Introduction
As a type of hematopoietic system disease, acute
leukemia (AL) is characterized by the malignant transformation of
hematopoietic stem and precursor cells within the bone marrow (BM)
or thymus (1). AL primarily
comprises of acute lymphoblastic (ALL), myeloblastic (AML),
undifferentiated (AUL) and mixed-lineage leukemia (AMLL) (2). AL has become a global health concern
due to its increasing incidence over the past decade (3). The worldwide incidence of AML has
gradually increased from 63.84×103 cases in 1990 to
119.57×103 cases in 2017, showing an increase of 87.3%
(4). The USA has diagnosed
approximately 5,960 new cases of ALL and reported 1,470
ALL-associated mortalities in 2018 (5). To date, allogeneic hematopoietic stem
cell transplantation (allo-HSCT) is the only potentially curative
treatment for AL (6). However,
allo-HSCT is associated with high-risk of non-relapse-associated
mortality and disease relapse, which are causes of mortality of
patients with AL who receive allo-HSCT treatment (7). It has been reported that the incidence
of relapse and risk of non-relapse mortality of core binding factor
AML are 19.8 and 22.5% for relapse and 20.9 and 23.3% for
non-relapse mortality, respectively (8). Therefore, reliable prognostic factors
are necessary to predict patient outcomes at the time of
transplantation.
Positron emission tomography (PET)/CT using the
radio-labeled glucose analog
18F-2′-deoxy-2′-fluorodeoxyglucose (18F-FDG)
has been widely used for diagnosis, staging and prognosis
prediction of malignant disease, including hematological malignancy
(9,10). Although a number of studies
concerning the prognostic value of 18F-FDG PET/CT in
patients with multiple myeloma or lymphoma undergoing HSCT have
been published (11–13), the prognostic value of
18F-FDG PET/CT imaging in patients with AL has yet to be
established. The present study aimed to determine whether
18F-FDG PET/CT performed before and/or after allo-HSCT
could be used to predict clinical outcomes in AL.
Materials and methods
Ethical approval
The present retrospective study (trial registration
no. ChiCTR1900024823) was approved by the Ethics Committee of the
First Affiliated Hospital of Soochow University (Suzhou, China;
approval no. 2019055), with a waiver of informed consent.
Patients
From January 2011 to January 2019, patients with AL
who underwent 18F-FDG PET/CT before or after allo-HSCT
were retrospectively enrolled in the present study. The following
inclusion criteria were used: Histologically confirmed as AL and
patients who underwent 18F-FDG PET/CT before or after
allo-HSCT. The following exclusion criteria were used: i) Patients
with lymphoma cell leukemia, ii) digital image data unavailable for
retrospective analysis, iii) patients who received granulocyte
colony-stimulating factor (G-CSF) therapy <1 month before PET/CT
scan and iv) time interval between day 0 of allo-HSCT and PET/CT
scan >12 months. Patients were followed-up for ≥4 months after
allo-HSCT. Follow-up data were collected through clinic or over the
phone, with an average follow-up of 25.2 months.
A total of 96 patients with AL were retrospectively
reviewed in the present study. A total of 24 patients were excluded
from the final analysis. Among these, 15 patients had lymphoma cell
leukemia, three received G-CSF therapy <1 month before PET/CT
scan and six underwent PET/CT scan >12 months before or after
allo-HSCT. A total of 72 patients [21 female, 51 male; mean age ±
standard deviation (SD), 31±13 years; range, 7–60 years] were
retrospectively enrolled in the final analysis.
Risk stratification of patients with AML was
primarily assessed according to European Leukemia Net (ELN) 2017
(14) and risk stratification of
patients with ALL was primarily assessed according to the NCCN
Guidelines (15). For patients with
insufficient information for NCCN 2019 and ELN 2017, risk
stratification was independently evaluated by two experienced
hematologists, and results were recorded by consensus. According to
NCCN 2019 Guidelines, ALL was divided into high-risk and low-risk,
while AML was divided into high-risk, intermediate-risk and
low-risk according to ELN 2017. Therefore, in order to better
analyze risk stratification, low-risk and intermediate-risk
patients with AML were combined as low-risk ones.
Image acquisition
18F-FDG PET/CT imaging was performed
using a standard whole-body protocol as previously described
(15). All patients were fasted for
≥6 h before 18F-FDG PET/CT examination. The baseline
blood glucose level was <11 mmol/l. At 60 min after
administration of 18F-FDG (dose, 0.12 mCi/kg),
18F-FDG PET/CT was performed using a Discovery STE
PET/CT scanner (General Electric Medical Systems, Milwaukee, WI,
USA). Transmission data were acquired via whole-body CT (140 kV;
120 mA; pitch, 1.75; transaxial FOV, 700 mm; slice thickness, 3.75
mm; rotation time, 0.8 sec). PET emission data in 3D-mode were
analyzed from the vertex of the skull to the proximal thigh, with
2–3 min per bed position. Transverse PET slices were reconstructed
using a standard iterative algorithm (ordered-subset expectation
maximization), with low-dose CT data utilized for image fusion and
attenuation correction, using the Xeleris workstation software (GE
Healthcare; ADW4.1).
Image analysis
18F-FDG PET/CT images were independently
evaluated by two experienced nuclear medicine physicians who were
blinded to the clinical information of all subjects, and the
results were recorded by consensus. Increased 18F-FDG
uptake in the BM and spleen was defined as diffuse and/or focal
18F-FDG uptake ≥ normal liver (16,17). The
region of interest located in the right lobe of the liver served as
the reference. Diffuse or focal 18F-FDG uptake above the
mediastinal blood pool or background uptake excluding infectious,
inflammatory or other neoplastic diseases was considered as other
PET-positive (18).
Statistical analysis
GraphPad Prism (version 5.0; GraphPad Software,
Inc.) and SPSS software (version 19.0; IBM, Corp.) were used for
statistical analysis. In order to assess the prognostic value of
18F-FDG PET/CT imaging, overall survival (OS) and
disease-free survival (DFS) were selected as the endpoints. OS was
defined as the period from the date of allo-HSCT to the date of
death. DFS was defined as the period from the date of allo-HSCT to
the date of relapse or death. OS and DFS were estimated via the
Kaplan-Meier method, and differences among groups were evaluated by
a log-rank test. The relation between factors associated with OS
and DFS was estimated using the Kaplan-Meier method, and the
log-rank test was used for univariate analysis. For multivariate
analysis, risk factors with statistical significance upon
univariate analysis were introduced into a Cox proportional hazards
model. P<0.05 was considered to indicate a statistically
significant difference.
Results
Characteristics of patients
A total of 72 patients (21 females, 51 males; mean
age ± SD, 31±13 years; range, 7–60 years) were enrolled between
January 2011 and January 2019. Of these 72 patients, 33 had ALL, 36
had AML and three had AMLL. In addition, 19 patients underwent
18F-FDG PET/CT before allo-HSCT, 46 underwent
18F-FDG PET/CT after allo-HSCT and seven underwent
18F-FDG PET/CT both before and after allo-HSCT.
Therefore, 79 examinations of 72 patients were enrolled in the
study. The median follow-up was 21 months. The white blood cell
count at diagnosis was >20×109/l in 27 patients. The
lactate dehydrogenase levels (>245 U/L) were high in 42 patients
(19). Detailed characteristics of
patients are presented in Table
I.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
Characteristics | N |
|---|
| Age, years |
|
|
≤20 | 20 |
|
>20 | 52 |
| Mean ±
standard deviation (range)a | 31±13 (7–60) |
| Sex |
|
|
Female | 21 |
|
Male | 51 |
| Acute leukemia
type |
|
|
Lymphoblastic | 33 |
|
Myeloblastic | 36 |
|
Mixed-lineage | 3 |
| De novo or
secondary |
|
| De
novo | 69 |
|
Secondary | 3 |
| Before or after
allogeneic hematopoietic stem cell transplantation |
|
|
Before | 19 |
|
After | 46 |
| Before
and after | 7 |
| Median
follow-up | 21 months |
| Lactate
dehydrogenase |
|
|
High | 42 |
|
Normal | 30 |
| White blood cell
count at diagnosis, ×109/l |
|
|
≤20 | 45 |
|
>20 | 27 |
| Risk
stratification |
|
|
Good | 20 |
|
Poor | 49 |
|
Unknown | 3 |
| Disease status |
|
|
Complete remission | 54 |
|
Non-remission | 18 |
| Pre-transplantation
MRD |
|
|
Positive | 43 |
|
Negative | 18 |
|
Post-transplantation MRD |
|
|
Positive | 22 |
|
Negative | 20 |
PET/CT results
A total of 63 examinations were 18F-FDG
PET positive, whereas 16 examinations were 18F-FDG PET
negative. Increased BM 18F-FDG uptake was observed in
24% (19/79) of examinations, including a homogeneous/diffuse
pattern throughout the body in 13 examinations and an
inhomogeneous/focal pattern or co-existing inhomogeneous/focal and
homogeneous/diffuse patterns in six examinations. Increased splenic
18F-FDG uptake was detected in 14% (11/79) of
examinations, including a homogeneous/diffuse pattern in 10
examinations and an inhomogeneous/focal pattern in one examination.
18F-FDG-avid lymph nodes were observed in 38% (30/79) of
examinations, including lymph nodes >1.5 cm in eight
examinations and lymph nodes ≤1.5 cm in 22 examinations. One
patient with lymph nodes <1.5 cm in short axis demonstrated
inflammation.
ENEMES involvement
was detected by PET/CT in 44% (35/79) of examinations. The most
common 18F-FDG-avid site was bone [16% (13/79) of
examinations]. Other 18F-FDG-avid sites included
nasopharynx (eight, including two inflammations), soft tissue
(seven), lung (five, including three inflammations), breast (four),
testes (three), brain (three, including one abscess), kidney,
heart, skin, pleura, parotid gland, liver (two each), adrenal,
uterus, eyelid and submandibular gland (one examination each).
Prognosis prediction of all PET/CT
examinations
Univariate and multivariate Cox regression analysis
of all 79 examinations was used to assess the effect of
18F-FDG PET/CT variables and clinical parameters on OS
and DFS (Tables II and III). In univariate analysis,
18F-FDG-avid lymph nodes >1.5 cm,
ENEMES involvement, disease status
and post-transplantation minimal residual disease (MRD) were
significantly associated with OS and DFS. However, only
ENEMES involvement [hazard ratio
(HR), 6.399; 95% CI, 1.843–22.224; P=0.003] and disease status (HR,
0.330; 95% CI, 0.128–0.848; P=0.021) were significantly associated
with OS in multivariate analysis and only post-transplantation MRD
was significantly associated with DFS (HR, 4.381; 95% CI,
1.594–12.040; P=0.004).
 | Table II.Univariate and multivariate analysis
of OS of all PET/CT examinations. |
Table II.
Univariate and multivariate analysis
of OS of all PET/CT examinations.
| A, Univariate
analysis |
|---|
|
|---|
| Parameters | P-value | Hazard ratio (95%
CI) |
|---|
| Sex, Male vs.
Female | 0.8524 | 0.9200
(0.3840–2.2070) |
| Age, >21
years | 0.5771 | 1.3620
(0.4600–4.0390) |
| Risk
stratification | 0.1642 | 1.8720
(0.7740–4.5270) |
| White blood cell
count, >20×109/l | 0.0772 | 2.1740
(0.9190–5.1420) |
| Elevated lactate
dehydrogenase, >245 U/l | 0.5077 | 0.7590
(0.3350–1.7170) |
| Acute leukemia
type | 0.1690 | 1.8250
(0.7740–4.3000) |
| PET-positive | 0.1234 | 2.0900
(0.8182–5.3370) |
| Increased BM
18F-FDG uptake | 0.4406 | 0.6850
(0.2620–1.7910) |
| Focal BM
18F-FDG uptake | 0.3840 | 1.2950
(0.0360–4.3590) |
| Increased splenic
18F-FDG uptake | 0.6955 | 0.8480
(0.3720–1.9340) |
|
18F-FDG-avid lymph nodes
>1.5 cm | 0.0140 | 2.8710
(1.0950–7.5290) |
|
ENEMES
involvement | 0.0003 | 4.5450
(2.0080–10.2900) |
| Disease status | 0.0009 | 0.1930
(0.0726–0.5100) |
| Pre-transplantation
MRD | 0.1737 | 2.1150
(0.7188–6.2240) |
|
Post-transplantation MRD |
0.0017a | 4.5940
(1.7740–11.9000) |
|
| B, Multivariate
analysis |
|
|
Parameters | P-value | Hazard ratio
(95% CI) |
|
|
ENEMES
involvement | 0.0030 | 6.3990
(1.8430–22.2240) |
|
18F-FDG-avid lymph nodes
>1.5 cm | 0.6620 | – |
| Disease status | 0.0210 | 0.3300
(0.1280–0.8480) |
|
Post-transplantation MRD | 0.1180 | – |
 | Table III.Univariate and multivariate analysis
of disease-free survival of all PET/CT examinations. |
Table III.
Univariate and multivariate analysis
of disease-free survival of all PET/CT examinations.
| A, Univariate
analysis |
|---|
|
|---|
| Parameters | P-value | Hazard ratio (95%
CI) |
|---|
| Sex, Male vs.
Female | 0.8031 | 0.900
(0.398–2.038) |
| Age, >21
years | 0.5537 | 1.339
(0.510–3.520) |
| Risk
stratification | 0.9173 | 0.958
(0.423–2.166) |
| White blood cell
count, >20×109/l | 0.4990 | 1.307
(0.602–2.840) |
| Elevated lactate
dehydrogenase, >245 U/l | 0.2326 | 0.632
(0.297–1.343) |
| Acute leukemia
type | 0.3659 | 1.426
(0.661–3.078) |
| PET-positive | 0.2950 | 1.621
(0.656–4.004) |
| Increased BM
18F-FDG uptake | 0.8546 | 0.920
(0.376–2.249) |
| Focal BM
18F-FDG uptake | 0.5620 | 1.567
(0.470–5.226) |
| Increased splenic
18F-FDG uptake | 0.2911 | 0.661
(0.306–1.426) |
|
18F-FDG-avid lymph nodes
>1.5 cm | 0.0080 | 3.743
(1.515–9.245) |
|
ENEMES
involvement | 0.0005 | 3.793
(1.782–8.071) |
| Disease status | 0.0091 | 0.285
(0.111–0.732) |
| Pre-transplantation
MRD | 0.3819 | 1.489
(0.610–3.634) |
|
Post-transplantation MRD | 0.0016 | 4.171
(1.722–10.100) |
|
| B, Multivariate
analysis |
|
|
Parameters | P-value | Hazard ratio
(95% CI) |
|
|
ENEMES
involvement | 0.0950 | – |
|
18F-FDG-avid lymph nodes
>1.5 cm | 0.2190 | – |
| Disease status | 0.2340 | – |
|
Post-transplantation MRD | 0.0040a | 4.381
(1.594–12.040) |
Kaplan-Meier analysis for OS of all 79 examinations
showed that patients with negative
ENEMES involvement and complete
remission (CR) had a better OS compared with patients with
non-remission (NR) and positive
ENEMES involvement (Fig. 1A and C, respectively). The median OS
was 28 months in patients with
ENEMES involvement; patients
without ENEMES involvement did not
reach the median OS (P<0.001). The median OS was 24 months in
patients with CR; patients with NR did not reach the median OS
(P<0.001). Kaplan-Meier analysis demonstrated that patients with
positive ENEMES involvement and NR
exhibited a shorter DFS compared with patients with negative
ENEMES involvement and CR
(Fig. 1B and D, respectively).
Kaplan-Meier analysis for OS showed that the patients with positive
post-transplantation MRD had a shorter OS (median OS, 24 months)
compared with patients in the negative post-transplantation MRD
group (median OS, not reached; P<0.01; Fig. 1E). Kaplan-Meier analysis for DFS
showed that patients with positive post-transplantation MRD had a
shorter DFS (median DFS, 29 months) compared with the patients in
the negative post-transplantation MRD group (median DFS, not
reached; P<0.01; Fig. 1F).
Prognosis prediction of examinations
after or before allo-HSCT
For 53 examinations after allo-HSCT,
18F-FDG-avid lymph nodes >1.5 cm (OS, P=0.010; DFS,
P=0.014), ENEMES involvement (OS,
P=0.0044; DFS, P=0.0002) (Fig. 2C and
D, respectively), disease status (OS, P=0.008; DFS, P=0.467)
(Fig. 2A and B respectively) and
post-transplantation MRD (OS, P=0.046; DFS, P=0.034) were all
univariately associated with OS and DFS (Tables IV and V). In multivariate analysis,
ENEMES involvement (OS: HR, 7.203;
95% CI, 1.510–34.369; P=0.013; DFS: HR, 3.671; 95% CI;
1.145–11.768; P=0.029) and disease status (OS: HR, 0.195; 95% CI,
0.050–0.762; P=0.019; DFS: HR, 0.278; 95% CI, 0.091–0.851; P=0.025)
were significantly associated with OS and DFS (Tables IV and V). For 26 examinations before allo-HSCT,
univariate analysis showed that
ENEMES involvement (P=0.0332),
post-transplantation MRD (P=0.0036) and disease status (P=0.0217)
were significantly associated with OS (Table VI). Multivariate Cox regression
analysis showed that only post-transplantation MRD was
significantly associated with OS (HR, 11.455; 95% CI, 1.336–98.179;
P=0.026) (Table VI).
Post-transplantation MRD was significantly associated with DFS
(P=0.0065) (Table VII).
 | Table IV.Univariate and multivariate analysis
of OS after allo-HSCT. |
Table IV.
Univariate and multivariate analysis
of OS after allo-HSCT.
| A, Univariate
analysis. |
|---|
|
|---|
| Parameters | P-value | Hazard ratio (95%
CI) |
|---|
| Sex, Male vs.
Female | 0.4427 | 1.48400
(0.54190–4.06200) |
| Age, >21
years | 0.9861 | 1.013000
(0.22840–4.49700) |
| Risk
stratification | 0.1832 | 1.95600
(0.72830–5.25600) |
| White blood cell
count, >20×109/l | 0.1070 | 3.33000
(1.24000–8.94500) |
| Elevated lactate
dehydrogenase, >245 U/l | 0.6205 | 0.79020
(0.31100–2.00700) |
| Acute leukemia
type | 0.3711 | 0.65000
(0.25300–1.67100) |
| Increased bone
marrow 18F-FDG uptake | 0.8303 | 0.81620
(0.12750–5.22700) |
| Increased splenic
18F-FDG uptake | 0.7893 | 0.87170
(0.31830–2.38700) |
|
18F-FDG-avid lymph nodes
>1.5 cm | 0.0100a | 0.11100
(0.02100–0.57500) |
|
ENEMES
involvement | 0.0044 | 3.87800
(1.52600–9.85400) |
| Disease status | 0.0080a | 0.20440
(0.06316–0.66130) |
| Pre-transplantation
MRD | 0.0743 | 2.83200
(0.90290–8.88200) |
|
Post-transplantation MRD | 0.0463 | 3.24300
(1.01900–10.32000) |
|
| B, Multivariate
analysis |
|
|
Parameters | P-value | Hazard ratio
(95% CI) |
|
|
ENEMES
involvement | 0.0130 | 7.20300
(1.51000–34.36900) |
|
18F-FDG-avid lymph nodes
>1.5 cm | 0.8920 | – |
| Disease status | 0.0190 | 0.19500
(0.05000–0.76200) |
 | Table V.Univariate and multivariate analysis
of disease-free survival after allo-HSCT. |
Table V.
Univariate and multivariate analysis
of disease-free survival after allo-HSCT.
| A, Univariate
analysis |
|---|
|
|---|
| Parameters | P-value | Hazard ratio (95%
CI) |
|---|
| Sex, Male vs.
Female | 0.2981 | 1.6600
(0.6391–4.3110) |
| Age, >21
years | 0.9243 | 0.9405
(0.2654–3.3320) |
| Risk
stratification | 0.8912 | 1.0650
(0.4308–2.6340) |
| White blood cell
count, >20×109/l | 0.3043 | 1.5590
(0.6680–3.6400) |
| Elevated lactate
dehydrogenase, >245 U/l | 0.1586 | 0.5393
(0.2286–1.2720) |
| Acute leukemia
type | 0.4395 | 0.7129
(0.3023–1.6810) |
| Increased bone
marrow 18F-FDG uptake | 0.2394 | 3.6020
(0.4259–30.4600) |
| Increased splenic
18F-FDG uptake | 0.4104 | 0.6315
(0.2114–1.8870) |
|
18F-FDG-avid lymph nodes
>1.5 cm | 0.0143 | 0.1980
(0.0560–0.7060) |
|
ENEMES
involvement | 0.0002 | 5.03700
(2.1550–11.7700) |
| Disease status | 0.0467 | 0.3375
(0.1157–0.9842) |
| Pre-transplantation
MRD | 0.5118 | 1.4060
(0.5081–3.8890) |
|
Post-transplantation MRD | 0.0335 | 3.0720
(1.0920–8.6460) |
|
| B, Multivariate
analysis |
|
|
Parameters | P-value | Hazard ratio
(95% CI) |
|
|
ENEMES
involvement | 0.0290 | 3.6710
(1.1450–11.7680) |
|
18F-FDG-avid lymph nodes
>1.5 cm | 0.0630 | – |
| Disease status | 0.0250 | 0.2780
(0.0910–0.8510) |
 | Table VI.Univariate and multivariate analysis
of OS before allo-HSCT. |
Table VI.
Univariate and multivariate analysis
of OS before allo-HSCT.
| A, Univariate
analysis |
|---|
|
|---|
| Parameters | P-value | Hazard ratio (95%
CI) |
|---|
| Sex, Male vs.
Female | 0.08140 | 0.21240
(0.037180–1.21300) |
| Age, >21
years | 0.54090 | 1.77200
(0.28310–11.10000) |
| Risk
stratification | 0.62150 | 1.71400
(0.25320–11.61000) |
| White blood cell
count, >20×109/l | 0.72150 | 0.69950
(0.09806–4.99100) |
| Elevated lactate
dehydrogenase, >245 U/l | 0.86300 | 0.86450
(0.16540–4.51900) |
| Acute leukemia
type | 0.33770 | 3.16700
(0.29990–33.45000) |
| Increased bone
marrow 18F-FDG uptake | 0.45810 | 0.52100
(0.09307–2.91600) |
| Increased splenic
18F-FDG uptake | 0.90260 | 1.12300
(0.17450–7.23000) |
|
18F-FDG-avid lymph nodes
>1.5 cm | 0.77450 | 0.31300
(0.01500–6.40900) |
|
ENEMES
involvement |
0.03320a | 6.16500
(1.15600–32.88000) |
| Disease status |
0.02170a | 0.11280
(0.01750–0.72740) |
| Pre-transplantation
MRD | 0.62630 | 0.50040
(0.03083–8.12000) |
|
Post-transplantation MRD |
0.00360a | 14.58000
(2.40600–88.37000) |
|
| B, Multivariate
analysis |
|
|
Parameters | P-value | Hazard ratio
(95% CI) |
|
|
ENEMES
involvement | 0.32900 | – |
| Disease status | 0.69100 | – |
|
Post-transplantation MRD |
0.02600a | 11.45500
(1.33600–98.17900) |
 | Table VII.Univariate analysis of disease-free
survival before allo-HSCT. |
Table VII.
Univariate analysis of disease-free
survival before allo-HSCT.
| Parameters | P-value | Hazard ratio (95%
CI) |
|---|
| Sex, Male vs.
Female | 0.3185 | 0.42490
(0.07905–2.28400) |
| Age, >21
years | 0.4728 | 1.90700
(0.32730–11.11000) |
| Risk
stratification | 0.7556 | 0.75070
(0.12340–4.56700) |
| White blood cell
count, >20×109/l | 0.1786 | 0.26510
(0.03831–1.83500) |
| Elevated lactate
dehydrogenase, >245 U/l | 0.8126 | 0.82120
(0.16100–4.18700) |
| Acute leukemia
type | 0.9503 | 1.06000
(0.17150–6.54600) |
| Increased BM
18F-FDG uptake | 0.8113 | 1.22500
(0.23190–6.46900) |
| Increased splenic
18F-FDG uptake | 0.8397 | 0.84530
(0.16590–4.30600) |
|
18F-FDG-avid lymph nodes
>1.5 cm | 0.1130 | 0.17000
(0.01400–2.09100) |
|
ENEMES
involvement | 0.6853 | 1.40400
(0.27180–7.25700) |
| Disease status | 0.0633 | 0.14470
(0.01882–1.11300) |
| Pre-transplantation
MRD | 0.6146 | 1.63900
(0.23960–11.21000) |
|
Post-transplantation MRD | 0.0065 | 15.91000
(2.17100–116.60000) |
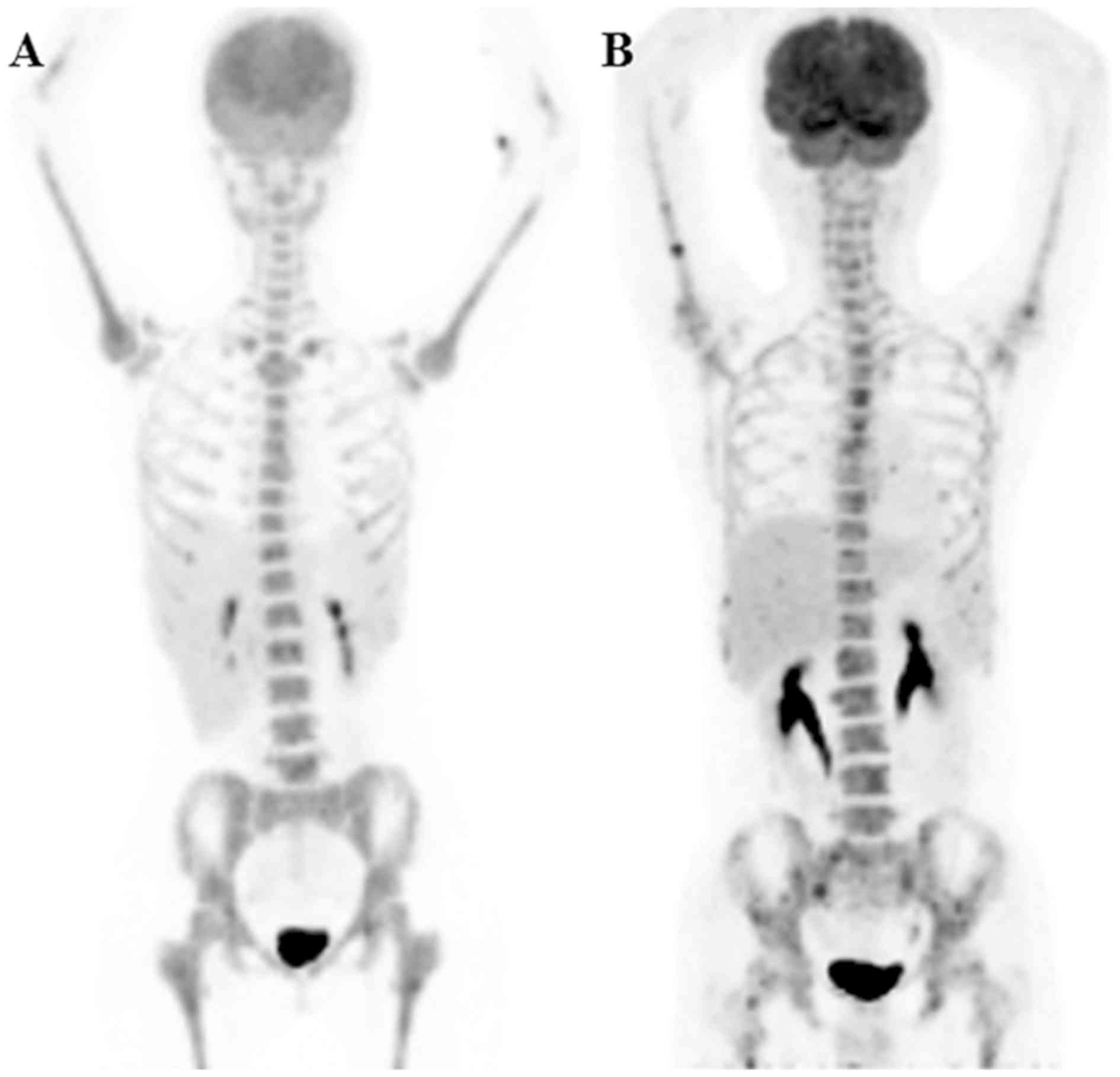
An example of diffuse homogeneous BM uptake and
co-existence of focal and diffuse BM uptake is shown in Fig. 3. Fig.
4 presents an example of 18F-FDG PET/CT examinations
with splenic and lymph node uptake. Figs. 5 and 6
present examples of 18F-FDG PET/CT examinations with
ENEMES site uptake.
Discussion
18F-FDG PET/CT is not regularly used in
the assessment of leukemia (20).
However, a number of clinical studies and case reports have
demonstrated the potential of 18F-FDG PET/CT in the
diagnosis of leukemic bone marrow infiltration and extramedullary
disease (EMD), evaluation of granulocytic sarcoma, detection of
Richter's syndrome and assessment of graft vs. host disease
(21–23). The present study aimed to investigate
the prognostic value of 18F-FDG PET/CT in patients with
AL treated with allo-HSCT. Although increased 18F-FDG
uptake by BM, spleen and lymph nodes was observed in patients with
AL, none of these factors were independent predictors of OS. Only
ENEMES involvement was
significantly associated with OS.
Since AL is a hematological malignancy that
originates from BM, increased BM uptake of 18F-FDG can
be observed in patients with AL (24). However, increased BM
18F-FDG uptake can also be observed in benign etiologies
and other types of malignant infiltration (25,26).
Jeong et al (27), performed
a meta-analysis to evaluate the prognostic value of
18F-FDG BM uptake in patients with a number of types of
solid tumor and found that patients with a low level of
18F-FDG BM uptake have a longer OS compared with those
with high levels of 18F-FDG BM uptake. Abe et al
(28) demonstrated that patients
with peripheral T cell lymphoma with BM involvement detected by
PET/CT exhibited a significantly shorter OS compared with those
without BM involvement, even among patients with negative BM
histology. However, certain studies have drawn the opposite
conclusion that high levels of 18F-FDG BM uptake have no
impact on survival, which is consistent with the results of the
present study (29,30). However, none of the aforementioned
studies investigated patients with AL. Elevated 18F-FDG
BM uptake in patients with AL may be different from that in other
patients, because BM is the source of leukemic cells and the
primary site of leukemia. Moreover, elevated 18F-FDG BM
uptake may be associated with reactive myelopoiesis and
inflammation, which occurs more frequently in patients with AL
(31).
The spleen is a primary location of extramedullary
AL (10). In numerous studies
involving patients with a different types of cancer, splenic
18F-FDG uptake has been demonstrated to be an
independent prognostic factor for predicting recurrence of cancer
or OS (32,33). However, the present study
demonstrated that elevated splenic 18F-FDG uptake had no
impact on prognosis of patients with AL. It is well known that the
spleen functions as a coordinator of immune response, a filter of
the circulating blood, and a reservoir for circulating cells and
platelets (34). Additionally, the
spleen has several responsibilities, including hemoglobin
degradation, hematopoiesis and iron recovery and plasma volume
regulation (35). For patients with
solid tumors, splenic metabolism primarily reflects the systemic
inflammatory response to cancer (36). However, splenic metabolism on
18F-FDG PET/CT may represent the complex processes of
hematopoiesis, which reflects both systemic inflammation and
hematological imbalance (32).
There is debate concerning the prognostic
significance of EMD in AL. A number of studies involving patients
with AL have reported that EMD is an independent prognostic factor
for OS (37,38), whereas other studies have
demonstrated that EMD has no impact on prognosis (39,40). EMD
in these studies was diagnosed by clinical examination. Since not
all extramedullary sites are easily detectable, EMD may have been
under-diagnosed. 18F-FDG PET/CT is a sensitive imaging
modality for diagnosing EMD in AL (7,19). Kumar
et al (41) compared
18F-FDG PET/CT and CT in terms of response and prognosis
of patients with AML with EMD and found that PET/CT can identify
more lesions and cases of metabolically progressive disease
compared with CT alone, thus affecting management. In accordance
with these results, the present study demonstrated that
ENEMES involvement detected by
18F-FDG PET/CT could serve as an adverse prognostic
factor of patients with AL before and/or after allo-HSCT.
There are a number of limitations in the present
study. First, it was a retrospective study with a relatively small
number of patients. Further prospective studies with larger sample
size are necessary to confirm the findings of the present study.
Second, the time interval between allo-HSCT and PET/CT scans was
heterogeneous, ranging from 0–12 months. PET/CT scans before and
after allo-HSCT were only performed in seven patients. Since
18F-FDG PET/CT is not regularly used in the assessment
of leukemia, there are no conclusive data on the optimum interval
between allo-HSCT and PET/CT scans. Moreover, the retrospective
nature of the present study did not permit regulation of the time
interval between allo-HSCT and PET/CT evaluation. Further multiple
time point studies are required to identify the optimum time point
for PET/CT scans. Finally, not all positive
ENEMES lesions, particularly EMD
in lymph nodes, were confirmed by histopathology.
The present data indicated that 18F-FDG
PET/CT imaging serves a key prognostic role in the evaluation of
patients with AL before and/or after allo-HSCT.
ENEMES involvement detected by
18F-FDG PET/CT may identify patients with AL with an
unfavorable outcome. Prospective clinical studies with larger
cohorts are required to conclusively define the prognostic role of
18F-FDG PET/CT in patients with AL treated with
allo-HSCT.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural
Science Foundation of China (grant no. 81601522), Medical Youth
Talent Project of Jiangsu Province (grant no. QNRC2016749) and
Suzhou People's Livelihood Science and Technology Project (grant
no. SYS2019038).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
ZXZ, YYZ and SMD conceived the study and wrote and
revised the manuscript. JW, TTZ and JHL reviewed, collected and
analyzed the data. BZ, QRL and SMD designed the study and acquired
the data. All authors contributed to the drafting of the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present retrospective study (trial registration
no. ChiCTR1900024823) was approved by the Ethics Committee of the
First Affiliated Hospital of Soochow University (Suzhou, China;
approval no. 2019055), with a waiver of informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gacha-Garay MJ, Niño-Joya AF, Bolaños NI,
Abenoza L, Quintero G, Ibarra H, Gonzalez JM, Akle V and
Garavito-Aguilar ZV: Pilot study of an integrative new tool for
studying clinical outcome discrimination in acute leukemia. Front
Oncol. 9:2452019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takahashi K, Wang F, Morita K, Yan Y, Hu
P, Zhao P, Zhar AA, Wu CJ, Gumbs C, Little L, et al: Integrative
genomic analysis of adult mixed phenotype acute leukemia delineates
lineage associated molecular subtypes. Nat Commun. 9:26702018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lennmyr E, Karlsson K, Ahlberg L, Garelius
H, Hulegårdh E, Izarra AS, Joelsson J, Kozlowski P, Moicean A,
Tomaszewska-Toporska B, et al: Survival in adult acute
lymphoblastic leukaemia (ALL): A report from the Swedish ALL
registry. Eur J Haematol. 103:88–98. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yi M, Li A, Zhou L, Chu Q, Song Y and Wu
K: The global burden and attributable risk factor analysis of acute
myeloid leukemia in 195 countries and territories from 1990 to
2017: Estimates based on the global burden of disease study 2017. J
Hematol Oncol. 13:722020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Malard F and Mohty M: Acute lymphoblastic
leukaemia. Lancet. 395:1146–1162. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hangai M, Urayama KY, Tanaka J, Kato K,
Nishiwaki S, Koh K, Noguchi M, Kato K, Yoshida N, Sato M, et al:
Allogeneic stem cell transplantation for acute lymphoblastic
leukemia in adolescents and young adults. Biol Blood Marrow
Transplant. 25:1597–1602. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tan J, Wang Y, Yu SJ, Ma YY, Lei HY and
Liu QF: Prognostic factors on graft-versus-host disease-free and
relapse-free survival after allogeneic hematopoietic stem cell
transplantation for adults with acute leukemia. Leuk Res. 59:1–7.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Halaburda K, Labopin M, Mailhol A, Socié
G, Craddock C, Aljurf M, Beelen D, Cornelissen JJ, Bourhis JH,
Labussière-Wallet H, et al: Allogeneic stem cell transplantation in
second complete remission for core binding factor acute myeloid
leukemia: A study from the acute leukemia working party of the
European society for blood and marrow transplantation.
Haematologica. 105:1723–1730. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Panebianco M, Bagni O, Cenfra N, Mecarocci
S, Ortu La Barbera E, Filippi L, Codacci-Pisanelli G, Biondi T,
Laghi A and Cimino G: Comparison of 18F FDG PET-CT AND CECT in
pretreatment staging of adults with Hodgkin's lymphoma. Leuk Res.
76:48–52. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou WL, Wu HB, Wang LJ, Tian Y, Dong Y
and Wang QS: Usefulness and pitfalls of F-18-FDG PET/CT for
diagnosing extramedullary acute leukemia. Eur J Radiol. 85:205–210.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stolzenburg A, Lückerath K, Samnick S,
Speer M, Kneer K, Schmid JS, Grigoleit GU, Hofmann S, Beer AJ,
Bunjes D, et al: Prognostic value of [18F]FDG-PET/CT in
multiple myeloma patients before and after allogeneic hematopoietic
cell transplantation. Eur J Nucl Med Mol Imaging. 45:1694–1704.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qiao W, Zhao J, Wang C, Wang T and Xing Y:
Predictive value of (18)F-FDG hybrid PET/CT for the clinical
outcome in patients with non-Hodgkin's lymphoma prior to and after
autologous stem cell transplantation. Hematology. 15:21–27. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Magnusson E, Cao Q, Linden MA, Frolich J,
Anand V, Burns LJ and Bachanova V: Hematopoietic cell
transplantation for mantle cell lymphoma: Predictive value of
pretransplant positron emission tomography/computed tomography and
bone marrow evaluations for outcomes. Clin Lymphoma Myeloma Leuk.
14:114–121. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Döhner H, Estey E, Grimwade D, Amadori S,
Appelbaum FR, Büchner T, Dombret H, Ebert BL, Fenaux P, Larson RA,
et al: Diagnosis and management of AML in adults: 2017 ELN
recommendations from an international expert panel. Blood.
129:424–447. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brown PA, Wieduwilt M, Logan A, DeAngelo
DJ, Wang ES, Fathi A, Cassaday RD, Litzow M, Advani A, Aoun P, et
al: Guidelines insights: Acute lymphoblastic leukemia, version
1.2019. J Natl Compr Canc Netw. 17:414–423. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alam MS, Fu L, Ren YY, Wu HB, Wang QS, Han
YJ, Zhou WL, Li HS and Wang Z: 18F-FDG super bone marrow uptake: A
highly potent indicator for the malignant infiltration. Medicine
(Baltimore). 95:e55792016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
St-Pierre F, Broski SM, LaPlant BR, Ristow
K, Maurer MJ, Macon WR, Habermann TM, Ansell SM, Thompson CA,
Micallef INM, et al: Detection of extranodal and spleen involvement
by FDG-PET imaging predicts adverse survival in untreated
follicular lymphoma. Am J Hematol. 94:786–793. 2019.PubMed/NCBI
|
|
18
|
Papajík T, Mysliveček M, Urbanová R,
Buriánková E, Kapitáňová Z, Procházka V, Turcsányi P, Formánek R,
Henzlová L, Flodr P, et al: 2-[18F]fluoro-2-deoxy-D-glucose
positron emission tomography/computed tomography examination in
patients with chronic lymphocytic leukemia may reveal richter
transformation. Leuk Lymphoma. 55:314–319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Albano D, Mazzoletti A, Spallino M, Muzi
C, Zilioli VR, Pagani C, Tucci A, Rossetti C, Giubbini R and
Bertagna F: Prognostic role of baseline 18F-FDG PET/CT metabolic
parameters in elderly HL: A two-center experience in 123 patients.
Ann Hematol. 99:1321–1330. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Valls L, Badve C, Avril S, Herrmann K,
Faulhaber P, O'Donnell J and Avril N: FDG-PET imaging in
hematological malignancies. Blood Rev. 30:317–331. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou M, Chen Y, Liu J and Huang G: A
predicting model of bone marrow malignant infiltration in
18F-FDG PET/CT images with increased diffuse bone marrow
FDG uptake. J Cancer. 9:1737–1744. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Michallet AS, Sesques P, Rabe KG, Itti E,
Tordot J, Tychyj-Pinel C, Baseggio L, Subtil F, Salles G, Dupuis JM
and Conte MJ: An 18F-FDG-PET maximum standardized uptake value
>10 represents a novel valid marker for discerning Richter's
Syndrome. Leuk Lymphoma. 57:1474–1477. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stölzel F, Röllig C, Radke J, Mohr B,
Platzbecker U, Bornhäuser M, Paulus T, Ehninger G, Zöphel K and
Schaich M: 18F-FDG-PET/CT for detection of
extramedullary acute myeloid leukemia. Haematologica. 96:1552–1556.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arslan F, Yilmaz M, Cakir T and Mert A:
Significant contribution of Fluorodeoxyglucose positron emission
tomography/computed tomography (FDG PET/CT) in a case of acute
lymphoblastic leukemia presenting with fever of unknown origin.
Intern Med. 53:789–791. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Su K, Nakamoto Y, Nakatani K, Kurihara K,
Hayakawa N and Togashi K: Diffuse homogeneous bone marrow uptake of
FDG in patients with acute lymphoblastic leukemia. ClinNucl Med.
38:e33–e34. 2013.
|
|
26
|
Derlin T, Alchalby H, Bannas P, Veldhoen
S, Apostolova I, Triviai I, Bengel FM and Kröger N: Assessment of
bone marrow inflammation in patients with myelofibrosis: An
18F-fluorodeoxyglucose PET/CT study. Eur J Nucl Med Mol Imaging.
42:696–705. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jeong SY, Kim SJ, Pak K, Lee SW, Ahn BC
and Lee J: Prognostic value of 18F-fluorodeoxyglucose bone marrow
uptake in patients with solid tumors: A meta-analysis. Medicine
(Baltimore). 97:e128592018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abe Y, Kitadate A, Usui Y, Narita K,
Kobayashi H, Miura D, Takeuchi M, O'uchi E, O'uchi T and Matsue K:
Diagnostic and prognostic value of using 18F-FDG PET/CT for the
evaluation of bone marrow involvement in peripheral T-cell
lymphoma. Clin Nucl Med. 44:e336–e341. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khan AB, Barrington SF, Mikhaeel NG, Hunt
AA, Cameron L, Morris T and Carr R: PET-CT staging of DLBCL
accurately identifies and provides new insight into the clinical
significance of bone marrow involvement. Blood. 122:61–67. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hong J, Lee Y, Park Y, Kim SG, Hwang KH,
Park SH, Jeong J, Kim KH, Ahn JY, Park S, et al: Role of FDG-PET/CT
in detecting lymphomatous bone marrow involvement in patients with
newly diagnosed diffuse large B-cell lymphoma. Ann Hematol.
91:687–695. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen Y, Zhou M, Liu J and Huang G:
Prognostic value of bone marrow FDG uptake pattern of PET/CT in
newly diagnosed diffuse Large B-cell lymphoma. J Cancer.
9:1231–1238. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yoon HJ, Kim BS, Moon CM, Yoo J, Lee KE
and Kim Y: Prognostic value of diffuse splenic FDG uptake on PET/CT
in patients with gastric cancer. PLoS One. 13:e01961102018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim SY, Moon CM, Yoon HJ, Kim BS, Lim JY,
Kim TO, Choe AR, Tae CH, Kim SE, Jung HK, et al: Diffuse splenic
FDG uptake is predictive of clinical outcomes in patients with
rectal cancer. Sci Rep. 9:13132019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mebius RE and Kraal G: Structure and
function of the spleen. Nat Rev Immunol. 5:606–616. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sharma Poudel B and Karki L: Abnormal
hepatic function and splenomegaly on the newly diagnosed acute
leukemia patients. JNMA J Nepal Med Assoc. 46:165–169.
2007.PubMed/NCBI
|
|
36
|
Nam HY, Kim SJ, Kim IJ, Kim BS, Pak K and
Kim K: The clinical implication and prediction of diffuse splenic
FDG uptake during cancer surveillance. Clin Nucl Med. 35:759–763.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sakellari I, Gavriilaki E, Batsis I,
Mallouri D, Gavriilaki M, Apostolou C, Iskas M, Voutiadou G,
Bouziana S, Bousiou Z, et al: Isolated extramedullary relapse as a
poor predictor of survival after allogeneic hematopoietic cell
transplantation for acute leukemia. Biol Blood Marrow Transplant.
25:1756–1760. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Støve HK, Sandahl JD, Abrahamsson J,
Asdahl PH, Forestier E, Ha SY, Jahnukainen K, Jónsson ÓG, Lausen B,
Palle J, et al: Extramedullary leukemia in children with acute
myeloid leukemia: A population-based cohort study from the nordic
society of pediatric hematology and oncology (NOPHO). Pediatr Blood
Cancer. 64:2017. View Article : Google Scholar
|
|
39
|
Kobayashi R, Tawa A, Hanada R, Horibe K,
Tsuchida M and Tsukimoto I; Japanese childhood AML cooperative
study group, : Extramedullary infiltration at diagnosis and
prognosis in children with acute myelogenous leukemia. Pediatr
Blood Cancer. 48:393–398. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bisschop MM, Révész T, Bierings M, van
Weerden JF, van Wering ER, Hählen K and van der Does-van den Berg
A: Extramedullary infiltrates at diagnosis have no prognostic
significance in children with acute myeloid leukaemia. Leukemia.
15:46–49. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kumar R, Harish N, Sharma A, Gupta RK,
Sharma A, Bakhshi S, Patel C, Thankarajan AS and Bal C: Comparison
of FDG PET/CT and CT in response assessment and prognosis of
patients with extra medullary disease in acute myeloid leukemia. J
Nucl Med. 60 (Suppl 1):S12512019.
|