptosis. The findings of the present
study suggest that PTTG11 plays an important role in glioma
progression by regulating cell proliferation and apoptosis. PTTG11
may be a potential therapeutic target for blocking glioma cell
proliferation.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the
Fundamental Research Funds for the Central Universities (grant no.
20720180042), the Health Science Research Personnel Training
Program of Fujian Province (grant no. 2016-CXB-12) and the Natural
Science Foundation of Fujian, China (grant no. 2016D019).
Availability of data and materials
All data used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LC, XJ and LY designed the experiments and wrote the
manuscript. LX, GW, JW and LL were involved in the design of the
study and performed the experiments. HZ, SC, MZ and CS performed
the statistical analysis. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Branger DF, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues
P and Ellison DW: The 2016 world health organization classification
of tumors of the central nervous system: A summary. Acta
Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hawkins-Daarud A, Rockne RC, Anderson AR
and Swanson KR: Modeling tumor-associated edema in gliomas during
anti-angiogenic therapy and its impact on imageable tumor. Front
Oncol. 3:662013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin L, Cai J and Jiang C: Recent advances
in targeted therapy for glioma. Curr Med Chem. 24:1365–1381. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Omuro A and DeAngelis LM: Glioblastoma and
other malignant gliomas: A clinical review. JAMA. 310:1842–1850.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Navarro L, Gil-Benso R, Megías J,
Muñoz-Hidalgo L, San- Miguel T, Callaghan RC, González-Darder JM,
López-Ginés C and Cerdá-Nicolás MJ: Alteration of major vault
protein in human glioblastoma and its relation with EGFR and PTEN
status. Neuroscience. 297:243–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Furnari FB, Fenton T, Bachoo RM, Mukasa A,
Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, et al:
Malignant astrocytic glioma: Genetics, biology, and paths to
treatment. Genes Dev. 21:2683–2710. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Inda MM, Bonavia R, Mukasa A, Narita Y,
Sah DW, Vandenberg S, Brennan C, Johns TG, Bachoo R, Hadwiger P, et
al: Tumor heterogeneity is an active process maintained by a mutant
EGFR-induced cytokine circuit in glioblastoma. Genes Dev.
24:1731–1745. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Muñoz-Hidalgo L, San-Miguel T, Megías J,
Monleón D, Navarro L, Roldán P, Cerdá-Nicolás M and López-Ginés C:
Somatic copy number alterations are associated with EGFR
amplification and shortened survival in patients with primary
glioblastoma. Neoplasia. 22:10–21. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Benito R, Gil-Benso R, Quilis V, Perez M,
Gregori-Romero M, Roldan P, Gonzalez-Darder J, Cerdá-Nicolas M and
Lopez-Gines C: Primary glioblastomas with and without EGFR
amplification: Relationship to genetic alterations and
clinicopathological features. Neuropathology. 30:392–400. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akizuki K, Sekine M, Kogure Y, Kameda T,
Shide K, Koya J, Kamiunten A, Kubuki Y, Tahira Y, Hidaka T, et al:
TP53 and PTEN mutations were shared in concurrent germ cell tumor
and acute megakaryoblastic leukemia. BMC Cancer. 20:52020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Solbach C, Roller M, Fellbaum C, Nicoletti
M and Kaufmann M: PTTG1 mRNA expression in primary breast cancer: A
prognostic marker for lymph node invasion and tumor recurrence.
Breast. 13:80–81. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Y, Zhou LP, Ma P, Sui CG, Meng FD, Tian
X, Fu LY and Jiang YH: Relationship of PTTG1 expression with tumor
invasiveness and microvessel density of pituitary adenomas: A
meta-analysis. Genet Test Mol Biomarkers. 18:279–285. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ramaswamy S, Ross KN, Lander ES and Golub
TR: A molecular signature of metastasis in primary solid tumors.
Nat Genet. 33:49–54. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cui LS, Lin T, Xu LX, Wang GL, Lin JB,
Feng SP, Cao Y, Cao Y, Song ZM and Jin X: The effect of
down-regulated gene PTTG11 on proliferation, apoptosis, migration
and invasion of human glioma cell SHG44. China Oncol. 29:338–344.
2019.
|
|
15
|
Johnston PA and Grandis JR: STAT3
signaling: Anticancer strategies and challenges. Mol Interv.
11:18–26. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tu Y, Zhong Y, Fu J, Cao Y, Fu G, Tian X
and Wang B: Activation of JAK/STAT signal pathway predicts poor
prognosis of patients with gliomas. Med Oncol. 28:15–23. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tan MSY, Sandanaraj E, Chong YK, Lim SW,
Koh LW, Ng WH, Tan NS, Tan P, Ang BT and Tang C: A STAT3-based gene
signature stratifies glioma patients for targeted therapy. Nat
Commun. 10:36012019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ouedraogo ZG, Biau J, Kemeny JL, Morel L,
Verrelle P and Chautard E: Role of STAT3 in genesis and progression
of human malignant gliomas. Mol Neurobiol. 54:5780–5797. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang N, Ahn SH, Kong DS, Lee HW and Nam
DH: The role of STAT3 in glioblastoma progression through dual
influences on tumor cells and the immune microenvironment. Mol Cell
Endocrinol. 451:53–65. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Siddiquee K, Zhang S, Guida WC, Blaskovich
MA, Greedy B, Lawrence HR, Yip ML, Jove R, McLaughlin MM, Lawrence
NJ, et al: Selective chemical probe inhibitor of Stat3, identified
through structure-based virtual screening, induces antitumor
activity. Proc Natl Acad Sci USA. 104:7391–7396. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou C, Tong Y, Wawrowsky K and Melmed S:
PTTG1 acts as a STAT3 target gene for colorectal cancer cell growth
and motility. Oncogene. 33:851–861. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sarper SE, Inubushi T, Kurosaka H, Minagi
HO, Kuremoto KI, Sakai T, Taniuchi I and Yamashiro T: Runx1-Stat3
signaling regulates the epithelial stem cells in continuously
growing incisors. Sci Rep. 8:109062018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Segatto I, Berton S, Sonego M, Massarut S,
Perin T, Piccoli E, Colombatti A, Vecchione A, Baldassarre G and
Belletti B: Surgery-Induced wound response promotes stem-like and
tumor-initiating features of breast cancer cells, via STAT3
signaling. Oncotarget. 5:6267–6279. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Su W, Guo C, Wang L, Wang Z, Yang X, Niu
F, Tzou D, Yang X, Huang X, Wu J, et al: LncRNA MIR22HG abrogation
inhibits proliferation and induces apoptosis in esophageal
adenocarcinoma cells via activation of the STAT3/c-Myc/FAK
signaling. Aging (Albany NY). 11:4587–4596. 2019.PubMed/NCBI
|
|
25
|
Guha P, Gardell J, Darpolor J, Cunetta M,
Lima M, Miller G, Espat NJ, Junghans RP and Katz SC: STAT3
inhibition induces bax-dependent apoptosis in liver tumor
myeloid-derived suppressor cells. Oncogene. 38:533–548. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chuang PY and He JC: JAK/STAT signaling in
renal diseases. Kidney Int. 78:231–234. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Banerjee K and Resat H: Constitutive
activation of STAT3 in breast cancer cells: A review. Int J Cancer.
138:2570–2578. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhi T, Jiang K, Xu X, Yu T, Zhou F, Wang
Y, Liu N and Zhang J: ECT2/PSMD14/PTTG11 axis promotes the
proliferation of glioma through stabilizing E2F1. Neuro Oncol.
21:462–473. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luwor RB, Baradaran B, Taylor LE, Iaria J,
Nheu TV, Amiry N, Hovens CM, Wang B, Kaye AH and Zhu HJ: Targeting
stat3 and smad7 to restore TGF-beta cytostatic regulation of tumor
cells in vitro and in vivo. Oncogene. 32:2433–2441. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Boelaert K, McCabe CJ, Tannahill LA,
Gittoes NJ, Holder RL, Watkinson JC, Bradwell AR, Sheppard MC and
Franklyn JA: Pituitary tumor transforming gene and fibroblast
growth factor-2 expression: Potential prognostic indicators in
differentiated thyroid cancer. J Clin Endocrinol Metab.
88:2341–2347. 2003. View Article : Google Scholar : PubMed/NCBI
|