Introduction
Gastric cancer (GC) is the third leading cause of
cancer-associated mortality worldwide and the high incidence of GC
poses a major threat to public health globally (1). At present, GC may be cured by radical
resection; however, for patients with advanced-stage disease that
can no longer receive radical surgical treatment, the median
survival time after treatment with trastuzumab plus chemotherapy is
only 13.8 months, the improvement of which poses a challenge to
scientists and oncologists (2).
Therefore, it is necessary to identify patients with poor prognosis
as soon as possible, which will help doctors optimize treatment
programs and improve the prognosis and quality of life of patients
with advanced GC. The Union for International Cancer Control TNM
classification system is one of the most common methods used by
clinicians to evaluate the prognosis of patients with GC (3). However, this system has certain
limitations, such as the fact that patients with the same tumor
stage may have different clinical outcomes (4,5).
Therefore, it is imperative to identify other independent
prognostic markers for GC to further classify patients who have
been staged according to the TNM system into subgroups with good
and poor prognosis. The addition of other well-proven markers
should enable doctors to treat patients with poor prognosis in a
more timely and effective manner.
Tumorigenesis is a multifactorial process.
Hypercoagulability, inflammation and malnutrition promote the
occurrence, development, recurrence and metastasis of tumors and
are associated with poor treatment outcomes (6–11).
Analysis of existing data has demonstrated that conditions of
hypercoagulability, inflammation and poor nutritional status are
independent prognostic factors for poor prognosis of patients with
GC. As a marker reflecting coagulation function nutritional and the
inflammatory status of patients, several studies have used the
fibrinogen-to-albumin ratio (FAR) or albumin-to-fibrinogen ratio
(AFR) to evaluate the prognosis of various cancer patients,
including those with GC, and have found that increased FAR or
decreased AFR levels were associated with poor prognosis in cancer
patients (12–17). Another critical role of FAR or AFR is
to assess the effect of the chemotherapy regimen in a particular
population to guide the selection of an optimal therapy (18–20).
However, there are currently few reports on the pretreatment FAR
being used as a marker to predict progression-free survival (PFS)
and overall survival (OS) in patients with GC undergoing first-line
chemotherapy.
The aim of the present study was to investigate
whether the pretreatment FAR may serve as a multifunctional marker
for predicting PFS and OS in patients with advanced GC receiving
first-line chemotherapy.
Materials and methods
Patient characteristics
The data of patients with non-resectable GC from the
Cancer Hospital of China Medical University (Shenyang, China)
between January 2014 and January 2019 were retrospectively
evaluated. All patients included were required to fulfill the
following criteria: i) The diagnosis was pathologically confirmed;
ii) the TNM stage for patients with GC unable to undergo radical
resection was considered only as stage III–IV; iii) the patients
included in the data collection received no adjuvant
chemoradiotherapy; iv) no severe coagulation disorders, nutritional
therapy, acute infections or other inflammatory conditions prior to
specimen collection; and v) blood sample collection performed
within 1 week prior to the initial first-line chemotherapy. A total
of 273 patients with advanced GC meeting the inclusion criteria
were included in the present retrospective study, 183 of whom were
male and 90 were female. The study protocol was approved by the
Cancer Hospital of China Medical University Ethics Committee
(Shenyang, China).
Clinical data collection and
follow-up
In all patients included in the study, computed
tomography, magnetic resonance imaging or other accepted imaging
examinations were used to determine the presence of metastatic
lymph nodes and organs. The reference range for plasma fibrinogen,
albumin and hemoglobin was 2–4, 35–55 and 115–155 g/l,
respectively. The accepted normal range for carcinoembryonic
antigen, CA19-9 and CA72-4 was 0–5 ng/ml, 0–37 U/ml and 0–6 U/ml,
respectively. The FAR was calculated as follows: FAR=fibrinogen
(g/l)/albumin (g/l) ×100%.
The primary chemotherapeutic regimens for patients
receiving first-line chemotherapy are as follows: SOX (oxaliplatin
+ S1)/CapeOX (oxaliplatin + capecitabine), FOLFOX (oxaliplatin +
leucovorin + 5-fluorouracil) and DCF (docetaxel + cisplatin +
5-fluorouracil)/DOF (docetaxel + oxaliplatin + 5-fluorouracil). A
total of 137 patients received SOX/CapeOX regimen, 49 patients
received FOLFOX regimen, 36 patients received DCF/DOF regimen and
51 patients received S1 or capecitabine monotherapy. The efficacy
of treatment was evaluated every 2–3 cycles. The Response
Evaluation Criteria in Solid Tumors version 1.1 were employed to
estimate the response to chemotherapy (21). Patients with first-line chemotherapy
failure were followed up every 2–3 months. The latest follow-up
date was December 2019. PFS and OS were considered as the primary
endpoints. PFS was defined as the time from start of first-line
chemotherapy until the first observed progression or the last
follow-up visit without progression, while OS was defined as the
time from the beginning of first-line chemotherapy to death from
any cause or the end of follow-up.
Statistical analysis
Data analysis was performed using SPSS v21 (IBM,
Corp.). Receiver operating characteristic (ROC) curve analysis was
applied to calculate the cut-off values of the FAR, fibrinogen and
albumin. Fisher's exact test and the χ2 test were
employed to evaluate the association between the FAR and the
clinicopathological characteristics. Univariate and multivariate
data analyses were conducted to identify potential predictors of
PFS and OS. The Kaplan-Meier method was used to construct survival
curves. The survival of patients was compared with the log-rank
test. P<0.05 was considered to indicate a statistical
significant difference.
Results
Optimal cut-off value of the FAR
In addition to the FAR, the present study also
assessed fibrinogen and albumin to determine whether the FAR is
superior to either fibrinogen or albumin alone in predicting the
prognosis of patients with GC. The area under the ROC curve (AUC)
is considered to indicate the overall diagnostic power of a model,
with a larger AUC indicating better diagnostic power of the
prognostic predictor. In order to assess the ability of the FAR to
discriminate between patients with advanced GC with different
prognosis, ROC curve analysis was performed to determine the
optimal cut-off values of the FAR, fibrinogen and albumin, and the
AUC values of these three indicators were further compared. The
median overall survival of 339 days was used as the fixed variable
and the levels of FAR, fibrinogen and albumin as the test variables
to determine the cut-off values. The optimal cut-off values of the
FAR, fibrinogen and albumin were 10.033, 3.795 and 40.550,
respectively. The FAR had a higher AUC value (0.690; 95% CI:
0.628–0.752; P<0.001) compared with fibrinogen (0.657; 95% CI:
0.593–0.721; P<0.001) and albumin (0.660; 95% CI: 0.596–0.724;
P<0.001; Fig. 1), which indicated
that the FAR is likely superior to fibrinogen and albumin alone in
predicting the prognosis of patients with GC. The cut-off values of
the FAR, fibrinogen and albumin were set as 10.03, 3.80 and 40.55,
respectively, in the further analyses and patients with FAR
>10.03, fibrinogen ≥3.80 and albumin ≥40.55 comprised the
high-FAR group, whereas the remaining patients comprised the
low-FAR group.
Association of clinicopathological
characteristics with the FAR
Of the 273 patients included in the present study,
183 (67.03%) were male and 90 (32.97%) were female. The median age
was 60 years (range, 52–65 years) and the median body mass index
was 21.6 kg/m2 (range, 19.6–23.6 kg/m2).
There were 80 (29.30%) patients with peritoneal metastasis and 220
(80.59%) patients with stage IV disease. Patients with a CA72-4
level >6 U/ml and hemoglobin <115 g/l accounted for 57.51 and
38.46% of the total, respectively (Table
I).
 | Table I.Association between pretreatment FAR
and clinicopathological characteristics. |
Table I.
Association between pretreatment FAR
and clinicopathological characteristics.
| Variable | All | Low FAR | High FAR | P-value |
|---|
| Total | 273 (100.0) | 175 (64.10) | 98 (35.90) |
|
| Age (years) |
|
|
| 0.057 |
|
<60 | 135 (49.45) | 79 (45.14) | 56 (57.14) |
|
|
≥60 | 138 (50.55) | 96 (54.86) | 42 (42.86) |
|
| Sex |
|
|
| 0.726 |
|
Male | 183 (67.03) | 116 (66.29) | 67 (68.37) |
|
|
Female | 90 (32.97) | 59 (33.71) | 31 (31.63) |
|
| Body mass index
(kg/m2) |
|
|
| 0.965 |
|
<18.5 or >25 | 72 (26.37) | 46 (26.29) | 26 (26.53) |
|
|
18.5–25 | 201 (73.63) | 129 (73.71) | 72 (73.47) |
|
| ECOG PS score |
|
|
| 0.695 |
|
0-1 | 228 (83.52) | 145 (82.86) | 83 (84.69) |
|
|
>1 | 45 (16.48) | 30 (17.14) | 15 (15.31) |
|
| Histological
differentiation |
|
|
| 0.050 |
|
High/moderate | 72 (26.37) | 53 (30.29) | 19 (19.39) |
|
|
Poor/mucinous | 201 (73.63) | 122 (69.71) | 79 (80.61) |
|
| Number of organs
with metastasis |
|
|
| 0.486 |
|
0-1 | 180 (65.93) | 118 (67.43) | 62 (63.27) |
|
|
>1 | 93 (34.07) | 57 (32.57) | 36 (36.73) |
|
| Peritoneal
metastasis |
|
|
| 0.010 |
|
Yes | 80 (29.30) | 42 (24.00) | 38 (38.78) |
|
| No | 193 (70.70) | 133 (76.00) | 60 (61.22) |
|
| TNM stage |
|
|
| 0.025 |
|
III | 53 (19.41) | 41 (23.43) | 12 (12.24) |
|
| IV | 220 (80.59) | 134 (76.57) | 86 (87.76) |
|
| CEA (ng/ml) |
|
|
| 0.691 |
| ≤5 | 152 (55.68) | 99 (56.57) | 53 (54.08) |
|
|
>5 | 121 (44.32) | 76 (43.43) | 45 (45.92) |
|
| CA19-9 (U/ml) |
|
|
| 0.264 |
|
≤37 | 168 (61.54) | 112 (64.00) | 56 (57.14) |
|
|
>37 | 105 (38.46) | 63 (36.00) | 42 (42.86) |
|
| CA72-4 (U/ml) |
|
|
| 0.027 |
| ≤6 | 116 (42.49) | 83 (47.43) | 33 (33.67) |
|
|
>6 | 157 (57.51) | 92 (52.57) | 65 (66.33) |
|
| Hemoglobin
(g/l) |
|
|
| 0.031 |
|
<115 | 105 (38.46) | 59 (33.71) | 46 (46.94) |
|
|
≥115 | 168 (61.54) | 116 (66.29) | 52 (53.06) |
|
There were certain differences in the
clinicopathological characteristics between the low-FAR and the
high-FAR groups. An elevated FAR was significantly associated with
peritoneal carcinomatosis (P=0.010), stage IV cancer (P=0.025),
increased CA72-4 levels (P=0.027) and anemia (P=0.031). The
clinicopathological characteristics of the patients are further
described in detail in Table I.
Prognostic factors indicating patient
survival
As presented in Table
II, univariate analyses revealed that metastasis in >1 organ
(P=0.003), peritoneal metastasis (P<0.001), TNM stage IV
(P=0.026) and increased CA72-4 levels (P=0.001) were significantly
associated with shorter PFS, while increased albumin (P=0.003) and
reduced fibrinogen (P<0.001) and FAR (P<0.001) levels were
significantly associated with longer PFS. On multivariate analysis,
only peritoneal metastasis (P=0.042), CA72-4 levels (P=0.013),
albumin (P=0.048) and FAR (P=0.020) were indicated to be
independently associated with PFS. As the P-value of FAR (P=0.020)
was lower compared with that of fibrinogen (P=0.231) and albumin
(P=0.048) alone, the FAR was considered as significantly more
effective than either fibrinogen or albumin alone in predicting PFS
in patients with GC.
 | Table II.Associations of PFS with FAR and
other clinicopathological factors. |
Table II.
Associations of PFS with FAR and
other clinicopathological factors.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age >60
years | 0.988 | 0.778–1.255 | 0.921 |
|
|
|
| Male sex | 1.140 | 0.884–1.471 | 0.313 |
|
|
|
| Body mass
index |
| <18.5 or >25
kg/m2 | 0.940 | 0.717–1.231 | 0.651 |
|
|
|
| ECOG PS score
>1 | 1.295 | 0.940–1.784 | 0.114 | 1.164 | 0.832–1.629 | 0.376 |
| Histological
differentiation, poor/mucinous | 1.144 | 0.873–1.500 | 0.329 |
|
|
|
| Metastasis in >1
organ | 1.464 | 1.138–1.883 | 0.003 | 1.232 | 0.920–1.651 | 0.162 |
| Peritoneal
metastasis | 1.813 | 1.388–2.367 | <0.001 | 1.370 | 1.011–1.857 | 0.042 |
| TNM stage IV | 1.412 | 1.042–1.913 | 0.026 | 1.060 | 0.759–1.481 | 0.731 |
| CEA >5
ng/ml | 1.162 | 0.913–1.480 | 0.223 |
|
|
|
| CA19-9 >37
U/ml | 0.972 | 0.761–1.243 | 0.823 |
|
|
|
| CA72-4 >6
U/ml | 1.506 | 1.178–1.926 | 0.001 | 1.375 | 1.068–1.770 | 0.013 |
| Hemoglobin <115
g/l | 0.919 | 0.718–1.176 | 0.502 |
|
|
|
| Fibrinogen <3.8
g/l | 0.567 | 0.441–0.729 | <0.001 | 0.805 | 0.564–1.149 | 0.231 |
| Albumin ≥40.55
g/l | 0.697 | 0.547–0.887 | 0.003 | 0.770 | 0.595–0.998 | 0.048 |
| FAR ≤10.03 | 0.468 | 0.360–0.608 | <0.001 | 0.638 | 0.436–0.932 | 0.020 |
As presented in Table
III, metastasis to >1 organ (P=0.030), peritoneal metastasis
(P<0.001) and CA72-4 levels >6 U/ml (P=0.033) were identified
as risk factors adversely affecting the OS of patients with GC,
while an age of >60 years (P=0.040), fibrinogen <3.8 g/l
(P<0.001), albumin ≥40.55 g/l (P<0.001) and FAR ≤10.03
(P<0.001) were indicated to be protective factors favorably
affecting the OS of patients with GC on univariate analysis. The
results of the multivariate analysis demonstrated that the OS of
patients with GG was independently associated with peritoneal
metastasis (P=0.019), albumin (P=0.016) and FAR (P=0.002). FAR
(P=0.002) was superior to fibrinogen (P=0.938) and albumin
(P=0.016) in predicting the OS of patients with GC.
 | Table III.Associations of OS with FAR and other
clinicopathological factors. |
Table III.
Associations of OS with FAR and other
clinicopathological factors.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age >60
years | 0.778 | 0.612–0.989 | 0.040 | 0.869 | 0.674–1.120 | 0.278 |
| Male sex | 1.136 | 0.881–1.465 | 0.324 |
|
|
|
| Body mass
index |
| <18.5 or >25
kg/m2 | 1.014 | 0.774–1.329 | 0.921 |
|
|
|
| ECOG PS score
>1 | 1.304 | 0.946–1.798 | 0.105 | 1.196 | 0.858–1.668 | 0.291 |
| Histological
differentiation, poor/mucinous | 1.268 | 0.968–1.661 | 0.084 | 1.096 | 0.832–1.444 | 0.516 |
| Metastasis in >1
organ | 1.326 | 1.028–1.709 | 0.030 | 1.166 | 0.884–1.537 | 0.277 |
| Peritoneal
metastasis | 1.932 | 1.478–2.524 | <0.001 | 1.441 | 1.063–1.955 | 0.019 |
| TNM stage IV | 1.188 | 0.879–1.605 | 0.263 |
|
|
|
| CEA >5
ng/ml | 1.123 | 0.884–1.428 | 0.342 |
|
|
|
| CA19-9 >37
U/ml | 1.072 | 0.839–1.369 | 0.578 |
|
|
|
| CA72-4 >6
U/ml | 1.301 | 1.021–1.656 | 0.033 | 1.224 | 0.951–1.574 | 0.116 |
| Hemoglobin <115
g/l | 0.932 | 0.729–1.192 | 0.575 |
|
|
|
| Fibrinogen <3.8
g/l | 0.628 | 0.490–0.804 | <0.001 | 0.986 | 0.695–1.399 | 0.938 |
| Albumin ≥40.55
g/l | 0.635 | 0.498–0.810 | <0.001 | 0.725 | 0.559–0.941 | 0.016 |
| FAR ≤10.03 | 0.463 | 0.358–0.599 | <0.001 | 0.568 | 0.394–0.819 | 0.002 |
Survival analysis according to
pretreatment FAR
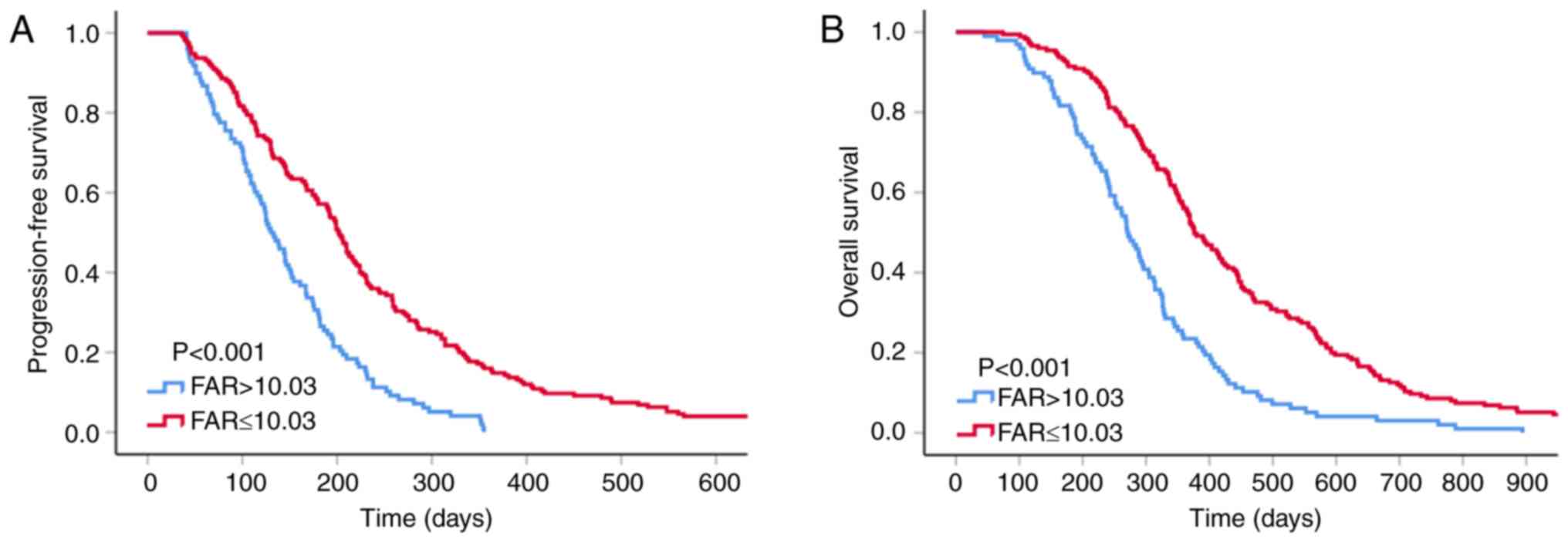
The median PFS and OS of the patients included in
the present study were 174 and 339 days, respectively. The low-FAR
group had longer median PFS and OS compared with the high-FAR group
(202 vs. 130 days, P<0.001; and 376 vs. 270 days, P<0.001,
respectively; Fig. 2).
Survival analysis for the FAR
according to the prognostic factor subtypes of GC
Previous studies demonstrated that distant
metastasis, peritoneal infiltration, TNM stage IV disease and
increased CA72-4 levels are crucial characteristics indicating
unfavorable prognosis in patients with GC (22–24).
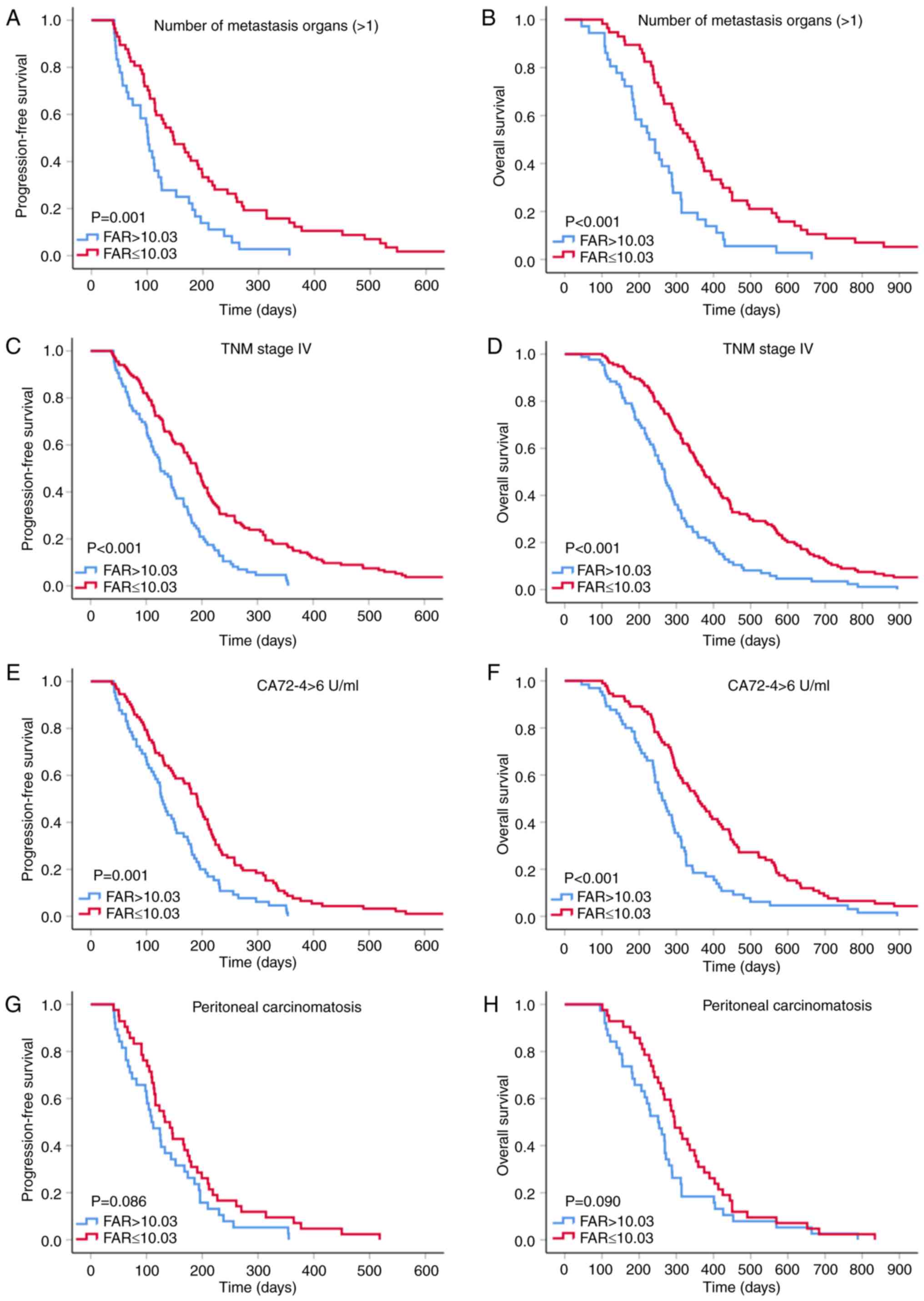
Therefore, a subgroup analysis was performed to investigate whether
the pretreatment FAR is able to further predict the outcomes of
patients with GC with poor prognosis (Fig. 3). The results suggested improved PFS
and OS in the low-FAR group within the subgroups with >1
metastatic organ (P=0.001 and P<0.001), stage IV cancer (all
P<0.001) and elevated CA72-4 levels (P=0.001 and P<0.001).
Although GC patients with low FAR within the peritoneal invasion
subgroup tended to have longer PFS and OS, there was no statistical
insignificance (all P>0.05) and this result may have been
obtained due to the small sample size (n=80).
For patients with normal albumin and fibrinogen
levels, clinicians may be prone to overlooking the coagulation,
inflammation and nutritional status that may indicate the prognosis
of such patients. Thus, a subgroup analysis was performed to
investigate whether the FAR is able to provide a clue as to the
prognosis of patients with normal albumin and fibrinogen indices.
The results suggested that low FAR within normal plasma fibrinogen
and albumin level subgroups of patients with GC was an indicator of
longer PFS and OS (all P<0.001; Fig.
4).
Discussion
Previous studies have reported the importance of the
FAR in predicting the prognosis of different types of cancer
(25–29). Zhang et al (30) indicated that a reduced AFR was an
independent predictor of poor OS in surgical stage II and III GC.
In the present study, it was determined that patients with GC and a
high FAR had shortened OS. In other words, the previous and the
present study suggested that patients with elevated fibrinogen
levels and decreased albumin levels had poor prognosis. Therefore,
the conclusion regarding the prognostic value of AFR and FAR for
predicting the OS of patients with GC in these two studies are
similar. However, the patient characteristics of the present cohort
are different from those in the previous study: The patients of the
present study had stage III–IV unresectable GC and all patients
received first-line chemotherapy. To the best of our knowledge, the
present study was the first study to indicate that the FAR may be
an independent predictor of first-line chemotherapy-associated PFS
and long-term OS in patients with advanced GC. Furthermore, the
prognostic value of the FAR was more powerful compared with that of
either fibrinogen or albumin alone. The most noteworthy result of
the present study was that the subgroup analysis results
demonstrated that the FAR may be a cost-effective and accessible
marker for patients with poor prognosis to further predict the
clinical outcomes of these populations. Furthermore, the FAR may be
a reliable indicator for predicting the prognosis of patients with
normal albumin and fibrinogen levels that may otherwise not draw
the physicians' attention. Therefore, physicians may be able to
formulate personalized treatment patterns for patients with GC with
different prognosis by effectively applying the FAR.
There is increasing and credible evidence that
cancer-associated hypercoagulability, inflammation and malnutrition
are highly prevalent among cancer patients. These abnormal
conditions may weaken the response of tumor patients to treatment
and promote the occurrence, development, metastasis and
exacerbation of cancer (31–36). The potential mechanism may be as
follows: First, patients with malignant tumors may occasionally be
in a hypercoagulable state, which may manifest as venous
thromboembolism and disseminated intravascular coagulation.
Although thrombosis may not be common among patients with advanced
cancer, systemic hypercoagulability is frequently observed. The
coagulation cascade has a crucial role in tumor growth. Components
involved in the hypercoagulable state provide a stable framework
for the tumor extracellular matrix, which sets the conditions for
angiogenesis, adhesion, migration and invasion of tumor cells. The
interaction of coagulation cytokines may also impede the
cytotoxicity of immune cells against tumor cells (37). Furthermore, cytokines may also induce
tumor cell proliferation and invasion by mediating the adhesion
between leukocytes and endothelial cells, which promotes
inflammation (38).
Second, inflammation is a hallmark of cancer
(39). Inflammation may lead to
mutagenesis, predisposing to the accumulation of mutations in tumor
protein 53 and other cancer-associated genes and chronic
inflammation that induces tissue damage may disrupt the barrier
function, expose the stem cell region to environmental carcinogens
or facilitate stem cells to be eroded by genotoxic compounds, which
may trigger tumor formation (40).
Kim et al (41) reported that
tumor necrosis factor (TNF)-α and Toll-like receptor family
member-2 (TLR2) may be essential for cancer metastasis. Cancer
cells may trigger macrophage activity, which promotes the
production of TNF-α by activating TLR2. The extracellular matrix
proteoglycan versican, which is elevated in tumors, augments
metastatic cancer growth by triggering TLR2 complexes and causing
TNF-α release. These factors create an inflammatory
microenvironment, which promotes metastatic growth (41).
Finally, malnutrition is universal among cancer
patients receiving oncological treatment. Patients suffering from
malnutrition have lower tumor treatment completion rates, poorer
quality of life and more complications, which ultimately leads to
lower survival rates (42).
Malnutrition may cause fluctuation in the bone marrow stromal
microenvironment, debilitate hematopoiesis and trigger thymic
atrophy through apoptosis of immature CD4/CD8 double-positive
thymocytes, thereby promoting a decline in immunity, which
facilitates the proliferation of tumor cells (43).
Previous studies have demonstrated that
anti-coagulant therapy, anti-inflammatory treatment and nutritional
support may reduce susceptibility to cancer, prevent disease
exacerbation and improve the clinical outcome (44–46). As
a reliable prognostic indicator, the FAR relies on fibrinogen and
albumin. Fibrinogen is an acute-phase protein produced by the liver
and its plasma levels increase under hypercoagulable and
inflammatory conditions (47–49). A
large body of evidence indicated that fibrinogen-related
coagulation dysfunction is tightly linked to cancer angiogenesis,
invasion, progression and metastasis (50,51).
Reliable data have demonstrated that elevated fibrinogen levels are
associated with poor prognosis of patients with cancer, including
GC (52,53). Albumin is also produced by liver
cells. The tumor-associated proinflammatory cytokines TNF-α and
interleukin-6 are able to inhibit albumin production by hepatocytes
(54). The decrease in plasma
albumin levels indicates a high degree of inflammation, poor
nutritional status and poor treatment efficacy (55,56).
Albumin has also been investigated for its potential value in
predicting shorter survival in a number of cancer types, including
GC (57,58).
As mentioned above, albumin and fibrinogen are
important prognostic indicators for patients with GC, but their
accuracy may be compromised by certain factors, such as dehydration
or fluid retention. The results of the present ROC curve analysis
demonstrated that the AUC of the FAR (0.690) was higher compared
with that of fibrinogen (0.657) and albumin (0.660), and the
P-value of FAR (PFS: P=0.020; OS: P=0.002) was lower compared with
that of fibrinogen (PFS: P=0.231; OS: P=0.938) and albumin (PFS:
P=0.048; OS: P=0.016) on multivariate analyses, which indicated
that the prognostic value of the FAR was superior to that of either
fibrinogen or albumin alone.
Based on the results of the present study,
clinicians may use the FAR to distinguish patients with poor
prognosis and personalize treatment to improve their quality of
life and prolong survival. There were certain limitations to the
present study. First, as this was a single-center retrospective
study, the usefulness of the FAR requires verification by
multicenter large-scale studies. Furthermore, a total of 273
patients included in the present study, which is a small sample
size and may be insufficient to draw definitive conclusions.
In conclusion, the FAR prior to first-line
chemotherapy may help identify specific patient populations that
are likely to benefit from chemotherapy and is an independent
predictor of PFS and OS; therefore, it may be used as an
innovative, dependable prognostic index for patients with
advanced-stage GC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Key
R&D Program of China (grant no. 2018YFC1311600), the Scientific
Research Foundation for the Introduction of Talents, Liaoning
Cancer Hospital & Institute (grant no. Z1702), the Science and
Technology Planning Project of Liaoning Province of China (grant
no. 201800449) and the Science and Technology Planning Project of
Shenyang (grant no. 191124088).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ analyzed the patient data and was a major
contributor in writing the manuscript. LZ, ZW, JX, ZZ, HL, YW, QD,
HP, QW, FB, FL and JZ performed the retrospective analysis of
patients. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This project was approved by the Ethical Committee
of the Cancer Hospital of China Medical University (Shenyang,
China). Due to the retrospective nature of this study the Ethics
Committee waived written informed consent of the included
patients.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fang C, Wang W, Deng JY, Sun Z, Seeruttun
SR, Wang ZN, Xu HM, Liang H and Zhou ZW: Proposal and validation of
a modified staging system to improve the prognosis predictive
performance of the 8th AJCC/UICC pTNM staging system for gastric
adenocarcinoma: A multicenter study with external validation.
Cancer Commun (Lond). 38:672018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoon JY, Sigel K, Martin J, Jordan R,
Beasley MB, Smith C, Kaufman A, Wisnivesky J and Kim MK: Evaluation
of the prognostic significance of TNM staging guidelines in lung
carcinoid tumors. J Thorac Oncol. 14:184–192. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shao Y, Geng Y, Gu W, Ning Z, Huang J, Pei
H and Jiang J: Assessment of lymph node ratio to replace the pn
categories system of classification of the tnm system in esophageal
squamous cell carcinoma. J Thorac Oncol. 11:1774–1784. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khorana AA and Fine RL: Pancreatic cancer
and thromboembolic disease. Lancet Oncol. 5:655–663. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miller GJ, Bauer KA, Howarth DJ, Cooper
JA, Humphries SE and Rosenberg RD: Increased incidence of neoplasia
of the digestive tract in men with persistent activation of the
coagulant pathway. J Thromb Haemost. 2:2107–2114. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arends J, Bodoky G, Bozzetti F, Fearon K,
Muscaritoli M, Selga G, van Bokhorst-de van der Schueren MA, von
Meyenfeldt M; DGEM (German Society for Nutritional Medicine), ;
Zürcher G, et al: ESPEN guidelines on enteral nutrition:
Non-surgical oncology. Clin Nutr. 25:245–259. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bozzetti F, Arends J, Lundholm K,
Micklewright A, Zurcher G and Muscaritoli M; ESPEN: ESPEN
Guidelines on parenteral nutrition: Non-surgical oncology. Clin
Nutr. 28:445–454. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu Q, Yan Y, Gu S, Mao K, Zhang J, Huang
P, Zhou Z, Chen Z, Zheng S, Liang J, et al: A novel
inflammation-based prognostic score: The fibrinogen/albumin ratio
predicts prognoses of patients after curative resection for
hepatocellular carcinoma. J Immunol Res. 2018:49254982018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang YY, Liu ZZ, Xu D, Liu M, Wang K and
Xing BC: Fibrinogen-albumin ratio index (fari): A more promising
inflammation-based prognostic marker for patients undergoing
hepatectomy for colorectal liver metastases. Ann Surg Oncol.
26:3682–3692. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu WY, Zhang HH, Xiong JP, Yan XB, Bai Y,
Lin JZ, Long JY, Zheng YC, Zhao HT and Sang XT: Prognostic
significance of the fibrinogen-to-albumin ratio in gallbladder
cancer patients. World J Gastroenterol. 24:3281–3292. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li SQ, Jiang YH, Lin J, Zhang J, Sun F,
Gao QF, Zhang L, Chen QG, Wang XZ and Ying HQ:
Albumin-to-fibrinogen ratio as a promising biomarker to predict
clinical outcome of non-small cell lung cancer individuals. Cancer
Med. 7:1221–1231. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zou YX, Qiao J, Zhu HY, Lu RN, Xia Y, Cao
L, Wu W, Jin H, Liu WJ, Liang JH, et al: Albumin-to-fibrinogen
ratio as an independent prognostic parameter in untreated chronic
lymphocytic leukemia: A retrospective study of 191 cases. Cancer
Res Treat. 51:664–671. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
You X, Zhou Q, Song J, Gan L, Chen J and
Shen H: Preoperative albumin-to-fibrinogen ratio predicts severe
postoperative complications in elderly gastric cancer subjects
after radical laparoscopic gastrectomy. BMC Cancer. 19:9312019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu W, Ye Z, Fang X, Jiang X and Jiang Y:
Preoperative albumin-to-fibrinogen ratio predicts chemotherapy
resistance and prognosis in patients with advanced epithelial
ovarian cancer. J Ovarian Res. 12:882019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ying J, Zhou D, Gu T, Huang J and Liu H:
Pretreatment albumin/fibrinogen ratio as a promising predictor for
the survival of advanced non small-cell lung cancer patients
undergoing first-line platinum-based chemotherapy. BMC Cancer.
19:2882019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang L, Zhang J, Wang Y, Dong Q, Piao H,
Wang Q, Zhou Y and Ding Y: Potential prognostic factors for
predicting the chemotherapeutic outcomes and prognosis of patients
with metastatic colorectal cancer. J Clin Lab Anal. 33:e229582019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Singh P, Toom S and Huang Y: Anti-claudin
18.2 antibody as new targeted therapy for advanced gastric cancer.
J Hematol Onco. 10:1052017. View Article : Google Scholar
|
|
23
|
Thomassen I, van Gestel YR, van Ramshorst
B, Luyer MD, Bosscha K, Nienhuijs SW, Lemmens VE and de Hingh IH:
Peritoneal carcinomatosis of gastric origin: A population-based
study on incidence, survival and risk factors. Int J Cancer.
134:622–628. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shimada H, Noie T, Ohashi M, Oba K and
Takahashi Y: Clinical significance of serum tumor markers for
gastric cancer: A systematic review of literature by the task force
of the japanese gastric cancer association. Gastric Cancer.
17:26–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun DW, An L and Lv GY: Albumin-fibrinogen
ratio and fibrinogen-prealbumin ratio as promising prognostic
markers for cancers: An updated meta-analysis. World J Surg Oncol.
18:92020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu M, Pan Y, Jia Z, Wang Y, Yang N, Mu J,
Zhou T, Guo Y, Jiang J and Cao X: Preoperative plasma fibrinogen
and serum albumin score is an independent prognostic factor for
resectable stage II–III Gastric Cancer. Dis Markers.
2019:90608452019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y, Yang JN, Cheng SS and Wang Y:
Prognostic significance of FA score based on plasma fibrinogen and
serum albumin in patients with epithelial ovarian cancer. Cancer
Manag Res. 11:7697–7705. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Y and Xiao G: Prognostic
significance of the ratio of fibrinogen and albumin in human
malignancies: A meta-analysis. Cancer Manag Res. 11:3381–3393.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun SY, Chen PP, Meng LX, Li L, Mo ZX, Sun
CH, Wang Y and Liang FH: High preoperative plasma fibrinogen and
serum albumin score is associated with poor survival in operable
esophageal squamous cell carcinoma. Dis Esophagus. 322019.doi:
10.1093/dote/doy057.
|
|
30
|
Zhang J, Li SQ, Liao ZH, Jiang YH, Chen
QG, Huang B, Liu J, Xu YM, Lin J, Ying HQ and Wang XZ: Prognostic
value of a novel FPR biomarker in patients with surgical stage II
and III gastric cancer. Oncotarget. 8:75195–75205. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Batschauer AP, Figueiredo CP, Bueno EC,
Ribeiro MA, Dusse LM, Fernandes AP, Gomes KB and Carvalho MG:
D-dimer as a possible prognostic marker of operable hormone
receptor-negative breast cancer. Ann Oncol. 21:1267–1272. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tsimafeyeu IV, Demidov LV, Madzhuga AV,
Somonova OV and Yelizarova AL: Hypercoagulability as a prognostic
factor for survival in patients with metastatic renal cell
carcinoma. J Exp Clin Cancer Res. 28:302009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dolan RD, Laird BJA, Horgan PG and
McMillan DC: The prognostic value of the systemic inflammatory
response in randomised clinical trials in cancer: A systematic
review. Crit Rev Oncol Hematol. 132:130–137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bilen MA, Martini DJ, Liu Y, Lewis C,
Collins HH, Shabto JM, Akce M, Kissick HT, Carthon BC, Shaib WL, et
al: The prognostic and predictive impact of inflammatory biomarkers
in patients who have advanced-stage cancer treated with
immunotherapy. Cancer. 125:127–134. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schütte K, Tippelt B, Schulz C, Röhl FW,
Feneberg A, Seidensticker R, Arend J and Malfertheiner P:
Malnutrition is a prognostic factor in patients with hepatocellular
carcinoma (HCC). Clin Nutr. 34:1122–1127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maasberg S, Knappe-Drzikova B, Vonderbeck
D, Jann H, Weylandt KH, Grieser C, Pascher A, Schefold JC, Pavel M,
Wiedenmann B, et al: Malnutrition predicts clinical outcome in
patients with neuroendocrine neoplasia. Neuroendocrinology.
104:11–25. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Palumbo JS, Talmage KE, Massari JV, La
Jeunesse CM, Flick MJ, Kombrinck KW, Jirousková M and Degen JL:
Platelets and fibrin(ogen) increase metastatic potential by
impeding natural killer cell-mediated elimination of tumor cells.
Blood. 105:178–185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ridker PM, MacFadyen JG, Thuren T, Everett
BM, Libby P and Glynn RJ: Effect of interleukin-1β inhibition with
canakinumab on incident lung cancer in patients with
atherosclerosis: Exploratory results from a randomised,
double-blind, placebo-controlled trial. Lancet. 390:1833–1842.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mantovani A: Cancer: Inflaming metastasis.
Nature. 457:36–37. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Greten FR and Grivennikov SI: Inflammation
and cancer: Triggers, mechanisms, and consequences. Immunity.
51:27–41. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim S, Takahashi H, Lin WW, Descargues P,
Grivennikov S, Kim Y, Luo JL and Karin M: Carcinoma-produced
factors activate myeloid cells through TLR2 to stimulate
metastasis. Nature. 457:102–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Marin Caro MM, Laviano A and Pichard C:
Nutritional intervention and quality of life in adult oncology
patients. Clin Nutr. 26:289–301. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Savino W and Dardenne M: Nutritional
imbalances and infections affect the thymus: Consequences on
T-cell-mediated immune responses. Proc Nutr Soc. 69:636–643. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Spek CA, Versteeg HH and Borensztajn KS:
Anticoagulant therapy of cancer patients: Will patient selection
increase overall survival? Thromb Haemost. 114:530–536. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu CY, Wu MS, Kuo KN, Wang CB, Chen YJ and
Lin JT: Effective reduction of gastric cancer risk with regular use
of nonsteroidal anti-inflammatory drugs in Helicobacter
pylori-infected patients. J Clin Oncol. 28:2952–2957. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Klek S, Scislo L, Walewska E, Choruz R and
Galas A: Enriched enteral nutrition may improve short-term survival
in stage IV gastric cancer patients: A randomized, controlled
trial. Nutrition. 36:46–53. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lin Y, Liu Z, Qiu Y, Zhang J, Wu H, Liang
R, Chen G, Qin G, Li Y and Zou D: Clinical significance of plasma
D-dimer and fibrinogen in digestive cancer: A systematic review and
meta-analysis. Eur J Surg Oncol. 44:1494–1503. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Davalos D and Akassoglou K: Fibrinogen as
a key regulator of inflammation in disease. Semin Immunopathol.
34:43–62. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ferrigno D, Buccheri G and Ricca I:
Prognostic significance of blood coagulation tests in lung cancer.
Eur Respir J. 17:667–673. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Palumbo JS, Kombrinck KW, Drew AF, Grimes
TS, Kiser JH, Degen JL and Bugge TH: Fibrinogen is an important
determinant of the metastatic potential of circulating tumor cells.
Blood. 96:3302–3309. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Im JH, Fu W, Wang H, Bhatia SK, Hammer DA,
Kowalska MA and Muschel RJ: Coagulation facilitates tumor cell
spreading in the pulmonary vasculature during early metastatic
colony formation. Cancer Res. 64:8613–8619. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yu X, Hu F, Yao Q, Li C, Zhang H and Xue
Y: Serum fibrinogen levels are positively correlated with advanced
tumor stage and poor survival in patients with gastric cancer
undergoing gastrectomy: A large cohort retrospective study. BMC
Cancer. 16:4802016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Perisanidis C, Psyrri A, Cohen EE,
Engelmann J, Heinze G, Perisanidis B, Stift A, Filipits M, Kornek G
and Nkenke E: Prognostic role of pretreatment plasma fibrinogen in
patients with solid tumors: A systematic review and meta-analysis.
Cancer Treat Rev. 41:960–970. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Pfensig C, Dominik A, Borufka L, Hinz M,
Stange J and Eggert M: A new application for albumin dialysis in
extracorporeal organ support: Characterization of a putative
interaction between human albumin and proinflammatory cytokines
IL-6 and TNFα. Artif Organs. 40:397–402. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Diakos CI, Charles KA, McMillan DC and
Clarke SJ: Cancer-related inflammation and treatment effectiveness.
Lancet Oncol. 15:e493–e503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Paccagnella A, Morassutti I and Rosti G:
Nutritional intervention for improving treatment tolerance in
cancer patients. Curr Opin Oncol. 23:322–330. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gupta D and Lis CG: Pretreatment serum
albumin as a predictor of cancer survival: A systematic review of
the epidemiological literature. Nutr J. 9:692010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ouyang X, Dang Y, Zhang F and Huang Q: Low
serum albumin correlates with poor survival in gastric cancer
patients. Clin Lab. 64:239–245. 2018. View Article : Google Scholar : PubMed/NCBI
|