Introduction
According to global cancer reports, there are
~850,000 new liver cancer cases and 840,000 liver cancer-associated
deaths worldwide in 2019 (1,2). The incidence is higher in Asia compared
with western countries, especially China, where liver cancer is the
sixth most common tumor and the second leading cause of
cancer-associated mortality in 2019 (2). Hepatocellular carcinoma (HCC) accounts
for ~90% of primary liver cancer and is primarily associated with
chronic hepatitis B virus (HBV) (3)
and hepatitis C virus (HCV) infection (4), followed by excessive drinking and
non-alcoholic fatty-associated liver disease (5). According to the Barcelona Clinic Liver
Cancer (BCLC) staging criteria and liver function, liver
transplantation or resection are the most common treatments
(6). Additionally, radiofrequency
ablation (RFA) therapies are feasible for early stage disease.
However, unfortunately, HCC is characterized by a rapid onset and
is invasive and fast-growing (6,7). HCC is
associated with a high recurrence rate and fatality rate when
combined with a history of cirrhosis, which results in the majority
of patients losing the opportunity for surgery when considering
social or economic factors, such as poor allocation of medical
resources and low income in deprived region (8). Moreover, the 5-year survival rate is
~12.5% (8). The development of RFA
and transarterial chemotherapy (TACE) offers an additional
treatment option for HCC. These local intervention treatments have
been widely accepted because several studies have confirmed the
potential benefits of combination therapy with targeted agents and
cellular immune therapies (9–11). Thus,
subsequent studies have demonstrated the efficacy of targeted
therapies based on tyrosine protein kinase inhibitors (TKIs) or
multikinase inhibitors in HCC, such as sorafenib, lenvatinib,
regorafenib and cabozantinib (7,12–14);
however, these agents fail to improve survival and so the overall
results are still unsatisfactory (15).
The successful application of immunotherapy in
malignant melanoma in the US in 2011 (16), as well as its application in other
types of cancer, and a deeper understanding of the mechanisms
underlying HCC pathogenesis have led researchers to investigate
whether immunotherapy could be applied in clinical trials of HCC.
Immune checkpoints mainly are comprised of the programmed death
receptor 1 (PD-1)/programmed death ligand 1 (PD-L1) and cytotoxic T
lymphocyte-associated protein 4 (CTLA-4) (17). To some extent, these checkpoints
enhance antitumor immunity as well as exaggerate immune system
activation (17). Among the
available inhibitors, PD-1 inhibitors are currently thought to be
the most promising (18–20). Based on two phase II clinical trials,
CheckMate 040 and KEYNOTE-224 in which both nivolumab and
pembrolizumab yielded promising results as second line agents after
first-line sorafenib treatment (21,22), the
Food and Drug Administration (FDA) approved nivolumab in 2017 and
pembrolizumab in 2018 as second-line therapies for HCC (23,24).
Evidence suggests that compared with the placebo, the PD-1
treatment group had notable potency and improved overall survival
(OS) as first-line compared with second-line treatment. However,
the latest data from the 2019 American Society of Clinical Oncology
(ASCO) were discouraging (25).
Overall, PD-1/PD-L1 inhibitors are challenging the role of TKIs as
first-line treatments for HCC.
Immune recognition, tolerance and escape of
HCC
Normal liver tissue is exposed to a variety of
antigens, including toxins and intestinal microbial products
(26–28). Liver cancer always occurs in the
context of chronic inflammation, which is an immunosuppressive
environment mediated by hepatocytes (27,29).
Under these inflammatory conditions, the inhibition of
antigen-specific immune monitoring is partially mediated by changes
in the molecular expression of immunosuppressive checkpoints and
dendritic cell function, increasing regulatory T cell numbers and
the release of immunosuppressive cytokines, such as interleukin-10
(IL-10) and transforming growth factor (TGF)-β (30–32).
Long-term exposure to antigens can also lead to the overexpression
of immunosuppressive checkpoint molecules on T cells, thus
resulting in energy failure or cell exhaustion (26,31).
Meanwhile, HCC creates an immunosuppressive microenvironment
through the expression of immunosuppressive factors that inhibit
antigen presentation and the immune response, thus preventing an
effective antitumor response and permitting further escape of
immune surveillance (26,31). Through the abnormal expression of
antigens, secretion of metabolites and cytokines and the change of
the immune microenvironment, liver cancer cells can escape
antitumor responses, resulting in immune escape of the tumor
(33). The process of immune escape
involves immunosuppressive factors, such as TGF-β and IL-10,
induction of dendritic cell (DC) apoptosis, changes in T cell
subtypes and decreased interferon-γ levels or its receptor
expression. In detail, TGF-β has a dual regulatory effect on tumors
(34). During the initial stages of
tumorigenesis, TGF-β inhibits cell proliferation and initiates cell
differentiation or apoptosis. However, during the progressive
stage, this effect is lost, resulting in immune inhibition,
stimulation of angiogenesis and induction of epithelia or
mesenchymal transformation. This provides a favorable
microenvironment for tumor cell proliferation, invasion and
metastasis (31). IL-10, which plays
a role in immunosuppression and promotes the immune escape of HCC
cells, belongs to the Th2 type family of cytokines. For example,
IL-10 can decrease the expression of the major histocompatibility
complex II (MHC-II) and costimulatory molecules, such as CD80/86,
thus weakening the functions of antigen-presenting cells (APCs)
that kill tumor cells or indirectly kill tumor cells by activating
T cells (30). As a result, HCC
occurs.
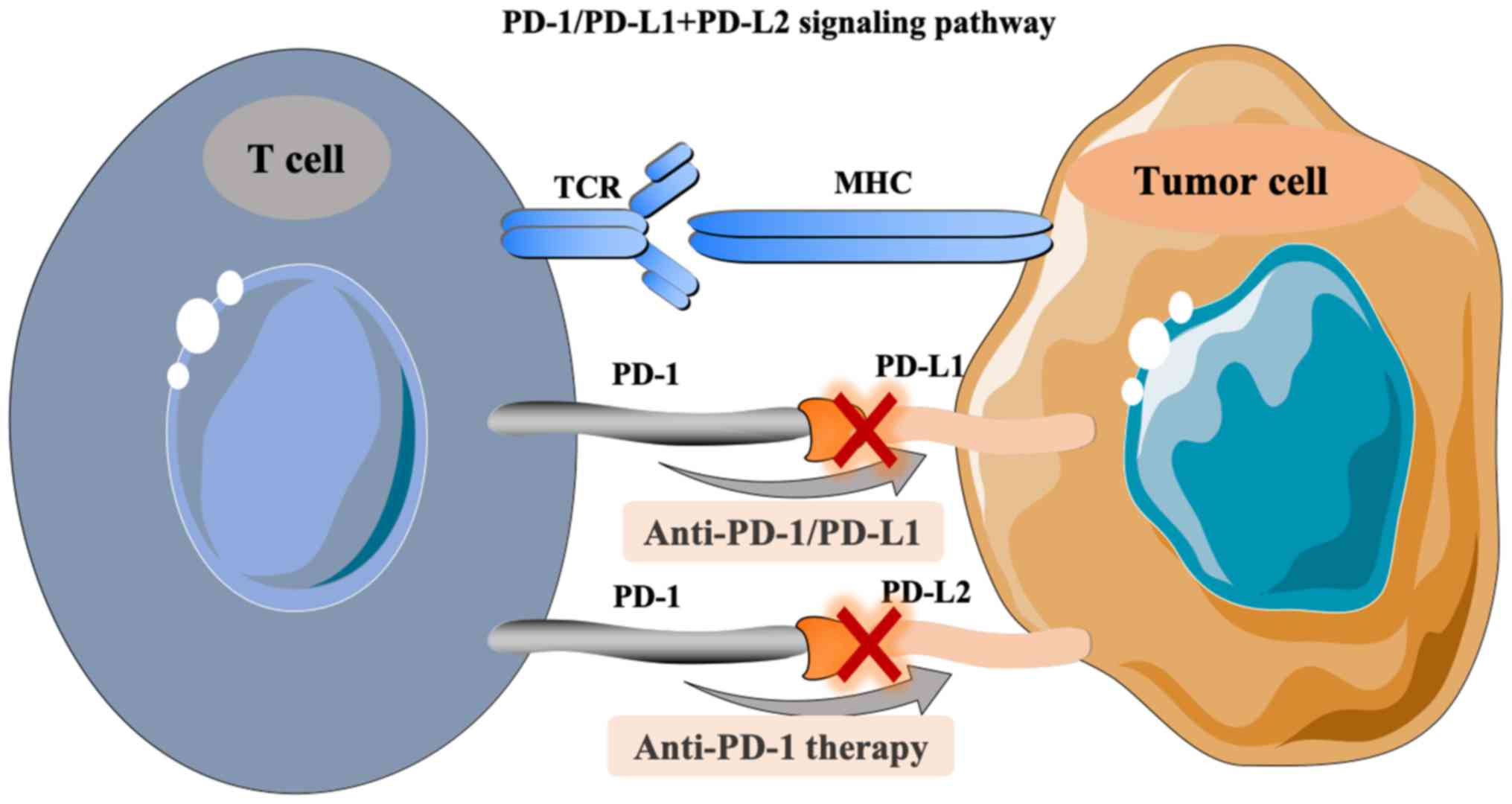
The role of PD1/PD-L1 in immune tolerance
and immune escape of HCC
ICIs are a type of membrane binding molecule that
play an important role in immune escape (35). In HCC, the common ICIs include
PD-1/PD-L1, CTLA-4, lymphocyte activating gene 3 protein (LAG-3)
and mucin domain molecule 3 (Tim-3) (26). PD-1, a member of the CD28 superfamily
of proteins, is a costimulatory receptor expressed on immune cells,
such as T, B and natural killer cells, but it is primarily
expressed on the surface of activated T cells [CD8+ T cells, T
regulatory cells (Tregs) or myeloid-derived suppressor cells]
(35,36). PD-L1 is a type I transmembrane
protein composed of 290 amino acids, which can be upregulated on
the surface of activated T cells, DCs and macrophages (37,38). As
an immunosuppressive receptor with a negative regulatory role, PD-1
always transmits a coinhibitory signal together with the T cell
antigen receptor (TCR) after binding with PD-L1 and PD-L2 on the
surface of activated T cells. Downstream signals gradually suppress
the expression of genes required for T cell activation, which then
induces tumor immune escape (39).
In detail, the pathway composed of PD-1 and its ligands, PD-L1 and
PD-L2, has a key role in maintaining peripheral immune tolerance
(40). In turn, tumor cells also
utilize this pathway to escape from T cell-induced immunity. PD-1
and PD-L1 or PD-L2, as two pairs of costimulatory signals,
constitute the same pathway of PD-1-mediated signaling, which
induces T cell activation and immune regulation by inhibiting cell
proliferation (41,42). When the PD-1/PD-L1 signaling pathway
is activated, it reduces the damage of the immune response to
surrounding tissues and helps prevent the occurrence of autoimmune
diseases (40). Similarly, the
activation of this pathway stimulates the binding of PD-L1
expressed by HCC cells to PD-1 on the surface of tumor infiltrating
lymphocytes, inducing the apoptosis of specific cytotoxic T
lymphocytes (CTL) that target the tumor. This decreases the
activity of T cells in the local microenvironment, and thus
mediates immune escape (40). PD-L1
expression is upregulated in HCC (43). Some studies have demonstrated that
this phenomenon induces the inherent expression of PD-L1 in tumor
cells through the phosphatidylinositol 3 kinase- protein kinase B
(PI3K-AKT) epidermal growth factor receptor and ALK tyrosine kinase
receptor/STAT3 signaling pathways, thus regulating the expression
of cell cycle checkpoint proteins and cell proliferation proteins
(33,34). Finally, the above process inhibits T
cell proliferation (17,44). Therefore, PD-1/PD-L1 is a key
interaction involved in the immune escape in liver cancer tumors
(26,36,39). In
addition, the PD-1/PD-L1 antibody can suppress this immune escape
ability, permitting cytotoxic T lymphocyte-mediated antitumor
responses by blocking the binding of PD-L1 and PD-1 (26,35,36). The
treatment principle related to the PD-1/PD-L1 signaling pathway is
shown in Fig. 1.
Application of PD-1/PD-L1 inhibitors in
HCC
At present, the most commonly used PD-1 inhibitors
are nivolumab, pembrolizumab, camrelizumab and sintilimab, while
the common PD-L1 inhibitors include durvalumab and atezolizumab
(37). Several guidelines have
recommended immunotherapy of HCC. Among them, the National
Comprehensive Cancer Network guidelines have recommended nivolumab
and pembrolizumab as second-line treatment options for advanced HCC
(45). The European Society of
Oncology (ESMO) guidelines have recommended nivolumab as first-line
and second-line options for HCC, and pembrolizumab has been
recommended as a second-line option (6).
Monotherapy studies
Nivolumab
The approval of nivolumab in September 2017 was
based on the CheckMate 040 trial (NCT01658878), which was a phase
I/II single-arm, multi-center, multi-cohort clinical trial
involving patients with advanced HCC (46). The patients enrolled onto the study
included those who had or had not received sorafenib treatment and
were HBV or HCV infected or not infected. In addition, the study
had five cohorts: Cohorts 1 and 2 underwent the dose escalation
phase (0.1–10 mg/kg nivolumab was given once every 2 weeks) and
dose expansion phase (3 mg/kg nivolumab was given once every 2
weeks until the disease progressed or toxic reactions could not be
tolerated), respectively. The two cohorts included 262 patients
with unresectable advanced HCC, including 182 patients who had
previously received sorafenib treatment and 80 patients who had
not, of whom 48 and 214 were in cohort 1 and 2, respectively. The
results show that the objective response rate (ORR) was 16–19% and
the median overall survival (mOS) time was 15.6 months. The ORR and
mOS of the dose escalation group were 15% and 15 months,
respectively, and those in the dose expansion group were 20% and
15.6 months, respectively. However, for other patients who had not
received sorafenib as a first-line agent, the ORR and mOS were
20–23% and 28.6 months, respectively. It is noteworthy that in this
trial, there were 85 patients from Asia; and the mOS of the total
population in this trial was 14.9, 14.8 months for patients with
HBV and HCV and 16.9 months for patients without these viruses. The
study indicated that patients can benefit from nivolumab,
regardless of hepatitis history. Moreover, the mOS of patients with
stable disease (SD) and partial response (PR) were 17.5 and 27.4
months, respectively, further suggesting that those with SD may
continue to benefit after immunotherapy (47). Furthermore, no viral recurrence was
observed in the patients with HBV/HCV infection.
Another global multicenter, randomized phase III
clinical trial, CheckMate 459 (NCT02576509), was designed to
observe the efficacy and safety of nivolumab as a first-line
treatment for unresectable HCC. The study compared the efficacy of
nivolumab with sorafenib to determine the safety and efficacy of
nivolumab (48). Compared with
sorafenib, the preliminary OS difference failed to reach the
pre-set statistical significance threshold value [hazard ratio
(HR)=0.84; P=0.0419] and showed significant OS improvement
(HR=0.85; 95% confidence interval, 0.72–1.02; P=0.0752). The
12-month OS rate in the nivolumab group was 59.7%, while that of
the sorafenib group was 55.1%. However, those patients in sorafenib
group showed a notable trend towards OS prolongation.
The CheckMate 9DX trial (NCT03383458) focused on
adjuvant immunotherapy after surgery (49). It aims to evaluate the efficacy of
adjuvant nivolumab treatment compared with a placebo in patients
with high risks of recurrence after HCC surgery, and it was
estimated to be completed in 2025.
Pembrolizumab
The FDA approved the use of pembrolizumab in
November 2018 based on the results of the phase II single-arm,
non-randomized trial, KEYNOTE-224 (NCT02702414) (50), which included 104 patients with HCC
who had been previously treated with sorafenib. One patient
achieved a complete response (CR), 17 patients achieved PR and 44
patients achieved SD. In addition, for the total population, the
ORR was 17%, and the disease control rate (DCR) was 62%. Moreover,
the median progression free survival (PFS) time was 4.9 months,
while the OS time was 12.9 months.
In 2019, the American Society of Clinical Oncology
(ASCO) meeting reported the results of the KEYNOTE-240 study
(NCT02702401), which was a randomized, placebo-controlled phase III
clinical study that evaluated the efficacy of pembrolizumab
compared with the best supportive treatments, such as symptomatic
palliation in patients with advanced HCC (51). In total, 413 patients were randomly
assigned to the pembrolizumab group (n=278) or the placebo group
(n=135) at a 2:1 ratio. The results showed that after a median
follow-up of 13.8 months, 10.1% of the experimental group and 3.0%
of the placebo group were still receiving therapy. Compared with
the placebo group, the OS time in the test group was prolonged by 3
months (13.9 months vs. 10.6 months; HR=0.78; unilateral P=0.0238),
but this figure did not reach the established statistical
difference (25). However, in the OS
and PFS subgroup analyses, the majority of subgroups observed an
obvious prolonging trend for pembrolizumab, thus suggesting
clinical benefits. Furthermore, the ORRs of the pembrolizumab group
and placebo group were 18.3% (95% CI, 14.0–23.4) and 4.4% (95% CI,
1.6–9.4), respectively. Regarding safety, common adverse events
included an increase in the levels of transaminase and bilirubin,
fatigue, pruritus, loss of appetite and diarrhea. The incidence of
grade 3/4 toxicities was relatively low. Another phase III
(NCT03062358), randomized trial is currently being conducted.
Camrelizumab
At the ESMO meeting in October 2018, a multicenter,
open, randomized, parallel-controlled, phase II clinical trial
(NCT02989922) in China that enrolled 220 patients (data for 217
patients can be accessed) was reported. In detail, patients were
randomly assigned to two subgroups at the ratio of 1:1 and received
intravenous camrelizumab (3 mg/kg) every 2 weeks (q2w, n=111) and
every 3 weeks (q3w, n=109), respectively. The overall ORR was 13.8%
(30/217), and the 6-month OS rate was 74.7% (52).
In September 2019, the data of the NCT02989922 study
were updated at the meeting of the Chinese Society of Clinical
Oncology (CSCO) (52). The ORR of
all subjects was 14.7%, while the 6-month OS rate was 74.4%.
Meanwhile, the most common adverse event was reactive capillary
epithelial proliferation (RCREP), the incidence of which was ~66.8%
(52).
In April 2020, Qin et al updated the latest
results of NCT02989922 in China (53). By November 2018, the median follow-up
time was 12.5 months. The results showed that both the q2w and q3w
subgroups showed a higher ORR of 11.9 and 17.6%, respectively, and
the ORR was 14.7% among all patients. In addition, the 6-month and
12-month OS rates were 74.4 and 55.9%, respectively. As for safety,
the most common treatment-associated adverse events (AEs) was still
RCREP with an incidence of 67%. The incidence of grade 3–4 AEs was
22%. Both the ORR and the incidence of AEs were similar to that of
other immune drugs, which is why camrelizumab was approved as the
first second-line ICI agent for the treatment of HCC in China
(53).
Combination therapy studies
Camrelizumab plus apatinib
A phase I clinical trial (NCT02942329) investigating
the combination of camrelizumab and apatinib [a TKI selectively
acting on vascular endothelial growth factor receptor (VEGFR)2] was
reported at the ASCO meeting in June 2018 (54). The results showed that the ORR of 14
patients was ~50%, and the DCR was 85.7%, which indicated that the
binding of PD-1 with antiangiogenic drugs may have a synergistic
effect in advanced HCC. The primary and most serious complication
was hypertension.
Camrelizumab plus chemotherapy
In September 2018, the CSCO conference reported the
results of a study performed by Qin et al. This was a phase
II clinical trial investigating first-line treatment with
camrelizumab combined with the FOLFOX regimen (fluorouracil+calcium
folinate+oxaliplatin) in HCC. In 22 patients, the ORR was 27.3%,
and the DCR was 72.7%. Furthermore, a phase III clinical study
(NCT03605706), required to further confirm the efficacy and safety,
is recruiting participants and is estimated to complete in 2021.
However, the results have not yet been reported. The primary
outcome is OS.
Moreover, Qin et al registered a multicenter
phase II trial to assess the efficacy and safety of camrelizumab
combined with FOLFOX4 as a first-line treatment in advanced HCC
(55). As a result, the ORR and DCR
of 34 patients were 26.5 and 79.4%, respectively. Although the mOS
level was not achieved, the safety of combinational therapy was
controllable, and the local control profiles of tumor were good
mainly reflected in DCR.
Atezolizumab plus bevacizumab
Atezolizumab targets PD-L1 (56). A phase IB clinical study of
atezolizumab combined with bevacizumab for the treatment of
advanced HCC, the GO30140 study (NCT02715531) (56), was reported at the ASCO conference in
2018. In 23 assessable patients, the ORR was 65% and the DCR was
95%.
In November 2019, the IMbrave 150 study, a global
multi-center and open-label phase III trial that enrolled 501
patients with unresectable HCC who did not receive systemic
therapies, was reported at the ESMO Asian conference (Abstract:
LBA3) (57). The patients were
randomly administered atezolizumab combined with bevacizumab or
sorafenib at a 2:1 ratio until unacceptable toxicity or no further
clinical benefits were observed. The latest results showed that the
median PFS time in the atezolizumab group was 6.8 months (range,
5.7–8.3 months), while that of the sorafenib group was 4.3 months
(range, 4.0–5.6 months).
At the Liver Cancer Summit 2020, which is organized
by the European Association for the Study of the Liver, Qin et
al presented the Chinese subgroup results of the IMbrave trial,
which confirmed the previous global results (58). Among 194 Chinese patients who had
poorer prognostic factors compared with the global data, 133
patients were randomly assigned to the combination group
(atezolizumab plus bevacizumab) and 61 to the sorafenib group. The
median follow-up time of the combined group and the sorafenib group
was 7.2 and 5.6 months, respectively. The mPFS was 5.7 vs. 3.2
months, and the ORR was 25 vs. 7%, which was consistent with the
global results. Moreover, the incidence of toxicity was relatively
low.
Pembrolizumab plus lenvatinib
In the REFLECT study, which aimed to compare the
efficacy of lenvatinib and sorafenib in unresectable patients with
advanced HCC, lenvatinib was shown not to be inferior to sorafenib;
therefore, lenvatinib was approved as a first-line agent for
advanced HCC (59). The 2018 ASCO
meeting reported a phase IB trial that enrolled 30 patients to
evaluate the safety and efficacy of pembrolizumab and lenvatinib in
advanced HCC (60). Among the 26
patients who could be included for evaluation, results showed that
one patient achieved CR, 10 achieved PR and 15 achieved SD.
Moreover, the ORR was 42.3%, and the PFS time reached 9.69 months.
In 2019, the American Association for Cancer Research updated the
data. Compared with the previous results in the 2018 ASCO meeting
(60), the number of patients
achieving CR was three, while 15 patients achieved PR according to
the modified Response Evaluation Criteria in Solid Tumors (RECIST)
standards (61). Indeed, these data
showed encouraging trends, in particular, the increase of patients
who reached PR (from 10 to 15). Therefore, a global phase III
randomized, controlled clinical study investigating first-line
treatment for advanced HCC using pembrolizumab and lenvatinib has
been launched, with pending results.
In 2019, Llovet et al updated the results
concerning combination therapy of pembrolizumab and lenvatinib in
advanced HCC (62). The results
showed that tolerance of the combination of agents was acceptable
and only 5% of patients had to terminate treatment because of grade
3–4 AEs. The ORR and DCR was 31 and 49%, respectively, and the
1-year survival rate was 40%.
Nivolumab plus lenvatinib
At the ASCO-GI 2020 meeting, Kudo et al
(63) reviewed the data of a phase
IB trial that focused on the efficacy of nivolumab combined with
lenvatinib in advanced HCC. The study enrolled 30 patients who were
randomly divided into two groups: One group (n=6) had multidrug
resistance and the other group (n=24) had no previous treatments.
The first part of this study explored the dose tolerance of
combinational agents via DLT evaluation. The results showed that no
DLT was observed in six patients. Among the 30 patients, the ORR
was 76.7% and the DCR was 96.7%. Moreover, ~10% of patients
achieved a complete response (CR). However, the second part of this
study enrolled 24 patients with no prior systematic therapy for
unresectable HCC who all received nivolumab (200 mg) plus
lenvatinib (12 mg or 8 mg, according to the weight of patient), and
for these patients the ORR was 79.2%.
Sintilimab plus IBI305
The ORIENT 32 study (NCT03794440) is an open-label,
multi-centre trial in China in which patients are randomized to
receive a combination of sintilimab and recombinant anti-VEGF
humanized monoclonal antibody (IBI305) vs. sorafenib (23). However, the results have not yet been
published.
The role of CTLA-4 in immune tolerance and
immune escape of HCC
CTLA-4 is a type of transmembrane receptor on T
cells that is primarily expressed on the surface of activated T
cells (42). The activation of T
lymphocytes requires the activation of two signaling pathways,
including the binding of the T cell receptor (TCR) and MHC-peptide
complex, which is presented by APCs and the B7 molecule to the
costimulatory molecule CD28 on the surface of T cells (17). CTLA-4, which always shares the B7
molecular ligand of the APC with CD28, is highly expressed in Tregs
and activated T lymphocytes, inducing the unresponsiveness of T
cells to negatively regulate the immune response (19). The overexpression of CTLA-4 in HCC
leads to uncontrolled growth of the tumor (64). CTLA-4 inhibitors can block the
activation of the CTLA-4 pathway, enhance the activation and
proliferation of T cells and then lower Treg-mediated
immunosuppression (17). The
treatment principle concerning the CTLA-4 signaling pathway is
shown in Fig. 2.
Applications of CTLA-4 inhibitors in
HCC
Stimulated by the TCR, CTLA-4 can be jointly
employed with B7 molecules to produce inhibition signals, thus
inhibiting the activation of T cells and then decreasing their
ability to recognize tumor antigens. This promotes the occurrence
and development of tumors (64).
CTLA-4 inhibitors restore the immune activity of T cells by
blocking the binding of CTLA-4 to B7 molecules (17,21,64), and
inhibitors include tremelimumab and ipilimumab.
Tremelimumab is a type of human immunoglobulin G 2
monoclonal antibody that blocks the signaling pathway involving
CTLA-4 (65). Sangro et al
reported a small-sample phase II clinical trial of tremelimumab in
2013 (NCT01008358), in which 21 patients with HCV-associated HCC
who received tremelimumab (15 mg/kg; every 90 days; maximum dose, 4
times) were recruited (65). There
were 17 patients who were incorporated into the evaluation. The
study demonstrated that tremelimumab has antitumor effects and
certain antiviral activity. In detail, the partial response (PR)
rate was 17.6% (3/17), and the DCR was 76.4% (13/17). Besides, the
mOS was 8.2 months, and the time to progression was 6.48 months.
Moreover, no serious adverse events were observed.
PD-1/PD-L1 inhibitors combined with
CTLA-4 inhibitors in HCC
Combination of nivolumab and
ipilimumab
Based on the CheckMate 040 study (NCT01658878)
(66), cohort 4 was used to explore
the safety and efficacy of the combination of nivolumab and
ipilimumab in patients with HCC previously treated with sorafenib
(67). This trial included 148
patients, ~88% of whom had vascular invasion or extrahepatic
metastasis and 91% of whom were diagnosed as having BCLC stage C
disease. According to the results, the ORR was 31% and the DCR was
49%. The OS of patients who received maintenance therapy (nivolumab
1 mg/kg, ipilimumab 3 mg/kg and sequential nivolumab therapy 240
mg) was 22.8 months. The safety profile analysis suggested that
this combination of double immunosuppressive agents was well
tolerated as there were relatively few grade 3/4
treatment-associated AEs, and novel AEs were observed in the
experimental group after the increase in ipilimumab dose. According
to the encouraging data of CheckMate 040 study, in March 2020, the
combination of nivolumab and ipilimumab was approved for patients
with HCC who previously treated with sorafenib by FDA (68).
Durvalumab monotherapy or combination
therapy with tremelimumab
In advance, the HIMALAYA study (NCT03298451)
(69), an open-label, multicenter
and randomized phase III study investigating durvalumab monotherapy
or combined therapy with tremelimumab vs. sorafenib in advanced
HCC, had planned to enroll 1,350 patients. In 2017, Kelley et
al (70) reported the early data
of 40 patients in a phase I trial investigating the combination of
durvalumab with tremelimumab in advanced HCC. Additionally, other
phase I/II trials (NCT03222076 and NCT03203304) have been designed
to evaluate the efficacy of a combination of immune checkpoint
blockers in advanced HCC (71–73).
Combination immunotherapy with locoregional
therapy in HCC
Similar to targeted drugs, local treatments can
mechanically reinforce the efficacy of ICIs by stimulating the
release of tumor-associated antigens from the tumor cells (29). In addition, the combination of
radiotherapy and chemotherapeutic agents is expected to increase
neoantigen release through DNA interference. This may lead to the
improved efficacy of ICIs, induce immunogenic cell death and
enhance the immune response by decreasing the number of
immunosuppressive cells, such as Tregs and myelogenous suppressor
cells (29,74).
Combination of TACE via drug-eluting
bead (DEB-)TACE and nivolumab
In 2018, the ASCO meeting reported a multicenter,
phase I trial of nivolumab with DEB-TACE in unresectable HCC that
aimed to evaluate the safety and tolerability of combined
therapies. The IMMUTACE study (NCT03572582) was a phase II trial in
Germany focusing on DEB-TACE and nivolumab (75).
Combination of TACE or RFA and
pembrolizumab
NCT03397654, a phase I/II study, was designed to
assess the safety and efficacy of the combination therapy of TACE
(using doxorubicin, 60 mg) plus pembrolizumab (200 mg, starting 30
or 45 days after TACE) that was repeatedly at three-week intervals
(73). The IMMULAB study
investigating the combination of local RFA plus pembrolizumab is
currently ongoing.
Combination of RFA and
tremelimumab
In 2017, Duffy et al (65) reported a study that totally enrolled
32 patients with HCC who received tremelimumab (two-dose level, 3.5
mg/kg and 10 mg/kg) every 4 weeks, and then followed by RFA. In
total, ~26% of patients achieved PR. In addition, the 6-month and
the 12-month PFS rates were 57.1 and 33.1%, respectively, whilst
the mOS was 12.3 months. Tables I
and II showed the current clinical
trials investigating ICIs in HCC.
 | Table I.Clinical trials investigating immune
checkpoint inhibitors monotherapy in hepatocellular carcinoma. |
Table I.
Clinical trials investigating immune
checkpoint inhibitors monotherapy in hepatocellular carcinoma.
| First author,
year | Therapy method | Target | Trial ID | Phase | Patient number | Lines of
Therapy | Endpoint | Status | (Refs.) |
|---|
| El-Khoueiry et
al, 2017 | Nivolumab (cohort
1) | PD-1 | NCT01658878 | I/II | 48 |
First/second-line | DLT/MTD | Completed | (46) |
| El-Khoueiry et
al, 2017 | Nivolumab (cohort
2) | PD-1 | NCT01658878 | I/II | 214 |
First/second-line | ORR | Completed | (46) |
| El-Khoueiry et
al, 2017 | Nivolumab (cohort
3) | PD-1 | NCT01658878 | I/II | 200 | First-line | ORR | Completed | (46) |
| Sangro et
al, 2016 | Nivolumab vs.
sorafenib | PD-1 | NCT02576509 | III | 726 | First-line | OS | Completed | (48) |
|
|
|
| CheckMate 459 |
|
|
|
|
|
|
| Exposito et
al, 2019 | Nivolumab vs.
placebo | PD-1 | NCT03383458 | III | 530 | Adjuvant | PFS | Recruiting | (49) |
|
|
|
| CheckMate 9DX |
|
|
|
|
|
|
| Zhu et al,
2018 | Pembrolizumab | PD-1 | NCT02702414 | II | 104 | Second-line | ORR/DCR | Completed | (50) |
|
|
|
| KEYNOTE-224 |
|
|
|
|
|
|
| Finn et al,
2019 | Pembrolizumab vs.
placebo | PD-1 | NCT02702401 | III | 413 | Second-line | OS/PFS | Recruiting | (25) |
|
|
| KEYNOTE-240 |
|
|
|
|
|
|
| Hilmi et al,
2019 | Pembrolizumab | PD-1 | NCT03062358 | III | 450 | Second-line | OS | Recruiting | (72) |
|
|
|
| KETNOTE-394 |
|
|
|
|
|
|
| Gao et al,
2019 | Pembrolizumab | PD-1 | NCT03211416 | I/II | 27 | First-line | ORR | Recruiting | (90) |
| Qin et al,
2020 | Camrelizumab | PD-1 | NCT02989922 | II | 220 | Second-line | ORR | Completed | (53) |
| Sangro et
al, 2013 | Tremelimumab | CTLA-4 | NCT01008358 | II | 21 | First-line | DCR | Recruiting | (65) |
 | Table II.Clinical trials investigating immune
checkpoint inhibitors combination therapy in hepatocellular
carcinoma. |
Table II.
Clinical trials investigating immune
checkpoint inhibitors combination therapy in hepatocellular
carcinoma.
| Author, year | Therapy method | Trial ID | Phase | Patients
number | Lines of
therapy | Endpoint | Status | (Refs.) |
|---|
| Qin et al,
2018 | Camrelizumab +
Apatinib | NCT02942329 | I | 14 | Second-line | ORR | Recruiting | (52) |
| Pishvaian et
al, 2018 | Atezolizumab +
Bevacizumab | NCT02715531
(GO30140) | IB | 23 | Second-line | ORR/DCR | Recruiting | (56) |
| Qin et al,
2020 | Atezolizumab +
Bevacizumab | NCT03434379 | III | 480 | First-line | OS/PFS | Recruiting | (58) |
| Llovet et
al, 2019 | Pembrolizumab +
Lenvatinib | NCT03006926 | I | 104 | Second-line | DLT/AEs | Recruiting | (62) |
| Yau et al,
2019 | Nivolumab +
Ipilimumab | NCT01658878 (cohort
4) | I/II | 148 | Second-line | AEs | Recruiting | (66) |
| Abou-Alfa et
al, 2018 | Durvalumab +
Tremelimumab | NCT03298451
(HIMALAYA) | III | 1,350 | First-line | OS | Recruiting | (69) |
Current concerns
At present, the concerns pertaining to HCC therapy
are as following.
Response of potential immunotherapy
biomarkers in HCC
According to the CheckMate 040 trial, it was
reported that patients with HCC can benefit from nivolumab
regardless of a history of hepatitis or PD-L1 expression in tumor
cells. There is no significant difference in the efficacy between
Asian patients and global patients, such as the OS and PFS trends
(46,47). Therefore, in contrast to the previous
hypothesis that PD-L1 was a predictive biomarker (76), it is not necessary to detect PD-L1
expression in tumor tissue when choosing nivolumab as a second-line
treatment in HCC (46). Previous
studies (REACH and REACH-2 study) have indicated that the baseline
level of AFP is associated with the efficacy of ramucirumab
(CYRAMZA trial) (77,78). However, the CheckMate 040 and the
KEYNOTE-224 studies reported that the efficacy of nivolumab and
pembrolizumab cannot be predicted, suggesting that there are no
effective biomarkers to predict the treatment response of PD-1
inhibitors in HCC (50,66). At present, the primary immunotherapy
biomarker factors undergoing further research are microsatellite
stability, tumor mutation load, immune cell status (CD4+ or CD8+ T
cells) (29,79), immunosuppressive receptors (TIM-3 and
LAG-3) (29,80) and immunosuppressive enzymes
(Indoleamine 2,3-dioxygenase, adenosine pathway) (79). Therefore, specific biomarkers that
can be used to predict clinical efficacy of PD-1 inhibitors are
needed.
The loss of mismatch repair (MMR) genes can
typically cause the accumulation of mismatch in the process of DNA
replication, leading to the occurrence of microsatellite
instability (MSI) (81). MSI is
common in gastrointestinal tumors such as gastric adenocarcinoma
(in 15–20% of cases) and colorectal adenocarcinoma (in 12–15% of
cases), especially in hereditary nonpolyposis hereditary colorectal
cancer or Lynch syndrome, which normally characterized by deficient
MMR and/or microsatellite instability-high (MSI-H) (82). As previous studies have reported,
patients with MSI-H treated with immunotherapies always show a
higher response rate and improved efficacy compared with those
patients without MSI-H. Therefore, several immunotherapeutic
agents, such as pembrolizumab, have been approved to treat solid
tumors including HCC (83). However,
the incidence of MSI-H in HCC is ~2% (84–86).
Also, the response rate of patients with HCC to pembrolizumab is
quite low; 2–2.4% (84,86). Moreover, the association between MSI
and immune checkpoints are unclear and thus need further
investigating.
Evaluation standard of immunotherapy
response
Unlike traditional chemotherapy or radiotherapy,
considering the delay of therapeutic benefits and durable
long-lasting effects of immunotherapy due to the initial
recruitment of activated T cells to the tumor site before the
initiation of antitumor activity, the assessment standard can be
modified and optimized through short-term and long-term efficacy
evaluation based on the RECIST guidelines. This enables
investigators to accurately track and grasp the change of tumor
during therapy (39,87). In addition, prior studies
investigating the application of ICIs in HCC mainly addressed their
use as second-line therapy (50,53,72).
Hence, the use of PD-1 agents as first-line treatment should be
addressed in future clinical trials.
Conclusions
In the past 10 years, sorafenib has been the only
targeted drug approved by the FDA as a first-line treatment agent
for advanced HCC (7). The overall
prognosis of HCC remains quite poor (15). Despite the gradual emergence of TKIs,
including regorafenib and lenvatinib (12–14), or
local interventional therapies (9–11),
substantial therapeutic advances in HCC are still lacking.
Therefore, the discovery of immunotherapy, especially ICIs,
provides new avenues for the comprehensive and systemic treatment
of advanced HCC. Mechanistically, TKIs affect antigen presentation
and the microenvironment, thereby enhancing or dampening the immune
response by stimulating the release of tumor-associated antigens
(7). Similarly, anti-angiogenesis
drugs, such as bevacizumab, can also inhibit tumor growth by
reducing the blood supply of tumor (12–14).
Thus, TKIs or anti-angiogenesis agents can be synergistic with
immunotherapeutic drugs. In addition, there are the potential
benefits of the synergistic effects of TACE or RFA combined with
immunotherapy (9–11). Therefore, combination treatments, not
just limited to immunotherapy agents but also TKIs,
anti-angiogenesis drugs and locoregional therapies, appears to be a
novel and promising strategy.
Several of the latest published datasets presented
in the ASCO meeting, although discouraging, indicated that the
inclusion of ICIs in combination therapies rendered them relatively
more effective, especially in terms of OS and PFS time (25,55,66). For
example, the negative results in KEYNOTE-240 may be due to the
following reasons. First of all, there was an inappropriate study
design related to this trial. For example, ~47.4% of the placebo
group subsequently received antitumor treatments, and ~10.4% of
them received PD-1/PD-L1 inhibitor treatments, which may have
affected the final results (the P-value did not reach statistical
significance). Hence, the effect of immunotherapy on placebo group
cannot be ignored. In addition, the survival data for the placebo
group were superior to those in other trials owing to the strict
enrollment selection; that is, this study excluded numerous
patients with macrovascular invasion that was regarded as one risk
factor of HCC prognosis. Furthermore, the OS and the PFS were both
set as endpoints resulting in higher requirements for achieving
significant results. Besides, this study did not recruit Chinese
patients who were the population with a high incidence of HCC;
however, another study, KEYNOTE-394, is recruiting Chinese
patients. Similar to KEYNOTE-240, CheckMate 459 also presented the
negative results, mainly in the OS and the PFS which failed to
reach the statistical difference (HR=0.85; P=0.0752). However, the
nivolumab group showed notably prolonged OS time compared with the
sorafenib group (16.4 vs. 14.7 months, P=0.0752). Also, this study
suggested that the response to nivolumab was associated with PD-L1
expression. In particular, those with PD-L1 ≥1% had higher response
rate compared with those with PD-L1 <1%. Therefore, PD-L1 may be
a predictive biomarker for the response to nivolumab treatment.
Further phase III trials should be conducted in the
future to investigate the efficacy of combination therapies.
Additional research is also needed to determine biomarkers to
predict clinical efficacy. Similarly, the concern of selecting
suitable patients should be addressed, such as the PD-L1 status. It
is speculated that patients with PD-L1 (+) may be benefit from
immunotherapies. Owing to the delayed effects of immunotherapies,
the evaluation standards should take into account as well.
Additionally, HCC typically accompanies chronic HBV
or HCV infection, especially in Asian countries, such as China
(88). In 2019, Fisicaro et
al reported that in patients with chronic hepatitis B
infection, numbers of specific T cells are low and these cells are
easily exhausted (89). Thus, these
T cells, such as CD8+ T cells, always show a suppressive effect,
which is due to environmental changes triggered by inflammation and
dysregulation of immune receptor expression, including upregulation
of multiple co-inhibitory receptors (89). Controlling the infection via
anti-HBV/anti-HCV treatment would spontaneously induce an extensive
and powerful response of antigen-specific T cells. Also, in
patients HCC with HBV or HCV infection, the PD-1/PD-L1 signaling
pathway can cause similar deactivation of specific CD8+ cells
(89). Therefore, specifically
blocking the PD-1/PD-L1 or B7-CTLA-4 signaling pathway via ICIs
theoretically can restore the activity of T cells, therefore this
may be helpful in controlling the virus and tumor progression in
these patients. Therefore, patients with HBC/HVC-associated HCC may
have an improved response to immunotherapies compared to those
without hepatitis virus infection. However, the application of ICIs
in hepatitis virus infection is quite limited (5,89). This
is possibly associated with the poor selectivity of ICIs and the
liver damage mediated by the suppression of normal liver tissue
function via co-inhibitory pathways; therefore, one potential
solution is to silence the inhibitory paths and to restore the
activity and function of virus-specific T cells such as CD8+ T
cells. In the future, more research is needed to investigate
virus-associated and non-viral HCC.
Overall, using ICIs as the first-line treatment or
combined with other therpies such as local regional methods and
targeted agents in future trial is a noteworthy point. Also,
seeking potential biomarkers contributes to predicting the
therapeutic effect and filtering suitable participants for
immunotherapies. In conclusion, immunotherapy in HCC is indeed
promising but challenging.
Acknowledgements
The authors would like to thank Dr FangYun Yang
(Department of Abdominal Oncology, Cancer Center, West China
Hospital, West China Medical School, Sichuan University) and Dr
Biao Yang (Departments of Gastroenterology, West China Hospital,
West China Medical School, Sichuan University) for their
support.
Funding
This study was sponsored by the Department of
Science & Technology of Sichuan Province of China (2017SZ0014)
to LZY.
Availability of data and materials
Not applicable.
Authors' contributions
ZYL contributed to the conception and idea of this
article. ZZ and BY contributed to the literature search. ZZ wrote
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
ICIs
|
immune checkpoint inhibitors
|
|
PD-1
|
programmed death receptor 1
|
|
PD-L1
|
programmed death ligand 1
|
|
CTLA-4
|
cytotoxic T lymphocyte-associated
protein 4
|
|
HBV
|
hepatitis B virus
|
|
HCV
|
hepatitis C virus
|
|
BCLC
|
Barcelona Clinic Liver Cancer
|
|
RFA
|
radiofrequency ablation
|
|
TACE
|
transarterial chemotherapy
|
|
TKIs
|
tyrosine protein kinase
|
|
FDA
|
Food and Drug Administration
|
|
TGF-β
|
transforming growth factor-β
|
|
TCR
|
T cell antigen receptor
|
|
ASCO
|
American Society of Clinical
Oncology
|
|
ESMO
|
European Society of Oncology
|
|
CSCO
|
Chinese Society of Clinical
Oncology
|
|
ORR
|
objective response rate
|
|
mOS
|
median overall survival
|
|
SD
|
stable disease
|
|
PR
|
partial response
|
|
DCR
|
disease control rate
|
|
PFS
|
progression free survival
|
|
AEs
|
adverse events
|
|
RCREP
|
reactive capillary epithelial
proliferation
|
|
CR
|
Complete response
|
|
VEGF
|
vascular endothelial growth
factor
|
|
DEB-TACE
|
drug-eluting bead transarterial
chemoembolization
|
|
MMR
|
mismatch repair
|
|
MSI
|
microsatellite instability
|
References
|
1
|
McGlynn KA, Petrick JL and London WT:
Global Epidemiology of Hepatocellular Carcinoma: An emphasis on
demographic and regional variability. Clin Liver Dis. 19:223–238.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller KD, Nogueira L, Mariotto AB,
Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL and Siegel
RL: Cancer treatment and survivorship statistics, 2019. CA Cancer J
Clin. 69:363–385. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zeng H, Chen W, Zheng R, Zhang S, Ji JS,
Zou X, Xia C, Sun K, Yang Z, Li H, et al: Changing cancer survival
in China during 2003–15: A pooled analysis of 17 population-based
cancer registries. Lancet Glob Health. 6:e555–e567. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kanwal F, Kramer J, Asch SM, Chayanupatkul
M, Cao Y and El-Serag HB: Risk of hepatocellular cancer in HCV
patients treated with direct-acting antiviral agents.
Gastroenterology. 153:996–1005.e1. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tan AT, Yang N, Lee Krishnamoorthy T, Oei
V, Chua A, Zhao X, Tan HS, Chia A, Le Bert N, Low D, et al: Use of
expression profiles of HBV-DNA integrated into genomes of
hepatocellular carcinoma cells to select T cells for immunotherapy.
Gastroenterology. 156:1862–1876.e9. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vogel A, Cervantes A, Chau I, Daniele B,
Llovet JM, Meyer T, Nault JC, Neumann U, Ricke J, Sangro B, et al:
Hepatocellular carcinoma: ESMO clinical practice guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 30:871–873. 2019.
View Article : Google Scholar
|
|
7
|
Benson AB III, D'Angelica MI, Abbott DE,
Abrams TA, Alberts SR, Saenz DA, Are C, Brown DB, Chang DT, Covey
AM, et al: NCCN guidelines insights: Hepatobiliary cancers, version
1.2017. J Natl Compr Canc Netw. 15:563–573. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bruix J, Reig M and Sherman M:
Evidence-based diagnosis, staging, and treatment of patients with
hepatocellular carcinoma. Gastroenterology. 150:835–853. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kudo M, Ueshima K, Ikeda M, Torimura T,
Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, et al:
Randomised, multicentre prospective trial of transarterial
chemoembolisation (TACE) plus sorafenib as compared with TACE alone
in patients with hepatocellular carcinoma: TACTICS trial. Gut.
69:1492–1501. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cui J, Wang N, Zhao H, Jin H, Wang G, Niu
C, Terunuma H, He H and Li W: Combination of radiofrequency
ablation and sequential cellular immunotherapy improves
progression-free survival for patients with hepatocellular
carcinoma. Int J Cancer. 134:342–351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lencioni R, Llovet JM, Han G, Tak WY, Yang
J, Guglielmi A, Paik SW, Reig M, Kim DY, Chau GY, et al: Sorafenib
or placebo plus TACE with doxorubicin-eluting beads for
intermediate stage HCC: The SPACE trial. J Hepatol. 64:1090–1098.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abou-Alfa GK, Meyer T, Cheng AL,
El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park
JW, et al: Cabozantinib in patients with advanced and progressing
hepatocellular carcinoma. N Engl J Med. 379:54–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bruix J, Qin S, Merle P, Granito A, Huang
YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al:
Regorafenib for patients with hepatocellular carcinoma who
progressed on sorafenib treatment (RESORCE): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet. 389:56–66.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Greten TF, Lai CW, Li G and
Staveley-O'Carroll KF: Targeted and immune-based therapies for
hepatocellular carcinoma. Gastroenterology. 156:510–524. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maur M, Tomasello C, Frassoldati A, Dieci
MV, Barbieri E and Conte P: Posterior reversible encephalopathy
syndrome during ipilimumab therapy for malignant melanoma. J Clin
Oncol. 30:e76–e78. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Buchbinder EI and Desai A: CTLA-4 and PD-1
Pathways: Similarities, differences, and implications of their
inhibition. Am J Clin Oncol. 39:98–106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sprinzl MF and Galle PR: Current progress
in immunotherapy of hepatocellular carcinoma. J Hepatol.
66:482–484. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prieto J, Melero I and Sangro B:
Immunological landscape and immunotherapy of hepatocellular
carcinoma. Nat Rev Gastroenterol Hepatol. 12:681–700. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang L and Wang FS: Clinical immunology
and immunotherapy for hepatocellular carcinoma: Current progress
and challenges. Hepatol Int. 13:521–533. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kudo M: Immuno-oncology in hepatocellular
carcinoma: 2017 Update. Oncology. 93 (Suppl 1):S147–S159. 2017.
View Article : Google Scholar
|
|
22
|
Waidmann O: Recent developments with
immunotherapy for hepatocellular carcinoma. Expert Opin Biol Ther.
18:905–910. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Johnston MP and Khakoo SI: Immunotherapy
for hepatocellular carcinoma: Current and future. World J
Gastroenterol. 25:2977–2989. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tian M, Shi Y, Liu W and Fan J:
Immunotherapy of hepatocellular carcinoma: Strategies for
combinatorial intervention. Sci China Life Sci. 62:1138–1143. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Finn RS, Ryoo BY, Merle P, Kudo M,
Bouattour M, Lim HY, Breder VV, Edeline J, Chao Y, Ogasawara S, et
al: Results of KEYNOTE-240: Phase 3 study of pembrolizumab (Pembro)
vs best supportive care (BSC) for second line therapy in advanced
hepatocellular carcinoma (HCC). J Clin Oncol. 37 (15
Suppl):S40042019. View Article : Google Scholar
|
|
26
|
Eggert T and Greten TF: Tumor regulation
of the tissue environment in the liver. Pharmacol Ther. 173:47–57.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nishida N and Kudo M: Immunological
microenvironment of hepatocellular carcinoma and its clinical
implication. Oncology. 92 (Suppl 1):S40–S49. 2017. View Article : Google Scholar
|
|
28
|
Tiegs G and Lohse AW: Immune tolerance:
What is unique about the liver. J Autoimmun. 34:1–6. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huz JI, Melis M and Sarpel U: Spontaneous
regression of hepatocellular carcinoma is most often associated
with tumour hypoxia or a systemic inflammatory response. HPB
(Oxford). 14:500–505. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Crispe IN: Liver antigen-presenting cells.
J Hepatol. 54:357–365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang Y, Han QJ and Zhang J:
Hepatocellular carcinoma: Mechanisms of progression and
immunotherapy. World J Gastroenterol. 25:3151–3167. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Josefowicz SZ, Lu LF and Rudensky AY:
Regulatory T cells: Mechanisms of differentiation and function.
Annu Rev Immunol. 30:531–564. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gong J, Chehrazi-Raffle A, Reddi S and
Salgia R: Development of PD-1 and PD-L1 inhibitors as a form of
cancer immunotherapy: A comprehensive review of registration trials
and future considerations. J Immunother Cancer. 6:82018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zou W, Wolchok JD and Chen L: PD-L1
(B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms,
response biomarkers, and combinations. Sci Transl Med.
8:328rv42016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Calderaro J, Rousseau B, Amaddeo G, Mercey
M, Charpy C, Costentin C, Luciani A, Zafrani ES, Laurent A, Azoulay
D, et al: Programmed death ligand 1 expression in hepatocellular
carcinoma: Relationship with clinical and pathological features.
Hepatology. 64:2038–2046. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wei F, Zhong S, Ma Z, Kong H, Medvec A,
Ahmed R, Freeman GJ, Krogsgaard M and Riley JL: Strength of PD-1
signaling differentially affects T-cell effector functions. Proc
Natl Acad Sci USA. 110:E2480–E2489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
El Dika I, Khalil DN and Abou-Alfa GK:
Immune checkpoint inhibitors for hepatocellular carcinoma. Cancer.
125:3312–3319. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Noonan A and Pawlik TM: Hepatocellular
carcinoma: An update on investigational drugs in phase I and II
clinical trials. Expert Opin Investig Drugs. 28:941–949. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mizukoshi E and Kaneko S: Immune cell
therapy for hepatocellular carcinoma. J Hematol Oncol. 12:522019.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liang SC, Latchman YE, Buhlmann JE,
Tomczak MF, Horwitz BH, Freeman GJ and Sharpe AH: Regulation of
PD-1, PD-L1, and PD-L2 expression during normal and autoimmune
responses. Eur J Immunol. 33:2706–2716. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang S, Bajorath J, Flies DB, Dong H,
Honjo T and Chen L: Molecular modeling and functional mapping of
B7-H1 and B7-DC uncouple costimulatory function from PD-1
interaction. J Exp Med. 197:1083–1091. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Elhag OA, Hu XJ, Wen-Ying Z, Li X, Yuan
YZ, Deng LF, Liu DL, Liu YL and Hui G: Reconstructed
adeno-associated virus with the extracellular domain of murine PD-1
induces antitumor immunity. Asian Pac J Cancer Prev. 13:4031–4036.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Intlekofer AM and Thompson CB: At the
bench: Preclinical rationale for CTLA-4 and PD-1 blockade as cancer
immunotherapy. J Leukoc Biol. 94:25–39. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Taube JM, Klein A, Brahmer JR, Xu H, Pan
X, Kim JH, Chen L, Pardoll DM, Topalian SL and Anders RA:
Association of PD-1, PD-1 ligands, and other features of the tumor
immune microenvironment with response to anti-PD-1 therapy. Clin
Cancer Res. 20:5064–5074. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Benson AB 3rd, D'Angelica MI, Abbott DE,
et al: NCCN Guidelines Insights: Hepatobiliary Cancers, Version
1.2017. J Natl Compr Canc Netw. 15:563–573. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
El-Khoueiry AB, Sangro B, Yau T, Crocenzi
TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, et al:
Nivolumab in patients with advanced hepatocellular carcinoma
(CheckMate 040): An open-label, non-comparative, phase 1/2 dose
escalation and expansion trial. Lancet. 389:2492–2502. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yau T, Hsu C, Kim TY, Choo SP, Kang YK,
Hou MM, Numata K, Yeo W, Chopra A, Ikeda M, et al: Nivolumab in
advanced hepatocellular carcinoma: Sorafenib-experienced Asian
cohort analysis. J Hepatol. 71:543–552. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sangro B, Park JW, Cruz CMD, Anderson J,
Lang L, Neely J, Shaw JW and Cheng AL: A randomized, multicenter,
phase 3 study of nivolumab vs sorafenib as first-line treatment in
patients (pts) with advanced hepatocellular carcinoma (HCC):
CheckMate-459. J Clin Oncol. 34 (15 Suppl):TPS41472016. View Article : Google Scholar
|
|
49
|
Exposito MJ, Akce M, Alvarez J, Assenat E,
Balart L, Baron A, Decaens T, Heurgue-Berlot A, Martin A, Paik S,
et al: Abstract No. 526 CheckMate-9DX: Phase 3, randomized,
double-blind study of adjuvant nivolumab vs placebo for patients
with hepatocellular carcinoma (hcc) at high risk of recurrence
after curative resection or ablation. J Vasc Interv Radiology.
30:S227–S228. 2019. View Article : Google Scholar
|
|
50
|
Zhu AX, Finn RS, Edeline J, Cattan S,
Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A,
et al: Pembrolizumab in patients with advanced hepatocellular
carcinoma previously treated with sorafenib (KEYNOTE-224): A
non-randomised, open-label phase 2 trial. Lancet Oncol. 19:940–952.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Finn RS, Chan SL, Zhu AX, Knox JJ, Cheng
AL, Siegel AB, Bautista O and Kudo M: Phase 3, randomized study of
pembrolizumab (pembro) vs best supportive care (BSC) for
second-line advanced hepatocellular carcinoma (HCC): KEYNOTE-240. J
Clin Oncol. 35 (15 Suppl):TPS41432017. View Article : Google Scholar
|
|
52
|
Qin SK, Ren ZG, Meng ZQ, Chen ZD, Chai XL,
Xiong JP, Bai YX, Yang L, Zhu H, Fang WJ, et al: LBA27A randomized
multicentered phase II study to evaluate SHR-1210 (PD-1 antibody)
in subjects with advanced hepatocellular carcinoma (HCC) who failed
or intolerable to prior systemic treatment. Ann Oncol. 29 (Suppl
8):mdy424.029. 2018. View Article : Google Scholar
|
|
53
|
Qin SK, Ren Z, Meng Z, Chen Z, Chai X,
Xiong J, Bai Y, Yang L, Zhu H, Fang W, et al: Camrelizumab in
patients with previously treated advanced hepatocellular carcinoma:
A multicentre, open-label, parallel-group, randomised, phase 2
trial. Lancet Oncol. 21:571–580. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xu JM, Zhang Y, Jia R, Wang Y, Liu R,
Zhang G, Zhao C, Zhang Y, Zou J and Wang Q: Anti-programmed death-1
antibody SHR-1210 (S) combined with apatinib (A) for advanced
hepatocellular carcinoma (HCC), gastric cancer (GC) or
esophagogastric junction (EGJ) cancer refractory to standard
therapy: A phase 1 trial. J Clin Oncol. 36 (15 Suppl):S40752018.
View Article : Google Scholar
|
|
55
|
Qin S, Chen Z, Liu Y, Xiong J, Ren Z, Meng
Z, Gu S, Wang L, Zou J; Jinling Hospital, ; et al: A phase II study
of anti-PD-1 antibody camrelizumab plus FOLFOX4 or GEMOX systemic
chemotherapy as first-line therapy for advanced hepatocellular
carcinoma or biliary tract cancer. J Clin Oncol. 37 (15
Suppl):S40742019. View Article : Google Scholar
|
|
56
|
Pishvaian MJ, Lee M, Ryoo BY, Stein S, Lee
KH, Stein W, Spahn J, Shao H, Liu B and Iizuka K: LBA26Updated
safety and clinical activity results from a phase Ib study of
atezolizumab + bevacizumab in hepatocellular carcinoma (HCC). Ann
Oncol. 29 (Suppl 8):viii718–viii719. 2018. View Article : Google Scholar
|
|
57
|
Cheng AL, Qin S, Ikeda M, Galle P, Ducreux
M, Zhu A, Kim TY, Merle P, Kaseb A, Li D, et al: IMbrave150:
Efficacy and safety results from a ph III study evaluating
atezolizumab (atezo)+ bevacizumab (bev) vs sorafenib (Sor) as first
treatment (tx) for patients (pts) with unresectable hepatocellular
carcinoma (HCC). Ann Oncol. 30:ix186–ix187. 2019. View Article : Google Scholar
|
|
58
|
Qin SK, Ren ZG, Feng Y, et al: Efficacy
and safety of atezolizumab + bevacizumab vs sorafenib in Chinese
patients with unresectable HCC in the phase III IMbrave150 study.
EASL Liver Cancer Summit 2020. OP02-03. Ann Oncol. 30:v8752020.
|
|
59
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ikeda M, Sung MW, Kudo M, Kobayashi M,
Baron AD, Finn RS, Kaneko S, Kraljevic S, Ishikawa K, Siegel AB, et
al: A phase 1b trial of lenvatinib (LEN) plus pembrolizumab (PEM)
in patients (pts) with unresectable hepatocellular carcinoma
(uHCC). J Clin Oncol. 36 (15 Suppl):S40762018. View Article : Google Scholar
|
|
61
|
Jeon MY, Lee HW, Kim BK, Park JY, Kim DY,
Ahn SH, Han KH, Baek SE, Kim HS, Kim SU and Park MS:
Reproducibility of European Association for the Study of the liver
criteria and modified response evaluation criteria in solid tumors
in patients treated with sorafenib. Liver Int. 38:1655–1663. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Llovet J, Shepard KV, Finn RS, Ikeda M,
Sung M, Baron DA, Kudo M, Okusaka T, Kobayashi M, Kumada H, et al:
A phase Ib trial of lenvatinib (LEN) plus pembrolizumab (PEMBRO) in
unresectable hepatocellular carcinoma (uHCC): Updated results. Ann
Oncol. 30:v286–v287. 2019. View Article : Google Scholar
|
|
63
|
Kudo M, Ikeda M, Motomura K, Okusaka T,
Kato N, Dutcus CE, Hisai T, Suzuki M, Ikezawa H, Iwata T, et al: A
phase 1b study of lenvatinib plus nivolumab in patients with
unresectable hepatocellular carcinoma (Study 117). J Clin Oncol. 38
(4 Suppl):S5132020. View Article : Google Scholar
|
|
64
|
Agdashian D, ElGindi M, Xie C, Sandhu M,
Pratt D, Kleiner DE, Figg WD, Rytlewski JA, Sanders C, Yusko EC, et
al: The effect of anti-CTLA4 treatment on peripheral and
intra-tumoral T cells in patients with hepatocellular carcinoma.
Cancer Immunol Immunother. 68:599–608. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sangro B, Gomez-Martin C, de la Mata M,
Iñarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, Larrea E,
Alfaro C, Sarobe P, et al: A clinical trial of CTLA-4 blockade with
tremelimumab in patients with hepatocellular carcinoma and chronic
hepatitis C. J Hepatol. 59:81–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yau T, Kang YK, Kim TY, El-Khoueiry AB,
Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, et al:
Nivolumab (NIVO) + ipilimumab (IPI) combination therapy in patients
(pts) with advanced hepatocellular carcinoma (aHCC): Results from
CheckMate 040. J Clin Oncol. 37 (15 Suppl):S40122019. View Article : Google Scholar
|
|
67
|
Finkelmeier F, Waidmann O and Trojan J:
Nivolumab for the treatment of hepatocellular carcinoma. Expert Rev
Anticancer Ther. 18:1169–1175. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Opdivo Prescribing Information. Opdivo
U.S. Product Information. Last updated. March. 2020, Princeton, NJ:
Bristol Myers Squibb Company;
|
|
69
|
Abou-Alfa GK, Chan SL, Furuse J, Galle PR,
Kelley RK, Qin S, Armstrong J, Darilay A, Vlahovic G, Negro A and
Sangro B: A randomized, multicenter phase 3 study of durvalumab (D)
and tremelimumab (T) as first-line treatment in patients with
unresectable hepatocellular carcinoma (HCC): HIMALAYA study. J Clin
Oncol. 36 (15 Suppl):TPS41442018. View Article : Google Scholar
|
|
70
|
Kelley RK, Abou-Alfa GK, Bendell JC, Kim
TY, Borad MJ, Yong WP, Morse M, Kang YK, Rebelatto M, Makowsky M,
et al: Phase I/II study of durvalumab and tremelimumab in patients
with unresectable hepatocellular carcinoma (HCC): Phase I safety
and efficacy analyses. J Clin Oncol. 35 (15 Suppl):S40732017.
View Article : Google Scholar
|
|
71
|
Choi C, Yoo GS, Cho WK and Park HC:
Optimizing radiotherapy with immune checkpoint blockade in
hepatocellular carcinoma. World J Gastroenterol. 25:2416–2429.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hilmi M, Neuzillet C, Calderaro J, Lafdil
F, Pawlotsky JM and Rousseau B: Angiogenesis and immune checkpoint
inhibitors as therapies for hepatocellular carcinoma: current
knowledge and future research directions. J Immunother Cancer.
7:3332019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Xu W, Liu K, Chen M, Sun JY, McCaughan GW,
Lu XJ and Ji J: Immunotherapy for hepatocellular carcinoma: Recent
advances and future perspectives. Ther Adv Med Oncol.
11:17588359198626922019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Loosen SH, Schulze-Hagen M, Bruners P,
Tacke F, Trautwein C, Kuhl C, Luedde T and Roderburg C: Sarcopenia
is a negative prognostic factor in patients undergoing
transarterial chemoembolization (TACE) for hepatic malignancies.
Cancers (Basel). 11:15032019. View Article : Google Scholar
|
|
75
|
Harding JJ, Erinjeri JP, Tan BR, Reiss KA,
Mody K, Khalil D, Yarmohammadi H, Nadolski G, Giardina JD, Capanu
M, et al: A multicenter pilot study of nivolumab (NIVO) with drug
eluting bead transarterial chemoembolization (deb-TACE) in patients
(pts) with liver limited hepatocellular carcinoma (HCC). J Clin
Oncol. 36 (15 Suppl):TPS41462018. View Article : Google Scholar
|
|
76
|
Chen CL, Pan QZ, Zhao JJ, Wang Y, Li YQ,
Wang QJ, Pan K, Weng DS, Jiang SS, Tang Y, et al: PD-L1 expression
as a predictive biomarker for cytokine-induced killer cell
immunotherapy in patients with hepatocellular carcinoma.
Oncoimmunology. 5:e11766532016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhu AX, Kang YK, Yen CJ, Finn RS, Galle
PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, et al:
Ramucirumab after sorafenib in patients with advanced
hepatocellular carcinoma and increased α-fetoprotein concentrations
(REACH-2): A randomised, double-blind, placebo-controlled, phase 3
trial. Lancet Oncol. 20:282–296. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhu AX, Park JO, Ryoo BY, Yen CJ, Poon R,
Pastorelli D, Blanc JF, Chung HC, Baron AD, Pfiffer TE, et al:
Ramucirumab versus placebo as second-line treatment in patients
with advanced hepatocellular carcinoma following first-line therapy
with sorafenib (REACH): A randomised, double-blind, multicentre,
phase 3 trial. Lancet Oncol. 16:859–870. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Sideras K, Biermann K, Verheij J,
Takkenberg BR, Mancham S, Hansen BE, Schutz HM, de Man RA,
Sprengers D, Buschow SI, et al: PD-L1, Galectin-9 and CD8+
tumor-infiltrating lymphocytes are associated with survival in
hepatocellular carcinoma. Oncoimmunology. 6:e12733092017.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X,
Liu J, Shi L, Liu C, Wang G and Zou W: Tim-3/galectin-9 signaling
pathway mediates T-cell dysfunction and predicts poor prognosis in
patients with hepatitis B virus-associated hepatocellular
carcinoma. Hepatology. 56:1342–1351. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Eso Y, Shimizu T, Takeda H, Takai A and
Marusawa H: Microsatellite instability and immune checkpoint
inhibitors: Toward precision medicine against gastrointestinal and
hepatobiliary cancers. J Gastroenterol. 55:15–26. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Latham A, Srinivasan P, Kemel Y, Shia J,
Bandlamudi C, Mandelker D, Middha S, Hechtman J, Zehir A,
Dubard-Gault M, et al: Microsatellite instability is associated
with the presence of lynch syndrome pan-cancer. J Clin Oncol.
37:286–295. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Le DT, Durham JN, Smith KN, Wang H,
Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et
al: Mismatch repair deficiency predicts response of solid tumors to
PD-1 blockade. Science. 357:409–413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kawaoka T, Ando Y, Yamauchi M, Suehiro Y,
Yamaoka K, Kosaka Y, Fuji Y, Uchikawa S, Morio K, Fujino H, et al:
Incidence of microsatellite instability-high hepatocellular
carcinoma among Japanese patients and response to pembrolizumab.
Hepatol Res. 50:885–888. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Goumard C, Desbois-Mouthon C, Wendum D,
Calmel C, Merabtene F, Scatton O and Praz F: Low levels of
microsatellite instability at simple repeated sequences commonly
occur in human hepatocellular carcinoma. Cancer Genomics
Proteomics. 14:329–339. 2017.PubMed/NCBI
|
|
86
|
Ando Y, Yamauchi M, Suehiro Y, Yamaoka K,
Kosaka Y, Fuji Y, Uchikawa S, Kodama K, Morio K, Fujino H, et al:
Complete response to pembrolizumab in advanced hepatocellular
carcinoma with microsatellite instability. Clin J Gastroenterol.
Feb 4–2020.(Online ahead of print). View Article : Google Scholar
|
|
87
|
Scheiner B, Kirstein MM, Hucke F,
Finkelmeier F, Schulze K, von Felden J, Koch S, Schwabl P, Hinrichs
JB, Waneck F, et al: Programmed cell death protein-1
(PD-1)-targeted immunotherapy in advanced hepatocellular carcinoma:
Efficacy and safety data from an international multicentre
real-world cohort. Aliment Pharmacol Ther. 49:1323–1333. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Boni C, Barili V, Acerbi G, Rossi M,
Vecchi A, Laccabue D, Penna A, Missale G, Ferrari C and Fisicaro P:
HBV immune-therapy: from molecular mechanisms to clinical
applications. Int J Mol Sci. 20:27542019. View Article : Google Scholar
|
|
90
|
Gao L, Yang X, Yi C and Zhu H: Adverse
events of concurrent immune checkpoint inhibitors and
antiangiogenic agents: A systematic review. Front Pharmacol.
10:11732019. View Article : Google Scholar : PubMed/NCBI
|