Introduction
Cancer is a complex and intrinsically heterogeneous
disease (1). An increased
understanding of the mechanisms underlying the progression of human
cancer may aid the identification of novel therapeutic targets.
Next generation sequencing methods have enabled the development of
a series of public databases, including The Cancer Genome Atlas
(TCGA) (2) and the Gene Expression
Profiling Interactive Analysis (GEPIA) (3) and Genotype-Tissue Expression databases
(4), which may be used to
investigate the heterogeneity and complexity of cancer. Gastric
cancer (GC) is one of the most common tumors of the digestive
system. The prognosis of patients with locally advanced GC is poor,
and the 5-year survival rate is <50% (5). In the past decades, studies have
focused on identifying novel diagnostic and prognostic biomarkers
for several types of human cancer, including GC. For example, TIMP
metallopeptidase inhibitor 2 (TIMP2) was identified as a prognostic
marker for GC, and increased expression of TIMP2 was correlated
with a shorter survival time in patients with GC (6). However, effective biomarkers to improve
the survival time of patients with GC are still required.
Collagen type VIII α 1 chain (COL8A1) encodes one of
the two α chains of collagen type VIII (7), which had been revealed to play a role
in atherogenesis and vascular remodeling, presumably through
modification of cell migration and proliferation (8,9). COL8A1
is produced in the cornea, aortic endothelial cells and
intraglomerular mesangial cells (10,11).
COL8A1 was found to promote the growth of smooth muscle cells,
suggesting that it serves important roles in regulating cell
biology (7). Moreover, previous
studies have revealed that COL8A1 may be implicated in cancer
progression. Di et al (12)
used weighted gene co-expression network analysis to report that
COL8A1 was an important stage-associated gene in bladder cancer.
Shang et al (13) reported
that COL8A1 was associated with the development and diagnosis of
colon cancer. Furthermore, the knockdown of COL8A1 suppressed cell
growth and invasion of hepatocarcinoma cells (14). However, despite the aforementioned
studies, the expression pattern and molecular functions of COL8A1
in human cancer remain largely unclear.
The present study evaluated the mRNA levels of
COL8A1 across different human cancer types and investigated the
association between COL8A1 expression and survival time using TCGA
database. Integrated analysis revealed that COL8A1 was upregulated
across human cancer types, including GC. Bioinformatics analysis
showed that COL8A1 was involved in regulating the cell cycle and
DNA replication. Furthermore, increased expression of COL8A1 was
associated with advanced stage and poor overall survival (OS) time
in patients with GC. Additionally, silencing of COL8A1
significantly suppressed the proliferation and migration of GC
cells in vitro. To the best of our knowledge, the present
study is the first to demonstrate that COL8A1 may serve as a
potential biomarker for GC.
Materials and methods
Public database analysis
COL8A1 expression data in Adrenocortical carcinoma
(ACC), Bladder Urothelial Carcinoma (BLCA), Breast invasive
carcinoma (BRCA), Cervical squamous cell carcinoma and endocervical
adenocarcinoma (CESC), Cholangio carcinoma (CHOL), Colon
adenocarcinoma (COAD), Lymphoid Neoplasm Diffuse Large B-cell
Lymphoma (DLBC), Esophageal carcinoma (ESCA), Glioblastoma
multiforme (GBM), Head and Neck squamous cell carcinoma (HNSC),
Kidney Chromophobe (KICH), Kidney renal clear cell carcinoma
(KIRC), Kidney renal papillary cell carcinoma (KIRP), Acute Myeloid
Leukemia (LAML), Brain Lower Grade Glioma (LGG), Liver
hepatocellular carcinoma (LIHC), Lung adenocarcinoma (LUAD), Lung
squamous cell carcinoma (LUSC), Mesothelioma (MESO), Ovarian serous
cystadenocarcinoma (OV), Pancreatic adenocarcinoma (PAAD),
Pheochromocytoma and Paraganglioma (PCPG), Prostate adenocarcinoma
(PRAD), Rectum adenocarcinoma (READ), Sarcoma (SARC), Skin
Cutaneous Melanoma (SKCM), Stomach adenocarcinoma (STAD),
Testicular Germ Cell Tumors (TGCT), Thyroid carcinoma (THCA),
Thymoma (THYM), Uterine Corpus Endometrial Carcinoma (UCEC),
Uterine Carcinosarcoma (UCS), Uveal Melanoma (UVM) datasets were
downloaded from the GEPIA database (gepia.cancer-pku.cn/detail.php)
on April 28, 2019.
The GSE15459 (15),
GSE29272 (16), GSE51105 (17), GSE62254 (18), GSE15459 (19) and GSE14210 (20) datasets and the Kaplan-Meier Plotter
database (21) were analyzed to
determine the association between COL8A1 expression and overall
survival time in patients with GC.
Cell culture
The human GC cell line AGS was purchased from the
American Type Culture Collection and cultured in Dulbecco's
modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.)
Lentivirus transfection
The short hairpin (sh)RNA targeting human COL8A1
(5′-TGTATAACGGCAGACAGAA-3′) and the negative control (NC) shRNA
(5′-TTCTCCGAACGTGTCACGT-3′) were designed and inserted into the
pGCSIL-GFP vector. The recombinant lentivirus was purchased from
Shanghai GeneChem Co., Ltd. Stable knockdown of COL8A1 was achieved
by transfecting the AGS cells with the lentiviral vector for 72
h.
Reverse-transcription quantitative PCR
(RT-qPCR)
RT-qPCR was performed as previously described
(22,23). The primer sequences used for qPCR
were as follows: COL8A1 forward, 5′-AGAACTACAACCCGCAGAC-3′ and
reverse, 5′-TTGAATAGAGCAACCCACA-3′; and GAPDH forward,
5′-GGGAGCCAAAAGGGTCAT-3′ and reverse, 5′-GAGTCCTTCCACGATACCAA-3′.
COL8A1 mRNA levels were quantified using the 2−ΔΔCq
method (24) and normalized to the
internal reference gene GAPDH.
Cell proliferation assay
Cell proliferation was assessed using the adherent
cell cytometry system Celigo® and analyzed using
Application Programing Interface (version 1.0; software). Briefly,
2,000 AGS cells transfected with shNC or shCOL8A1 were seeded in a
6-well plate. The number of cells was counted after 1, 2, 3, 4 or 5
days. The experiment was performed in triplicate.
Cell apoptosis
Flow cytometry was used to assess cell apoptosis.
Briefly, 5×105 AGS cells transfected with shNC and
shCOL8A1 were seeded into a 6-well plate and incubated for 48 h.
The cells were subsequently collected and washed twice with PBS.
Apoptosis was detected using a flow cytometer and an Annexin V-APC
Apoptosis Detection kit (eBioscience; Thermo Fisher Scientific,
Inc.).
Bioinformatics analysis
The Database for Annotation, Visualization and
Integrated Discovery (DAVID; david.ncifcrf.gov/home.jsp) was used for
bioinformatics analysis.
Western blotting
Western blotting was performed as previously
described (25). The primary
antibodies used in the present study included anti-COL8A1 (cat. no.
PA5-62731; Thermo Fisher Scientific, Inc.) and anti-GAPDH (cat. no.
sc-32233; Santa-Cruz Biotechnology, Inc.). A goat anti-mouse
secondary antibody (cat. no. sc-2005; Santa-Cruz Biotechnology,
Inc.) was used.
Statistical analysis
Statistical analyses were performed using SPSS
software (version 15.0; SPSS Inc.). Each experiment was performed
in triplicate. The data are expressed as the mean ± standard
deviation. The Student's t-test or Mann-Whitney U-test was used to
determine the association between COL8A1 expression and the
clinical characteristics of the tumors. Kaplan-Meier analysis
followed by the log-rank test and Cox regression analysis were used
to assess the association between COL8A1 expression and the OS time
of patients. P<0.05 was considered to indicate a statistically
significant difference.
Results
COL8A1 is upregulated across different
types of human cancer
The mRNA levels of COL8A1 in tumor and normal
tissues across human cancer types, including ACC, BLCA, BRCA, CESC,
CHOL, COAD, DLBC, ESCA, GBM, HNSC, KICH, KIRC, KIRP, LAML, LGG,
LIHC, LUAD, LUSC, MESO, OV, PAAD, PCPG, PRAD, READ, SARC, SKCM,
STAD, TGCT, THCA, THYM, UCEC, UCS, and UVM, were analyzed using
TCGA datasets. The results revealed that COL8A1 was significantly
upregulated in HNSC, KIRC, PRAD, CHOL, CESC, BRCA, READ, LUSC,
COAD, ESCA, SKCM, BLCA, THCA, STAD and LIHC tissues compared with
normal tissues (Fig. 1 and Table I), suggesting that COL8A1 may serve
as an oncogene.
 | Figure 1.COL8A1 expression is upregulated
across human cancer types. The present study analyzed the mRNA
levels of COL8A1 in tumor tissues and normal samples across human
cancer types, including (A) HNSC, (B) KIRC, (C) PRAD, (D) CHOL, (E)
BRCA, (F) READ, (G) COAD, (H) ESCA, (I) SKCM, (J) THCA, (K) STAD
and (L) LIHC. P<0.05 was considered to indicate a statistically
significant difference. *P<0.05; ***P<0.001. TCGA, The Cancer
Genome Atlas. |
 | Table I.COL8A1 expression is upregulated in
multiple types of human cancer. |
Table I.
COL8A1 expression is upregulated in
multiple types of human cancer.
| Cancer | Median (tumor) | Median
(normal) | P-value |
|---|
| ACC | 1.07 | 5.01 |
3.89×10−16 |
| BLCA | 5.14 | 8.19 |
5.82×10−1 |
| BRCA | 20.75 | 5.39 |
1.00×10−34 |
| CESC | 2.23 | 2.72 |
9.61×10−1 |
| CHOL | 5.86 | 0.32 |
8.37×10−1 |
| COAD | 7.54 | 5.87 |
3.85×10−4 |
| DLBC | 0.23 | 0.04 |
2.25×10−16 |
| ESCA | 7.82 | 2.41 |
2.01×10−19 |
| GBM | 5.19 | 0.19 |
2.23×10−33 |
| HNSC | 4.62 | 0.83 |
6.43×10−9 |
| KICH | 1.61 | 2.87 |
2.23×10−1 |
| KIRC | 23.36 | 4.06 |
2.43×10−35 |
| KIRP | 2.76 | 3.77 |
3.75×10−1 |
| LAML | 0.06 | 0.06 |
9.95×10−1 |
| LGG | 0.32 | 0.19 |
2.00×10−10 |
| LIHC | 0.76 | 0.25 |
1.80×10−10 |
| LUAD | 27.01 | 31.21 |
1.65×10−5 |
| LUSC | 13.43 | 31.81 |
1.39×10−37 |
| OV | 6.09 | 8.06 |
8.78×10−1 |
| PAAD | 25.99 | 0.34 |
3.26×10−68 |
| PCPG | 4.44 | 3.48 |
9.12×10−1 |
| PRAD | 7.28 | 8.78 |
7.99×10−1 |
| READ | 8.91 | 6.37 |
1.62×10−3 |
| SARC | 14.34 | 3.93 |
1.35×10−1 |
| SKCM | 1.78 | 1.82 |
4.49×10−5 |
| STAD | 13.43 | 1.48 |
4.93×10−36 |
| TGCT | 1.25 | 0.57 |
3.49×10−5 |
| THCA | 68.71 | 24.8 |
8.99×10−49 |
| THYM | 0.35 | 0.04 |
6.07×10−12 |
| UCEC | 1.70 | 3.29 |
5.71×10−2 |
| UCS | 2.64 | 3.29 |
3.71×10−1 |
COL8A1 has prognostic value across
human cancer types
The prognostic value of COL8A1 expression in human
cancer was evaluated using Kaplan-Meier analysis and the log-rank
test. Upregulated COL8A1 mRNA expression was significantly
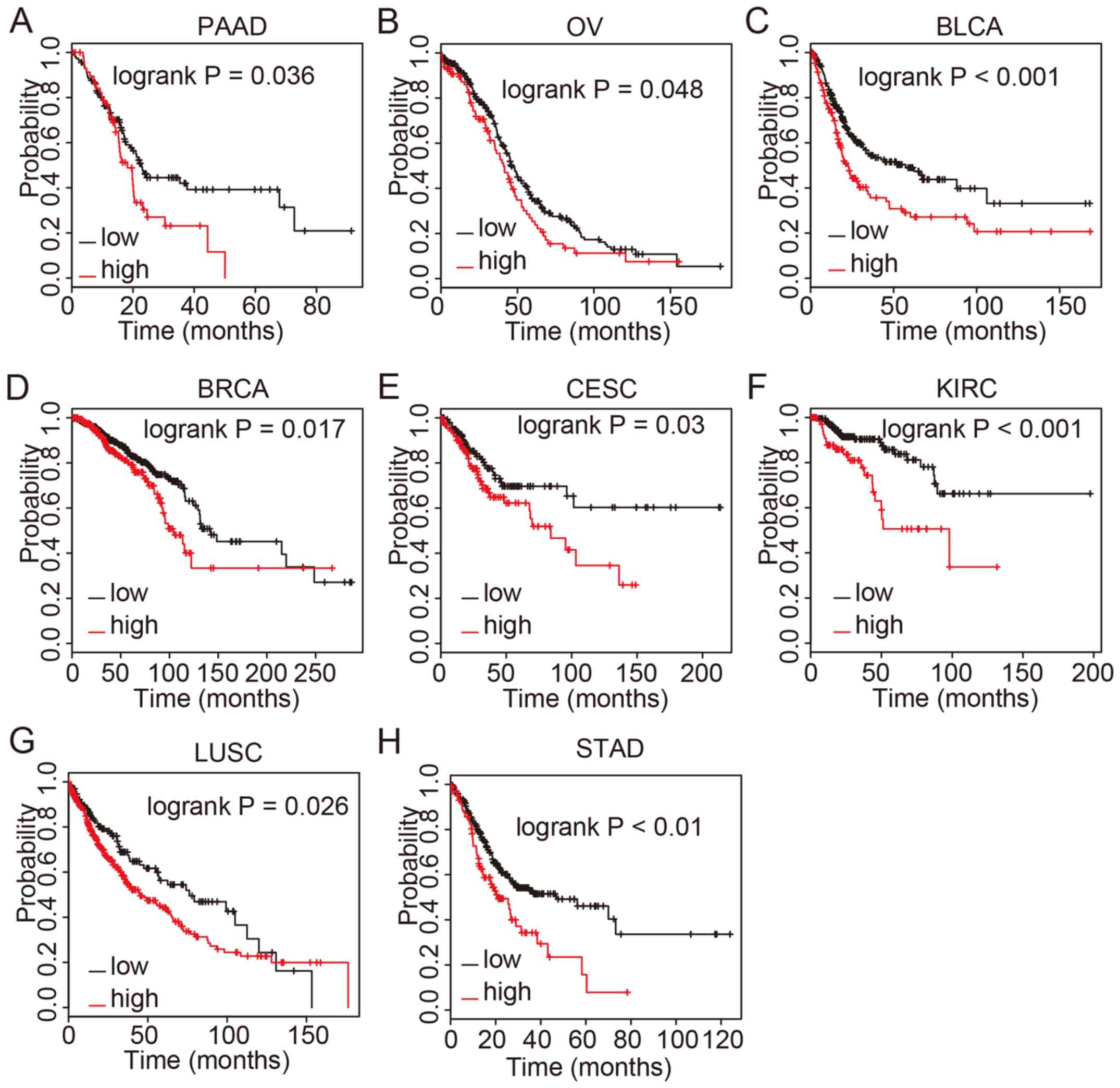
associated with OS time in several cancer types, including PAAD,
OV, BLCA, BRCA, CESC, KIRC, LUSC and STAD (Fig. 2). Patients with high COL8A1
expression had a significantly shorter OS time compared with those
with low expression. The results of these analyses, together with
previous reports investigating bladder and colon cancer, suggested
that COL8A1 may serve as a biomarker for cancer prognosis.
COL8A1 is upregulated in advanced GC
samples
The present study revealed that COL8A1 was
significantly upregulated in stage II–IV STAD compared with stage I
STAD samples, in stage III and IV OV compared with stage II OV
samples, in stage II and III ESCA compared with stage I ESCA
samples and in stage III and IV OV compared with stage II BLCA
samples (Fig. 3).
Upregulation of COL8A1 predicts a poor
prognosis in GC
The present study revealed that high COL8A1
expression was associated with a shorter OS time in patients with
GC. In order to further validate this, Kaplan-Meier survival curves
were used to analyze the association between COL8A1 levels and OS
time in GC samples. The GSE15459, GSE29272, GSE51105, GSE62254,
GSE15459 and GSE14210 datasets and the Kaplan-Meier Plotter
database were analyzed. Similarly to TCGA analysis, patients with
GC with a high COL8A1 expression level had a shorter OS time
compared with those with low expression. Interestingly, high COL8A1
expression was associated with a shorter OS time in both human
epidermal growth factor receptor 2 (HER2)-positive and negative GC
samples (Fig. 4).
Bioinformatics analysis reveals the
potential functions of COL8A1 in GC
The present study conducted co-expression and
bioinformatics analysis to reveal the potential roles of COL8A1 in
GC. TCGA STAD dataset was used to perform co-expression analysis
and a total of 1,461 genes with an absolute Pearson's correlation
coefficient >0.4 were identified as the potential targets of
COL8A1. Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene
Ontology (GO) analyses using DAVID were subsequently performed.
KEGG analysis revealed that COL8A1 was significantly
involved in regulating ‘PI3K-Akt signaling’, ‘cGMP-PKG signaling’,
‘Rap1 signaling’, ‘Ras signaling’, ‘MAPK signaling’, ‘hippo
signaling’, ‘TGF-β signaling’, ‘hedgehog signaling’, ‘cAMP
signaling’ and ‘Wnt signaling’ (Fig.
5B). GO analysis demonstrated that COL8A1 was associated with
the ‘regulation of cell adhesion’, ‘extracellular matrix
organization’, ‘angiogenesis’, ‘skeletal system development’,
‘regulation of cell proliferation’, ‘cell-matrix adhesion’,
‘extracellular matrix disassembly’, ‘wound healing’, ‘negative
regulation of angiogenesis’ and ‘positive regulation of cell
migration’ (Fig. 5A). There results
suggested that COL8A1 may participate in regulating GC cell
proliferation and migration.
Knockdown of COL8A1 expression in AGS
cells
RT-qPCR results revealed that COL8A1 was highly
expressed in AGS cells compared to MG-803 cells (Fig. 6A). A loss-of-function assay was
subsequently performed to explore the functions of COL8A1 in GC.
COL8A1 expression in AGS cells was knocked down using
lentivirus-mediated gene transfection. As shown in Fig. 6B and C, both the mRNA and protein
levels of COL8A1 were reduced in AGS cells following transfection
with shCOL8A1 compared with shNC.
Silencing of COL8A1 suppresses AGS
cell proliferation and colony formation
A Celigo® Cell Counting assay was used to
detect AGS proliferation following COL8A1 knockdown. The results
revealed that the proliferation rate of AGS cells transfected with
shCOL8A1 was significantly inhibited compared with cells
transfected with shNC. As indicated in Fig. 7A, the average cell number in the
shCOL8A1-transfected group decreased by about 61% compared with the
shNC-transfected group on day 5.
The colony formation assay revealed similar results
to the cell proliferation assay. As shown in Fig. 7, shCOL8A1-transfected cells formed
fewer and smaller colonies compared with the shNC-transfected
group. Statistical analysis further confirmed that knockdown of
COL8A1 significantly reduced the number of colonies (Fig. 7B-C; P<0.001). Collectively, these
results suggested that knockdown of COL8A1 inhibited the
proliferation of AGS cells.
COL8A1 knockdown promotes the
apoptosis of GC cells
Bioinformatics analysis revealed that COL8A1 was
involved in regulating apoptosis-related pathways. Therefore, the
effect of COL8A1 knockdown on the apoptosis of AGS cells was
investigated. As shown in Fig. 8A,
the activity of caspase 3 and 7 was significantly increased
following COL8A1 knockdown in AGS cells compared with the shNC
group. Moreover, we detected the protein levels of cleaved
caspase-3 using western blot assay. The results showed that the
levels of cleaved caspase-3 were significantly up-regulated after
COL8A1 knockdown in GC cells (Fig.
8B). These results suggested that COL8A1 knockdown
significantly induces apoptosis of AGS cells (Fig. 8C). Furthermore, Annexin V-APC
staining revealed that COL8A1 knockdown significantly promoted the
apoptosis of AGS cells compared with the shNC group (Fig. 8D).
Discussion
COL8A1 is a collagen type VIII protein. Previous
studies have demonstrated that COL8A1 is upregulated in several
types of human cancer (12,26,27).
COL8A1 was found to be associated with tumor stage and prognosis in
patients with bladder (12) and
colon cancer (13). Furthermore,
knockdown of COL8A1 inhibited the growth and invasion of
hepatocellular carcinoma (14).
Additionally, COL8A1 was reported to be involved in the regulation
of GC progression (26,28). To the best of our knowledge, the
present study was the first to demonstrate that COL8A1 was
upregulated in several types of cancer, including HNSC, KIRC, PRAD,
CHOL, CESC, BRCA, READ, LUSC, COAD, ESCA, SKCM, BLCA, THCA, STAD
and LIHC, and was associated with a shorter OS time. These results
suggested that COL8A1 may serve as a potential oncogene in human
cancer.
GC is one of the most common tumors of the digestive
system and is associated with a poor prognosis (29,30).
Previous studies have investigated potential biomarkers for GC.
Gastric cancer metastasis-associated long non-coding RNA was
upregulated in patients with GC and was associated with metastasis
(31). Upregulation of erythrocyte
membrane protein band 4.1 like 5 was correlated with poor prognosis
and a shorter survival time in patients with GC (32). However, despite the aforementioned
advances, there is still an urgent need to identify effective
biomarkers for GC. The present study analyzed a series of public
databases, including TCGA and the Kaplan-Meier Plotter, to reveal
that COL8A1 was upregulated in advanced GC samples compared with
stage I GC samples. Moreover, the results demonstrated that
patients with GC with high COL8A1 expression had a shorter OS time
compared with those with low expression. Interestingly, a high
expression of COL8A1 was associated with a shorter OS time in both
HER2-positive and negative GC samples. These results suggested that
COL8A1 may serve as a potential biomarker for GC.
The present study performed co-expression, GO and
KEGG pathway analyses to explore the potential roles of COL8A1 in
GC. The results revealed that COL8A1 was involved in regulating
several proliferation and metastasis-related biological processes,
including ‘cell adhesion’, ‘regulation of cell proliferation’, and
‘positive regulation of cell migration’. KEGG pathways analysis
showed that COL8A1 was associated with the regulation of the
PI3K-Akt, mitogen-activated protein kinase (MAPK), hippo and
transforming growth factor β signaling pathways. Previous studies
have demonstrated that the PI3K-Akt signaling played an important
role in regulating GC cell cycle, proliferation, and chemotherapy
resistance (33). Furthermore, the
MAPK signaling pathway was associated with the regulation of GC
metastasis (34). The present study
performed a loss-of-function assay to validate the influence of
COL8A1 on GC cell proliferation and migration. Collectively, the
results suggested that COL8A1 serves as an oncogene in GC by
promoting the proliferation and suppressing apoptosis of GC
cells.
In conclusion, the present study revealed that
COL8A1 was upregulated in several types of human cancer and was
associated with a shorter OS time. Bioinformatics analysis revealed
that COL8A1 was involved in regulating cell proliferation and
metastasis. Experimental validation of COL8A1 expression
demonstrated that silencing of COL8A1 significantly decreased GC
cell proliferation in vitro. Therefore, COL8A1 may serve as
a potential therapeutic target or prognostic biomarker for GC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ and YC designed the study. YS, WG and PW
collected and analyzed public data. JZ and LL conducted
bioinformatics analysis. JZ, GC, ZC, GL and GZ performed the
experiments. JZ and LZ performed the statistical analyses. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Allison KH and Sledge GW: Heterogeneity
and cancer. Oncology (Williston Park). 28:772–778. 2014.PubMed/NCBI
|
|
2
|
Hutter C and Zenklusen JC: The cancer
genome atlas: Creating lasting value beyond its data. Cell.
173:283–285. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
GTEx Consortium: Human genomics. The
genotype-tissue expression (GTEx) pilot analysis: Multitissue gene
regulation in humans. Science. 348:648–660. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao B, Zhang J, Chen X, Xu H and Huang B:
Mir-26b inhibits growth and resistance to paclitaxel chemotherapy
by silencing the CDC6 gene in gastric cancer. Arch Med Sci.
15:498–503. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang W, Zhang Y, Liu M, Wang Y, Yang T, Li
D, Ding F, Bai G and Li Q: TIMP2 is a poor prognostic factor and
predicts metastatic biological behavior in gastric cancer. Sci Rep.
8:96292018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li X, Wang Z, Tong H, Yan Y and Li S:
Effects of COL8A1 on the proliferation of muscle-derived satellite
cells. Cell Biol Int. 42:1132–1140. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Plenz GA, Deng MC, Robenek H and Volker W:
Vascular collagens: Spotlight on the role of type VIII collagen in
atherogenesis. Atherosclerosis. 166:1–11. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ooshima A and Muragaki Y: Collagen
metabolism in atherogenesis. Ann NY Acad Sci. 598:582–584. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aldave AJ, Bourla N, Yellore VS, Rayner
SA, Khan MA, Salem AK and Sonmez B: Keratoconus is not associated
with mutations in COL8A1 and COL8A2. Cornea. 26:963–965. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aldave AJ, Rayner SA, Salem AK, Yoo GL,
Kim BT, Saeedian M, Sonmez B and Yellore VS: No pathogenic
mutations identified in the COL8A1 and COL8A2 genes in familial
Fuchs corneal dystrophy. Invest Ophthalmol Vis Sci. 47:3787–3790.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Di Y, Chen D, Yu W and Yan L: Bladder
cancer stage-associated hub genes revealed by WGCNA co-expression
network analysis. Hereditas. 156:72019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shang J, Wang F, Chen P, Wang X, Ding F,
Liu S and Zhao Q: Co-expression network analysis identified COL8A1
is associated with the progression and prognosis in human colon
adenocarcinoma. Dig Dis Sci. 63:1219–1228. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao Y, Jia L, Mao X, Xu H, Wang B and Liu
Y: siRNA-targeted COL8A1 inhibits proliferation, reduces invasion
and enhances sensitivity to D-limonence treatment in
hepatocarcinoma cells. IUBMB Life. 61:74–79. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Subhash VV, Yeo MS, Wang L, Tan SH, Wong
FY, Thuya WL, Tan WL, Peethala PC, Soe MY, Tan DSP, et al:
Anti-tumor efficacy of Selinexor (KPT-330) in gastric cancer is
dependent on nuclear accumulation of p53 tumor suppressor. Sci Rep.
8:122482018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li WQ, Hu N, Burton VH, Yang HH, Su H,
Conway CM, Wang L, Wang C, Ding T, Xu Y, et al: PLCE1 mRNA and
protein expression and survival of patients with esophageal
squamous cell carcinoma and gastric adenocarcinoma. Cancer
Epidemiol Biomarkers Prev. 23:1579–1588. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brasacchio D, Busuttil RA, Noori T,
Johnstone RW, Boussioutas A and Trapani JA: Down-regulation of a
pro-apoptotic pathway regulated by PCAF/ADA3 in early stage gastric
cancer. Cell Death Dis. 9:4422018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cristescu R, Lee J, Nebozhyn M, Kim KM,
Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, et al: Molecular
analysis of gastric cancer identifies subtypes associated with
distinct clinical outcomes. Nat Med. 21:449–456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ooi CH, Ivanova T, Wu J, Lee M, Tan IB,
Tao J, Ward L, Koo JH, Gopalakrishnan V, Zhu Y, et al: Oncogenic
pathway combinations predict clinical prognosis in gastric cancer.
PLoS Genet. 5:e10006762009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim HK, Choi IJ, Kim CG, Kim HS, Oshima A,
Yamada Y, Arao T, Nishio K, Michalowski A and Green JE: Three-gene
predictor of clinical outcome for gastric cancer patients treated
with chemotherapy. Pharmacogenomics J. 12:119–127. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nagy Á, Lánczky A, Menyhárt O and Győrffy
B: Validation of miRNA prognostic power in hepatocellular carcinoma
using expression data of independent datasets. Sci Rep. 8:92272018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao X, Hao S, Wang M, Xing D and Wang C:
Knockdown of pseudogene DUXAP8 expression in glioma suppresses
tumor cell proliferation. Oncol Lett. 17:3511–3516. 2019.PubMed/NCBI
|
|
23
|
Peng C, Li X, Yu Y and Chen J: LncRNA
GASL1 inhibits tumor growth in gastric carcinoma by inactivating
the Wnt/β catenin signaling pathway. Exp Ther Med. 17:4039–4045.
2019.PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jin H, Zhang Q, Zhang S, Liu H, Man Z and
Wang Y: Effects of claudin-1 downregulation on the physiological
processes of gallbladder cancer SGC996 cells. Oncol Lett.
17:1688–1694. 2019.PubMed/NCBI
|
|
26
|
Liu X, Wu J, Zhang D, Bing Z, Tian J, Ni
M, Zhang X, Meng Z and Liu S: Identification of potential key genes
associated with the pathogenesis and prognosis of gastric cancer
based on integrated bioinformatics analysis. Front Genet.
9:2652018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Z, Chen G, Wang Q, Lu W and Xu M:
Identification and validation of a prognostic 9-genes expression
signature for gastric cancer. Oncotarget. 8:73826–73836. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen J, Wang X, Hu B, He Y, Qian X and
Wang W: Candidate genes in gastric cancer identified by
constructing a weighted gene co-expression network. Peer J.
6:e46922018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhuo W, Liu Y, Li S, Guo D, Sun Q, Jin J,
Rao X, Li M, Sun M, Jiang M, et al: Long noncoding RNA GMAN,
up-regulated in gastric cancer tissues, is associated with
metastasis in patients and promotes translation of ephrin a1 by
competitively binding GMAN-AS. Gastroenterology. 156:676–691. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jeong MH, Park SY, Lee SH, Seo J, Yoo JY,
Park SH, Kim MJ, Lee S, Jang S, Choi HK, et al: EPB41L5 mediates
TGFβ-induced metastasis of gastric cancer. Clin Cancer Res.
25:3617–3629. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Almhanna K, Strosberg J and Malafa M:
Targeting AKT protein kinase in gastric cancer. Anticancer Res.
31:4387–4392. 2011.PubMed/NCBI
|
|
34
|
Yang M and Huang CZ: Mitogen-activated
protein kinase signaling pathway and invasion and metastasis of
gastric cancer. World J Gastroenterol. 21:11673–11679. 2015.
View Article : Google Scholar : PubMed/NCBI
|