Introduction
Gastric cancer is one of the most common cancers
worldwide (1). Its incidence varies
by region, with a high representation in East Asia, Eastern Europe,
and South America. Surgery is currently the sole curative treatment
option for patients with advanced gastric cancer; however, a
substantial number of patients experience disease recurrence
(2,3). Adjuvant chemotherapy (AC) such as S-1
monotherapy or combination therapy with capecitabine and
oxaliplatin (XELOX) has been the standard of treatment following
gastrectomy with D2 dissection for pathologic stages II or III
(4,5). However, the optimal time for
postoperative chemotherapy initiation after surgery is yet to be
established. A significantly short time interval (TI) between
surgery and AC can affect the patient's recovery from surgery and
is more likely to cause problems including surgical wound
complications. In contrast, a long TI between surgery and AC leads
to a high risk of cancer recurrence due to growth of microscopic
metastases. In the real world, delays in AC administration after
surgery are common for various medical conditions such as
postoperative complications, a decline in the patient's physical
status as well as other nonmedical reasons such as low patient
compliance, delayed consultation with medical oncologist, or
economic issues (6,7). In our hospital, AC is routinely
administered within 4–6 weeks of surgery.
According to the 2017 Japan Gastric Cancer
Guidelines, AC, particularly in the case of S-1 chemotherapy, is
empirically recommended within 6 weeks of surgery (8). The Korean Practice Guidelines for
Gastric Cancer 2018 were recently published following an
evidence-based multidisciplinary approach, but did not include a
recommended time point for the start of AC after gastrectomy
(9). There were also no specific
comments that focused on the time of AC administration in the
pivotal phase 3 trials that have confirmed the role of AC in
gastric cancer (10,11). In a recent phase 3 trial evaluating
the role of perioperative chemotherapy with or without
immunotherapy, a TI for AC was defined at 4–10 weeks following
surgery (12). Due to the fact that
a precise cutoff value has not been established for the delayed
time in AC administration, we analyzed the survival rate according
to a predefined TI between surgery and the start of AC and
attempted to define an optimal TI. In cases where delays were
observed, we also addressed the reasons for this occurrence.
Patients and methods
Patients
Clinical data from patients diagnosed with stage
II–III gastric adenocarcinoma who received AC after gastrectomy
with D2 lymph node dissection between 2009 and 2016 in Kyung Hee
University Hospital were reviewed retrospectively. Among these,
patients eligible for analysis with accurate records were
evaluated. Patients' data included demographics, TNM staging, types
of chemotherapeutic agents, and TI between surgery and the start of
AC. TI was defined as the period from the date of surgery to the
start of AC. Staging of cancer was based on the guidelines
established by the American Joint Committee on Cancer 7th edition.
The protocol for the study was reviewed and approved by the
Institutional Review Board (IRB) of Kyung Hee University Hospital
(approval no. KHUH 2020-01-044). All analyses and writing of the
manuscript were in accordance with the Declaration of Helsinki.
Data collection and statistical
analyses
This study aimed to investigate the clinical effect
of TI on disease recurrence and survival. Therefore, we first
searched for variables that affected the clinical outcomes in these
patients. We subsequently analyzed the impact of TI on disease
recurrence and overall survival (OS) after adjusting for other
variables using the Cox regression analysis. We investigated
whether the initially planned AC was successfully completed, and
reasons for delays in AC administration when TI was over 4 weeks
were also determined. To compare the effect of TI between the two
groups, the TIs were dichotomized based on the predetermined times
of 3, 4, 5, 6, 7, or 8 weeks and on the median value of TI for each
patient. OS was defined as the period from the date of surgery to
the last follow-up or death from any cause. Disease-free survival
(DFS) was defined as the period from the date of surgery to the
time of cancer recurrence or death from any cause. Survival
outcomes were estimated using the Kaplan-Meier survival curves. A
log-rank test was performed when a significant difference between
the survival curves for each of the groups was observed. In case of
the effect of the types of adjuvant chemotherapy on the survival
outcomes, firth's penalized maximum likelihood bias reduction
method for Cox regression was used. When statistically significant
factors were observed in the univariate analysis, multivariate
analysis using a Cox regression model were performed. A P-value
<0.05 was considered statistically significant. All statistical
analyses were performed using the Statistical Package for the
Social Sciences version 23 package (International Business Machines
Corporation) and R 3.5.3 software (https://cran.r-project.org).
Results
Patients characteristics
A total of 172 patients were identified in this
study. Among these, 97 patients (56.4%) were diagnosed with stage
II gastric adenocarcinoma and 75 patients (43.6%) with stage III
gastric adenocarcinoma. All patients included in this study
underwent surgical resection with D2 lymph node dissection. The
median age of the patient population was 51.5 years, with a higher
proportion of male patients (n=124, 72%). Six types of chemotherapy
were used as follows: XELOX (capecitabine and oxaliplatin), TS-1,
FP (5-fluorouracil plus cisplatin), TS-1/Cisplatin, FOLFOX
(oxaliplatin, leucovorin plus 5-fluorouracil) and doxifluridine in
27.9, 50.0, 13.9, 3.4, 2.9, and 1.7% of patients, respectively. The
median follow-up duration was 40.8 (range, 3–109) months.
Recurrence was observed in a total of 68 patients (39.5%), with
most of the patients presenting with distant metastases (n=62 out
of 68, 91.1%). Patients' characteristics are summarized in Table I, and TI distribution is shown in
Fig. 1. The median TI was 4.1
(range, 2.1-9.8) weeks. Based on this observation, we compared the
groups based on TI <4 weeks (n=66, 38.4%) and TI ≥4 weeks
(n=106, 61.6%). The majority of patients (n=123, 71.5%) had
received chemotherapy within 3 to 5 weeks of surgery, with few
patients receiving chemotherapy within 3 weeks or after 8
weeks.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Variables | N | % |
|---|
| Age (year) |
|
|
| Median (range) | 51.5 | (21–82) |
|
<60 | 88 | 51.2 |
| ≥60 | 84 | 48.8 |
| Sex |
|
|
| Male | 124 | 72.1 |
|
Female | 48 | 27.9 |
| Stage |
|
|
| II | 97 | 56.4 |
| III | 75 | 43.6 |
| Type of adjuvant
chemotherapy |
|
|
|
XELOX | 48 | 27.9 |
| TS-1 | 86 | 50.0 |
| FP | 24 | 13.9 |
|
TS-1/Cisplatin | 6 | 3.4 |
|
FOLFOX | 5 | 2.9 |
|
Doxifluridine | 3 | 1.7 |
| Median (range) | 4.1 | (2.1-9.8) |
| Recurrence |
|
|
| No | 104 | 60.4 |
| Yes | 68 | 39.6 |
| Pattern of
recurrencea |
|
|
|
Loco-regional | 6 | 8.9 |
|
Distant | 62 | 91.1 |
| Death |
|
|
| No | 116 | 67.4 |
|
Yes | 56 | 32.6 |
| Planned adjuvant
chemotherapy was completed |
|
|
| No | 98 | 57.0 |
|
Yes | 74 | 43.0 |
| Total | 172 | 100 |
Clinical factors affecting survival
outcomes
Following a univariate analysis, the clinical
factors that showed a significant effect on the survival outcome
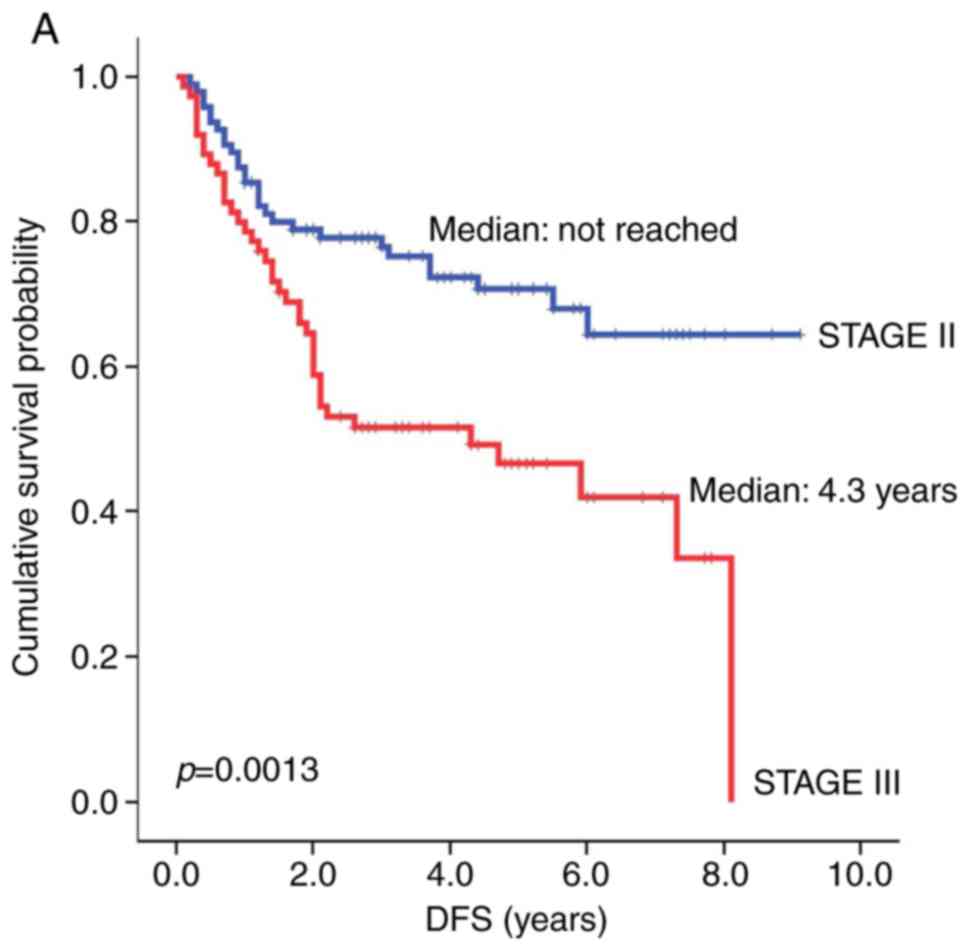
included tumor stage and TI. The median DFS for patients with stage
III gastric adenocarcinoma was significantly shorter than that for
patients with stage II gastric adenocarcinoma [4.3 years vs. not
reached (NR), respectively; hazard ratio (HR)=2.22, P=0.0013,
Fig. 2A]. OS showed a similar
statistical trend toward patients with stage II vs. III gastric
adenocarcinoma (NR vs. 7.0 years, respectively; HR=2.01, P=0.0098,
Fig. 2B). Similarly, a TI of greater
than or less than 4 weeks had a significant impact on patients'
survival. Patients who started chemotherapy 4 weeks or more after
surgery showed a significantly shorter median DFS compared to
patients who had started chemotherapy within 4 weeks of surgery
(6.0 vs. 8.1 years, respectively; HR=1.80, P=0.0277, Fig. 3A). The median OS in patients with TI
≥4 weeks was also shorter than that in patients with TI <4 weeks
(7.0 vs. NR years, HR=2.15, P=0.0133, Fig. 2B). Other clinical factors such as
sex, age, and whether the planned chemotherapy was completed had no
significant effect on either DFS or OS (Table II). After adjusting for the effect
of tumor stage in the multivariate analysis, TI ≥4 weeks still
showed a significantly worse effect on reducing both DFS and OS
(DFS: HR=1.737, P=0.040; OS: HR=1.939, P=0.018, Table III). In addition to the median
value of TI (4 weeks), we also compared DFS and OS based on a
different TI from 3 to 8 weeks. However, only a TI of 4 weeks
discriminated between DFS and OS.
 | Table II.Univariate analysis of disease-free
survival and overall survival based on clinical factors. |
Table II.
Univariate analysis of disease-free
survival and overall survival based on clinical factors.
|
|
| DFS | OS |
|---|
|
|
|
|
|
|---|
| Variable | N | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age |
|
| 0.3672 |
| 0.7545 |
|
<60 | 88 | 1 |
| 1 |
|
|
≥60 | 84 | 1.56
(0.97-2.53) |
| 1.35
(0.80-2.29) |
|
| Sex |
|
| 0.657 |
| 0.4181 |
|
Male | 124 | 1 |
| 1 |
|
|
Female | 48 | 1.60
(0.97–2.53) |
| 1.27
(0.72–2.24) |
|
| Stage |
|
| 0.0013a |
| 0.0098 |
| II | 97 | 1 |
| 1 |
|
|
III | 75 | 2.22
(1.37-3.60) |
| 2.01
(1.18-3.40) |
|
| Type of adjuvant
chemotherapya |
|
|
|
|
|
|
XELOX | 48 | 1 |
| 1 |
|
|
TS-1 | 86 | 0.60
(0.34-1.08) | 0.0872 | 0.65
(0.34-1.27) | 0.2059 |
| FP | 24 | 1.29
(0.65-2.57) | 0.4723 | 1.67
(0.80-3.49) | 0.1760 |
|
TS-1/cisplatin | 6 | 0.90
(0.27-3.00) | 0.8594 | 1.34
(0.41-4.46) | 0.6303 |
|
FOLFOX | 5 | 0.86
(0.22-3.33) | 0.8315 | 0.65
(0.11-3.85) | 0.6330 |
|
Doxifluridine | 3 | 0.42
(0.03-6.15) | 0.5274 | 0.55
(0.03-9.67) | 0.6798 |
| Planned adjuvant
chemotherapy was completed |
|
| 0.0969 |
| 0.2675 |
| No | 98 | 1 |
| 1 |
|
|
Yes | 74 | 1.50
(0.93–2.43) |
| 1.35
(0.80–2.28) |
|
| Time interval |
|
| 0.0277 |
| 0.0133 |
| <4
weeks | 67 | 1 |
| 1 |
|
| ≥4
weeks | 105 | 1.8
(1.067-3.045) |
| 2.15
(1.173-3.939) |
|
 | Table III.Multivariate analysis of DFS and OS
based on stage and TI of 4 weeks. |
Table III.
Multivariate analysis of DFS and OS
based on stage and TI of 4 weeks.
|
| DFS | OS |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Stage (III vs.
II) | 2.163
(1.331-3.515) | 0.002 | 1.939
(1.143-3.290) | 0.014 |
| Time interval
(<4 weeks vs. ≥4 weeks) | 1.737
(1.026-2.939) | 0.040 | 1.939
(1.143-3.290) | 0.018 |
Reasons for delayed adjuvant
chemotherapy
Reasons for delays in the administration of
chemotherapy included postoperative complications (i.e.,
intra-abdominal abscesses, anastomotic site leakage, paralytic
ileus, or wound infections), inadequate physical condition to start
chemotherapy (i.e., general weakness or poor oral intake), and
others (i.e., low patient compliance, economic status, or delays
due to patient's unavailability). Considering the retrospective
nature of this study, obtaining the reasons for delaying AC was
challenging. However, the most common causes for delays of longer
than 4 weeks in administering AC were surgical complications
(n=29/105, 26.6%), followed by a poor general condition by the
patient (n=13/105, 12.3%). All reasons for delayed AC are listed in
Table IV. For surgical
complications, the types of complications were investigated in more
detail (Supplementary Table). The median DFS of patients who
experienced surgical complication was insignificantly shorter than
that of patients without surgical complication (7.3 vs. 8.1 years,
HR=1.34, P=0.326). The median OS also tended to be numerically
shorter without statistical significance in patients who
experienced postoperative complications than that in patients who
did not, although the median values in both groups were not reached
(HR=1.52, P=0.185).
 | Table IV.Reasons for delayed chemotherapy (≥4
weeks). |
Table IV.
Reasons for delayed chemotherapy (≥4
weeks).
| Reason for delayed
chemotherapy | N (%) |
|---|
| Postoperative
complications (i.e., intra-abdominal abscess, anastomosis site
leakage, ileus, wound infection) | 29 (51) |
| Inadequate
condition to start chemotherapy (i.e., general weakness, poor oral
intake) | 13 (23) |
| Other conditions
requiring hospitalization, excluding postoperative
complications | 12 (21) |
| Personal condition
(i.e., low patient's compliance, economic status, delayed due to
foreign residence) | 3 (5) |
| Total | 57 (100) |
Discussion
AC is typically administered to eradicate residual
cancer and invisible micrometastases that may remain after surgery.
Previous studies using animal models have shown that surgical
resection of primary tumors increased the number of circulating
tumor cells and promoted the proliferation of residual cells
(13). Surgery has also been shown
to promote the production of oncogenic growth factors such as
transforming growth factor-α and to significantly reduce the
immunotherapeutic effect of interleukin-2 and lymphokine-activated
killer cells (14). Cellular
proliferation of cancer cells progresses rapidly initially and then
progressively. Therefore, when the tumor burden is minimal
following surgery, it is expected that AC should be administered as
soon as possible. However, a significantly early AC start can
affect the patient's recovery such as wound healing and may cause
adverse effects when the patient's general condition has not been
fully recovered. Conversely, beginning AC significantly late can
increase the risk of recurrence due to regrowth of microscopic or
indolent foci of viable tumor cells. As a result, several studies
have evaluated the optimal timing for AC administration,
particularly in patients with colon or breast cancer.
In colon cancer, early AC administration has been
shown to improve OS compared to late administration, using various
time points from 60 days to 8 weeks (15,16).
Another study showed that AC started within 4 to 8 weeks improved
survival compared to later starts of longer than 8 weeks (17). A previous meta-analysis has also
shown that relative OS decreases by 14% for every 4-week delay in
the initiation of AC (18). Early AC
within 20 days improved DFS, whereas initiation of AC within 21
days of surgery was not associated with OS or DFS in patients with
early breast cancer, suggesting there is some complexity and
ambiguity in the optimal time of AC after tumor resection surgery
(19,20). Similar reports in gastric cancer are
insufficient; thus, the appropriate timing of AC administration in
patients with gastric cancer is yet to be established. In a Korean
retrospective study, AC within 28 days led to significant
improvement in 10-year OS, suggesting the early initiation of AC
after gastrectomy (21). However,
the chemotherapeutic agents in the study (5-FU, mitomycin C, and
polysaccharide-K) are not the standard agents used recently.
Another Korean study showed AC administered within 8 weeks instead
of 4 weeks of surgery improved survival outcomes (22). Interestingly, the results of the
analysis of subgroups who were able to start AC within 4 weeks
because of minimally invasive surgery (i.e., laparoscopic or
robot-assisted gastrectomy) showed a significantly better OS and
relapse-free survival compared to subgroups who started AC after 4
weeks. Conversely, other reports showed no survival benefit for
patients who received AC within 4 weeks of surgery (23). A study in Taiwan showed that starting
AC within 8 weeks of a gastrectomy resulted in an improved 5-year
recurrent-free survival rate, possibly contributing to an improved
OS (24). Another study on AC using
S-1 chemotherapy, from Japan, found that the timing of AC was not
associated with OS (25). However,
others reported that S-1 administration within 6 weeks of surgery
was associated with a decrease in recurrence rates and an increase
in survival time (26). A recent
meta-analysis showed a survival benefit when AC was started within
6 to 8 weeks of surgery. However, when AC was started after 8
weeks, a 20% increased risk of death was observed (27). Notwithstanding, this meta-analysis
did not evaluate solely gastric cancer, but also included other
types of cancer such as colorectal or pancreatic cancer. Taken
together, it is important to set an optimal TI for AC delivery,
despite differences in patient's recovery after surgery, surgery
methods, types of AC, and tumor patterns. It has been not exactly
known why the results of previous studies are inconsistent with
each other. Considering that prospective comparative studies have
not yet been conducted, it is inevitable to interpret them in
consideration of the number of samples, methods of statistical
analysis, and potential biases in each study. However, as reported
in the aforementioned study (22)
that early AC within 4 weeks due to minimally invasive surgical
technique led a better survival outcomes, it may be reasonable to
recommend starting AC as early as possible (i.e., within 4 weeks)
as long as the patient's condition improves sufficiently after
surgery and there is no reason to delay the administration of
AC.
In this study, we dichotomized the TI as less than
vs. equal or more than 4 weeks based on the median value of TI
within our study population (4.1 months). Additionally, we further
examined the effect of various TIs set arbitrarily every 1 week to
evaluate whether there were significant differences based on the
different TIs. We found that OS and DFS were greater in the group
with a TI lower than 4 weeks than those in the group with a TI
higher than 4 weeks. In addition to TI, TNM stage was another
significant factor that affected patients' survival in our
multivariate analysis (stage III vs. II, HR=2.22. 95% CI:
1.37-3.60). This is consistent with the results from previous
studies (10,11,20) and
may suggest that our study population provides a significant
representation of the general population in terms of disease
characteristics and the natural course of disease, despite the
retrospective nature of our cohort. Therefore, the significance of
the 4-week TI obtained in this study may be considered as a
reliable TI for the general population as well. Additionally, this
study included solely patients who received gastrectomy with D2
lymph node dissection, unlike previous studies that included
patients undergoing both D1 and D2 dissections. Since D2 dissection
has become a standard technique, our study may be more
appropriately applied to the real-world clinical practice (28,29).
Besides its retrospective nature, our study has some
limitations. First, preexisting comorbidity data for patients were
missing. Comorbidities are important factors to consider as they
can affect the recovery from surgery, start of chemotherapy, and
patients' long-term survival. We were not able to investigate the
effects of comorbidities in this instance. However, as patients
included here were sufficiently healthy to withstand a radical
surgery, even if other comorbidities were observed, it is possible
to assume that the overall health status of our patients was
satisfactory. In addition to the comorbidities that had already
been diagnosed, it has been found that postoperative complications
have a significantly negative impact on survival, including both on
OS and disease-specific mortality, in patients with gastric cancer
(30). In fact, when comparing HR,
there was a tendency of worse long-term survival outcomes according
to the immediate postoperative complications. The fact that
statistical significance was not observed in this study population
might be related to the insufficient sample size. Second, although
the Korean study mentioned earlier showed the association between
the possibility of early AC and minimally invasive surgical
procedures, we did not investigate the surgical methods used. A
study reported that a laparoscopic approach was associated with a
reduced recovery time and allowed for a shortened TI to AC
administration (31). Other studies
have shown that this approach was not associated with the time of
AC start and that although it allowed for earlier discharge
following surgery, its benefits did not last longer than in open
surgery post-discharge (23). Third,
98 of the 172 patients (57%) did not complete the planned AC.
However, these patients were equally distributed in both groups of
patients with TI ≥4 weeks and <4 weeks; hence, the potential
effect might be offset. Finally, a selection bias may be present in
our data considering that this study was conducted in a single
center and with a small number of patients.
In conclusion, this study suggests that AC should be
initiated within 4 weeks of surgery with D2 resection in patients
with gastric cancer. Delays longer than 4 weeks in AC
administration for any reason may be detrimental to patients'
survival.
Supplementary Material
Supporting Data
Acknowledgements
The current study was presented at European Society
for Medical Oncology (ESMO) Asia 2018 Congress, 23 Nov-25 Nov 2018
in Singapore, and published as abstract no. 1199 in Annals of
Oncology (2018) 29 (suppl_9): ix46-ix66. 10.1093/annonc/mdy432.
Funding
No funding was received.
Availability of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CHM designed the study. GTA and CHM collected
patient data and performed the initial statistical analysis. SJJ
performed the detailed statistical analysis. GTA was a major
contributor in writing the manuscript. CHM supervised the written
manuscript. SKB, HJK and JJH provided the clinical information for
patients included in the analysis. SKB, HJK and JJH interpreted the
results and wrote the first draft of the discussion section. All
authors read and approved the final manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The current study was approved by Kyung Hee
University Hospital IRB (approval no. KHUH 2020-01-044).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gunderson LL: Gastric cancer-patterns of
relapse after surgical resection. Semin Radiat Oncol. 12:150–161.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
D'Angelica M, Gonen M, Brennan MF,
Turnbull AD, Bains M and Karpeh MS: Patterns of initial recurrence
in completely resected gastric adenocarcinoma. Ann Surg.
240:808–816. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Noh SH, Park SR, Yang HK, Chung HC, Chung
IJ, Kim SW, Kim HH, Choi JH, Kim HK, Yu W, et al: Adjuvant
capecitabine plus oxaliplatin for gastric cancer after D2
gastrectomy (CLASSIC): 5-year follow-up of an open-label,
randomised phase 3 trial. Lancet Oncol. 15:1389–1396. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sakuramoto S, Sasako M, Yamaguchi T,
Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi
Y, Imamura H, et al: Adjuvant chemotherapy for gastric cancer with
S-1, an oral fluoropyrimidine. N Engl J Med. 357:1810–1820. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Becerra AZ, Aquina CT, Mohile SG, Tejani
MA, Schymura MJ, Boscoe FP, Xu Z, Justiniano CF, Boodry CI, Swanger
AA, et al: Variation in delayed time to adjuvant chemotherapy and
disease-specific survival in stage III colon cancer patients. Ann
Surg Oncol. 24:1610–1617. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chavez-MacGregor M, Clarke CA,
Lichtensztajn DY and Giordano SH: Delayed initiation of adjuvant
chemotherapy among patients with breast cancer. JAMA Oncol.
2:322–329. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Japanese Gastric Cancer Association, .
Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric
Cancer. 20:1–19. 2017. View Article : Google Scholar
|
|
9
|
Guideline Committee of the Korean Gastric
Cancer Association (KGCA), Development Working Group & Review
Panel, . Korean Practice Guideline for gastric cancer 2018: An
evidence-based, multi-disciplinary approach. J Gastric Cancer.
19:1–48. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sasako M, Sakuramoto S, Katai H, Kinoshita
T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T and
Ohashi Y: Five-year outcomes of a randomized phase III trial
comparing adjuvant chemotherapy with S-1 versus surgery alone in
stage II or III gastric cancer. J Clin Oncol. 29:4387–4393. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bang YJ, Kim YW, Yang HK, Chung HC, Park
YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY, et al: Adjuvant
capecitabine and oxaliplatin for gastric cancer after D2
gastrectomy (CLASSIC): A phase 3 open-label, randomised controlled
trial. Lancet. 379:315–321. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bang YJ, Van Cutsem E, Fuchs CS, Ohtsu A,
Tabernero J, Ilson DH, Hyung WJ, Strong VE, Goetze TO, Yoshikawa T,
et al: KEYNOTE-585: Phase III study of perioperative chemotherapy
with or without pembrolizumab for gastric cancer. Future Oncol.
15:943–952. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gunduz N, Fisher B and Saffer EA: Effect
of surgical removal on the growth and kinetics of residual tumor.
Cancer Res. 39:3861–3865. 1979.PubMed/NCBI
|
|
14
|
Ono I, Gunji H, Suda K, Iwatsuki K and
Kaneko F: Evaluation of cytokines in donor site wound fluids. Scand
J Plast Reconstr Surg Hand Surg. 28:269–273. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bayraktar UD, Chen E, Bayraktar S, Sands
LR, Marchetti F, Montero AJ and Rocha-Lima CM: Does delay of
adjuvant chemotherapy impact survival in patients with resected
stage II and III colon adenocarcinoma? Cancer. 117:2364–2370. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Des Guetz G, Nicolas P, Perret GY, Morere
JF and Uzzan B: Does delaying adjuvant chemotherapy after curative
surgery for colorectal cancer impair survival? A meta-analysis. Eur
J Cancer. 46:1049–1055. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Klein M, Azaquoun N, Jensen BV and Gögenur
I: Improved survival with early adjuvant chemotherapy after colonic
resection for stage III colonic cancer: A nationwide study. J Surg
Oncol. 112:538–543. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Biagi JJ, Raphael MJ, Mackillop WJ, Kong
W, King WD and Booth CM: Association between time to initiation of
adjuvant chemotherapy and survival in colorectal cancer: A
systematic review and meta-analysis. JAMA. 305:2335–2342. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Colleoni M, Bonetti M, Coates AS,
Castiglione-Gertsch M, Gelber RD, Price K, Rudenstam CM, Lindtner
J, Collins J, Thürlimann B, et al: Early start of adjuvant
chemotherapy may improve treatment outcome for premenopausal breast
cancer patients with tumors not expressing estrogen receptors. The
International Breast Cancer Study Group. J Clin Oncol. 18:584–590.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shannon C, Ashley S and Smith IE: Does
timing of adjuvant chemotherapy for early breast cancer influence
survival? J Clin Oncol. 21:3792–3797. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kang SY, Ahn MS, Song GW, Choi YW, Lee HW,
Jeong SH, Park JS, Cho YK, Han SU, Sheen SS, et al: Does the timing
of adjuvant chemotherapy for gastric cancer influence patient
outcome? Acta Oncol. 54:1231–1234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park HS, Jung M, Kim HS, Kim HI, An JY,
Cheong JH, Hyung WJ, Noh SH, Kim YI, Chung HC and Rha SY: Proper
timing of adjuvant chemotherapy affects survival in patients with
stage 2 and 3 gastric cancer. Ann Surg Oncol. 22:224–231. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee Y, Min SH, Park KB, Park YS, Kim JW,
Ahn SH, Kim JW, Park DJ, Lee KW and Kim HH: Effect of early
adjuvant chemotherapy on survival of advanced gastric cancer
patients: A propensity score-matched analysis. J Gastric Cancer.
18:58–68. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang SM, Chen YC, Chen WY, Yang LY, Tsan
DL, Tsang NM, Yap WK, Tsai CS, Leung WM, Hong JH, et al: Optimal
timing for postsurgical adjuvant therapy in patients with gastric
cancer: A propensity score matching study. J Cancer. 10:332–340.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fujitani K, Kurokawa Y, Takeno A, Endoh S,
Ohmori T, Fujita J, Yamasaki M, Takiguchi S, Mori M and Doki Y;
Osaka University Clinical Research Group for Gastroenterological
Surgery, : Time to initiation or duration of S-1 adjuvant
chemotherapy; which really impacts on survival in stage II and III
gastric cancer? Gastric Cancer. 21:446–452. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamamoto M, Sakaguchi Y, Kinjo N,
Yamaguchi S, Egashira A, Minami K, Ikeda Y, Morita M, Toh Y and
Okamura T: S-1 adjuvant chemotherapy earlier after surgery
clinically correlates with prognostic factors for advanced gastric
cancer. Ann Surg Oncol. 23:546–551. 2016. View Article : Google Scholar
|
|
27
|
Petrelli F, Zaniboni A, Ghidini A, Ghidini
M, Turati L, Pizzo C, Ratti M, Libertini M and Tomasello G: Timing
of adjuvant chemotherapy and survival in colorectal, gastric, and
pancreatic cancer. A systematic review and meta-analysis. Cancers
(Basel). 11:5502019. View Article : Google Scholar
|
|
28
|
Degiuli M, Sasako M, Calgaro M, Garino M,
Rebecchi F, Mineccia M, Scaglione D, Andreone D, Ponti A and Calvo
F; Italian Gastric Cancer Study Group, : Morbidity and mortality
after D1 and D2 gastrectomy for cancer: Interim analysis of the
Italian Gastric Cancer Study Group (IGCSG) randomised surgical
trial. Eur J Surg Oncol. 30:303–308. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Degiuli M, Sasako M, Ponti A and Calvo F:
Survival results of a multicentre phase II study to evaluate D2
gastrectomy for gastric cancer. Br J Cancer. 90:1727–1732. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kubota T, Hiki N, Sano T, Nomura S, Nunobe
S, Kumagai K, Aikou S, Watanabe R, Kosuga T and Yamaguchi T:
Prognostic significance of complications after curative surgery for
gastric cancer. Ann Surg Oncol. 21:891–898. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kaito A, Kinoshita T, Shitara K, Shibasaki
H and Nishida T: Timing of initiation of adjuvant chemotherapy for
gastric cancer: A case-matched comparison study of laparoscopic vs.
open surgery. Eur J Surg Oncol. 43:801–807. 2017. View Article : Google Scholar : PubMed/NCBI
|