Introduction
Prostate cancer (PCa) is a common malignant tumor in
the male genitourinary system. According to estimates by American
Cancer Society, in the United States, 164,690 new cases of PCa were
diagnosed and PCa accounted for nearly 19% of all male newly
diagnosed cancers in 2018 (1).
Surgery, endocrine therapy, radiation and chemotherapy are the
current preferred therapeutic approaches (2). For some patients with advanced PCa,
endocrine therapy is the treatment of choice (3), and this delays disease progression
temporarily. Nevertheless, eventually PCa treatment becomes
difficult due to the development of castration-resistant prostate
cancer (CRPC) (4,5).
The androgen receptor (AR) is considered to be the
most important factor in the progression of PCa in CRPC (6,7). AR is a
member of the nuclear receptor superfamily of proteins and is a
class of nuclear transcription factors that facilitate entry into
cells of testosterone and double-hydrogen testosterone to form
androgen-androgen receptor complexes (8). AR and heat shock protein (HSP90)
dissociate and are transported to the prostate, seminal vesicle and
epididymis the nuclei of target tissues, such as the skeletal
muscle system, which modulates activation and inhibition of target
genes, thereby mediating the effects of androgens (9,10).
Studies have shown that abnormal expression of AR is one of the key
factors in the progression of CRPC (11–13).
Prostate specific antigen (PSA) has been suggested as a molecular
target for prostate cancer because it is not only active in
prostate tissues, but also plays a key role in the signaling
pathway of prostate cancer (14).
The combination of androgens and AR induces the dissociation of AR
from HSP90 and stimulates AR phosphorylation to activate the
activity of downstream proteins (15). These proteins activate or inhibit
target genes, which regulate the proliferation, survival and
production of PSA in prostate cells (16).
ARV7 is a truncated AR that functions as an AR
shear-variant (AR-V). Alike AR, ARV7 retains an
NH2-terminal domain (NTD) and DNA-binding domain (DBD),
but lacks the full-length ligand-binding domain (LBD) of AR. The
binding of androgen to AR LBD allows the ligand-binding receptor to
enter the nucleus and then transcriptionally regulate
androgen-responsive genes. Therefore, ARV7 is not available to bind
to the androgen ,and regulates the transcriptional activation of
downstream target genes in the absence of androgens (17,18).
ARV7 is considered to play an important role in the process of
castration resistance in PCa invasion, metastasis, hormone therapy
resistance and biochemical recurrence (18).
Resveratrol (Res) is a natural polyphenol compound
containing a stilbene moiety. The formula is
C14H12O3. It is found in several
plants, including grapes and blueberries (19). A previous clinical pharmacological
study has demonstrated that the cell proliferation antigen Ki-60
expression in tumor tissues is significantly decreases after
patients are treated with Res, which means that Res may have
potential antitumor effects (20).
The antitumor mechanism of Res is mainly through cell cycle arrest
and the induction of apoptosis, including upregulation of p21
cyclin-dependent kinase inhibitor 1 (Cip1/WAF1), Bax/Bcl-2, Bak and
p53 and activation of caspases signaling pathways (21). Several in vitro and in
vivo studies have shown that Res inhibits the proliferation of
several cancer types, including breast cancer, leukemia, colon
cancer, lung cancer, liver cancer and thyroid cancer (22,23). In
addition, studies have shown that resveratrol inhibits cell
proliferation by interfering with AR synthesis in prostate cancer
cells (24,25).
The present study investigated the effects of Res
in vitro on proliferation and apoptosis in PCa cell lines
(LNCaP and LNCaP-B). Alterations in the expression of AR protein
and the levels of AKT phosphorylation were analyzed. Finally, the
effects of Res on PCa cell proliferation and apoptosis via ARV7 and
the PI3K/AKT signaling pathway were measured.
Materials and methods
Cell culture
The human PCa cell line LNCaP and human normal
prostate cell line RWPE-1 were obtained from Shanghai Institute of
Biochemistry and Cell Biology. Both cell lines purchased are
certified by the American Type Culture Collection. PCa cells were
routinely cultured in RPMI-1640 medium supplemented with 10% (v/v)
fetal bovine serum (FBS) and RWPE-1 cells were cultured according
to the manufacture's direction in K-SFM medium (all Gibco; Thermo
Fisher Scientific, Inc.), penicillin 100 U/ml and streptomycin 100
U/ml (both Beijing Solarbio Science & Technology Co., Ltd.) at
37°C under an atmosphere of 5% CO2 in humidified
air.
Reagents and antibodies
Resveratrol (Res), docetaxel (PC) and bicalutamide
were purchased from Sigma-Aldrich; Merck KGaA and were dissolved in
100% DMSO to create stock solutions of 50 mM and stored at −20°C.
These were subsequently diluted in RPMI-1640 medium to the desired
concentration for the experiments and the final concentration of
DMSO that was given to the cells was 0.05%. Antibodies against AKT
(cat. no. 4685), phosphorylated (p-)AKT (cat. no. 4058) were
obtained from Cell Signaling Technology, Inc. Antibodies against AR
(cat. no. 133273), ARV7 (cat. no. 15656) and GAPDH (cat. no. 9485)
were purchased from Abcam. GAPDH was used as a loading control. The
goat anti-rabbit and anti-mouse IgG-HRP secondary antibodies were
purchased from Wuhan Boster Biological Technology, Ltd. The AKT
pathway activator (phen) was purchased from Selleck Chemicals,
primers (ARV7 and GAPDH) were purchased from Angen Biotech Co.,
Ltd. SYBR® Premix Ex Taq™ II (Tli RNaseH Plus) and the
PrimeScript™ RT Reagent kit with gDNA Eraser (Perfect Real Time)
were purchased from Bao Biological Engineering Co., Ltd.
(http://www.takara.com.cn).
Lipofectamine® 2000 was purchased from Thermo Fisher
Scientific, Inc., and the miRcute miRNA First-Strand cDNA Synthesis
kit was purchased from Tiangen Biochemical Technology Co., Ltd.
Construction of the hormone-resistant
PCa cell line (LNCaP-B) and induction of ARV7 over-expression in
LNCaP-B cells
LNCaP cells in the androgen-dependent logarithmic
growth phase were digested with trypsin and inoculated in a medium
containing 10% non-androgen (activated charcoal-treated) FBS and
cultured at 37°C with 5% CO2. After 10 generations of
culture, bicalutamide (1.0 µM) was added to the culture medium for
20 generations, and then the concentration of bicalutamide was
increased to 5.0 µM to continue subculture. By 6 months of
subculture, hormone-resistant prostate cancer cells had been
constructed successfully.
The ARV7 overexpression vector was based on the
PCDNA3.1 plasmid (Guangzhou RiboBio Co., Ltd.). The primers were
designed and the RNA fragment of the ARV7 gene was obtained
using the cell genome cDNA as a template. The fragment was inserted
into multiple cloning sites downstream of the plasmid PCDNA3.1 to
obtain the wild-type ARV7-overexpression vector. The PCDNA3.1-ARV7
plasmid and DH5α competent cells mixed culture PCDNA3.1-ARV7. The
constructed PCDNA3.1-ARV7 vector and PCDNA3.1 vector were
introduced into LNCaP-B cells, respectively, using Lipofectamine
2000 reagent according to the manufacturer's instructions, and ARV7
protein expression was verified using western blotting.
Cell viability assay
Cell viability was determined using an MTT assay.
Briefly, the PCa cell lines LNCaP and LNCaP-B (5×103
cells/well) were seeded in 96-well plates. Cells were cultured
overnight (37°C, 5% CO2), and the cells were incubated
with various concentrations of RES (25, 50, 100 and 200 µM)
dissolved in DMSO and PC (5 µM) and various concentrations (200
µl/wells) of RES, RES+ARV7 and RES+phen for 12, 24, 48 and 72 h (at
37°C in 5% CO2). DMSO (0.05%) was used as the negative
control (NC) group. MTT (0.5 mg/ml, 20 µl/well) was added to each
well 4 h in advance. After 4 h incubation (at 37°C in 5%
CO2), DMSO (150 µl/well) was added to each well, and the
cells were measured at 492 nm using a Multiskan Ascent microplate
photometer. Viability was expressed as the percent viable cells
compared with vehicle-treated control cells that were arbitrarily
assigned as 100% viability.
Flow cytometric analysis for apoptosis
assay
A flow cytometric assay was conducted to analyze the
apoptosis of PCa cells. Cells plated in 6-cm plates were treated
with various concentrations of Res (50 and 100 µM), PC (5 µM),
RES+ARV7 and RES+phen. After 24-h treatment, cells were washed with
phosphate buffer saline and harvested. The apoptosis assay was
performed according to the manufacturer's protocol of the Annexin
V/7-ADD apoptosis kit (BD Pharmingen™; BD Biosciences). The
sedimented cells were resuspended with 500 ul binding buffer based
on the instructions. Subsequently, 5 µl Annexin V-7AAD and 5 µl
Annexin V-APC were added to the cell suspension and cells were
incubated at room temperature for 15 min in the dark. The stained
cells were analyzed using BD FACSCanto II flow cytometry (BD
Pharmingen™; BD Biosciences) and the data analyses were performed
using SPSS 19.0 software (IBM, Corp.).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from LNCaP and LNCaP-B cells
treated with various drugs in each group, using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). The concentration and
purity of RNA were measured using UV spectrophotometry (Thermo
Fisher Scientific, Inc.). One microgram of the total RNA was used
as a template and was reverse transcribed into cDNA using
PrimeScript™ RT Reagent kit with gDNA Eraser (Perfect Real Time) to
obtain a template of the reaction system. The condition for reverse
transcription was at 37°C for 15 min, followed by 85°C for 5 sec.
The expression levels of AR, ARV7 and GAPDH in the cells were
analyzed using two-step fluorescence quantitative PCR. A 10-µl
reaction system was generated. The qPCR reaction was performed
using the LightCycler 480 II DNA Amplifier according to the
manufacturer's instructions. The reaction conditions were as
follows: Pre-denaturation at 95°C for 2 min followed by 95°C for 15
sec, and 60°C for 30 sec was performed for a total of 40 cycles.
After completion of the reaction, the relative expression levels of
AR and ARV7 in the cells were expressed using 2−ΔΔCq
normalization (26). The designed
specific primers for each gene are listed in Table I.
 | Table I.The primer sequences of the reverse
transcription quantitative-PCR. |
Table I.
The primer sequences of the reverse
transcription quantitative-PCR.
| Gene | Primer sequence
(5′→3′) |
|---|
| AR | F:
AGGGCAGATCTTGTCCACCG |
|
| R:
TTGCTCAGAAGAGTTCAACA |
| ARV7 | F:
AGGGCAGATCTTGTCCACCG |
|
| R:
TTGCTCAGAAGAGTTCAACA |
| GAPDH | F:
CGGAGTCAACGGATTTGGTCGTAT |
|
| R:
AGCCTTCTCCATGGTGGTGAAGAC |
Western blot analysis
PCa cells were treated with various concentrations
of Res or PC for 24 h. Cells were lysed in RIPA lysis buffer
(Beyotime Institute of Biotechnology) and total proteins were
extracted at 4°C. The total protein concentrations were determined
using the BCA assay (Beyotime Institute of Biotechnology). The
proteins (20 µg/lane) were loaded on a 12% gel, and resolved using
SDS-PAGE with a constant voltage of 80 V. Then the proteins were
transferred to PVDF membranes (EMD Millipore) using the Bio-Rad
PowerPac Basic Power Supply system. Membranes were blocked in 5%
skimmed milk in Tris-Buffered Saline Tween-20, followed by
incubation with primary antibodies overnight at 4°C. The dilutions
of ARV7, Bax, Bcl-2, AR, AKT, p-AKT and GAPDH were all 1:1,000.
Blots were then probed with the IgG-HRP secondary antibody
(1:1,000) at room temperature for 1 h, and protein bands were
detected using chemiluminescence reagent (EMD Millipore), under the
Tanon5200 chemiluminescent imaging system (Tanon Science and
Technology Co., Ltd.). ImageJ 1.8.0 software (National Institutes
of Health) was used to quantify western blotting images.
Statistical analysis
All the data analyses were performed using SPSS 19.0
software. All experiments were repeated at least three times and
P<0.05 was considered to indicate a statistically significant
difference. All experiments were performed in triplicate. Data are
expressed as the mean ± SD. A Student's unpaired t-test was used
for determining the differences between two groups and for more
than two group comparisons one-way ANOVA test with a Tukey's post
hoc test was used.
Results
Effects of Res on proliferation of PCa
cells and effects of ARV7 overexpression on proliferation of
LNCaP-B cells
Various concentrations of Res (25, 50, 100 and 200
µM) and PC (5 µM) were used to treat LNCaP and LNCaP-B cells for
24, 48 and 72 h. The proliferative capacities of both cell types
were significantly inhibited compared with the control, and the
effects were time- and dose-dependent (Fig. 1A and B). The results suggested that
Res inhibited the proliferation of PCa cells. Nevertheless,
following administration of relatively various concentrations of
Res (25, 50, 100 and 200 µM) to RWPE-1 cells for 12, 24, 48 and 72
h, the viability of PCa cells was significantly lower compared with
that of normal cells after Res treatment after 48 and 72 h
(Fig. 1C). To further investigate
the molecular mechanism of ARV7 inhibition in PCa cells mediated by
Res, an overexpression vector (PCDNA3.1-ARV7) of ARV7 was
constructed and transfected it into LNCaP-B cells. Western blot
analysis verified that, compared with cells transfected with empty
vector (PCDNA3.1), ARV7 protein levels in cells transfected with
ARV7 overexpression vector (ARV7) increased significantly (Fig. 1D). It was demonstrated that the
Res+ARV7 group and the Res+phen group restored the inhibitory
effect of Res on the proliferation of LNCaP-B cells compared with
Res treatment alone (Fig. 1E). This
suggests that effect of Res on the proliferation of LNCaP-B cells
may be associated with the ARV7 and AKT pathways.
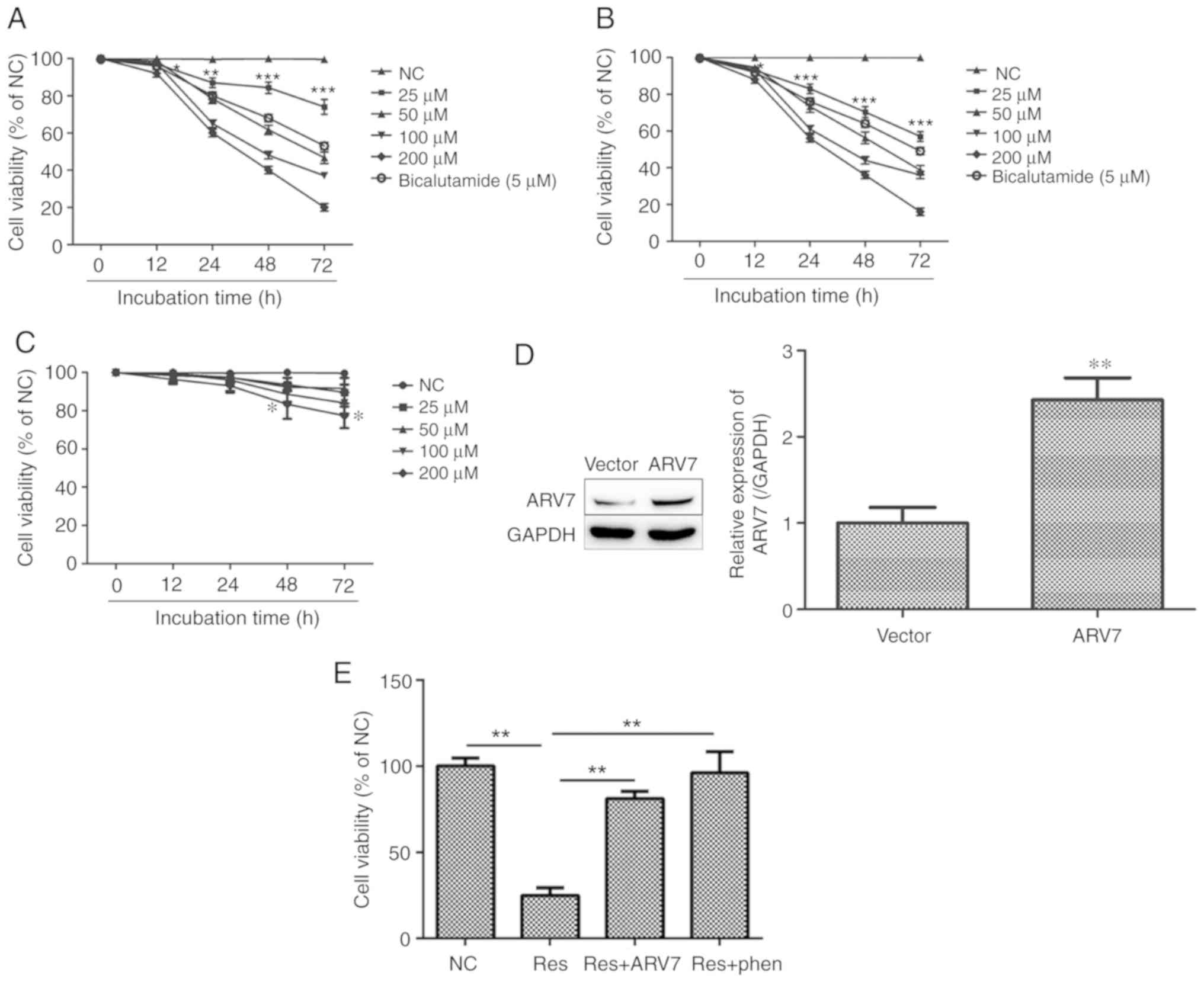 | Figure 1.Res inhibits LNCaP and LNCaP-B cell
proliferation and the effects of ARV7 overexpression on
proliferation of LNCaP-B cells. (A and B) The cell viability of
LNCaP and LNCaP-B cells exhibited a significant decrease in a dose-
and time-dependent manner, which was measured using an MTT assay.
(C) The effect of Res on the cell viability of RWPE-1 cells was
significantly less compared with PCa cells. (D) Western blot
analysis verified that, compared with cells transfected with empty
vector, ARV7 protein levels in cells transfected with the
ARV7-overexpression vector increased significantly. (E) Compared
with the Res+ARV7 group and the Res+phen group, Res group shows
stronger inhibition in LNCaP-B cells. The data are presented as the
mean ± SD for three independent experiments, *P<0.05,
**P<0.01 and ***P<0.001, vs. cells treated with DMSO
(control). Res, resveratrol; ARV7, AR splicing variant 7; Vector,
empty vector; phen; AKT pathway activator. |
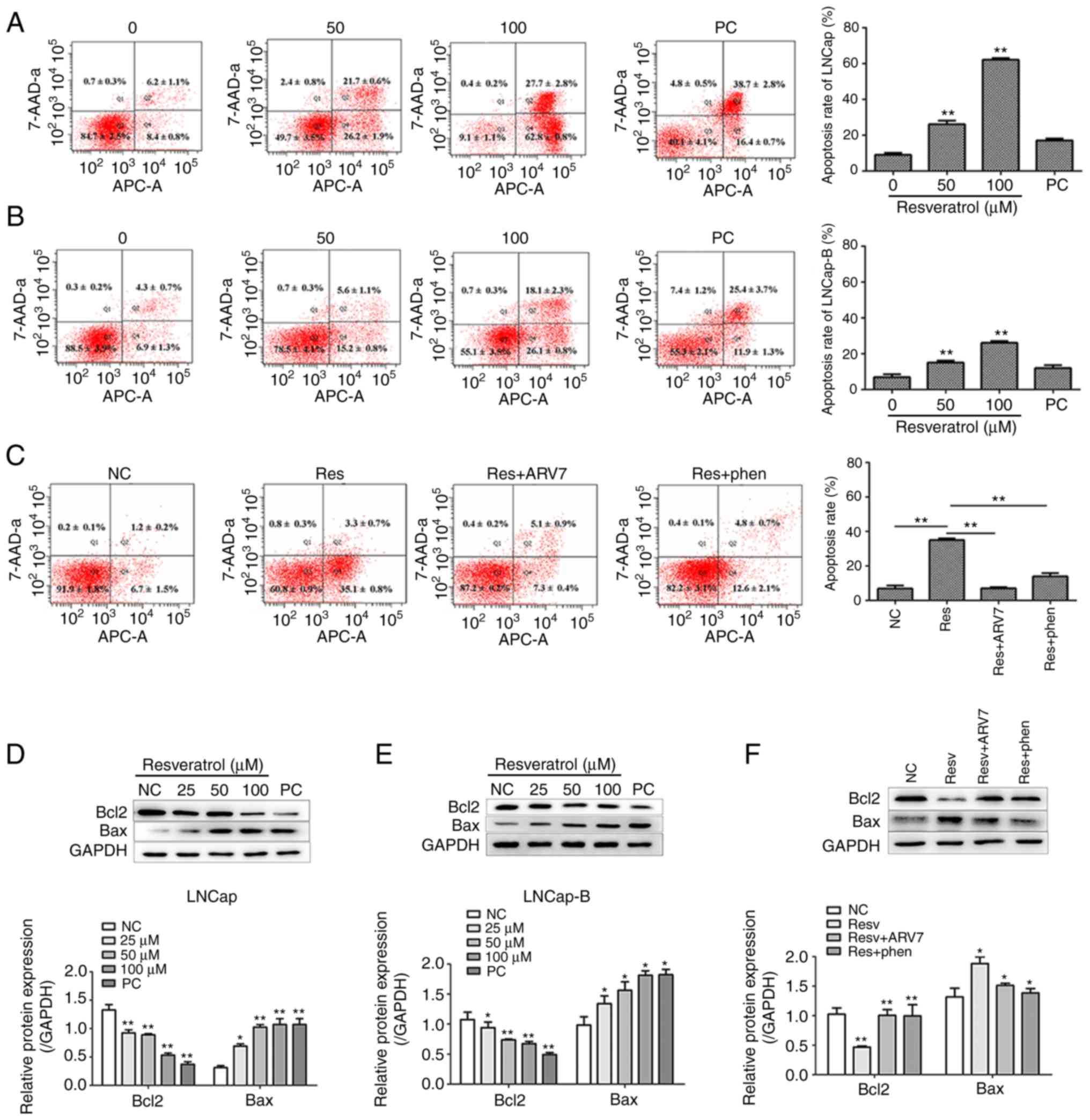
Effects of Res on the apoptosis of PCa
cells and the effect of ARV7 on apoptosis of LNCaP-B cells induced
by Res
Flow cytometry was used to measure the effects of
various concentrations of Res on apoptosis in LNCaP and LNCaP-B.
Compared with the NC group, after treatment of LNCaP and LNCaP-B
cells with various concentrations of Res (50 and 100 µM) for 24 h,
the apoptosis level of LNCaP cells increased by approximately 33.3
and 75.9%, respectively, and the apoptosis level of LNCaP-B cells
increased by approximately 9.6 and 33%, respectively. (Q1, Q2, Q3,
Q4 represented dead cells, late apoptotic cells, viable cells, and
early apoptotic cells, respectively.) Therefore, the total number
of early and late apoptotic cells in the two cell lines was
significantly higher compared with those in the control group. The
effect of Res on apoptosis of LNCaP and LNCaP-B cells was
concentration-dependent (P<0.05 and P<0.01; Fig. 2A and B, respectively).
The effects of overexpression of ARV7 on Res-induced
apoptosis in LNCaP-B cells were also studied. Upregulation of ARV7
suppressed Res-induced apoptosis by approximately 26%. It was
reported that Res+ARV7 and Res+phen treatment reversed Res-induced
apoptosis in LNCaP-B cells (Fig.
2C). In order to further study the relevant molecular
mechanisms, the levels of apoptosis-associated proteins were
measured using western blotting. The results showed that all
concentrations of Res increased the expression levels of
pro-apoptotic proteins Bax and the expression levels of
antiapoptotic protein Bcl2 were lower compared with those in
control group (Fig. 2D and E). In
addition, compared with the effect of Res on PCa cells, Res+ARV7
and Res+phen treatment increased the expression of antiapoptotic
protein Bcl2 and decreased the expression of pro-apoptotic protein
Bax (Fig. 2F). These results
suggested that Res not only inhibits the proliferation of PCa
cells, but also induces apoptosis. These results also suggested
that the pro-apoptotic effect of Res on LNCaP-B cells is associated
with the ARV7 and AKT pathway signaling pathways.
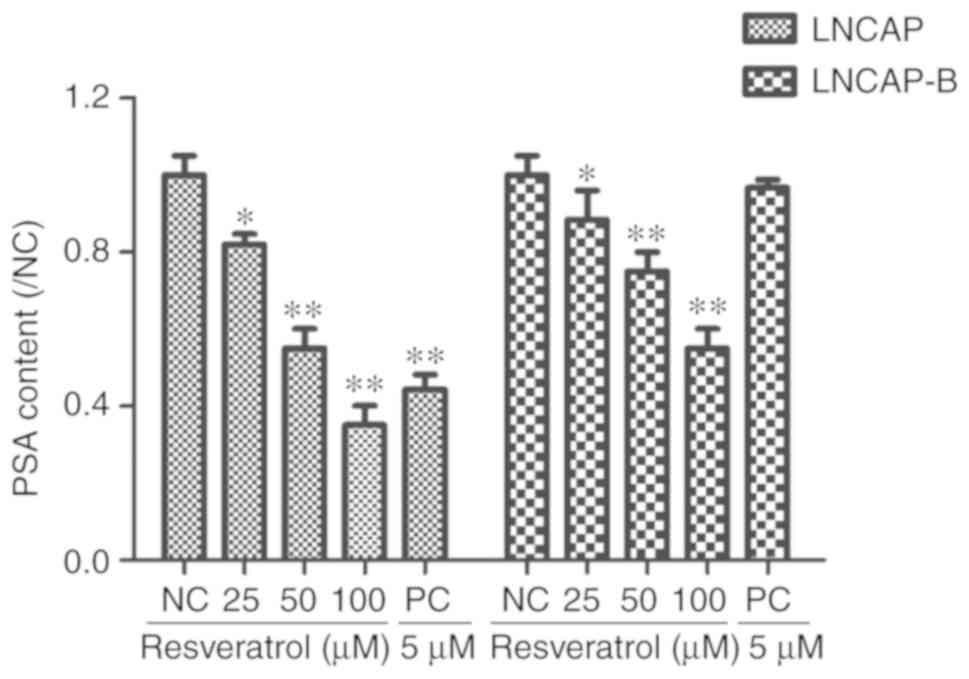
Effect of Res on secretion of PSA by
PCa cells
Chemiluminescence detection showed that various
concentrations of Res (25, 50 and 100 µM) and PC (5 µM) applied to
LNCaP and LNCaP-B cells for 24 h, caused PSA content in both cell
lines to be significantly lower compared with that of the control
group (*P<0.05 and **P<0.01). Furthermore, it was also
revealed that Res decreased PSA levels in LNCaP and LNCaP-B cells
in a concentration-dependent manner (Fig. 3). This suggested that Res inhibited
the secretion of PSA by LNCaP and LNCaP-B cells.
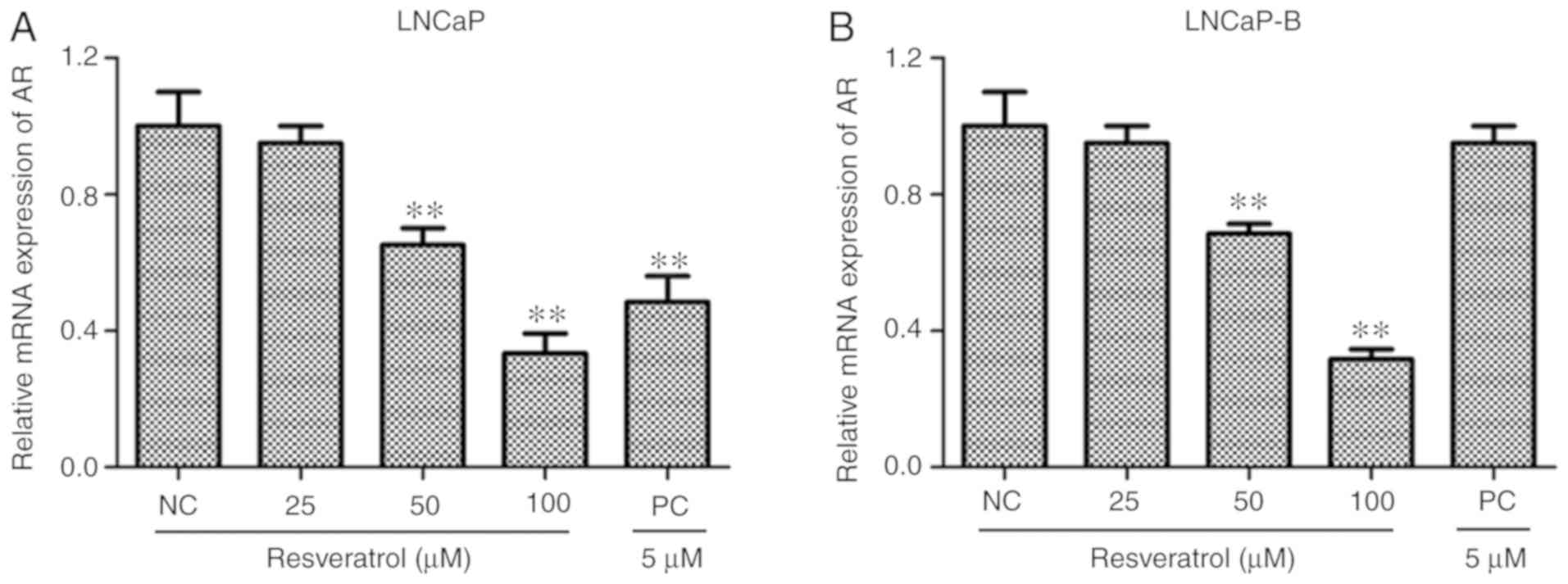
Effect of Res on AR mRNA levels in PCa
cells
The effect of various concentrations of Res on mRNA
levels in PCa cells was measured using RT-qPCR. It was demonstrated
that, after treatment of LNCaP and LNCaP-B cells with various
concentrations of Res (25, 50 and 100 µM) and PC (5 µM) for 24 h,
expression levels of AR mRNA in the two PCa cells were
significantly lower compared with those of the control group
(Fig. 4A and B). It was also
reported found that, with increasing Res concentrations, the effect
of Res on the downregulation of AR mRNA levels in PCa cells
was dose-dependent. These results indicated that Res inhibited the
expression levels of AR in LNCap and LNCap-B cells in a
dose-dependent manner.
Effect of Res on AR and ARV7 protein
levels in PCa cells
The effect of various concentrations of Res on
levels of AR protein in PCa cells was measured using western
blotting. Expression levels of AR protein in the two PCa cells were
significantly lower compared with those in the control group after
treatment of LNCaP and LNCaP-B cells with various concentrations of
Res (25, 50 and 100 µM) for 24 h. It was also shown that Res was
associated with decreases of AR expression in PCa cells in a
concentration-dependent manner (Fig. 5A
and B). Western blot analysis showed that Res was associated
with lower levels of AR protein in LNCaP and LNCaP-B cells.
Studies have shown that PCa castration-resistance is
associated with expression levels of ARV7 (27,28).
Therefore, expression levels of ARV7 in LNCaP and LNCaP-B cells
were examined using western blotting. ARV7 was expressed at low
levels in LNCaP cells but positive in LNCaP-B (Fig. 5C). This suggested that the hormone
resistance of LNCaP-B may be associated with high expression of
ARV7. The effect of Res on ARV7 expression was further examined and
it was revealed that Res inhibited ARV7 expression (Fig. 5D). This suggested that the inhibitory
effect of Res on hormone-resistant LNCaP-B cells may be associated
with the inhibition of ARV7 expression.
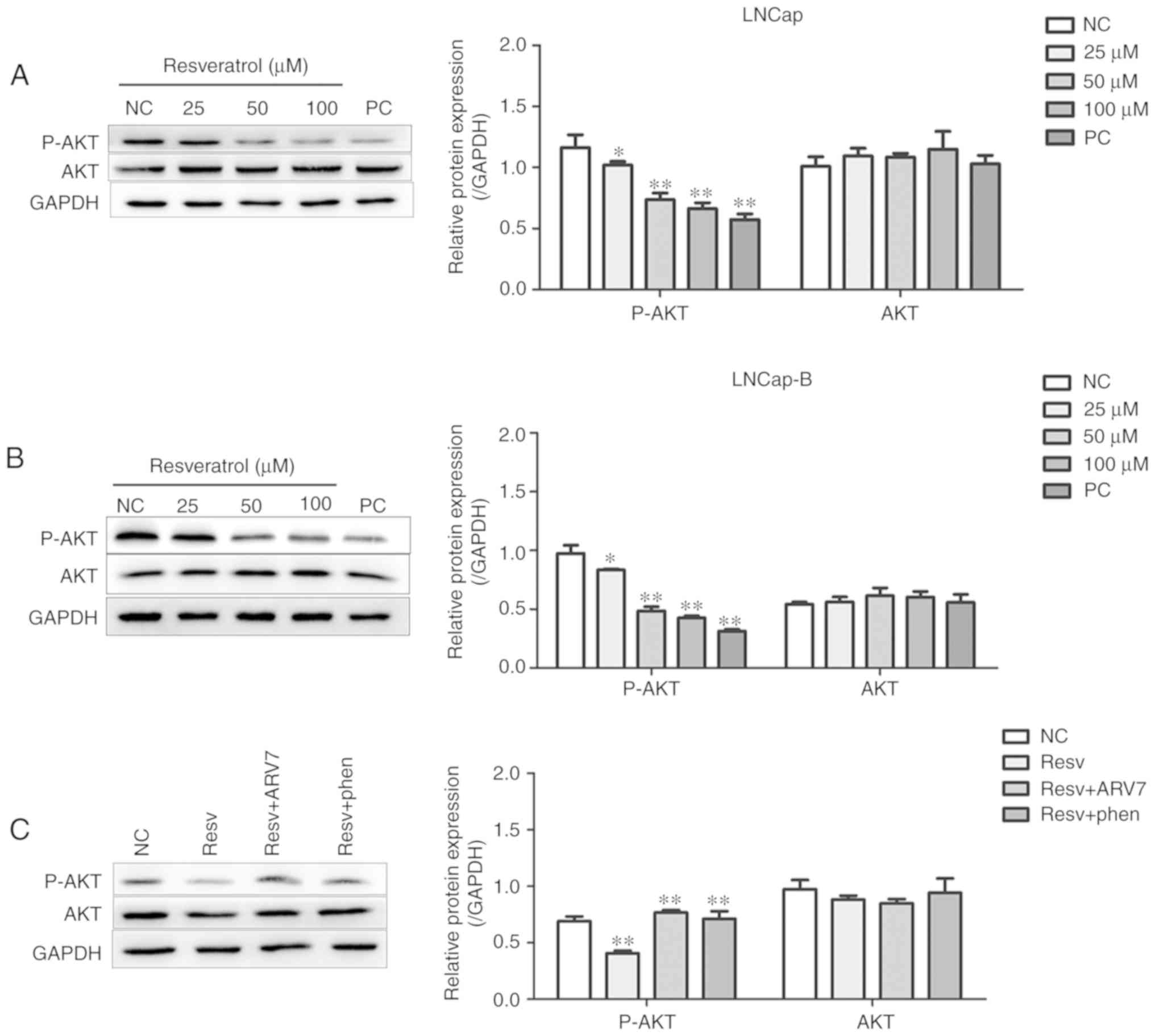
Effect of Res on the AKT signaling
pathway in PCa cells and the effect of ARV7 on the AKT signaling
pathway in LNCaP-B cells
Western blotting was used to measure the effect of
Res on the AKT signaling pathway in PCa cells. After treatment of
LNCaP and LNCaP-B cells with various concentrations of Res (25, 50
and 100 µM) for 24 h, levels of p-AKT in the two PCa cells were
significantly lower compared with those in the control group
(Fig. 6A and B). Res inhibited the
AKT phosphorylation in a concentration-dependent manner. These
results suggested that Res may have inhibited AKT phosphorylation
in LNCaP and LNCaP-B cells.
The molecular mechanism of Res-mediated ARV7
inhibition in PCa was further investigated. Compared with the Res
group, the Res+ARV7 and Res+AKT pathway activator groups showed
significantly higher levels of p-AKT (Fig. 6C). These results suggested that
overexpression of ARV7 significantly decreased Res-induced
phosphorylation of AKT.
Discussion
Advanced PCa is very common (29). It is usually treated with castration
combined with anti-androgen medications; however, after a certain
period of treatment, castration-resistant PCa often develops.
Studies have demonstrated that about 90% of patients with PCa
eventually develop into castration-resistant PCa and the average
survival time is only 16–18 months (30,31). For
castration-resistant PCa, despite the fact that comprehensive
treatment with chemotherapy and other treatments can prolong
progression-free survival (PFS), these treatments ultimately fail
to effectively prevent the progression of the disease (32). Therefore, improved treatments for
castration-resistant PCa need to be developed.
Res has a structure similar to diethylstilbestrol
and possesses estrogenic activity; it is considered to be a natural
phytoestrogen (33). Kuwajerwala
et al (34) showed that Res
inhibited the proliferation of PCa cells. Res is expected to become
a drug for clinical treatment of PCa. The present study
demonstrated that Res significantly inhibited the proliferation of
LNCaP and LNCaP-B cells in a concentration range of 25–100 µM. This
effect had a dual dependence on time and dose. In addition, the
number of early apoptotic cells and late apoptotic cells in the two
cell lines increased significantly after Res was applied to LNCaP
and LNCaP-B cells for 24 h. Moreover, the effect of Res on
apoptosis of LNCaP and LNCaP-B cells also increased in a
concentration-dependent manner. These results suggested that Res
inhibits proliferation in androgen-dependent PCa LNCaP cells and
hormone-resistant PCa LNCaP-B cells, possibly achieved by inducing
apoptosis in LNCaP and LNCaP-B cells.
The prostate is a target organ of androgens, which
are the most important factors affecting the growth of PCa.
Androgens bind to the AR and activate the AR signaling pathway,
thereby regulating PCa cell proliferation and apoptosis (35). A previous study have shown that
castration resistance in PCa involves abnormalities regarding the
AR, for example the AR signaling pathway remains activated
(36). In addition, another study
have shown that the AR is highly expressed in castration-resistant
PCa and there is amplification of AR mRNA (37). Therefore, antagonizing or blocking AR
activity remains key in castration treatment of PCa. PSA has been
suggested as a molecular target for prostate cancer because it is
not only active in prostate tissues, but also plays a key role in
the signaling pathway of PCa (14).
In the current study, the effects of various concentrations of Res
on PSA secretion and AR expression in PCa LNCaP and LNCaP-B cells
were examined, and on the levels of AKT phosphorylation in PCa
cells. It was shown that various concentrations of Res
significantly inhibited secretion of PSA by LNCaP and LNCaP-B
cells, inhibited the expression of AR mRNA and protein, and
inhibited AKT phosphorylation. This inhibition was
concentration-dependent. These data suggested that AR expression
changes and abnormal signaling pathways are important factors in
the treatment of hormone-resistant PCa cells, as well as
hormone-dependent PCa, and the emergence of AR-Vs is one of the
important AR changes.
A Previous study have shown that ARV7 mRNA and
protein levels are significantly increased in hormone-independent
PCa cells and clinical specimens, and are associated with
postoperative recurrence, distant metastasis and shortened survival
time (38). It is also hypothesized
that the structural features of AR-cleavage variants may activate
downstream target genes through androgen-independent pathways to
effect proliferation of PCa and eventually develop into
androgen-independent PCa (39). This
suggests that ARV7 may play an important role in
castration-resistant PCa. Moreover, it present study reported that
ARV7 expression was low in LNCaP cells and higher in LNCaP-B cells,
suggesting that the hormone resistance of LNCaP-B cells may be
associated with high expression of ARV7. This result was consistent
with the results of Guo et al (27), who suggested that ARV7 played an
important role in castration-resistant PCa. The current study
further examined the effect of Res on ARV7 expression and showed
that Res inhibited ARV7 expression. It was also further
demonstrated that inhibition of LNCaP-B cells by Res may be
associated with the inhibition of ARV7 expression.
A previous review suggested that the PI3K/AKT
signaling pathway is an important pathway affecting biological
processes, including cell proliferation, cycle progression,
migration and angiogenesis (40).
Liao et al (41) showed that
the intensity of AKT expression in prostate tumor tissues is
positively correlated with PSA levels, and that increased PSA
expression is associated with tumor progression. Another study
showed that the activation of the PI3K/AKT pathway promotes the
anti-apoptotic effect of PCa cells and the tumor progression
(42). AKT is frequently activated
in PCa cells, providing proliferation and survival signals, and
regulating AR activity through phosphorylation (43). In line with these studies, the
present results suggested that Res inhibits the AKT phosphorylation
in LNCaP and LNCaP-B cells, and that the regulation of AR
expression has a significant effect on hormone resistance. Res+ARV7
treatment elevated the levels of p-AKT, suggesting that
overexpression of ARV7 reversed the inhibition effect of Res on AKT
phosphorylation.in PCa. The inhibitory effect of
Res on the AKT pathway is associated with ARV7. The effect of
overexpression of ARV7 on Res inhibition of PCa proliferation and
apoptosis was also investigated. Compared with the Res group, the
Res+ARV7 and Res+phen groups restored the inhibitory effect of Res
on LNCaP-B cell proliferation. It was shown that Res inhibited the
proliferation of LNCaP-B cells in association with the ARV7 and AKT
pathways. The apoptosis assay showed that the Res+ARV7 and Res+phen
groups restored the proapoptotic effect of Res in LNCaP-B cells,
and that this effect was associated with the ARV7 and AKT
pathways.
Taken together, the present data suggested that Res
inhibits the proliferative capacity of androgen-dependent LNCaP
cells and hormone-resistant LNCaP-B cells, and induced apoptosis in
both of these PCa cell lines. Res also inhibited the secretion of
PSA. These effects may be associated with Res inhibiting AKT
phosphorylation in LNCaP and LNCaP-B cells and by regulation of AR
mRNA and protein expression levels. It was also demonstrated that
Res induced proliferation and apoptosis in LNCaP-B cells, possibly
by causing decreased expression of ARV7 and inhibiting the
activation of the AKT pathway. One limitation of the present study
is that it was limited to in vitro data. Therefore, in the
future, in vivo studies should be considered. In summary,
these findings show the value of investigating the anticancer
effects of Res and may provide a preliminary theoretical basis for
its clinical application in the treatment of PCa.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Funds of China (grant no. 81272833).
Availability of data and materials
All data generated or analyzed during this study
were included in this published article.
Authors' contributions
MSY and JJL designed the experiments. MSY, HST, ZL,
XJC and SHL performed the experiments. MSY, JRM and JJL were
involved in data collection and statistical analyses. MSY, SHL, JRM
and JJL wrote the article and prepared figures. JJL provided
guidance and the financial support. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Park JW, Jang WS, Koh DH, Ham WS, Rha KH,
Hong SJ and Choi YD: Impact of early salvage androgen deprivation
therapy in localized prostate cancer after radical prostatectomy: A
propensity score matched analysis. Yonsei Med J. 59:580–587. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharifi N, Gulley JL and Dahut WL:
Androgen deprivation therapy for prostate cancer. JAMA.
294:238–244. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scher HI, Beer TM, Higano CS, Anand A,
Taplin ME, Efstathiou E, Rathkopf D, Shelkey J, Yu EY, Alumkal J,
et al: Antitumour activity of MDV3100 in castration-resistant
prostate cancer: A phase 1–2 study. Lancet. 375:1437–1446. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taneja SS: Re: Abiraterone in metastatic
prostate cancer without previous chemotherapy. J Urol. 190:8802013.
View Article : Google Scholar
|
|
6
|
Shafi AA, Yen AE and Weigel NL: Androgen
receptors in hormone-dependent and castration-resistant prostate
cancer. Pharmacol Ther. 140:223–238. 2013. View Article : Google Scholar
|
|
7
|
Ryan CJ and Tindall DJ: Androgen receptor
rediscovered: The new biology and targeting the androgen receptor
therapeutically. J Clin Oncol. 29:3651–3658. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tilley WD, Buchanan G, Hickey TE and
Bentel JM: Mutations in the androgen receptor gene are associated
with progression of human prostate cancer to androgen independence.
Clin Cancer Res. 2:277–285. 1996.PubMed/NCBI
|
|
9
|
He B, Gampe RJ Jr, Kole AJ, Hnat AT,
Stanley TB, An G, Stewart EL, Kalman RI, Minges JT and Wilson EM:
Structural basis for androgen receptor interdomain and coactivator
interactions suggests a transition in nuclear receptor activation
function dominance. Mol Cell. 16:425–438. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shang Y, Myers M and Brown M: Formation of
the androgen receptor transcription complex. Mol Cell. 9:601–610.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Montgomery RB, Mostaghel EA, Vessella R,
Hess DL, Kalhorn TF, Higano CS, True LD and Nelson PS: Maintenance
of intratumoral androgens in metastatic prostate cancer: A
mechanism for castration- resistant tumor growth. Cancer Res.
68:4447–4454. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Waltering KK, Helenius MA, Sahu B, Manni
V, Linja MJ, Jänne OA and Visakorpi T: Increased expression of
androgen receptor sensitizes prostate cancer cells to low levels of
androgens. Cancer Res. 69:8141–8149. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen CD, Welsbie DS, Tran C, Baek SH, Chen
R, Vessella R, Rosenfeld MG and Sawyers CL: Molecular determinants
of resistance to antiandrogen therapy. Nat Med. 10:33–39. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moradi A, Srinivasan S, Clements J and
Batra J: Beyond the biomarker role: Prostate-specific antigen (PSA)
in the prostate cancer microenvironment. Cancer Metastasis Rev.
38:333–346. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reebye V, Querol Cano L, Lavery DN, Brooke
GN, Powell SM, Chotai D, Walker MM, Whitaker HC, Wait R, Hurst HC
and Bevan CL: Role of the HSP90-associated cochaperone p23 in
enhancing activity of the androgen receptor and significance for
prostate cancer. Mol Endocrinol. 26:1694–1706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin CY, Jan YJ, Kuo LK, Wang BJ, Huo C,
Jiang SS, Chen SC, Kuo YY, Chang CR and Chuu CP: Elevation of
androgen receptor promotes prostate cancer metastasis by induction
of epithelial-mesenchymal transition and reduction of KAT5. Cancer
Sci. 109:3564–3574. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu R, Dunn TA, Wei S, Isharwal S, Veltri
RW, Humphreys E, Han M, Partin AW, Vessella RL, Isaacs WB, et al:
Ligand-independent androgen receptor variants derived from splicing
of cryptic exons signify hormone-refractory prostate cancer. Cancer
Res. 69:16–22. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hörnberg E, Ylitalo EB, Crnalic S, Antti
H, Stattin P, Widmark A, Bergh A and Wikström P: Expression of
androgen receptor splice variants in prostate cancer bone
metastases is associated with castration-resistance and short
survival. PLoS One. 6:e190592011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stewart JR, Artime MC and O'Brian CA:
Resveratrol: A candidate nutritional substance for prostate cancer
prevention. J Nutr. 133 (7 Suppl):2440S–2443S. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Patel KR, Brown VA, Jones DJ, Britton RG,
Hemingway D, Miller AS, West KP, Booth TD, Perloff M, Crowell JA,
et al: Clinical pharmacology of resveratrol and its metabolites in
colorectal cancer patients. Cancer Res. 70:7392–7399. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aggarwal BB, Bhardwaj A, Aggarwal RS,
Seeram NP, Shishodia S and Takada Y: Role of resveratrol in
prevention and therapy of cancer: Preclinical and clinical studies.
Anticancer Res. 24:2783–2840. 2004.PubMed/NCBI
|
|
22
|
Aluyen JK, Ton QN, Tran T, Yang AE,
Gottlieb HB and Bellanger RA: Resveratrol: Potential as anticancer
agent. J Diet. (Suppl 9):45–56. 2012. View Article : Google Scholar
|
|
23
|
Delmas D, Lancon A, Colin D, Jannin B and
Latruffe N: Resveratrol as a chemopreventive agent: A promising
molecule for fighting cancer. Curr Drug Targets. 7:423–442. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jang YG, Go RE, Hwang KA and Choi KC:
Resveratrol inhibits DHT-induced progression of prostate cancer
cell line through interfering with the AR and CXCR4 pathway. J
Steroid Biochem Mol Biol. 192:1054062019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mitchell SH, Zhu W and Young CY:
Resveratrol inhibits the expression and function of the androgen
receptor in LNCaP prostate cancer cells. Cancer Res. 59:5892–5895.
1999.PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo Z, Yang X, Sun F, Jiang R, Linn DE,
Chen H, Chen H, Kong X, Melamed J, Tepper CG, et al: A novel
androgen receptor splice variant is up-regulated during prostate
cancer progression and promotes androgen depletion-resistant
growth. Cancer Res. 69:2305–2313. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sobhani N, Generali D, D'Angelo A, Aieta M
and Roviello G: Current status of androgen receptor-splice variant
7 inhibitor niclosamide in castrate-resistant prostate-cancer.
Invest New Drug. 36:1133–1137. 2018. View Article : Google Scholar
|
|
29
|
Deng Y, Bi R, Zhu Z, Li S, Xu B, Rather WA
and Wang C: A surveillance, epidemiology and end results database
analysis of the prognostic value of organ-specific metastases in
patients with advanced prostatic adenocarcinoma. Oncol Lett.
18:1057–1070. 2019.PubMed/NCBI
|
|
30
|
Saad F, Chi KN, Finelli A, Hotte SJ, Izawa
J, Kapoor A, Kassouf W, Loblaw A, North S, Rendon R, et al: The
2015 CUA-CUOG Guidelines for the management of castration-resistant
prostate cancer (CRPC). Can Urol Assoc J. 9:90–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Penning TM: Mechanisms of drug resistance
that target the androgen axis in castration resistant prostate
cancer (CRPC). J Steroid Biochem Mol Biol. 153:105–113. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thakur A, Vaishampayan U and Lum LG:
Immunotherapy and immune evasion in prostate cancer. Cancers.
5:569–590. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jang M, Cai L, Udeani GO, Slowing KV,
Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta
RG, et al: Cancer chemopreventive activity of resveratrol, a
natural product derived from grapes. Science. 275:218–220. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kuwajerwala N, Cifuentes E, Gautam S,
Menon M, Barrack ER and Reddy GP: Resveratrol induces prostate
cancer cell entry into s phase and inhibits DNA synthesis. Cancer
Res. 62:2488–2492. 2002.PubMed/NCBI
|
|
35
|
Isaacs JT and Isaacs WB: Androgen receptor
outwits prostate cancer drugs. Nat Med. 10:26–27. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Scher HI and Sawyers CL: Biology of
progressive, castration-resistant prostate cancer: Directed
therapies targeting the androgen-receptor signaling axis. J Clin
Oncol. 23:8253–8261. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Thakur MK, Heilbrun LK, Sheng S, Stein M,
Liu G, Antonarakis ES, Vaishampayan U, Dzinic SH, Li X, Freeman S,
et al: A phase II trial of ganetespib, a heat shock protein 90
Hsp90) inhibitor, in patients with docetaxel-pretreated metastatic
castrate-resistant prostate cancer (CRPC)-a prostate cancer
clinical trials consortium (PCCTC) study. Invest New Drugs.
34:112–118. 2005. View Article : Google Scholar
|
|
38
|
Vellky JE, Bauman TM, Ricke EA, Huang W
and Ricke WA: Incidence of androgen receptor and androgen receptor
variant 7 coexpression in prostate cancer. Prostate. 79:1811–1822.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hsu CL, Liu JS, Wu PL, Guan HH, Chen YL,
Lin AC, Ting HJ, Pang ST, Yeh SD, Ma WL, et al: Identification of a
new androgen receptor (AR) co-regulator BUD31 and related peptides
to suppress wild-type and mutated AR-mediated prostate cancer
growth via peptide screening and X-ray structure analysis. Mol
Oncol. 8:1575–1587. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen H, Zhou L, Wu X, Li R, Wen J, Sha J
and Wen X: The PI3K/AKT pathway in the pathogenesis of prostate
cancer. Front Biosci (Landmark Ed). 21:1084–1091. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liao Y, Grobholz R, Abel U, Trojan L,
Michel MS, Angel P and Mayer D: Increase of AKT/PKB expression
correlates with gleason pattern in human prostate cancer. Int J
Cancer. 107:676–680. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pilling AB and Hwang C: Targeting
prosurvival BCL2 signaling through Akt blockade sensitizes
castration-resistant prostate cancer cells to enzalutamide.
Prostate. 79:1347–1359. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lin HK, Hu YC, Yang L, Altuwaijri S, Chen
YT, Kang HY and Chang C: Suppression versus induction of androgen
receptor functions by the phosphatidylinositol 3-kinase/Akt pathway
in prostate cancer LNCaP cells with different passage numbers. J
Biol Chem. 278:50902–50907. 2003. View Article : Google Scholar : PubMed/NCBI
|