Introduction
Cutaneous melanoma (CM) is one of the most
aggressive types of skin cancer and mainly affects the Caucasian
population (1). Epidemiological data
collected across the world demonstrate a steady increase in the
incidence of melanoma in recent decades. In the USA, the incidence
of CM in the Caucasian subpopulation increased from 20.9 in 1996 to
31.5 in 2017 (2) (the incidence
rates reported are per 100,000 individuals per year). In Germany,
the incidence of CM grew from 10.3 in 1976 to 13.3 in 2003
(3). In Finland, from 1953–2003 the
incidence of CM changed from 1.5 to 12.8 (4). The incidence of melanoma in Latvia has
increased from 5.1 new melanoma cases per 100,000 inhabitants in
1998 to 7.8 in 2008 (5). Although
this increase is mainly attributed to the early detection of the
disease, the number of patients diagnosed with advanced melanoma
and with cancer exhibiting metastatic potential is also growing
worldwide (6,7), with ~30% of patients with primary
melanoma developing metastasis (8,9).
Metastatic melanoma has a poor prognosis, with the median survival
time ranging between 8 and 18 months following diagnosis depending
on the tumor stage (10,11). The efficacy of the treatment of
metastatic melanoma in recent years has increased following the
successful application of small molecular inhibitors that target
specific oncogenic mutations, for example those within the
BRAF gene (12,13). Studies have also suggested that the
development of immunotherapy with checkpoint inhibitors, which
involves using monoclonal antibodies to cytotoxic T lymphocyte
antigen-4 and programmed death-1 receptor/ligand, may aid in the
treatment of this disease (14–16).
However, the efficacy of these treatments is limited by the
development of resistance to targeted therapy (12,13).
Furthermore, durable response to immunotherapy is restricted only
to a subset of patients (14–16).
The previously reported risk factors for a poor
outcome of melanoma include high Breslow thickness, ulceration,
high mitotic rate, high levels of lactate dehydrogenase,
lymphovascular invasion and microscopic and clinical satellites
(17–22). The formation of metastasis has also
been associated with old age, the presence of a number of primary
tumor localizations and a history of skin cancer or other types of
cancer (23–25). In the aforementioned studies, the
majority of melanomas were thin and rarely exceeded a Breslow
thickness of 4.0 mm; by contrast, melanomas in Latvia have been
reported to exhibit a median thickness of 6.0 mm (5). This may be due to the delays in seeking
medical assistance, which allows for a unique patient cohort where
patients with metastasis are well represented and the tumors
exhibit diverse features. This cohort presents opportunities for
identifying additional features that may be associated with
melanoma metastasis. In addition, to the best of our knowledge, the
current study is the first systematic study of metastatic melanoma
in the Latvian population. The aim of the present study was to
analyze patient data and tumor characteristics in order to identify
a set of parameters that may aid in predicting the probability and
timing of the onset of CM metastasis.
Materials and methods
Design and data sources
In the present retrospective case-control analysis,
patients with metastasis (T stages IIIA-IV) served as the cases
(the first occurrence of metastasis for the primary tumor was
considered), whereas patients with melanoma who did not develop
metastasis during the study period were used as the controls (T
stages IA-IIC). Staging was determined according to the guidelines
by the American Joint Committee of Cancer, 8th Edition
(11). Patient inclusion criteria
were: Histologically confirmed melanoma with metastasis
(corresponding to the T stage groups IIIA-IV) for cases and
histologically confirmed melanoma without metastasis (corresponding
to the T stage groups IA-IIC) for controls. Patients with multiple
primary melanomas where it was not possible to identify a single
original melanoma were excluded from the study. The cases and
controls were selected from a cohort of 2,026 patients with CM that
were treated at the Riga East University Hospital Latvian Oncology
Centre (REUHLOC), which is the largest oncological hospital in
Latvia, between January 1998 and December 2015. Of all melanoma
cases in Latvia, ~80% were referred to REUHLOC during this time
period. Metastasis was defined as in-transit metastasis (manifested
before regional lymph nodes), metastasis with regional lymph node
involvement or distant metastasis. A total of 647 patients in the
cohort developed metastasis. Individuals for whom metastasis was
detected at the time of the diagnosis of primary tumor or <6
months after the initial diagnosis were excluded from the analysis
due to a high probability of exhibiting metastasis in a subclinical
form at the time of the diagnosis. Following this exclusion, 309
cases remained and were used for subsequent analysis. For each
case, one control initially diagnosed in the same year was
selected; the chosen control patient was required to have been
followed up at least until the time when metastasis had been
detected in the case patient and not to have died prior to the
diagnosis of metastases. Age and sex were also considered when
matching a case patient with a control: Patients were divided into
age groups spanning 20 years each, and each patient was matched
with a control from the same age group and of the same sex. When
several controls were available, one control was selected at
random. A total of 278 cases were successfully matched using all
the criteria, and the total number of individuals included in the
case-control study was 556. The variables examined for an
association with the risk of developing metastasis were body mass
index (BMI) and tumor characteristics, including CM subtype,
predominant cell type in the lesion, Breslow thickness, presence of
ulceration, pigment and anatomic localization of the tumor. Tumor
localizations were grouped into the following regions: Head and
neck, limbs, hands and feet, and trunk. Among the CM subtypes,
superficial spreading melanoma, nodular melanoma and lentigo
malignant melanoma were distinguished. Of all predominant cell
types, epithelial, spindle and mixed cell types were discerned.
Statistical analysis
The associations between potential risk factors
(categorical features) and the probability of developing metastasis
were assessed using a Pearson's χ2 test. The
distributions of risk factors for male and female patients were
compared in the same way for case and control groups. If the number
of patients in one of the subgroups was small, a Fisher's exact
test was used. If the association with a factor was significant and
the factor had ≥3 discrete categories, pairwise comparisons between
the categories were performed using Fisher's exact test. The
differences in Breslow thickness, age and BMI (continuous features)
were also assessed using the non-parametric Mann-Whitney test. For
continuous features the mean ± standard deviation (mean ± SD) was
reported. Both Pearson's χ2 tests and Fisher's exact
tests were carried out using R software version 3.6.3. (functions
‘chisq.test’ and ‘fisher.test’ were used, respectively) (26). Odds ratios were calculated using the
R function ‘oddsratio’. Mann-Whitney test P-values were obtained by
the ‘wilcox.test’ in R. P<0.05 was considered to indicate a
statistically significant difference.
Multivariate models were constructed using several
risk factors associated with the presence or absence of metastases.
Logistic regression models were used in all multivariate analyses.
Multivariate models were constructed using stepwise regression,
where the candidate variables were Breslow thickness, BMI (both
analyzed as continuous variables), ulceration, pigment and tumor
localization. Multivariate models included patients with no missing
data for the analyzed variables (n=272; 136 cases and controls
matched for sex and age). CM subtype and predominant cell type in
the lesion were not included in the present analysis due to
insignificant P-values in the univariate analysis (Pearson's
χ2 test or Fisher's exact test P-values were regarded as
appropriate for the number of observations; the test that involved
all categories of a feature was referred to) and missing
observations. Models were built using the function ‘stepAIC’ from
the R package ‘MASS’, starting with a model without any factors
(27).
Within the group of patients with metastasis
(n=309), time from initial diagnosis until the diagnosis of
metastasis was compared for a variety of subgroups. The features
that were previously considered for the inclusion in the
multivariate models were analyzed. A negative binomial regression
model was fitted for each risk factor. The R function ‘glm.nb’ was
used for computations.
Patient survival was assessed using the Cox
proportional hazards model. The significance of the association
between metastasis and survival was examined using the log-rank
test, which was applied to the model. The R function ‘coxph’ from
the package ‘survival’ was used for the analysis (28). Kaplan-Meier curves were analyzed
using the function ‘survfit’ and were visualized using the R
package ‘ggfortify’ (29,30). To compare the estimated survival of
the present study with already published data the Surveillance,
Epidemiology and End Results (SEER) database (2) was used for the Caucasian
population.
Results
Patient characteristics
The number of newly diagnosed cases of metastasis
was demonstrated to consistently increase over time in the study
cohort (Fig. 1A). A total of 647 out
of 2,026 patients (31.9%) were indicated to develop metastasis, and
219 (10.8%) patients presented with metastasis at the time of
diagnosis. A total of 428 (21.1%) patients developed metastasis
during the study period, and of these, 119 patients (5.9%)
developed metastases within the first 6 months following treatment
and were subsequently excluded from the case-control study.
Therefore, a total of 309 patients with melanoma (15.3%), for which
metastases were diagnosed at ≥6 months after the beginning of
treatment, were included in the analysis. Of these, 278 patients
were matched with controls by age and sex, including 164 female
(59.0%) and 114 male (41.0%) patients. The mean age was 61.8±14.9
years for female and 61.1±13.6 years for male patients
(Mann-Whitney test, P=0.340). The majority of the patients (n=192
or 66.7%) developed metastasis within the first two years following
surgery (Fig. 1B).
Risk factors associated with melanoma
metastasis
The distribution of risk factors within the case and
control groups, and the associations between clinicopathological
features and metastasis are presented in Table I. Thick melanomas were observed in
the case and control groups, although the mean tumor thickness at
diagnosis was higher in patients with metastasis compared with that
in patients without metastasis (5.21 vs. 4.02 mm, respectively;
Mann-Whitney test, P=0.012). The likelihood of developing
metastasis for patients with melanoma Breslow thickness >4.00 mm
and those with lower Breslow thickness was also compared, as 4.00
mm is the threshold used for separating patients with melanoma T
stages IA-IIC and stages IIIA-IV; the likelihood of metastasis was
significantly higher in patients with melanoma Breslow thickness
>4.00 mm [odds ratio (OR), 1.59; 95% CI, 1.06-2.38; P=0.030].
Pairwise comparisons among four intervals of Breslow thickness
indicated that patients with melanomas with Breslow thickness
2.01-4.00 and >4 mm were more likely to develop metastasis
compared with patients with melanomas of 1.01-2.00 mm (P=0.009 and
P=4.94×10−4, respectively; Table SI). The presence of ulceration
significantly increased the risk of metastasis (OR, 1.66; 95% CI,
1.07-2.59; P=0.033), and the absence of pigment from melanoma
tissue was also associated with the likelihood of metastasis (OR,
2.14; 95% CI, 1.10-4.19; P=0.035; Table
I).
 | Table I.Risk factors for melanoma metastasis
(sex- and age-matched dataset, n=278). |
Table I.
Risk factors for melanoma metastasis
(sex- and age-matched dataset, n=278).
|
| Patients with
metastasis | Patients without
metastasis |
|
|
|---|
|
|
|
|
|
|
|---|
| Risk factor | N | % | N | % | OR (95% CI) |
P-valuea |
|---|
| Tumor
localization |
|
|
|
|
|
|
| Head
and neck | 28 | 10.1 | 41 |
14.7 |
| 0.400 |
|
Limbs | 113 | 40.6 | 104 |
37.4 |
|
|
|
Trunk | 117 | 42.1 | 115 |
41.4 |
|
|
| Hands
and feetd | 20 | 7.2 | 18 |
6.5 |
|
|
|
Totale | 278 | 100.0 | 278 | 100.0 |
|
|
| CM subtype |
|
|
|
|
|
|
|
Superficial spreading
melanoma | 15 | 20.8 | 8 |
11.1 |
| 0.219c |
| Nodular
melanoma | 55 | 76.4 | 60 |
83.3 |
|
|
| Lentigo
malignant melanoma | 2 | 2.8 | 4 |
5.6 |
|
|
|
Totale | 72 | 100.0 | 72 | 100.0 |
|
|
| Breslow thickness,
mm |
|
|
|
|
|
|
| Mean ±
SD | 5.21±6.17 | 4.02±4.95 | 0.012b |
|
|
|
|
Median | 4.0 | 2.5 |
|
|
|
|
|
≤1.0 | 54 | 25.1 | 55 |
25.5 |
| 0.005 |
|
1.0-2.0 | 23 | 10.7 | 49 |
22.8 |
|
|
|
2.0-4.0 | 55 | 25.6 | 50 |
23.3 |
|
|
|
>4.0 | 83 | 38.6 | 61 |
28.4 |
|
|
|
Totale | 215 | 100.0 | 215 | 100.0 |
|
|
| Ulceration |
|
|
|
|
|
|
|
Absent | 61 | 37.9 | 81 |
50.3 | Reference |
|
|
|
|
|
|
| 1.66
(1.07-2.59) |
|
|
Present | 100 | 62.1 | 80 |
49.7 |
| 0.033 |
|
Totale | 161 | 100.0 | 161 | 100.0 |
|
|
| Pigment |
|
|
|
|
|
|
|
Present | 195 | 87.4 | 209 |
93.7 | Reference |
|
|
|
|
|
|
| 2.14
(1.10-4.19) |
|
|
Absent | 28 | 12.6 | 14 |
6.3 |
| 0.035 |
|
Totale | 223 | 100.0 | 223 | 100.0 |
|
|
| Predominant cell
type in the lesion |
|
|
|
|
|
|
|
Epithelial | 121 |
|
67.6 | 118 |
65.9 | 0.139 |
|
Spindle | 21 |
|
11.7 | 33 |
18.4 |
|
|
Mixed | 37 |
|
20.7 | 28 |
15.7 |
|
|
Totale | 179 |
| 100.0 | 179 | 100.0 |
|
| BMI |
|
|
|
|
|
|
| Mean ±
SD | 28.64±5.56 | 28.20±5.93 |
| 0.462b |
|
|
|
Median | 27.5 | 27.8 |
|
|
|
|
|
18.61-25.00 | 40 |
|
27.0 | 44 |
29.7 | 0.869 |
|
25.01-30.00 | 57 |
|
38.5 | 54 |
36.5 |
|
|
>30.01 | 51 |
|
34.5 | 50 |
33.8 |
|
|
Totale | 148 |
| 100.0 | 148 | 100.0 |
|
Within the initial cohort of 309 individuals, which
were paired with controls only by the year of initial diagnosis and
the time of follow-up, male sex also appeared to be a significant
risk factor (OR, 1.53; 95% CI, 1.10-2.12; P=0.013). In addition,
the anatomical localization of melanoma was associated with the
development of metastasis (P=0.034; Table SII). To explore this further, the
differences in melanoma features between female and male patients
were examined. The anatomical localization of melanoma was the only
characteristic that was indicated to be significantly different for
male and female patients (Table
II). Localizations differed for both sexes among the patients
with metastasis (P=0.010) as well as among the control subjects
without metastasis (P=0.003; Table
SIII). Relative to those on limbs, male patients presented with
more cases of melanoma on their trunk, hands and feet, as well as
on the head and neck compared with female patients (Table III). Melanoma localization on the
trunk had significantly different frequencies for males and females
when individuals were stratified into groups by metastatic status,
and it differed by sex for patients without and with metastasis
(OR, 2.82; 95% CI, 1.61-4.96; P=3.26×10−4; and OR, 2.47;
95% CI, 1.43-4.25; P=1.22×10−3, respectively; Tables SIV and SV). The analysis of melanoma features
within the female and male cohorts separately demonstrated that
tumor Breslow thickness, ulceration and absence of pigment were
associated with metastasis in female patients, whereas similar
associations were not observed in the male cohort (Table II).
 | Table II.Differences between melanoma risk
factors in female and male patients (sex- and age-matched dataset,
n=278). |
Table II.
Differences between melanoma risk
factors in female and male patients (sex- and age-matched dataset,
n=278).
|
|
|
|
|
|
|
| Female |
| Male |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
|
| Female | Male |
|
| With
metastasis | Without
metastasis |
|
| With
metastasis | Without
metastasis |
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Risk factor | N | % | N | % | OR (95% CI) |
P-valuea | N | % | N | % | OR (95% CI) |
P-valuea | N | % | N | % | OR (95% CI) |
P-valuea |
|---|
| Tumor
localization |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Head
and neck | 40 |
12.2 | 29 |
12.7 |
| <0.001 | 15 |
9.1 | 25 |
15.3 |
| 0.401 | 13 |
11.4 | 16 |
14.0 |
| 0.902 |
|
Limbs | 155 |
47.3 | 62 |
27.2 |
|
| 80 |
48.8 | 75 |
45.7 |
|
| 33 |
29.0 | 29 |
25.5 |
|
|
|
Trunk | 113 |
34.4 | 119 |
52.2 |
|
| 58 |
35.4 | 55 |
33.5 |
|
| 59 |
51.7 | 60 |
52.6 |
|
|
| Hands
and feetd | 20 |
6.1 | 18 |
7.9 |
|
| 11 |
6.7 | 9 |
5.5 |
|
| 9 |
7.9 | 9 |
7.9 |
|
|
|
Totale | 328 | 100.0 | 228 | 100.0 |
|
| 164 | 100.0 | 164 | 100.0 |
|
| 114 | 100.0 | 114 | 100.0 |
|
|
| CM subtype |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Superficial spreading
melanoma | 15 |
16.3 | 8 |
15.4 |
| 0.693c | 10 |
21.7 | 5 |
10.9 |
| 0.191c | 5 |
19.2 | 3 |
11.5 |
| 0.465c |
| Nodular
melanoma | 72 |
78.3 | 43 |
82.7 |
|
| 35 |
76.1 | 37 |
80.4 |
|
| 20 |
76.9 | 23 |
88.5 |
|
|
| Lentigo
malignant melanoma | 5 |
5.4 | 1 |
1.9 |
|
| 1 |
2.2 | 4 |
8.7 |
|
| 1 |
3.9 | 0 |
0.0 |
|
|
|
Totale | 92 | 100.0 | 52 | 100.0 |
|
| 46 | 100.0 | 46 | 100.0 |
|
| 26 | 100.0 | 26 | 100.0 |
|
|
| Breslow thickness,
mm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mean ±
SD | 4.95±6.33 | 4.11±4.31 | 0.479b | 5.66±6.86 | 4.24±5.70 | 0.013b | 4.53±4.93 | 3.70±3.56 | 0.435b |
|
|
|
|
|
|
|
|
|
|
Median | 3.0 | 3.0 |
| 4.0 | 2.0 |
| 3.0 | 3.0 |
|
|
|
|
|
|
|
|
|
|
|
≤1.0 | 64 |
24.8 | 45 |
26.2 |
| 0.325 | 32 |
24.8 | 32 |
24.8 |
| 0.001 | 22 | 25.6 | 23 |
26.7 |
| 0.907 |
|
1.0-2.0 | 45 |
17.5 | 27 |
15.7 |
|
| 11 |
8.5 | 34 |
26.4 |
|
| 12 | 14.0 | 15 |
17.5 |
|
|
|
2.0-4.0 | 56 |
21.7 | 49 |
28.5 |
|
| 30 |
23.3 | 26 |
20.1 |
|
| 25 | 29.1 | 24 |
27.9 |
|
|
|
>4.0 | 93 |
36.0 | 51 |
29.6 |
|
| 56 |
43.4 | 37 |
28.7 |
|
| 27 | 31.3 | 24 |
27.9 |
|
|
|
Totale | 258 | 100.0 | 172 | 100.0 |
|
| 129 | 100.0 | 129 | 100.0 |
|
| 86 | 100.0 | 86 | 100.0 |
|
|
| Ulceration |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Absent | 78 |
40.2 | 64 |
50.0 | Ref |
| 31 |
32.0 | 47 |
48.5 | Ref |
| 30 |
46.9 | 34 |
53.1 | Ref |
|
|
Present | 116 |
59.8 | 64 |
50.0 | 0.67
(0.43-1.05) | 0.106 | 66 |
68.0 | 50 |
51.5 | 2.00
(1.12-3.59) | 0.028 | 34 |
53.1 | 30 |
46.9 | 1.28
(0.64-2.57) | 0.596 |
|
Totale | 194 | 100.0 | 128 | 100.0 |
|
| 97 | 100.0 | 97 | 100.0 |
|
| 64 | 100.0 | 64 | 100.0 |
|
|
| Pigment |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Present | 237 |
90.5 | 167 |
90.8 | Ref |
| 112 |
85.5 | 125 |
95.4 | Ref |
| 83 | 90.2 | 84 |
91.3 | Ref |
|
|
Absent | 25 |
9.5 | 17 |
9.2 | 0.97
(0.51-1.84) | 1.000c | 19 |
14.5 | 6 |
4.6 | 3.53
(1.36-9.16) | 0.010c | 9 | 9.8 | 8 |
8.7 | 1.14
(0.42-3.09) | 1.000c |
|
Totale | 262 | 100.0 | 184 | 100.0 |
|
| 131 | 100.0 | 131 | 100.0 |
|
| 92 | 100.0 | 92 | 100.0 |
|
|
| Predominant cell
type in the lesion |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Epithelial | 130 |
63.7 | 109 |
70.8 |
| 0.236 | 64 |
62.7 | 66 |
64.7 |
| 0.174 | 57 |
74.0 | 52 |
67.5 |
| 0.518 |
|
Spindle | 31 |
15.2 | 23 |
14.9 |
|
| 12 |
11.8 | 19 |
18.6 |
|
| 9 |
11.7 | 14 |
18.2 |
|
|
|
Mixed | 43 |
21.1 | 22 |
14.3 |
|
| 26 |
25.5 | 17 |
16.7 |
|
| 11 |
14.3 | 11 |
14.3 |
|
|
|
Totale | 204 | 100.0 | 154 | 100.0 |
|
| 102 | 100.0 | 102 | 100.0 |
|
| 77 | 100.0 | 77 | 100.0 |
|
|
| BMI |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mean ±
SD | 28.5±6.27 | 28.3±4.84 | 0.810b | 28.9±6.10 | 28.0±6.44 | 0.224b | 28.2±4.63 | 28.4±5.09 | 0.598b |
|
|
|
|
|
|
|
|
|
|
Median | 27.4 | 27.9 |
| 27.9 | 27.1 |
| 26.9 | 27.9 |
|
|
|
|
|
|
|
|
|
|
|
18.01-25.00 | 56 |
31.5 | 28 |
23.7 | 0.054 |
| 25 |
28.1 | 31 |
34.8 |
| 0.593 | 15 |
25.4 | 13 |
22.0 |
| 0.718 |
|
25.01-30.00 | 57 |
32.0 | 54 |
45.8 |
|
| 29 |
32.6 | 28 |
31.5 |
|
| 28 |
47.5 | 26 |
44.1 |
|
|
|
>30.01 | 65 |
36.5 | 36 |
30.5 |
|
| 35 |
39.3 | 30 |
33.7 |
|
| 16 |
27.1 | 20 |
33.9 |
|
|
|
Totale | 178 | 100.0 | 118 | 100.0 |
|
| 89 | 100.0 | 89 | 100.0 |
|
| 59 | 100.0 | 59 | 100.0 |
|
|
 | Table III.Pairwise comparisons of the
frequencies of tumor localization for female and male patients
(sex- and age-matched dataset, n=278). |
Table III.
Pairwise comparisons of the
frequencies of tumor localization for female and male patients
(sex- and age-matched dataset, n=278).
|
|
| Male |
|---|
|
|
|
|
|---|
|
|
| Limbs |
| Hands and
feetb |
| Trunk |
| Head and neck |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
|
| Tumor
localization | OR (95% CI) |
P-valuea | OR (95% CI) |
P-valuea | OR (95% CI) |
P-valuea | OR (95% CI) |
P-valuea |
|---|
| Female | Limbs |
|
| 2.25
(1.12-4.54) | 0.036 | 2.63
(1.78-3.89) | <0.001 | 1.81
(1.03-3.18) | 0.039 |
|
| Hands and
feetb |
|
|
|
| 1.17
(0.59-2.33) | 0.727 | 0.81
(0.36-1.79) | 0.685 |
|
| Trunk |
|
|
|
|
|
| 1.45
(0.84-2.50) | 0.217 |
|
| Head and neck |
|
|
|
|
|
|
|
|
Multivariate models built by stepwise regression
demonstrated that the only independently significant prognostic
factor for the development of metastasis was tumor ulceration, and
it was selected as it significantly improved the multivariate model
according to the overall model likelihood and the Akaike
Information Criterion (ulceration OR, 1.62; 95% CI,1.00-2.62;
P=0.051). Age and sex were incorporated in this model by the
matching of cases and controls.
Patient survival
The survival of patients exhibiting primary melanoma
differed significantly from those who developed metastasis [hazard
ratio (HR), 3.50; 95% CI, 2.72-4.51; P<2.00×10−16;
Fig. 2A]. Improved survival was also
observed in female compared with male patients (HR, 1.44; 95% CI,
1.14-1.81; P=1.94×10−3). However, this difference was
only observed in females without metastasis (HR, 1.86; 95% CI,
1.20-2.88; P=5.04×10−3), and was not observed once
metastasis had developed (HR, 1.05; 95% CI, 0.80-1.37; P=0.744;
Fig. 2B-D). The comparison of
patients' survival in the present study with the survival reported
in the SEER data base (2)
demonstrated that the 5-year survival after the initial diagnosis
was 61.1% in the cohort of the present study compared with 91.6%
for the Caucasian population in the SEER database. These
differences in survival were observed both for localized disease
(controls without metastasis; T stages IA-IIC) and for melanoma
with metastasis (cases; T stages IIIA-IV). The survival rates were
41.9% (controls) and 80.7% (cases) in the present study compared
with 65 and 98.3% in the SEER database for the respective groups
(Table IV) (2).
 | Table IV.Patient 5-year survival rates in the
local cohort and the SEER database (2). |
Table IV.
Patient 5-year survival rates in the
local cohort and the SEER database (2).
|
| 5-year survival
rate, % |
|---|
|
|
|
|---|
| Stage | Local cohort | SEER database |
|---|
| All melanomas | 61.1 | 91.6 |
| Localized disease
(T stage IA-IIC) | 80.7 | 98.3 |
| Metastasis
(regional and distant; T stage IIIA-IV) | 41.9 | 65.0 |
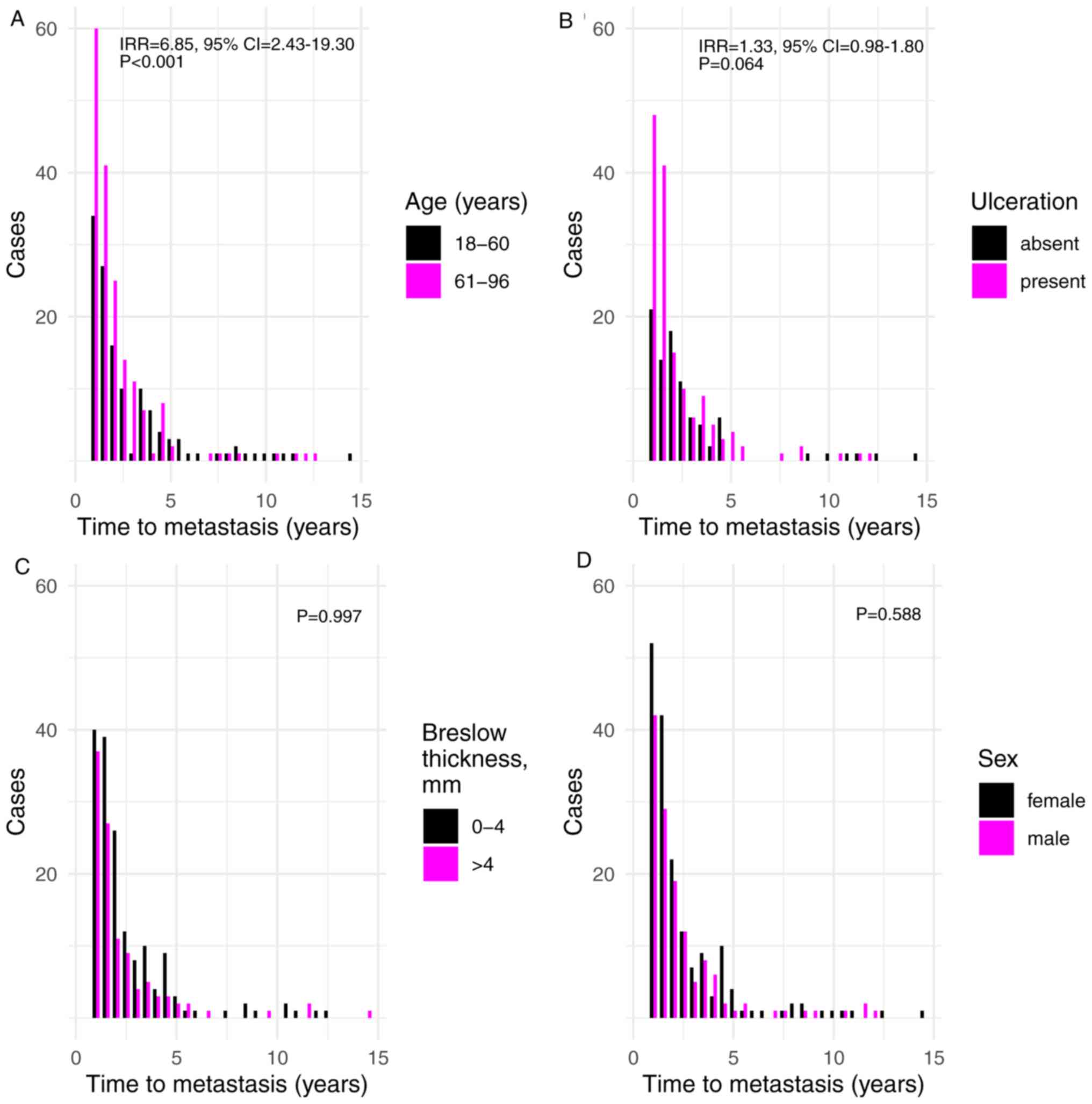
Time until metastasis since primary
diagnosis
The time until metastasis was diagnosed was also
considered in the current study. The results demonstrated that
increased age was associated with shorter time until metastasis
(incident rate ratio (IRR), 6.85; 95% CI, 2.43-19.30;
P=2.78×10−4) (Fig. 3A).
In addition, ulceration indicated a borderline significant
association with shorter time until metastasis (IRR=1.33, 95%
CI=0.98-1.80; P=0.064; Fig. 3B).
None of the remaining factors exhibited an association with shorter
time until metastasis, including tumor Breslow thickness and sex
(P=0.997 and P=0.588, respectively; Fig.
3C and D).
Discussion
The present study revealed an ascending trend for
the number of melanomas that progress towards metastasis from
1988–2015. This trend was consistent with the observations of other
studies, where a decline in late-stage melanomas was not observed,
and a decrease in the mortality caused by melanoma was also not
observed (1). The results of the
current study indicated that tumor ulceration exhibited the
strongest association with melanoma metastasis. Furthermore, tumor
ulceration was indicated to be the only independently significant
prognostic factor for the development of metastasis in the
multivariate model. Tumor ulceration has been previously reported
to be a prognostic factor for melanoma (17,18,21,22). In
the present study, ulceration was also nominally associated with a
shorter time until the development of metastasis. Tumor Breslow
thickness was another factor that exhibited a strong association
with melanoma metastasis; this measurement is an important hallmark
of melanoma progression, and it is recognized as one of the main
prognostic factors on which the current clinical staging of
melanoma is based (10,11). Patients in the cohort used in the
present study exhibited thicker melanomas with a median Breslow
thickness of 3.00 mm (4.00 mm in patients with metastasis and 2.50
mm in those without) compared with other studies reporting a median
thickness of 0.62 mm and a steady decrease of this metric (6,31).
However, the percentage of patients with primary melanoma who
developed metastasis was only slightly higher in the current study
compared with the previous literature at 31.9% vs. 30.0%,
respectively (8,9). In addition, Breslow thickness exhibited
no impact on the time when metastasis was diagnosed within the
present study. Tumor thickness may be a proxy of the stage of
primary tumor advancement, and the spreading of melanoma may be
independent of the thickness of primary tumor at the time of
diagnosis. This may mean that tumor Breslow thickness is not the
main determinant of the speed with which metastasis develops, and
that other mechanisms are responsible for this spread.
Alternatively, the lack of association between Breslow thickness
and the time of metastasis development may be due to the different
effects of the initial Breslow thickness on early and late
metastasis. Of note, the survival rate in the present cohort was
noticeably lower compared with that reported in the literature
(2). This might be explained by the
delays in seeking medical assistance and by the availability of
state-of-the-art treatment options during the study period
(1998–2015) in Latvia. In addition patients' age and mortality from
other diseases may have had an influence on this discrepancy,
especially because the difference was similar for patients with
metastasis and patients without metastasis (localized disease).
This observation emphasizes the requirement for identifying
additional patient and melanoma characteristics that may be
associated with disease progression.
Ulceration, tumor Breslow thickness and the absence
of pigment in melanoma tissues are known prognostic factors for
melanoma (17,21) and were associated with metastasis in
the cohort used in the present study. These features were
associated with the development of melanoma metastasis in female
patients, whereas similar associations were not observed in the
male cohort. However, male sex appeared to be a significant risk
factor for the development of melanoma metastasis. In 1969, Clark
et al (32) observed that
melanomas were more aggressive in male patients compared with those
in female patients. Numerous studies have consistently indicated
sex to be an independent prognostic factor for the development of
melanoma even after the adjustment for age, Breslow thickness,
histological subtype and body site (33–36),
ulceration (36–38), vascular invasion (39), mitotic rate (38) and sentinel lymph node positivity
(34,36,40). A
biological basis for this advantage in female survival has been
suggested (34,35). A number of factors have been
hypothesized to contribute to the increased female patient
survival, including sex-linked physiological differences in skin,
sex hormone levels, pregnancy, use of oral contraceptives and
hormone replacement therapy [reviewed in (41)] and the presence of oxidative stress
(42). However, the precise
biological mechanism underlying this phenomenon remains to be
determined. In the current study, no significant differences
between male and female patients in terms of age, tumor Breslow
thickness, histological subtype, pigment and ulceration were
observed. No discrepancies in these factors were observed when male
and female patients were compared separately within groups with and
without metastasis. However, it was demonstrated that melanoma
localization differed among sexes. A previous study has reported
similar results, revealing that among various risk factors, only
localization is not similarly distributed for both sexes (5). In the present study and in the
aforementioned studies, female patients exhibited more melanomas on
the limbs, whereas male patients had a number of melanomas on the
trunk (5). According to previous
studies, melanomas on the trunk exhibit a worse prognosis compared
with melanomas on the extremities (43–45). Age
is another factor that is often associated with a poor prognosis,
especially in male patients (46).
This was not confirmed in the current study, although it was
indicated that advanced age was associated with a shorter time
until the onset of metastasis in both sexes.
A major limitation of the current study was
acquiring data from a large referral center, which may not fully
represent the Latvian population. However, REUHLOC is the main
oncological hospital in the country, where the majority of patients
with melanoma are treated. A similar approach to data collection
has been used previously (47). In
addition, patients were not classified according to melanoma
sub-stages. The rationale behind this decision was as follows. It
is important to utilize as much of the available information about
patients and tumors as possible to improve the prediction of
metastases. Each conversion of a continuous feature, such as
Breslow thickness, into a discrete characteristic is associated
with loss of information and reduces the statistical power of
models where this feature is included. The consequences of
collapsing several features into one coarser trait are similar.
Thus, it is preferable to consider multiple features simultaneously
in a multivariate model. Using disease stages may be beneficial if
there were numerous outliers in the data. However, such trends were
not observed in the present study. The type of treatment received
by a patient was not incorporated into statistical models and is a
limitation of the present study. This was done as during the study
period no novel treatment options were available and all patients
in the cohort in the present study received standard treatments:
Patients with stage I and II disease were put under observation,
stage III melanoma patients mostly received interferon alpha
(IFN-α) and for stage IV patients the main treatment option was
chemotherapy with dacarbazine.
In conclusion, the present study demonstrated that
the main features associated with the speed of melanoma progression
and the development of metastasis in Latvia, despite the lower
5-year survival rates, are similar to those reported previously and
include tumor ulceration, absence of pigment in melanoma and tumor
Breslow thickness, although the latter is not associated with the
time at which metastasis is diagnosed. The present study also
indicated that an additional feature associated with melanoma
progression is male sex.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Ms. Anna Azaryan
(Vienna University, Austria) for critical reading and advice
regarding the manuscript and Ms. Irina Verhovcova (Latvian
Biomedical Research and Study Centre, Riga, Latvia) for technical
assistance in the manuscript preparation.
Funding
This work was supported by The European Regional
Development Fund (project no. 1.1.1.1/18/A/099).
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author upon a reasonable
request.
Authors' contributions
DP designed the study, interpreted the data and was
a major contributor in writing the manuscript. DR performed
statistical analyses, interpreted the data and was a major
contributor in writing the manuscript. IC conceived the study and
interpreted the data. KA, EK and AO acquired the data. All authors
read, edited and approved the final manuscript.
Ethics approval and consent to
participate
The Research Ethics Committee of the Institute of
Cardiology and Regenerative Medicine of University of Latvia
approved the study protocol.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CM
|
cutaneous melanoma
|
|
REUHLOC
|
the Riga East University Hospital
Latvian Oncology Centre
|
|
HR
|
hazard ratio
|
|
IRR
|
incident rate ratio
|
|
BMI
|
body mass index
|
References
|
1
|
Apalla Z, Lallas A, Sotiriou E, Lazaridou
E and Ioannides D: Epidemiological trends in skin cancer. Dermatol
Pract Concept. 7:1–6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
SEER*Explorer, . An interactive website
for SEER cancer statistics. Surveillance Research Program, National
Cancer Institute. June
26–2020
|
|
3
|
Lasithiotakis KG, Leiter U, Gorkievicz R,
Eigentler T, Breuninger H, Metzler G, Strobel W and Garbe C: The
incidence and mortality of cutaneous melanoma in southern Germany:
Trends by anatomic site and pathologic characteristics, 1976 to
2003. Cancer. 107:1331–1339. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stang A, Pukkala E, Sankila R, Söderman B
and Hakulinen T: Time trend analysis of the skin melanoma incidence
of Finland from 1953 through 2003 including 16,414 cases. Int J
Cancer. 119:380–384. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Azarjana K, Ozola A, Ruklisa D, Cema I,
Rivosh A, Azaryan A and Pjanova D: Melanoma epidemiology, prognosis
and trends in Latvia. J Eur Acad Dermatol Venereol. 27:1352–1359.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shaikh WR, Dusza SW, Weinstock MA,
Oliveria SA, Geller AC and Halpern AC: Melanoma thickness and
survival trends in the United States, 1989–2009. J Natl Cancer
Inst. 108:djv2942015.PubMed/NCBI
|
|
7
|
Whiteman DC, Green AC and Olsen CM: The
growing burden of invasive melanoma: Projections of incidence rates
and numbers of new cases in six susceptible populations through
2031. J Invest Dermatol. 136:1161–1171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Essner R, Lee JH, Wanek LA, Itakura H and
Morton DL: Contemporary surgical treatment of advanced-stage
melanoma. Arch Surg. 139:961–967. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oliaro A, Filosso PL, Bruna MC, Mossetti C
and Ruffini E: Pulmonary metastasectomy for melanoma. J Thorac
Oncol. 5 (6 Suppl 2):S187–S191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Balch CM, Gershenwald JE, Soong SJ,
Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG,
Ding S, et al: Final version of 2009 AJCC melanoma staging and
classification. J Clin Oncol. 27:6199–6206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gershenwald JE, Scolyer RA, Hess KR,
Sondak VK, Long GV, Ross MI, Lazar AJ, Faries MB, Kirkwood JM,
McArthur GA, et al: Melanoma staging: Evidence-based changes in the
American joint committee on cancer eighth edition cancer staging
manual. CA Cancer J Clin. 67:472–492. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sullivan RJ and Flaherty KT: Resistance to
BRAF-targeted therapy in melanoma. Eur J Cancer. 49:1297–1304.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rizos H, Menzies AM, Pupo GM, Carlino MS,
Fung C, Hyman J, Haydu LE, Mijatov B, Becker TM, Boyd SC, et al:
BRAF inhibitor resistance mechanisms in metastatic melanoma:
Spectrum and clinical impact. Clin Cancer Res. 20:1965–1977. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vilgelm AE, Johnson DB and Richmond A:
Combinatorial approach to cancer immunotherapy: Strength in
numbers. J Leukoc Biol. 100:275–290. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bhandaru M and Rotte A: Monoclonal
antibodies for the treatment of melanoma: Present and future
strategies. Methods Mol Biol. 1904:83–108. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weiss SA, Wolchok JD and Sznol M:
Immunotherapy of melanoma: Facts and hopes. Clin Cancer Res.
25:5191–5201. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Homsi J, Kashani-Sabet M, Messina JL and
Daud A: Cutaneous melanoma: Prognostic factors. Cancer Control.
12:223–229. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
In 't Hout FE, Haydu LE, Murali R,
Bonenkamp JJ, Thompson JF and Scolyer RA: Prognostic importance of
the extent of ulceration in patients with clinically localized
cutaneous melanoma. Ann Surg. 255:1165–1170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han D, Zager JS, Shyr Y, Chen H, Berry LD,
Iyengar S, Djulbegovic M, Weber JL, Marzban SS, Sondak VK, et al:
Clinicopathologic predictors of sentinel lymph node metastasis in
thin melanoma. J Clin Oncol. 31:4387–4393. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dummer R, Hauschild A, Lindenblatt N,
Pentheroudakis G and Keilholz U; ESMO Guidelines Committee, :
Cutaneous melanoma: ESMO clinical practice guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 26 (Suppl
5):v126–v132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Elder DE: Melanoma progression. Pathology.
48:147–154. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Portelli F, Galli F, Cattaneo L, Cossa M,
De Giorgi V, Forte G, Fraternali Orcioni G, Gianatti A, Indini A,
Labianca A, et al: The prognostic impact of the extent of
ulceration in clinical stage I–II melanoma patients: A multicenter
study of the Italian melanoma intergroup (IMI). Br J Dermatol. Apr
13–2020.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Balch CM, Soong SJ, Gershenwald JE,
Thompson JF, Coit DG, Atkins MB, Ding S, Cochran AJ, Eggermont AM,
Flaherty KT, et al: Age as a prognostic factor in patients with
localized melanoma and regional metastases. Ann Surg Oncol.
20:3961–3968. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brauer JA, Wriston CC, Troxel AB,
Elenitsas R, Shin DB, Guerry DP and Ming ME: Characteristics
associated with early and late melanoma metastases. Cancer.
116:415–423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Messeguer F, Agustí-Mejías A, Traves V,
Requena C, Alegre V, Guillén C, Oliver V and Nagore E: Mitotic rate
and subcutaneous involvement are prognostic factors for survival
after recurrence in patients with only locoregional skin metastasis
as the first site of recurrence from cutaneous melanoma. J Eur Acad
Dermatol Venereol. 27:436–441. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
R Core Team, . R: A language and
environment for statistical computing. Version 3.6.3. R Foundation
for Statistical Computing. (Vienna). 2012.
|
|
27
|
Venables WN and Ripley BD: Modern applied
statistics with S. (Fourth). Springer. (New York, NY). 2002.
View Article : Google Scholar
|
|
28
|
Therneau TM: A package for survival
analysis in S. Version 2.38. 2015.
|
|
29
|
Tang Y, Horikoshi M and Li W: ggfortify:
Unified interface to visualize statistical result of popular R
packages. The R Journal. 8:474–485. 2016. View Article : Google Scholar
|
|
30
|
Horikoshi M and Tang Y: ggfortify: Data
visualization tools for statistical analysis results.
|
|
31
|
Davies JR, Randerson-Moor J, Kukalizch K,
Harland M, Kumar R, Madhusudan S, Nagore E, Hansson J, Höiom V,
Ghiorzo P, et al: Inherited variants in the MC1R gene and survival
from cutaneous melanoma: A BioGenoMEL study. Pigment Cell Melanoma
Res. 25:384–394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Clark WH Jr, From L, Bernardino EA and
Mihm MC: The histogenesis and biologic behavior of primary human
malignant melanomas of the skin. Cancer Res. 29:705–727.
1969.PubMed/NCBI
|
|
33
|
Downing A, Newton-Bishop JA and Forman D:
Recent trends in cutaneous malignant melanoma in the Yorkshire
region of England; incidence, mortality and survival in relation to
stage of disease, 1993–2003. Br J Cancer. 95:91–95. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lasithiotakis K, Leiter U, Meier F,
Eigentler T, Metzler G, Moehrle M, Breuninger H and Garbe C: Age
and gender are significant independent predictors of survival in
primary cutaneous melanoma. Cancer. 112:1795–1804. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
de Vries E, Nijsten TE, Visser O,
Bastiaannet E, van Hattem S, Janssen-Heijnen ML and Coebergh JW:
Superior survival of females among 10 538 Dutch melanoma patients
is independent of Breslow thickness, histologic type and tumor
site. Ann Oncol. 19:583–589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mays MP, Martin RC, Burton A, Ginter B,
Edwards MJ, Reintgen DS, Ross MI, Urist MM, Stromberg AJ, McMasters
KM and Scoggins CR: Should all patients with melanoma between 1 and
2 mm Breslow thickness undergo sentinel lymph node biopsy? Cancer.
116:1535–1544. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Balch CM, Soong SJ, Gershenwald JE,
Thompson JF, Reintgen DS, Cascinelli N, Urist M, McMasters KM, Ross
MI, Kirkwood JM, et al: Prognostic factors analysis of 17,600
melanoma patients: Validation of the American joint committee on
cancer melanoma staging system. J Clin Oncol. 19:3622–3634. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Azzola MF, Shaw HM, Thompson JF, Soong SJ,
Scolyer RA, Watson GF, Colman MH and Zhang Y: Tumor mitotic rate is
a more powerful prognostic indicator than ulceration in patients
with primary cutaneous melanoma: An analysis of 3661 patients from
a single center. Cancer. 97:1488–1498. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nagore E, Oliver V, Botella-Estrada R,
Moreno-Picot S, Insa A and Fortea JM: Prognostic factors in
localized invasive cutaneous melanoma: High value of mitotic rate,
vascular invasion and microscopic satellitosis. Melanoma Res.
15:169–177. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Scoggins CR, Ross MI, Reintgen DS, Noyes
RD, Goydos JS, Beitsch PD, Urist MM, Ariyan S, Sussman JJ, Edwards
MJ, et al: Gender-related differences in outcome for melanoma
patients. Ann Surg. 243:693–698. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Roh MR, Eliades P, Gupta S, Grant-Kels JM
and Tsao H: Cutaneous melanoma in women. Int J Womens Dermatol. 3
(Suppl 1):S11–S15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Joosse A, De Vries E, Van Eijck CH,
Eggermont AM, Nijsten T and Coebergh JW: Reactive oxygen species
and melanoma: An explanation for gender differences in survival?
Pigment Cell Melanoma Res. 23:352–364. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Leiter U, Meier F, Schittek B and Garbe C:
The natural course of cutaneous melanoma. J Surg Oncol. 86:172–178.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Callender GG, Egger ME, Burton AL,
Scoggins CR, Ross MI, Stromberg AJ, Hagendoorn L, Martin RC II and
McMasters KM: Prognostic implications of anatomic location of
primary cutaneous melanoma of 1 mm or thicker. Am J Surg.
202:659–665. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gordon D, Smedby KE, Schultz I, Olsson H,
Ingvar C, Hansson J and Gillgren P: Sentinel node location in trunk
and extremity melanomas: Uncommon or multiple lymph drainage does
not affect survival. Ann Surg Oncol. 21:3386–3394. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lotz M, Budden T, Furney SJ and Virós A:
Molecular subtype, biological sex and age shape melanoma tumour
evolution. Br J Dermatol. Apr 13–2020.(Online ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Buljan M, Rajacić N, Vurnek Zivković M,
Blajić I, Kusić Z and Situm M: Epidemiological data on melanoma
from the referral centre in croatia (2002–2007). Coll Antropol. 32
(Suppl 2):S47–S51. 2008.
|