Introduction
Adult patients with acute B cell lymphoblastic
leukemia (B-ALL) often relapse following chemotherapy alone, and
the long-term survival rate is ~30% (1,2).
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a
radical treatment, and 50% of adult patients exhibit long-term
disease-free survival (DFS) after transplantation (3,4).
However, it is difficult to achieve remission via traditional
treatment methods in patients with relapsed/refractory (R/R) B-ALL
due to a high tumor burden and poor patient physical condition, and
these patients lose the best chance of receiving transplantation.
Previously, only 5% of patients with R/R B-ALL who underwent
conventional chemotherapy were able to undergo allo-HSCT (5,6). Minimal
residual disease (MRD) status has been demonstrated to be a strong
prognostic factor for adult patients with ALL (7). Logan et al (7) reported that MRD monitoring is useful
for determining the clinical indications for allogeneic HSCT in the
treatment of ALL in CR1. Patients with (MRD+) possess a
number of risks for transplantation, and the outcome for patients
with MRD+ who undergo allo-HSCT is worse compared with
that of patients without MRD (MRD−) (8–10).
Chimeric antigen receptor (CAR)-T cell therapy is a
novel cellular immunotherapy that uses genetically engineered T
cells that express tumor-specific CARs and combines the
antigen-antibody binding mechanism with the killing effect of T
cells (11,12), thus specifically killing tumors.
Anti-CD19 CAR-T (CART19) cell therapy can significantly improve the
remission and survival rate of patients with B-ALL (13,14). The
remission rate of CART19 cell therapy for patients with B-ALL can
be as high as 70–90% (15–17), but the duration period of CAR-T cells
in vivo is noteworthy, and in some cases recurrence occurs
soon after CAR-T cell therapy (18).
There is controversy over the benefits of bridging to allo-HSCT
after CAR-T cell therapy. For example, Park et al (19) evaluated the 19-28z CAR-T cell
infusion in adult patients with R/R B-ALL; 12/44 patients underwent
allo-HSCT, and the results demonstrated that allo-HSCT had no
significant effect on survival after infusion of CAR-T cells. In
addition, Summers et al (20)
evaluated the efficacy of SCRI-CAR19v1 for treating pediatric and
young adult patients with B-ALL; 17/50 patients underwent
allo-HSCT, and the results suggested that these patients had
improved leukemia-free survival.
The aim of the present study was to investigate the
feasibility, safety and efficacy of CART19 cell therapy bridging to
HSCT in adult patients with R/R B-ALL.
Patients and methods
Study design and patients
The present study was a phase I open-label clinical
trial aiming to evaluate the feasibility, safety and efficacy of
the CART19 bridging regimen followed by allo-HSCT for the treatment
of patients with R/R B cell leukemia (clinical trial no.
NCT03110640). Patients were screened and treated at The First
Affiliated Hospital of Wenzhou Medical University (Wenzhou, China)
between December 2016 and November 2017. A total of 4 patients were
not eligible for the CART19 cell clinical trial due to liver
dysfunction (n=2), abnormal renal function (n=1) and pneumonia
(n=1). The median age of the patients was 34.1 years (range, 16–57
years), and 4 patients were male and five were female. The
inclusion criteria were as follows: i) 5–70 years old and ii)
histologically confirmed CD19+ leukemia. Patients also
had to meet one of the following conditions: i) received at least
two prior combination chemotherapy regimens (not including single
agent monoclonal antibody therapy) and failed to achieve complete
remission (CR) or have disease recurrence; ii) creatinine
expression levels <2.5 mg/dl; iii) Alanine
aminotransferase/Aspartate aminotransferase <3× normal levels;
iv) bilirubin levels <2.0 mg/dl. In addition, female patients of
a reproductive age had to have a negative serum or urine pregnancy
test within 48 h before treatment infusion. Patients who met any of
the following criteria were excluded: i) Symptoms of central
nervous system, including central nervous system-leukemia; ii)
another malignant tumor; iii) active hepatitis B or C, or HIV; iv)
other diseases that may affect the outcome of the trial, such as
acute myocardial infarction; v) severe cardiovascular or
respiratory disease; vi) poorly controlled hypertension; vii) a
history of poorly controlled mental illness; viii) patients who had
taken immunosuppressive agents within one week of the start date
due to organ transplantation or another disease requiring
long-lasting treatment; ix) unstable pulmonary embolism, deep vein
thrombosis or other major arterial/venous thromboembolic events 30
days prior to the study; x) patients reaching a steady dose of
anticoagulant therapy 3 months ago before the study start date; xi)
pregnant or lactating women; and xii) patients with a disease
affecting their ability to provide informed consent. The protocol
was approved by The First Affiliated Hospital of Wenzhou Medical
University Institutional Review Board (approval no. 2016-220). All
patients provided written informed consent prior to enrollment.
Consent was obtained from the patients or their parents/legal
guardians if the patients were <18 years. All patients underwent
CART19 cell infusion after the standard lymphodepletion regimen
with fludarabine (25 mg/m2/day on days −4, −3 and −2)
and cyclophosphamide (1,000 mg/m2 on day −4). The
post-infusion responses and toxicities were closely monitored
daily. The expansion and persistence of CD19 CAR-T cells (the
copies of CD19 CAR DNA and the percentage of CD19 CAR T cells), as
well as MRD, were measured using flow cytometry (FCM) and
quantitative (q)PCR assays on days +1, +3, +9, +14, +21 and +28 and
every 30 days after CD19 CAR-T cell infusion. When no CART19 cells
could be detected in the blood and/or MRD recurrence was observed,
patients began HSCT therapy. Salvage therapy for no. 4 patient was
FLAG-IDA regimen which included fludarabine, cytarabine,
idarubicin, and G-CSF.
Generation of retroviral vectors,
CAR-T cell production and quality control of CART19 cells
The present study constructed γ-retroviral vectors
encoding second-generation BBζCD19-targeting CAR molecules
(4-1BB-engineered CAR-T cells) (Fig.
1C). To evaluate the functionality of these CAR constructs,
human primary T cells from 9 patients were transduced with CAR
vectors. All CAR PBMC populations were expanded under conditions to
generate CAR-T cells. Activated PBMCs were resuspended in 50 IU/ml
recombinant human IL-2 and incubated at 37°C for 7–14 days. During
ex vivo expansion, culture medium was replenished, and the
density of T cells was maintained at 0.5-1×106 cells/ml.
Routine screening of CART19 cell cultures was negative before
infusion. The methods for the generation of retroviral vectors,
CAR-T cell production and quality control of CART19 cells before
infusion are presented in our previous study (21). In this study, the PBMCs were from the
patient but not from the healthy donors, which was different from
our previous study (21). The
methods for the generation of retroviral vectors, CAR-T cell
production and quality control of CART19 cells before infusion are
accordant with our previous study (21).
CART19 cell treatment
The CART19 cell infusion day was set as study day 0.
Prior to CAR-T cell infusion, patients were administered 1,000
mg/m2 cyclophosphamide on day −4 and 25
mg/m2/day fludarabine on days −4, −3 and −2. On day 0,
CART19 cells at a target dose of 1×106 T cells/kg were
intravenously injected directly into the enrolled patients.
Management of cytokine release
syndrome (CRS)
Hemodynamic parameters, vital signs and peripheral
blood cytokine levels, including interferon γ (IFN-γ), interleukin
2 (IL-2), tumor necrosis factor α (TNF-α), IL-6, IL-10 and
C-reactive protein (CRP), were closely monitored on days +1, +3,
+9, +14, +21 and +28 and every 30 days after CD19 CAR-T cell
infusion. Supportive care for patients receiving CAR T cells was
also provided. If a patient met CRS criteria for tocilizumab
treatment (90 mmHg systolic blood pressure could not be maintained
with norepinephrine, oxygen requirement of ≥50% FiO2 for
≥2 h continuously, severe dyspnea potentially requiring mechanical
ventilation), 4–8 mg/kg tocilizumab was administered over 1 h with
the overall dose ≤800 mg. If a patient met CRS criteria for
corticosteroids (grade 3 neurological toxicity, with the exception
of headaches, lasting continuously for ≥24 h or grade 4 neurologic
toxicity of any duration), 10 mg dexamethasone was administered for
6 h via intravenous injection until toxicities improved to grade 1
or lower. All toxicities occurring within 30 days of CAR-T cell
therapy were graded for all 9 patients.
Assessment of CART19 cell expansion
and persistence
Genomic (g)DNA was isolated from peripheral blood
mononuclear cell (PBMC) samples using a Mini BEST Universal Genomic
DNA Extraction kit (Takara Biotechnology Co., Ltd.), quantified
using a spectrophotometer and stored at −80°C. The presence,
expansion and persistence of CAR-T cells in the blood and bone
marrow were assessed using quantitative (q)PCR (21). The analysis of CAR-T cell percentages
in PBMCs and bone marrow samples was performed using
multiparametric FCM (21). The CAR-T
cells were stained for CD45RA, CD45RO, CD3, CD4, CD8 and CD62L with
fluorescence-labelled antibodies (BioLegend). CAR detection was
monitored with polyclonal goat anti-mouse F(ab)2 antibodies
labelled with biotin (Jackson Immunoresearch Laboratories, Inc.)
and streptavidin labelled with BV421 (BioLegend) (21).
Assessment of serum cytokines, immune
effector molecules and MRD
Measurements of IL-6, granzyme B, TNF-α and IFN-γ
were performed by FCM using a BD Cytometric Bead Array (BD
Biosciences) according to the manufacturer's instructions. IL-15
and CRP expression levels were analyzed using the cytokines ELISA
kit (cat. no. ABIN4883305) from R&D Systems. MRD assessment was
measured at regular intervals using FCM and qPCR assays. MRD in the
bone marrow of all patients was assessed at the following time
points. The parameters varied with patients, owing to the
difference of their immunophenotype at initial diagnosis: On
enrolment, on day 1 and every 30 days after CD19 CAR-T cell
infusion. In addition, MRD in the peripheral blood of all patients
at days+1, +3, +9, +14, +21 and +28 and every 30 days after CD19
CAR-T cell infusion was analyzed. RT-PCR was performed to assess
BCR-ABL1 (p190) for Ph+ B-ALL (22).
Donor selection
A matched sibling donor was chosen as the first
treatment option. Patients were eligible for haploidentical SCT if
a matched sibling donor was unavailable. Donors and patients were
assessed for the degree of HLA matching. HLA-A and HLA-B typing
were performed by intermediate resolution DNA typing, whereas
HLA-DRB1 typing was performed using high-resolution DNA techniques.
The donor inclusion criteria were as follows: The donor was
HLA-matched with the patient, and the donor had no serious heart,
liver and kidney disease. Among the five donors, two were sibling
donors, and the other three were haploidentical HSCT donors. The
median age of the donors was 38 years (age range, 24–49 years),
four men and one woman. Leukapheresis was performed for patients
4–5 days after starting G-CSF based on the peripheral blood
CD34+ cell count (PB CD34). All donors tolerated the
stem cell (from bone marrow or/and peripheral blood) harvest and
leukapheresis procedures and had no severe side effects.
Conditioning regimen and
graft-versus-host disease (GVHD) prophylaxis
When no CART19 cells could be detected in the blood
and/or MRD recurrence was observed, patients began to receive HSCT.
The conditioning therapy for patients undergoing haploidentical
HSCT was a modified busulfan/cyclophosphamide (BUCY) regimen plus
thymoglobulin (ATG) consisting of cytarabine (4 g/m2/day
intravenously on days −10 and −9), BU (3.2 mg/kg/day on days −7 to
−4), CY (1.8 g/m2/day intravenously on days −5 and −4),
Methyl-CCNU semustine (250 mg/m2 orally once on day −3)
and ATG (5 mg/kg/day intravenously on days −5 to −2). The
conditioning therapy for patients undergoing sibling HSCT was BUCY,
consisting of BU (3.2 mg/kg/day on days −7 to −4), CY (1.8
g/m2/day intravenously on days −5 and −4) and Me-CCNU
(250 mg/m2 orally once on day −3). All patients received
cyclosporine A (5 mg/kg.d) and short-term methotrexate 15
mg/m2 +1, 10 mg/m2 +3,6,11 for GVHD
prophylaxis.
Engraftment, chimerism,MRD and GVHD
evaluation
For VHD evaluation, skin rash <25% within +100
days was defined as grade 1 skin aGVHD. The day of engraftment was
defined as maintenance of an absolute neutrophil count >500/Ul
for 3 consecutive days after HSCT. Chimerism was evaluated by
DNA-based HLA typing (for mismatched loci), PCR DNA fingerprinting
of short tandem repeats and chromosomal fluorescence in situ
hybridization (FISH) for the Y chromosome. MRD was assessed by FISH
and FCM. The MRD parameters by FCM varied with patients, owing to
the difference of their immunophenotype at initial diagnosis, and
MRD parameters by FISH for Y chromosome when the donor and patient
sex are different. MRD and chimerism were evaluated on days +30,
+60, +90 and +180 and years +1, +3 and +5. The methodology for
DNA-based HLA typing (for mismatched loci) was as follows: Genomic
DNA was extracted from human whole blood. PCR products were gel
purified and sequenced. Sequences were imported into the Sequencing
Based Typing software, GenDx SBTengine® to analyze
heterozygous nucleotide positions throughout the amplified region.
The methodology for PCR DNA fingerprinting of short tandem repeats
was as follows: DNA was isolated from the skin biopsies as well as
from a donor blood. PCR amplification of nine highly polymorphic
short tandem repeats was performed for each specimen and a unique
DNA ‘fingerprint’ was obtained from each. Thorough analysis
confirmed GVHD. The methodology for chromosomal fluorescence in
situ hybridization (FISH) for the Y chromosome was as follows:
Before hybridization, the DNA probe was labeled by various means.
The labeled probe and the target DNA were denatured. Combining the
denatured probe and target allows the annealing of complementary
DNA sequences.
Statistical analysis
All statistical analyses were performed using SPSS
version 19 (SPSS, Inc.) and data are presented as the mean ±
standard deviation. Differences between two groups were analyzed
using unpaired two-tailed Student's t-tests. Differences among
multiple groups were analyzed using one-way ANOVA and Bonferroni's
correction. OS and progression-free survival rate were analyzed
using Kaplan-Meier curves and log-rank tests. P<0.05 was
considered to indicate a statistically significant difference. All
experiments were performed in triplicate.
Results
Characterization of patients
Between November 2016 and November 2017, a total of
13 patients with CD19+ R/R B-ALL were recruited. A total
of 4 patients were excluded from receiving lymphodepletion
chemotherapy and CART19 cell infusion (Fig. 1). The other 9 patients received CAR-T
cell infusion. As presented in Table
I, all patients had R/R B-ALL, and 6 patients had detectable
leukemic cells in the bone marrow prior to CART19 cell infusion.
The median percentage of leukemic blasts in the bone marrow was
12.35% (range, 1.09-63.9%) of marrow blasts (Table I). One patient (patient 6) had
Ph+ ALL [relapsed with G250E and V299L mutations and an
additional abnormal karyotype: del(16),t(1,16)(q21,q12)]. Patient 6
did not achieve remission despite steroid-based chemotherapy and
TKI-targeted therapy, including dasatinib and nilotinib. Two
patients had asymptomatic central nervous system leukemia (CNSL)
(patients 6 and 7) before CART19 cell infusion.
 | Table I.Response, toxicities and prognosis
after CART19 therapy and SCT of patients with relapsed/refractory
B-cell acute lymphocytic leukemia. |
Table I.
Response, toxicities and prognosis
after CART19 therapy and SCT of patients with relapsed/refractory
B-cell acute lymphocytic leukemia.
|
|
|
|
| Infused CART19
cells | CRS | BM leukemia cell
clinical response | Stem cell
transplantation |
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Patient no. | Sex | Type | Age, years |
CART/CD3+ T cells, % | Dose,
×106/kg | Grade | Tocili | Steroid | Before CART19,
% | After 1 month,
% | SCT | Type | Side effects | 1-year outcome |
|---|
| 1 | M | Ph− | 24 | 59.3 | 1 | 1 | N | N | 63.90 | <0.01 | Y | haplo-HSCT | cGVHD | CR |
| 2 | M | Ph− | 38 | 46.5 | 1 | 1 | N | N | 10.60 | <0.01 | Y | haplo-HSCT | cGVHD | CR |
| 3 | F | Ph− | 43 | 61.6 | 1 | 0 | N | N | 5.20 | <0.01 | Y | Sibling SCT | N | CR |
| 4 | F | Ph− | 18 | 26 | 1 | 1 | N | N | 3.00 | <0.01 | Y | Sibling SCT | N | CR |
| 5 | M | Ph− | 16 | 60 | 1 | 2 | N | N | 1.09 | <0.01 | Y | haplo-HSCT | aGVHD grade I
skin | CR |
| 6 | F | Ph+ | 49 | 62 | 1 | 4 | Y | Y | 5.00 | <0.01 | N | N | NA | CR |
| 7 | F | Ph− | 40 | 50 | 1 | 3 | Y | N | 6.80 | <0.01 | N | N | NA | CR |
| 8 | F | Ph+ | 57 | 62 | 1 | 2 | N | N | 4.60 | <0.01 | N | N | NA | CR, Imatinib
maintenance |
| 9 | M | Ph− | 22 | 65.6 | 1 | 2 | N | N | 11 | <0.01 | N | N | Relapse | Relapsed and died
on day 123 |
Preclinical evaluations of CAR-T
cells
As presented in Table
I, a median of 54.78% (range, 26–65.6%) of CD3+ T
cells expressed CAR constructs equipped with signaling domains
consisting of 4-1BB. All 9 patients received the CART19 cell
infusion at a dose of 1×106 cells/kg.
Efficacy assessment of CAR-T cell
infusion
All patients achieved CR within 28 days of infusion
of CAR-T cells. One patient with Ph+ ALL who was
resistant to both dasatinib and nilotinib also achieved
MRD− remission. The 2 patients who had asymptomatic CNSL
maintained a blast-negative status in the cerebrospinal fluid and
MRD− remission in the bone marrow (BM).
Safety evaluation of CAR-T cell
infusion
A total of 8 patients experienced CAR-T cell-related
adverse effects of any grade, with grade 3 and grade 4 events
reported in 1 (11.1%) patient each. Three patients experienced
grade 2 CRS. CRS was fully reversible in all 5 patients and was
well managed with supporting therapy alone (n=3), supporting
therapy plus the anti-IL6 receptor antibody tocilizumab (n=1) and
supporting therapy plus tocilizumab and corticosteroids (n=1)
(Table I). The patient who
experienced grade 4 CRS had severe diffuse alveolar hemorrhage
after CAR-T cell infusion and recovered one week after high-dose
corticosteroid therapy, mechanical ventilation, tocilizumab and
supportive care (blood transfusion and oxygen inhalation.). The
patient achieved CR with MRD− on day 21 and had
maintained CR for 203 days at the end of the follow-up. Serum
levels of the cytokines IL15, IL6, granzyme B, TNF, IFN-γ and CRP
were assessed during CRS (Fig. 2).
The results showed that serum levels of cytokine IL-15 ranged from
3–43 pg/ml, while those of cytokine IL-6 ranged from 4–609 pg/ml.
Serum levels of cytokine granzyme B ranged from 1–156 pg/ml, while
those of TNF ranged from 3.2-59.1 pg/ml. Serum levels of cytokine
IFN-γ ranged from 1–482 pg/ml, while those of CRP ranged from 0–601
mg/l, and there was no significant difference in the peak levels
between the bridging HSCT group and the non-HSCT group (Fig. 3).
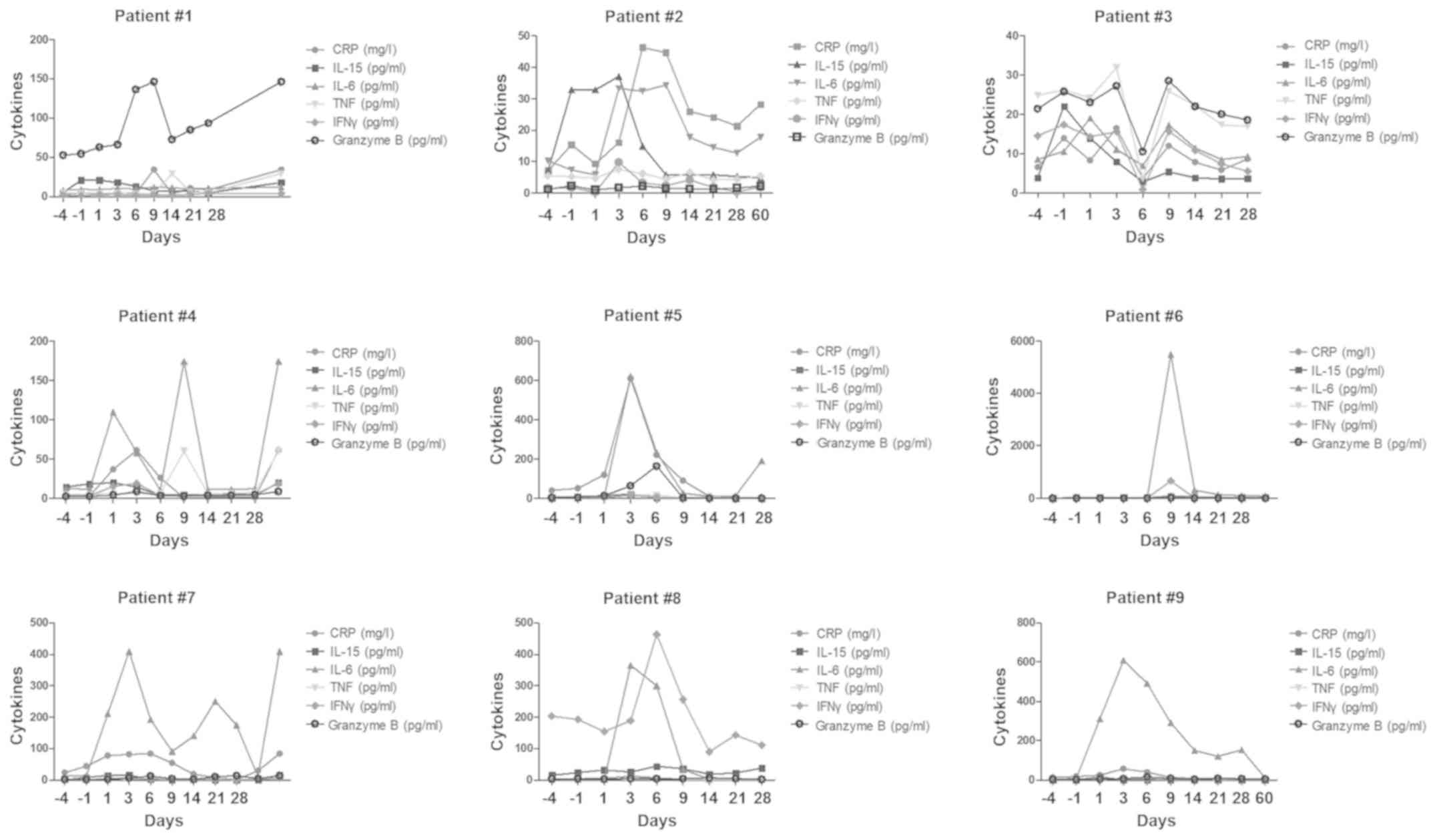 | Figure 2.Cytokine levels in each patient.
Intermittent monitoring of serum levels of cytokines IL15, IL6,
granzyme B, TNF, IFN-γ and CRP were assessed during CRS on day −4,
−1, +1, +3, +6, +9, +14, +21 and +28 following chimeric antigen
receptor T therapy with or without hematopoietic stem cell
transplantation for relapse/refractory B-cell lymphoblastic
leukemia. CRS, cytokine release syndrome; IL, interleukin; TNF,
tumor necrosis factor; IFN-γ, interferon γ; CRP, C-reactive
protein. |
There was no significant difference in the peak
levels in the BM/PB between the bridging HSCT group and the
non-HSCT group (Fig. 4A-D). After
treatment with CART19 cell infusion, all patients achieved CR and 2
out of 9 (22.2%) patients had severe complications (grade 4 CRS),
but CRS in these patients was reversed after administration of IL-6
receptor antagonists and glucocorticoid [1 of the 2 patients
received allo-HSCT and exhibited long-term survival; the other
patient with Ph+ ALL also had BCR-ABL1
(p190)+ B-ALL and secondary resistance to imatinib].
Steroid-based mutation detection of the ABL gene revealed the
presence of G250E and V299L mutations of one patient. Chromosomal
banding analysis of the bone marrow revealed additional abnormal
karyotypes, including deletion (16),t(1,16)(q21,q12). Although the
treatment regimen was changed to dasatinib (800 mg/day) and a
steroid-based regimen (idamycin 10 mg/m2, d1-3,
vincristine 4 mg d1,8,15,22 and prednisone 60 mg d1-28) for 3
months, patients were unable to achieve remission. Grade 4 CRS and
diffuse alveolar hemorrhage occurred after CD19 CAR-T cell
infusion, and the patient recovered within 1 week after high-dose
corticosteroid therapy, mechanical ventilation, tocilizumab and
supportive care. Imatinib was orally administered in the absence of
CAR-T cells after 3 months of CD19 CAR-T cell infusion, and this
patient also survived at the end of follow-up. The remaining 7
(77.7%) patients had complications of CRS of grades 0–2, and 2
patients with severe complications had higher MRD before the
infusion compared with the other patients and multiple relapses in
the past.
 | Figure 4.CART19 engraftment, expansion and
persistence in vivo. (A) Expression levels of CART19s in the
PB assessed using quantitative PCR on days 1, 3, 6, 9, 14,21, 28,
60 and 90 after infusion of CART19s in bridging HSCT group
(patients 1–5). (B) Expression levels of CART19s in PB assessed by
RT-qPCR on days 1, 3, 6, 9, 14, 21, 28, 60 and 90 after infusion of
CART19s in non-HSCT group (patients 6–9). (C) Box plot of the
association between peak expression levels of CART19s and CAR DNA
copies in the PB between the bridging HSCT and non-HSCT groups. (D)
Box plot of the association between peak expression levels of
CART19s and CAR DNA copies in the bone marrow between the bridging
HSCT and non-HSCT groups. CART, chimeric antigen receptor T; PB,
peripheral blood; CAR, chimeric antigen receptor; HSCT,
hematopoietic stem cell transplantation. |
Expansion and persistence of CART19
cells in the peripheral blood and bone marrow
In all 9 patients, the in vivo expansion of
CAR-T cells was monitored by qPCR and FCM. As presented in Fig. 4, all patients had detectable
circulating CAR-T cells. The peak in CAR-T cell expansion ranged
between days 6 and 9 (Fig. 4). The
median peak of CAR DNA copies was 2.61×105 (range,
3.16×104−6.34×105) copies/µg gDNA in the
blood and 6.48×103 (range, 302–2.13×104)
copies/µg gDNA in the bone marrow. The median peak of CAR DNA
copies was 3.92×105 (range,
1.78×104−6.34×105) copies/µg gDNA in the
blood and 10.9×103 (range,
2.61×103−21.3×103) copies/µg gDNA in the bone
marrow in the non-HSCT group, whereas the median peak of CART DNA
copies was 1.57×105 (range,
3.16×105−4.87×105) copies/µg gDNA in the
blood and 2.88×103 (range, 302–5.48×103)
copies/µg gDNA in the bone marrow in the bridging HSCT group. In
addition, CART19 cells were detectable in the blood and marrow
between 3 and 6 months. When no CART19 cells could be detected in
the blood and/or MRD recurrence was observed, patients began to
receive HSCT. Patient 4, who still had detectable levels of CAR-T
cells in bone marrow analyzed using qPCR (117 copies/μg DNA) at day
+90 following CAR-T cell infusion, relapsed with CD19−
leukemia (>20% blasts in the bone marrow) at the same time. This
patient then received salvage therapy (FLAG-IDA regimen which
included fludarabine, cytarabine, idarubicin and G-CSF) and matched
sibling donor SCT and maintained MRD− CR post-HSCT until
the end of the follow-up period.
Patient receiving SCT and donor
characteristics
Among the 5 patients who received allo-HSCT, there
were 4 patients with no detectable CART19 cells in the blood/bone
marrow on day +90 after CAR-T cell therapy and 1 patient who
relapsed with CD19− leukemia even with detectable levels
of CAR-T cells in the bone marrow analyzed using qPCR on day +90
following CAR-T cell infusion (Table
I).
Engraftment
All 5 patients achieved sustained myeloid
engraftment. Full donor chimerism was achieved in all 5 patients
after transplantation. The median time for myeloid recovery was 14
days (range, 12–16 days), and the median time for platelet recovery
was 15 days (range, 13–17 days).
GVHD
All patients with engraftment were eligible for the
assessment of acute (a)GVHD. One patient had grade 1 skin aGVHD
(Skin rash <25% within +100 days). A total of 5 patients
survived >100 days after transplantation and were evaluated for
cGVHD. Two of these patients developed limited cGVHD, whereas no
patients developed extensive cGVHD.
Outcome
As presented in Fig.
5, eight of the 9 patients with a median follow-up time of 338
days (range, 123–365 days) maintained CR. The 1-year allo-HSCT
group had an OS rate of 100% and a DFS rate of 100%. Two of the 4
patients in the untransplanted group had Ph+ B cell
acute lymphoblastic leukemia. Imatinib was administered orally in
the absence of CAR-T cells 3 months after CD19 CAR-T cell infusion,
and the patients had sustained remission. One patient refused
transplantation, and the bone marrow continued to recover. One
patient refused to undergo allo-HSCT, relapsed 6 months after CD19
CAR-T cell infusion, refused further chemotherapy and other
treatment, and died after 9 months. The 1-year OS rate of the
untransplanted group was 75%, and the DFS rate was 75%.
 | Figure 5.Outcome after CAR-T19 bridging with
HSCT. (A) Leukemia-free survival of all 9 patients. Survival
fractions were calculated using the Kaplan-Meier method, and lines
indicate censored patients. Group 1, Bridging HSCT. Group 2,
non-HSCT. (B) OS of all patients. Survival fractions were
calculated using the Kaplan-Meier method, and lines indicate
censored patients. Group 1, bridging HSCT. Group 2, non-HSCT. (C)
Flow chart of the outcome of all 9 patients, including stem cell
transplantation, donor and transplantation complications. The
patient who suffered relapse 3 months after CAR-T therapy died 1
month later, thus the leukemia-free survival changed at 3 months
while the OS curve changed at 4 months. CART, chimeric antigen
receptor T; MRD, minimal residual disease; HSCT, hematopoietic stem
cell transplantation; SCT, stem cell transplantation; a, acute; c,
chronic; GVHD, graft-versus-host disease; OS, overall survival. |
Discussion
The present study aimed to investigate the
feasibility, safety and efficacy of CD19 CAR-T cell therapy as a
bridge to allo-HSCT in the treatment of adult patients with R/R
B-ALL. The clinical outcomes of the enrolled patients were
positive.
The present study demonstrated that it is feasible
to determine the timing of transplantation by simultaneously
monitoring MRD and the CD19 CAR-T cell ratio or qPCR values.
Consistent with the results of previous studies (15,23–25),
strong expansion and long-term persistence of CD19 CAR-T cells were
observed in the present experiments. The duration was 3–6 months,
and CD19 CAR-T cell amplification peaked between days 6 and 9.
Patients with MRD− CR received allo-HSCT at a median
time of 104 days after infusion. Of note, CD19 CAR-T cells in the
PB of patients cannot be detected using FCM, but the CD19 CAR DNA
copy number can be detected using qPCR. A possible explanation for
this is that CARs antigen on the surface shed from the cell surface
which can't be detected by FCM, whereas CAR DNA of transduced T was
existent which can be detected via qPCR analysis (16). These findings are consistent with
those of Maude et al (16),
who reported that 3 patients did not respond to CTL019 after CAR-T
cell infusion, but CTL019 expression levels peaked at 6–14 days and
were detected using qPCR, whereas circulating CTL019 was not
detected by FCM. In the present study, at day +90 after CAR-T cell
infusion, there levels of CAR-T cells in the bone marrow were still
detectable using qPCR.
MRD− was a feasible predictor of survival
in the present study. This was consistent with the findings of Park
et al (17), who used
multiparameter FCM to evaluate MRD in BM samples from days 14–28
after CAR-T cell infusion after 19-41BB treatment and demonstrated
that post-treatment MRD status was a strong predictor of OS; the
6-month OS rate was 76% in the MRD− CR group and 14% in
the MRD+ CR group. The use of MRD monitoring to
determine the timing of transplantation requires analysis of CD19
antigen expression levels and must also take into account the fact
that B-ALL leukemia-initiating cells with stem cell-like
characteristics that do not express CD19 also affect the
therapeutic effect (26). In the
present study, the leukemia cells of the patient who had response
after CART19 cell treatment but relapsed after 3 months did not
express CD19, and the leukemia cells had transformed into acute
myeloid leukemia cells. The reason for recurrence may be that the
leukemia stem cell differentiation patterns in ALL are mostly
branched, rather than a single linear pattern (27,28), and
there may have been at least two CD18+ and
CD19− leukemia cell clones in the patient. After CAR-T
cell therapy killed CD19+ dominant clones in large
numbers, the original unaffected non-predominant myeloid leukemia
cell clones became the dominant clones, and the proliferation of
these cells led to leukemia recurrence. Another possibility is that
antigenic shift or antigenic drift occurred in the CD19+
leukemia cells. The appearance of stem cell markers (for example
CD34+) and myeloid cell markers would suggest that the
leukemia cells had escaped immune attack and surveillance, leading
to leukemia recurrence. Therefore, after CAR-T cell treatment, MRD
monitoring should not be limited to CD19+ cell
populations. It is particularly important to focus on the
immunophenotypic changes of CD19− cell populations and
other leukemia cell populations.
The results of the present study were consistent
with the findings of Hu et al (18), who confirmed that in CART19 cell
treatments for patients with R/R ALL in China, MRD and the number
of previous relapses after the pre-treatment regimen were
associated with the high risk of grade 3 CRS, while other risk
factors included age, sex and therapies prior to the FC
pre-treatment regimen (chemotherapy or allo-HSCT). CART19 cell
dosage and MRD were not associated with the risk of grade 3 CRS in
the present study. It was also observed that serum levels of CRP
were associated with the severity of CRS. Patients with grade 3 or
4 CRS had higher peak CRP expression levels after infusion of
CART19 cells compared with patients without CRS or with grade 1 or
2 CRS. In the case of patient 6, CRS was effectively improved
without a significant effect on CART19 cell expansion and
persistence using tocilizumab. These results are consistent with
recent published findings (18).
Luznik et al (6) reported that following the treatment of
hematologic malignancies with HLA-haploidentical HST, platelet and
median granulocyte engraftment times were 15 and 24 days,
respectively, and that 13% of patients experienced surgical
failure. The cumulative incidence of non-relapse mortality for
aGVHD was 15% at 1 year, and the recurrence rate was 51%. The OS
rate at 2 years after transplantation was 36%. In the present
study, patients who received allo-HSCT had a shorter platelet
recovery period and no graft failure, no aGVHD or extensive cGVHD.
Compared with previous stem cell transplantation studies (29–31), the
patients who underwent bridging transplantation after CART19 cell
infusion had a similar engraftment time and a similar incidence of
aGVHD or extensive cGVHD. The cumulative incidence of non-relapse
mortality for aGVHD was lower (0%) at 1 year, and the recurrence
rate was lower (0%). The OS at 1 year after transplantation was
higher (100%).
The results of the present study were consistent
with those of Summers et al (20), who evaluated the efficacy of
SCRI-CAR19v1 treatment in pediatric and young adult patients with
B-ALL, and 17/50 patients underwent allo-HSCT, suggesting an
improvement in leukemia-free survival rate in the patients who
underwent allo-HSCT compared with those who did not.
There were limitations to the present study. Due to
the short duration, the number of cases was small, and the
follow-up time was short. Despite the results already achieved, the
long-term effects of CAR-T bridged allo-HSCT treatment remain to be
determined. Determining the overall efficacy of this therapy in R/R
B-ALL requires a large number of patients and comparison with the
CD19 CAR-T cell treatment alone group by statistical analysis. In
addition, all patients received the same chemotherapy regimen
combination, dosage power and intensity (FC regimen, 1,000
mg/m2 cyclophosphamide on day −4 and 25
mg/m2/day fludarabine on days −4, −3 and −2). In the
future, it would be important to investigate the mortality and
morbidity rates and adverse side effects of other variations of
CAR-T therapy conditions, such as chemotherapy regimen
combinations, dosage power and intensity.
The present study demonstrated that in patients with
R/R B-ALL, the 1-year OS and DFS rates of the CD19 CAR T+HCT group
were improved compared with those of the CD19 CAR T therapy alone
group. For the patients with R/R B-ALL, whose CAR-T cells in
vivo do not survive for a long period, their recurrence
commonly occurred soon after CAR-T cell therapy. The bridging to
allo-HSCT after CAR-T cell therapy is feasible, safe and efficient
for these patients.
Acknowledgements
The authors would like to thank Dr Ying He, Dr
Fengyang Lin, Dr Bin Liang, Dr Liyuan Tang, Dr Shujuan Zhou, Dr
Yifen Shi, Dr Bin Liang, Dr Yan Zhuang, Dr Qiang Zhuang, Dr Laixi
Bi, Dr Qianying Zhang and Dr Xi Xu (The First Affiliated Hospital
of Wenzhou Medical University) for their treatment for the patients
before CAR-T therapy. In addition, the authors would like to thank
Dr Binbin Chen (The First Affiliated Hospital of Wenzhou Medical
University), Dr Ph Feng He, Dr Yarong Liu, Dr Zixiao Shi, Dr Tao
Jin, Dr Ronglin Xie, Dr Baofeng Wei and Dr Jing Chen for detecting
CAR DNA copies (R&D Department, Hrain Biotechnology Co.
Ltd.).
Funding
This study was supported by grants from The Natural
Science Foundation of Zhejiang province (grant nos. LY20H080003,
LQ16H080002,81100355, 81172613, 81300430, Y207422 and LY16H080006),
the Wenzhou Municipal Science and Technology Bureau (grant no.
2018ZY014) and The National Natural Science Foundation (grant nos.
81600167, LQ12H08002, Y2111000 and LY16H080007).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
SJ and KY designed the study. SJ, KY, SZ, HF and YM
performed the research. SJ, KY and YM wrote the manuscript. SJ, KY
and YM performed the statistical analysis and interpreted the data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The protocol was approved by The First Affiliated
Hospital of Wenzhou Medical University Institutional Review Board
(Wenzhou, China; approval no. 2016-220). All patients provided
written voluntary informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CAR-T bridged
|
chimeric antigen receptor
T-bridged
|
|
HSCT
|
hematopoietic stem cell
transplantation
|
|
allo-HSCT
|
allogeneic hematopoietic stem cell
transplantation
|
|
OS
|
overall survival
|
|
DFS
|
disease free survival
|
|
aGVHD
|
acute graft-versus-host disease
|
|
cGVHD
|
chronic graft-versus-host disease
|
|
R/R B-ALL
|
relapse/refractory B lymphoblastic
leukemia
|
|
PBMC
|
peripheral blood mononuclear cells
|
|
B-ALL
|
acute B lymphoblastic leukemia
|
|
CR
|
complete remission
|
|
MRD
|
minimal residual disease
|
|
CNSL
|
central nervous system leukemia
|
|
ATG
|
thymoglobulin
|
|
FISH
|
fluorescence in situ
hybridization
|
|
FCM
|
flow cytometry
|
|
CRS
|
cytokine release syndrome
|
References
|
1
|
Iacobucci I and Mullighan CG: Genetic
basis of acute lymphoblastic leukemia. J Clin Oncol. 35:975–983.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Terwilliger T and Abdul-Hay M: Acute
lymphoblastic leukemia: A comprehensive review and 2017 update.
Blood Cancer J. 7:e5772017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Styczynski J, Tallamy B, Waxman I, van de
Ven C, Milone MC, Shaw LM, Harrison L, Morris E, Satwani P, Bhatia
M, et al: A pilot study of reduced toxicity conditioning with BU,
fludarabine and alemtuzumab before the allogeneic hematopoietic SCT
in children and adolescents. Bone Marrow Transplant. 46:790–799.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu LP, Zhang XH, Wang FR, Mo XD, Han TT,
Han W, Chen YH, Zhang YY, Wang JZ, Yan CH, et al: Haploidentical
transplantation for pediatric patients with acquired severe
aplastic anemia. Bone Marrow Transplant. 52:381–387. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang J, Graves SS, Butts-Miwongtum T,
Sale GE, Storb R and Mathes DW: Long-term tolerance toward
Haploidentical Vascularized Composite Allograft Transplantation in
a Canine Model Using Bone Marrow or Mobilized Stem Cells.
Transplantation. 100:e120–e127. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luznik L, O'Donnell PV and Fuchs EJ:
Post-transplantation cyclophosphamide for tolerance induction in
HLA-haploidentical bone marrow transplantation. Semin Oncol.
39:683–693. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Logan AC, Zhang B, Narasimhan B, Carlton
V, Zheng J, Moorhead M, Krampf MR, Jones CD, Waqar AN, Faham M, et
al: Minimal residual disease quantification using consensus primers
and high-throughput IGH sequencing predicts post-transplant relapse
in chronic lymphocytic leukemia. Leukemia. 27:1659–1665. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bassan R, Spinelli O, Oldani E,
Intermesoli T, Tosi M, Peruta B, Rossi G, Borlenghi E, Pogliani EM,
Terruzzi E, et al: Improved risk classification for risk-specific
therapy based on the molecular study of minimal residual disease
(MRD) in adult acute lymphoblastic leukemia (ALL). Blood.
113:4153–4162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maude S and Barrett DM: Current status of
chimeric antigen receptor therapy for haematological malignancies.
Br J Haematol. 172:11–22. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pan J, Yang JF, Deng BP, Zhao XJ, Zhang X,
Lin YH, Wu YN, Deng ZL, Zhang YL, Liu SH, et al: High efficacy and
safety of low-dose CD19-directed CAR-T cell therapy in 51
refractory or relapsed B acute lymphoblastic leukemia patients.
Leukemia. 31:2587–2593. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Neelapu SS, Locke FL, Bartlett NL, Lekakis
LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T,
Lin Y, et al: Axicabtagene Ciloleucel CAR T-Cell Therapy in
Refractory Large B-Cell Lymphoma. N Engl J Med. 377:2531–2544.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tiberghien P, Deconinck E and Adotevi O:
More on Anti-CD19 CAR T Cells in CNS Diffuse Large-B-Cell Lymphoma.
N Engl J Med. 377:2101–2102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brentjens RJ, Davila ML, Riviere I, Park
J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska
M, et al: CD19-targeted T cells rapidly induce molecular remissions
in adults with chemotherapy-refractory acute lymphoblastic
leukemia. Sci Transl Med. 5:177ra382013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Davila ML, Riviere I, Wang X, Bartido S,
Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska
M, et al: Efficacy and toxicity management of 19-28z CAR T cell
therapy in B cell acute lymphoblastic leukemia. Sci Transl Med.
6:224ra252014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee DW, Kochenderfer JN, Stetler-Stevenson
M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M,
Shah NN, et al: T cells expressing CD19 chimeric antigen receptors
for acute lymphoblastic leukaemia in children and young adults: A
phase 1 dose-escalation trial. Lancet. 385:517–528. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maude SL, Teachey DT, Porter DL and Grupp
SA: CD19-targeted chimeric antigen receptor T-cell therapy for
acute lymphoblastic leukemia. Blood. 125:4017–4023. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park JH, Rivière I, Gonen M, Wang X,
Sénéchal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E, et
al: Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic
Leukemia. N Engl J Med. 378:449–459. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu Y, Wu Z, Luo Y, Shi J, Yu J, Pu C,
Liang Z, Wei G, Cui Q, Sun J, et al: Potent Anti-leukemia
Activities of Chimeric Antigen Receptor-Modified T Cells against
CD19 in Chinese Patients with Relapsed/Refractory Acute Lymphocytic
Leukemia. Clin Cancer Res. 23:3297–3306. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park J, Riviere I, Wang XY, Bernal Y,
Purdon T, Halton E, Wang Y, Curran KJ, Sauter CS, Sadelain M, et
al: Implications of Minimal Residual Disease Negative Complete
Remission (MRD-CR) and Allogeneic Stem Cell Transplant on Safety
and Clinical Outcome of CD19-Targeted 19-28z CAR Modified T Cells
in Adult Patients with relapsed, Refractory B-Cell ALL. Blood.
126:6822015. View Article : Google Scholar
|
|
20
|
Summers C, Annesley C, Bleakley M,
Dahlberg A, Jensen MC and Gardner R: Long Term Follow-up after
SCRI-CAR19v1 Reveals Late Recurrences As Well As a Survival
Advantage to Consolidation with HCT after CAR T Cell Induced
Remission. Blood. 132 (Supp. 1):9672018. View Article : Google Scholar
|
|
21
|
Cheng Z, Wei R, Ma Q, Shi L, He F, Shi Z,
Jin T, Xie R, Wei B, Chen J, et al: In vivo expansion and antitumor
activity of coinfused CD28- and 4-1BB-engineered CAR-T cells in
patients with B cell leukemia. Mol Ther. 26:976–985. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kochenderfer JN, Dudley ME, Feldman SA,
Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes
MS, Sherry RM, et al: B-cell depletion and remissions of malignancy
along with cytokine-associated toxicity in a clinical trial of
anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood.
119:2709–2720. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harb JG, Chyla BI and Huettner CS: Loss of
Bcl-x in Ph+ B-ALL increases cellular proliferation and
does not inhibit leukemogenesis. Blood. 111:3760–3769. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maude SL, Frey N, Shaw PA, Aplenc R,
Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et
al: Chimeric antigen receptor T cells for sustained remissions in
leukemia. N Engl J Med. 371:1507–1517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Porter DL, Hwang WT, Frey NV, Lacey SF,
Shaw PA, Loren AW, Bagg A, Marcucci KT, Shen A, Gonzalez V, et al:
Chimeric antigen receptor T cells persist and induce sustained
remissions in relapsed refractory chronic lymphocytic leukemia. Sci
Transl Med. 7:303ra1392015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grupp SA, Kalos M, Barrett D, Aplenc R,
Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, et
al: Chimeric antigen receptor-modified T cells for acute lymphoid
leukemia. N Engl J Med. 368:1509–1518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bozic I, Antal T, Ohtsuki H, Carter H, Kim
D, Chen S, Karchin R, Kinzler KW, Vogelstein B and Nowak MA:
Accumulation of driver and passenger mutations during tumor
progression. Proc Natl Acad Sci USA. 107:18545–18550. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brentjens RJ, Rivière I, Park JH, Davila
ML, Wang X, Stefanski J, Taylor C, Yeh R, Bartido S, Borquez-Ojeda
O, et al: Safety and persistence of adoptively transferred
autologous CD19-targeted T cells in patients with relapsed or
chemotherapy refractory B-cell leukemias. Blood. 118:4817–4828.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bachanova V, Sandhu K, Yohe S, Cao Q,
Burke MJ, Verneris MR and Weisdorf D: Allogeneic hematopoietic stem
cell transplantation overcomes the adverse prognostic impact of
CD20 expression in acute lymphoblastic leukemia. Blood.
117:5261–5263. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang XJ, Zhu HH, Chang YJ, Xu LP, Liu DH,
Zhang XH, Jiang B, Jiang Q, Jiang H, Chen YH, et al: The
superiority of haploidentical related stem cell transplantation
over chemotherapy alone as postremission treatment for patients
with intermediate- or high-risk acute myeloid leukemia in first
complete remission. Blood. 119:5584–5590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Villalobos IB, Takahashi Y, Akatsuka Y,
Muramatsu H, Nishio N, Hama A, Yagasaki H, Saji H, Kato M, Ogawa S,
et al: Relapse of leukemia with loss of mismatched HLA resulting
from uniparental disomy after haploidentical hematopoietic stem
cell transplantation. Blood. 115:3158–3161. 2010. View Article : Google Scholar : PubMed/NCBI
|



















