Introduction
The World Health Organization reported that 627,000
women died globally in 2018 due to breast cancer, and the rate of
new cases of breast cancer is predicted to increase in future years
(1). The risk of developing breast
cancer involves genetic factors, such as alterations in BRCA1/BRCA2
and hormonal exposure history, as well as unhealthy lifestyle
behaviours, such as smoking, drinking alcohol or the reduction of
melatonin levels and impaired cortisol secretion, which occurs due
to changes in the sleeping pattern of night shift workers (2,3).
Although there are >30 medicines available for breast cancer
therapy, research into the potency of naturally-derived compounds
remains vital, in order to pave the way for the discovery of novel
drugs to use in breast cancer therapy (3).
A number of naturally-derived drugs, including
paclitaxel and vincristine, have been applied in the clinical
therapy of breast cancer (3–5). One of the natural resource products
that is known as an anticancer therapy includes bee products
(6–10). The bee products commonly consumed
include honeycomb, honey, propolis, pollen, royal jelly and bee
venom. Similar to other characteristics of natural products, the
activity of each bee product depends on its bioactive constituents,
which are affected by the species of the bee, the geographical
origin of the beehive and the harvesting season of the products
(8,10).
The district of Luwu Utara, in the South Sulawesi
province in Indonesia, is one of the most productive apiculture
areas in Indonesia. The Trigona spp. is mainly cultivated to
obtain high-quality honey as the primary valuable product, while
the utilisation of its propolis and bee pollen is not as popular as
honey (11,12). As shown in Fig. 1, propolis is a mixture of bee saliva
with resinous material derived primarily from resins of leaf buds,
sap flows and other plant sources, and is used as the main material
to repair the beehive. The propolis of the Trigona spp. is
known to have a strong aroma compared with other propolis produced
in different Indonesian provinces (11). In contrast to propolis, bee pollen is
a granular material derived from the pollen of various flowers,
while honey results from a complex process involving the sugary
material secretions of plants, followed by enzymatic reaction and
water evaporation (6,8).
In the research of propolis, numerous previous
studies have utilised the ethanolic extract of propolis or another
organic fraction (6,7). However, the present study focused on
the effect of the water-soluble fraction of propolis and bee pollen
on the proliferation of the human breast cancer MCF-7 cell line.
Human epithelial keratinocytes (HaCaT) were also used in the
present study to estimate the toxicity of water-soluble propolis
and bee pollen in normal cells.
Caffeic acid phenethyl ester (CAPE) is known as one
of the bioactive compounds of propolis, mostly in the Baccharis
propolis (9,13). It has a mechanism of action to
decrease the expression levels of NF-κB in the apoptotic pathway of
pancreatic and colon cancer (14).
Another known mechanism of CAPE, as well as propolis, is to cause
an accumulation of acetylated histone proteins in MCF-7 estrogen
receptor-positive (ER+) cells, resulting in decreases in
ER and progesterone receptor (PR) expression in these cells, thus,
decreasing MCF-7 proliferation (7,9,14). Therefore, the present study also
aimed to identify the presence of CAPE in the examined products of
the Trigona spp.
Materials and methods
Samples
Propolis and bee pollen were collected from a
stingless bee of the Trigona spp. beehive in the district of
Luwu Utara (South Sulawesi, Indonesia). These bee products were
harvested from the same hive in September 2018. The products were
stored at 2–8°C in a lid-closed plastic container until further
use. CAPE with >98% purity was purchased from Santa Cruz
Biotechnology, Inc.
MCF-7 and HaCaT cell cultures
MCF-7 cells and HaCaT cells were provided by The
Faculty of Medicine, Padjadjaran University, Bandung-Indonesia.
MCF-7 cells were cultured in DMEM and HaCaT cells were cultured in
RPMI. Both cultures were supplemented with 10% FBS and 1%
penicillin-streptomycin solution (all Gibco; Thermo Fisher
Scientific, Inc.). The cells were cultured in T25 tissue culture
flasks at 37°C in a 5% CO2 incubator and were
subcultured every 2–3 days before they reached confluency to keep
the cultures healthy and actively growing. The experiments were
performed with cells at 70–80% confluence (15).
Preparation of water-soluble propolis
and bee pollen
The water-soluble propolis was prepared by grinding
10 g propolis with the aid of an equivalent amount of 70% ethanol.
This was transferred to a glass bottle containing 100 ml of 70%
ethanol. The bottle was protected from light and was left at room
temperature for 4 days with intermittent shaking. On the 5th day,
the solvent was collected and 100 ml fresh 70% ethanol was added to
continue the extraction process for a further 24 h while shaking
intermittently. This was repeated the next day. A total of 300 ml
solvent was collected after 7 days of extraction. The ethanol was
removed using a rotary evaporator at 40–60°C, at 40 rpm and
pressure of 200–250 bar (Rotavapor R-215; BÜCHI Labortechnik AG).
Once the ethanol was completely removed, the wax-like
water-insoluble formed and completely separated from the
water-soluble fraction. The water-soluble propolis was freeze-dried
in a Labconco freeze dryer (Thermo Fisher Scientific, Inc.) for
~40–41 h in 5 cycles. The initial process was to decrease the
temperature from room temperature to −40°C for ~5–6 h. After
reaching −40°C, the first cycle started by freezing at a constant
of −40°C for ~18 min. The subsequent cycles involved primary drying
at −34°C for ~16 h, first secondary drying at −23°C for ~6 h,
second secondary drying at 17°C for ~8–9 h and final drying at 25°C
for ~4–5 h. The freeze-dried water-soluble propolis was kept at
2–8°C until further use.
Bee pollen was prepared by dispersing it in water
1:10 (b/v) and filtered using Whatman® Grade 41 paper
(Cytiva). The solution was freeze-dried for 40–41 h using the same
protocol as the aforementioned preparation of water-soluble
propolis and kept at 2–8°C until further use.
Identification of CAPE by
high-performance liquid chromatography-ultraviolet (HPLC-UV) and
electrospray ionisation mass spectrometry (ESI-MS)
The HPLC-UV analysis was performed using a
Prominence SIL-20A autosampler equipped with a Shimadzu LC-20AT
pump (Shimadzu Corporation). Reverse-phase chromatography analyses
were performed using a Shodex RSpak RP18 (Showa Denko America,
Inc.), 4.6×150 mm ID × length, 6 µm particle size, 450 Å pore size
and 20 µl volume injection. The mobile phase consisted of Solvent A
[water: Formic acid (95:5)] and Solvent B (methanol). The linear
gradient system was conducted at a flow rate of 1.0 ml/min,
starting from 30% Solvent B for 15 min, increased to 40% Solvent B
at 20 min, 45% Solvent B at 30 min, 60% Solvent B at 50 min, 80%
Solvent B at 52 min and 80% Solvent B at 60 min. The CAPE standard
was prepared in methanol at 0.1 mg/ml. Samples and standards
solutions, as well as the mobile phase, were degassed and filtered
through a 0.45-µm membrane filter (EMD Millipore). The chromatogram
was recorded at 340 nm. Identification of the compounds was
performed by comparing their retention time and UV absorption
spectrum with those of the standards (9,16).
The ESI-MS method was performed using the
ACQUITY® triple quadrupole detector (Waters Corporation)
using cone source start at 41 V in positive mode and 43 V in
negative mode at capillary voltage 3.00 kV. The collision gas flow
rate was set at 0.20 and 0.25 ml/min for positive and negative
mode, respectively. The source and desolvation temperatures were
100 and 300°C, respectively. Mass spectra of water-soluble propolis
and bee pollen were further analysed in the negative ion mode
scanning from 0 to 1,000 m/z. Data were analysed using the
MassLynx™ 4.1 software (Waters Corporation).
Protein identification via
SDS-PAGE
A 12% polyacrylamide gel was used to identify the
presence of proteins in the water-soluble propolis, bee pollen,
honey and a commercial bee pollen powder (local company) as
additional information. Raw bee pollen, honey and commercial bee
pollen powder were dissolved in purified water to a concentration
of 0.1 mg/ml. The prior freeze-dried water-soluble propolis was
used as a sample. A total of 50 µl of each sample were mixed with
SDS loading buffer (0.756% Tris buffer pH 6.8 containing 2% SDS,
20% bromophenol blue, 10% glycerol and 0.715 M mercaptoethanol) and
heated at 90°C for 5 min. A total of 20 µl of samples and 2 µl of
marker (Precision Plus Protein™ Dual colour standard; Bio-Rad
Laboratories, Inc.) was loaded into each lane. Electrophoresis was
performed using a Mini-Protean® Tetra Cell apparatus
(Bio-Rad Laboratories, Inc.) at a constant voltage of 150 V for
45–60 min. The gel was then stained with Coomassie blue and the
bands were observed visually and documented by gel imaging system
(Azure Biosystems).
In vitro antioxidant activity assay
using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging
assay
The radical scavenging activity (RSA) of the bee
products was examined using the DPPH method. DPPH (0.05 mM) was
prepared by dissolving 10 mg DPPH reagent (Sigma-Aldrich; Merck
KGaA) in 250 ml acetonitrile: Methanol mixture (1:1, v/v), followed
by the addition of 250 ml acetate buffer (pH 5.5; 100 mM).
Concentrations were prepared in water as follows: 76, 152, 227 and
303 µg/ml of water-soluble propolis; 125, 250 and 500 µg/ml of bee
pollen; and 3.75, 7.5 and 15 mg/ml of honey. A quantity of 0.077 ml
of the samples was added to 3 ml DPPH solution and allowed to stand
for 15–30 min in the dark at room temperature. The absorbance was
measured at λmax 515–517 nm, using 0.05 mM DPPH solution
as a blank. The percentage of DPPH scavenging (% RSA) was estimated
using the following equation: % RSA =
[(A0-A1)/A0]x100, where
A0 is the absorbance of the initial DPPH solution as the
blank and A1 is the absorbance of the samples. The
half-maximal effective concentration (EC50) value was
calculated using GraphPad Prism 7.05 software (GraphPad Software,
Inc.).
In vitro antiproliferative assay in
MCF-7 cells
The antiproliferative activity of water-soluble
propolis and bee pollen was evaluated via MTT assay. The data was
analysed in normalization method to negative control (without
cells) and positive control (untreated cells). The dose-effect
curves were analysed using GraphPad Prism 7.05 software in
non-linear regression (sigmoidal) method. The antiproliferative
activity of honey was also determined in this experiment as
additional information. MCF-7 cells at a density of
2×104 cells/well were seeded in treated 96-well plates
and cultured for 24 h at 37°C with 5% CO2 to let the
cells adhere to the bottom of the plates. The cultures were then
treated with different concentrations (0.7, 1.3, 2.7, 5.3, 10.7,
21.4 and 42.7 mg/ml of water-soluble propolis, and 0.8, 1.7, 3.3,
6.6, 13.3, 26.5 and 53 mg/ml of bee pollen), and cultured for 24 h
at 37°C with 5% CO2. Each concentration was tested in
triplicate, and untreated cells were used as the positive control
and a well without cells as the negative control. After 24 h of
incubation, the medium was aspirated and replaced with 100 µl of
freshly prepared medium containing 0.5 mg/ml MTT reagent, followed
by further 3–4 h incubation at 37°C with 5% CO2. The MTT
solution was removed from the well and 100 µl DMSO was added in
each well to solubilise the formazan crystals. Finally, the
absorbance was measured at a wavelength of 450 nm using the
Multiscan® Ex multiplate reader (Thermo Labsystems).
In vitro antiproliferative assay in
HaCaT cells
The antiproliferative activity of water-soluble
propolis and bee pollen in HaCaT cells was evaluated via MTT assay
similar to in vitro antiproliferative assay in MCF-7;
however, cells were used at the density of 5×103
cells/well. The cultures were treated with water-soluble propolis
and bee pollen at concentrations of 0.4, 0.8, 1.6, 3.1, 6.3, 12.5
and 25 mg/ml, and cultured for 24 or 48 h at 37°C with 5%
CO2, in order to observed the effect of different
treatments over time.
Flow cytometric analysis
MCF-7 cells were seeded in a 6-well plate at a
density of 5×105 cells/well and were cultured for 24 h
at 37°C with 5% CO2. The cultures were treated with 10.8
mg/ml water-soluble propolis and 18.6 mg/ml bee pollen, and
incubated for 24 h at 37°C with 5% CO2. After 24 h of
treatment, cells were observed by inverted phase contrast
microscope (Olympus Corporation) at ×100 and ×200 magnifications.
The cells were harvested using 0.01% trypsin at 37°C for 5 min. The
cells were collected in 2-ml tubes and were washed twice with cold
PBS via centrifugation for 5 min at 300 × g at room temperature.
The cells were gently resuspended in 1X binding buffer at a
concentration of 1×106 cells/ml. Further analysis was
performed using the FITC-Annexin V apoptosis detection kit I (BD
Pharmingen; BD Biosciences), according to the manufacturers
protocol. Briefly, 100 µl of the samples solution (1×105
cells/ml) was transferred to a 5-ml culture tube. A total of 5 µl
FITC-Annexin V and 5 µl PI was added to the tube for 15 min in the
dark at room temperature. Finally, 400 µl 1X binding buffer was
added to the tubes. The samples were analysed immediately within 1
h by using flow cytometer (BD Accuri™ C6 instrument; BD
Biosciences) and were analysed by using BD Accuri™ C6 software
(version 1.0.264.21; BD Biosciences).
Statistical analysis
The in vitro experiments to measure the
antioxidant and antiproliferative activities in MCF-7 and HaCaT
cells were performed in triplicate (n=3) and repeated in three
independent experiments. The data were represented as the means ±
standard deviation. The antioxidant activity assay was analysed by
GraphPad Prism 7.05 software. The in vitro antiproliferative
assays in MCF-7 cells was analysed by GraphPad Prism 7.05 software
for the IC50 determination, followed by one-way ANOVA by
SPSS v23.0 (IBM Corp.). The in vitro antiproliferative assay
in HaCaT cells was analysed by GraphPad Prism 7.05 software,
followed by two-way ANOVA and Tukeys post hoc test by SPSS v23.0
(IBM Corp). The flow cytometric results were analysed by one-way
ANOVA followed by Tukeys post hoc test by SPSS v23.0. P<0.05 was
considered to indicate a statistically significant difference.
Results
Preparation of water-soluble propolis
and bee pollen
The freeze-dried water-soluble propolis and bee
pollen formed a solid, hard, flake-like material at 2–8°C. These
samples were easily dissolved in water and medium. They must be
stored at 2–8°C, as storing at room temperature for >8 h will
melt the flake-like material into a sticky viscous material.
Total antioxidant properties
The antioxidant activity analysis revealed that the
half-maximal effective concentration (EC50) values
against DPPH radicals of water-soluble propolis and bee pollen were
1.3±0.4 and 0.4±0.07 mg/ml, respectively, while the EC50
of honey was 6.2±0.6 mg/ml. This indicated that the antioxidant
activity of bee pollen was 3 times higher than that of
water-soluble propolis and 15 times higher than that of honey
(Fig. 2).
Identification of CAPE via HPLC-UV and
ESI-MS
The HPLC-UV analysis was performed to identify the
presence of CAPE in water-soluble propolis and bee pollen, as well
as in honey and raw propolis. However, the present study revealed
that honey and bee pollen of the Trigona spp. did not
contain CAPE, as no peak of CAPE was identified in its
chromatogram. Meanwhile, peak of CAPE was identified only in trace
values in raw propolis, as well as in water-soluble propolis,
estimated as 2 ppb. Further analysis was conducted using ESI-MS in
source positive ion and negative ion spectra to confirm the reason
for the absence or traces result in the HPLC-UV analysis. As shown
in Fig. 3, the source negative ion
spectra of CAPE (C17H16O4), which
has a molecular weight of 284.3 g/mol, was not identified as a
major bioactive constituent in water-soluble propolis nor in bee
pollen. The weak spectrum of CAPE was identified in bee pollen with
m/z 283.45. Mass spectra of water-soluble propolis in the negative
ion mode scanning from 0 to 1,000 m/z exhibited similar major
compounds to bee pollen, having a spectrum with m/z 195.26[M-H]-,
255.46[M-H]-, 279.5[M-H] and 339.38[M-H]-. Meanwhile, the mass
spectra of bee pollen acquired using electrospray in the negative
ion mode scanning from 0 to 1,000 m/z exhibited several major
compounds having a spectrum with m/z 195.26[M-H]-, 339.46[M-H]- and
431.36[M-H]. The major constituent was assumed to be flavonoids.
However, further experiments are required to identify the major
constituents in these natural products as observed in the ESI-MS
spectra.
Protein identification via
SDS-PAGE
The present study examined the presence of protein
primarily in water-soluble propolis and bee pollen, as well as in
honey, in the insoluble part of propolis (wax fraction), which was
collected during the preparation of the water-soluble propolis, and
in commercial bee pollen powder. As shown in Fig. 4, no protein bands appeared in the
lanes of honey, the wax fraction of propolis and the commercial bee
pollen powder. Notably, protein bands were observed in the lane of
bee pollen and water-soluble propolis. Two major bands were
identified with a size between 50 and 75 kDa. Further studies are
required to evaluate the characteristics of proteins or enzymes
present in bee pollen and water-soluble protein.
In vitro antiproliferative assay in
MCF-7 cells
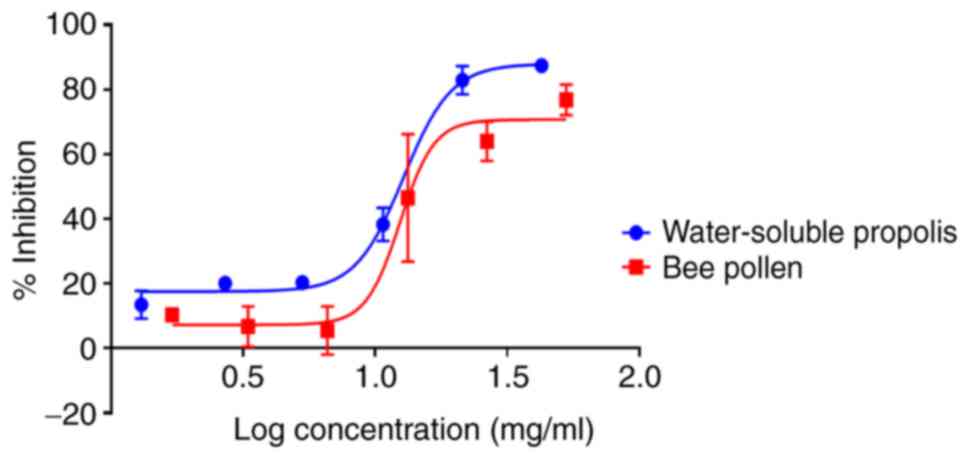
The MTT assay revealed that water-soluble propolis
and bee pollen induced inhibitory effects against MCF-7 cell
proliferation in a concentration-dependent manner after 24 h of
treatment. The samples exhibited significant activity (P<0.05)
with IC50 values of 10.8±0.06 and 18.6±0.03 mg/ml,
respectively (Fig. 5). Furthermore,
honey did not exhibit any cytotoxic activity against MCF-7 cell
proliferation after 24 h (data not shown).
In vitro antiproliferative assay in
HaCaT cells
The experiment revealed that HaCaT cell treatment
with water-soluble propolis for 24 h giving IC50 of
2.7±0.06 mg/ml. The cytotoxic effect was higher after 48 h of
treatment than 24 h, with an IC50 of <0.4 mg/ml.
Fig. 6 shows that there were
significant differences at concentrations of 0.4 (P<0.0001), 0.8
(P<0.001), 1.6 (P<0.01) and 25 mg/ml (P<0.05).
Nevertheless, the two-way ANOVA with Tukeys post hoc test revealed
that there was no significant difference between the treatment with
water-soluble propolis at 24 and 48 h (P>0.05) at concentrations
of 3.1, 6.3 and 12.5 mg/ml. By contrast, the treatment of cells
with bee pollen for 24 h indicated that bee pollen was less toxic
to the cells compared with water-soluble propolis at concentration
<25 mg/ml with an IC50 >50 mg/ml (estimated as
~975.2 mg/ml using GraphPad Prism 7.05 software). The
antiproliferative activity increased after 48 h of treatment at
higher concentrations, with an IC50 of 9.6±0.07
mg/ml.
Furthermore, the antiproliferative activities of the
samples were observed at 24 and 48 h. Regarding the IC50
values resulting from each treatment, there were significant
differences in antiproliverative activity between water-soluble
propolis and bee pollen at 24 h (P<0.05) and 48 h (P<0.05).
The results from Fig. 6 revealed
that there were significant differences between treatment with
water-soluble propolis and bee pollen at 24 h using concentrations
0.4, 0.8, 1.6, 3.1, 6.3, 12.5 and 25 mg/ml (P<0.0001).
Significant differences were also observed between treatment with
water-soluble propolis and bee pollen at 48 h using concentrations
0.4, 0.8, 1.6, 3.1, 6.3 and 12.5 mg/ml (P<0.0001).
Interestingly, a higher dose of bee pollen (25 mg/ml) resulted in
significant difference (P<0.05) compared with water-soluble
propolis at the same concentration of 25 mg/ml. The result revealed
that the antiproliferative activity of bee pollen increased in a
time- and dose-dependent manner.
Flow cytometric analysis
Flow cytometric analysis results were reported as
the percentage of live, necrotic or apoptotic cells (early or late
apoptosis). Fig. 7A represents a
summary of the flow cytometric analysis as a bar chart, exhibiting
a decrease of live cells and an increased proportion of apoptotic
cells after treatment with water-soluble propolis and bee pollen
compared with in the untreated cells used as a control. Untreated
MCF-7 cells represented a normal cell population, where most cells
(88.0%) were live cells and small proportions of cells were in
early apoptosis, late apoptosis or necrosis (0.2, 9.5 and 2.3%,
respectively; Fig. 7B). MCF-7 cells
treated with water-soluble propolis at the IC50
concentration (10.8 mg/ml) for 24 h exhibited a significant
decrease in live cells (P<0.001; Fig.
7A). Flow cytometric analysis revealed that the percentages of
live cells and late apoptotic cells were 8.2 and 89.8%,
respectively, while cells in early apoptosis and necrosis were 0.8
and 1.2%, respectively (Fig. 7C). In
the present study, MCF-7 cells treated with bee pollen at
IC50 concentration of 18.6 mg/ml, for 24 h exhibited a
notable result where a progression of cells towards late apoptosis.
Flow cytometric analysis of the sample treated with bee pollen
revealed that the percentage of live cells was 78.8%, and necrotic
cells represented 0.8% of the cell population. Compared with
untreated cells, the percentages of early and late apoptotic cells
increased to 3.5 and 16.9%, respectively. (Fig. 7D). Observation of the MCF-7 cell
culture after 24 h of treatment by inverted phase contrast
microscope at ×100 and ×200 magnification showed results similar to
the flow cytometric analysis. The results from the present study
demonstrated that MCF-7 cell treatment with water-soluble propolis
and bee pollen lead to decreased cell density (Fig. 8C and D). Furthermore, untreated cells
showed normal proliferation and reached confluency in 24 h
(Fig. 8A). As presented in Fig. 8B, untreated MCF-7 cells, which grew
as normal and healthy cells, had a cobblestone-like phenotype with
25–30 µm length. However, MCF-7 cells treated with bee pollen
presented with early apoptosis according to cell shrinkage to
rounded cells (Fig. 8D). Treatment
of MCF-7 cells with water-soluble propolis resulted in cells in
late apoptosis, where blebbing cells, formation of apoptopodia and
apoptotic bodies were observed (Fig.
8C).
Discussion
A number of natural products have been used as
traditional medicines to maintain the quality of life and to
contribute to the development of novel drugs. Natural
product-derived drug discoveries have demonstrated their
pharmacological activity, for example as cardiovascular (digoxin
obtained from Digitalis purpurea), anti-malaria (quinine
from Cinchona officinalis) and antineoplastic agents
(paclitaxel derived from the pacific yew tree Taxus
brevifolia) (4,5) Among natural products, bee products are
considered to have numerous pharmacological effects, such as
antimicrobial, antiviral, anti-inflammatory and anticancer
(6,7,9). The
unique characteristics of these products are mostly affected by the
species of the bee and geographical factors, as well as the
harvesting time (8,10). Therefore, understanding the specific
characteristics and pharmacological effects of bee products from
each region is essential.
During the initial study, it was revealed that the
ethanolic extract and water-insoluble propolis (wax fraction) had a
strong cytotoxic activity on MCF-7 cells, with IC50
values of 0.2±0.09 and 0.04±0.003 mg/ml, respectively; these in
vitro activities were higher than that using the water-soluble
propolis, which had an IC50 of 10.8±0.06 mg/ml
(P<0.05; data not shown). However, considering that the
water-insoluble propolis (wax fraction) contain resinous
substances, which are practically insoluble in water and
spontaneously form coagulation in an aqueous environment, this
could hamper the ethanolic extract and wax fraction to be further
developed in the future. Therefore, the water-soluble phase of the
extract was further investigated in the present study, in order to
elaborate the potency of water-soluble bioactive materials in
propolis which have polar or moderate polarity characteristics. It
was expected that the bioactive materials in water-soluble propolis
and bee pollen would fulfil class 1 (the bioactive materials have
high solubility and extensive metabolism) or class 2 (the bioactive
materials have low solubility, extensive metabolism) criteria of
the Biopharmaceutical Drug Disposition and Classification System
(17,18). Bioactive materials with class 1 or
class 2 characteristics are considered as promising candidates in
drug discovery and could be investigated in further research, since
they have good solubility and permeability in the gastrointestinal
membrane in oral administration (17,18).
Among the products examined, bee pollen had a high
antioxidant activity with EC50 against DPPH radicals of
0.4±0.06 mg/ml. This value is one-fourth that of vitamin C and is
considered as having mild antioxidant activity (19). Additionally, the water-soluble
propolis had lower antioxidant properties than bee pollen, with
EC50 of 1.3±0.4 mg/ml. Furthermore, honey was found to
have a low antioxidant properties of EC50 6.2±0.6
mg/ml.
To date, 500 compounds have been identified in
propolis (13). Flavonoid content is
commonly found in poplar propolis, which is produced in Europe,
North America and non-tropical regions of Asia, while the Brazilian
propolis, known as the Baccharis type, is abundant in cinnamic acid
derivatives (13). CAPE is known as
the bioactive compound found in New Zealand, Brazilian and Romanian
propolis; this natural phenolic compound is an ester of caffeic
acid, which is a hydroxycinnamic acid and phenethyl alcohol
(13,20). Using an HPLC-UV detector, the present
study revealed that CAPE was not the main bioactive compound in
Trigona spp. bee pollen, water-soluble propolis and honey.
CAPE was identified in trace values in raw propolis, as well as in
water-soluble propolis, estimated as 2 ppb. However, due to the
below detection limit of HPLC, the present results should be
confirmed using more specific LC-MS instruments. The preliminary
results using ESI-MS indicated that the spectrum of CAPE was
identified as weak in bee pollen. Several intensive spectrums
indicated that other compounds may serve a role in the
antiproliferative activity of water-soluble propolis and bee pollen
from the Trigona spp. SDS-PAGE was used to identify protein
constituents in samples. Two primary water-soluble proteins were
identified in bee pollen and water-soluble propolis between 50 and
75 kDa. These proteins were not identified in honey, wax propolis
or the commercial bee pollen powder. However, the specific proteins
remain unknown.
In contrast to manuka honey derived from the UK,
which has been reported to induce apoptosis at a concentration of
4.7% (w/v) in MCF-7 cells, and Tualang honey from Malaysia, which
also induced apoptosis in a breast cancer animal model, our
experimental results revealed that honey from the Trigona
spp. examined had no anticancer properties in MCF-7 cells (10,21).
Flow cytometric analysis of the MCF-7 cell line
treated for 24 h with water-soluble propolis and bee pollen at
their IC50 concentrations revealed the highest
distribution cell population in Annexin V and PI areas. This
condition explained that there were changes in cells physiology due
to treatments, which may lead to apoptosis. At early apoptosis
stage, the cells lose the plasma membrane asymmetry and
externalisation of phosphatidylserine to outer membrane occurs.
This allows cells to be labeled with Annexin V. The translocation
of phosphatidylserine results in loss of membrane cell integrity,
which allows PI to enter cells and bind to DNA (22,23).
This stage is known as the late apoptosis of the cell. The result
was confirmed by cell morphological observation using an inverted
phase contrast microscope. The MCF-7 cell line has been originally
isolated from breast tissue of a 69-year old Caucasian woman with
metastatic disease (24,25). The characterised cells display ER and
PR expression, which may represent the luminal A type of breast
cancer, which occurs in 80% of patients with breast cancer
(3,24). The untreated MCF-7 cells incubated
for 24 h exhibited normal, healthy and confluent cells, with a
cobblestone-like phenotype, a size of 25–30 µm and strong cell-cell
adhesion (26). On the other hand,
MCF-7 cells treated with bee pollen and water-soluble propolis
exhibited a marked morphological change in the apoptotic cells.
Characteristics of apoptotic cell morphology include chromatin
condensation and cell shrinking, the formation of rounded cells,
plasma membrane blebbing and disintegration of organelles, the
collapse of the nucleus and organelles, membrane protrusion
formation into apoptopodia and the formation of cell fragmentation
of apoptotic bodies (27,28). In the present study, treatment with
water-soluble propolis for 24 h induced typical changes of cells in
late apoptosis, such as membrane blebbing, formation of apoptopodia
and apoptotic bodies, while treatment with bee pollen for 24 h
resulted in the shrinkage of cells to form rounded cells.
Furthermore, water-soluble propolis and bee pollen treatment
resulted in a decrease in cell density. The population doubling
time of MCF-7 cells is ~38 h (15).
Since the significant activity of water-soluble propolis and bee
pollen to induce apoptosis was observed within 24 h, this indicated
that water-soluble propolis and bee pollen may have a cytotoxic
effect in MCF-7 cells. In the future, further study to investigate
the mechanism of action of water-soluble propolis and bee pollen
which contains of polar bioactive material will be important to
understand the potency of these natural products as anticancer
therapy.
The water-soluble propolis and bee pollen exhibited
similar antiproliferative activities in the MCF-7 cell line;
however, these products exhibited markedly different toxicity in
HaCaT cells. Water-soluble propolis was toxic to HaCaT cells after
24 h of treatment and even more after 48 h of treatment. Notably,
bee pollen resulted less toxic to the cells than water-soluble
propolis, with an IC50 >50 mg/ml after 24 h of
treatment. A longer treatment for 48 h revealed that the
antiproliferative activity of bee pollen was dose- and
time-dependent. The antiproliferative activity of bee pollen was
not observed after 24 and 48 h of treatment with ≤3.1 mg/ml.
Notably, this activity was observed after 48 h of treatment, with
an IC50 of 9.6±0.07 mg/ml. The current findings could be
a preliminary step for future investigation of bioactive compounds
in water-soluble propolis and bee pollen for the development of
breast anticancer drugs.
This study presented some limitations. Firstly, the
ESI-MS analysis was not completed with reference standard analysis
or library compounds information. Therefore, we were not able to
identify the presence of the estimated compounds in water-soluble
propolis or bee pollen. Secondly, the proteins present in
water-soluble propolis and bee pollen are still unknown. Thirdly,
current flow cytometric analysis was performed 24 h after treatment
at the IC50 concentration of water-soluble propolis,
where majority of the cell (89.9%) is already in the late apoptosis
stage. Therefore, shorter analysis treatment (6 or 12 h after
treatment) could be performed to obtain the profile of the
apoptosis stage. Forthly, the in vitro antiproliferative
assay in MCF-7 cells of water-soluble propolis and bee pollen were
conducted at different concentrations. Therefore, the comparison of
significant difference at each concentration of samples could not
be analysed.
In conclusion, water-soluble propolis and bee pollen
of the Trigona spp. from the district of Luwu Utara (South
Sulawesi, Indonesia) demonstrated antioxidant and antiproliferative
properties against MCF-7 cell proliferation. The antiproliferative
activity was observed using a cytotoxic analysis method after
treatment for 24 h, and the cytotoxic activity was identified as an
apoptosis mechanism. However, the bee products exerted a different
toxicity activity in the normal HaCaT cell line, in which the
water-soluble propolis resulted toxic, but the bee pollen resulted
less toxic. In contrast to propolis products from other countries,
CAPE was not the main bioactive compound. The present results
suggested that water-soluble propolis and bee pollen may have the
potential to be elaborated further as a breast anticancer therapy.
Further research is required to understand the bioactive compounds
in water-soluble propolis and bee pollen, and their potential
mechanisms of action as a breast anticancer therapy.
Acknowledgements
The authors would like to thank Dr Ahmad Faried and
the Cell Culture and Cytogenetic Laboratory of the Faculty of
Medicine of Padjadjaran University (Bandung, West Java, Indonesia)
for providing the MCF-7 and HaCaT cell lines.
Funding
The present study was supported by the Ministry of
Research, Technology & Higher Education, directorate general of
research and development strengthening (Indonesia; grant no.
1123s/UN6.O/LT/2019).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors contributions
EA performed the extracts preparation, antioxidant
activity, HPLC and ESI-MS analysis, protein identification,
apoptosis/flow cytometric analysis and the in vitro assays.
AD and AS evaluated the data, conceived the study and directed the
work. EA wrote the manuscript with comments from all authors. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
WHO, . Breast cancer WHO. 2019, https://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en/Dec
15–2019
|
|
2
|
Shah R, Rosso K and Nathanson SD:
Pathogenesis, prevention, diagnosis and treatment of breast cancer.
World J Clin Oncol. 5:283–298. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Amalia E, Diantini1 A and Subarnas A:
Overview of current and future targets of breast cancer medicines.
J Pharm Sci Res. 11:2385–2397. 2019.
|
|
4
|
Siddiqui AA, Iram F, Siddiqui S and Sahu
K: Role of natural products in drug discovery process. Int J Drug
Dev Res. 6:172–204. 2016.
|
|
5
|
Lichota A and Gwozdzinski K: Anticancer
activity of natural compounds from plant and marine environment.
Int J Mol Sci. 19:35332018. View Article : Google Scholar
|
|
6
|
Choudhari MK, Haghniaz R, Rajwade JM and
Paknikar KM: Anticancer activity of Indian stingless bee propolis:
An in vitro study. Evid Based Complement Alternat Med.
2013:9282802013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Omene C, Kalac M, Wu J, Marchi E, Frenkel
K and OConnor OA: Propolis and its active component, caffeic acid
phenethyl ester (CAPE), modulate breast cancer therapeutic targets
via an epigenetically mediated mechanism of action. J Cancer Sci
Ther. 5:334–342. 2013.PubMed/NCBI
|
|
8
|
Kustiawan PM, Puthong S, Arung ET and
Chanchao C: In vitro cytotoxicity of Indonesian stingless bee
products against human cancer cell lines. Asian Pac J Trop Biomed.
4:549–556. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mărghitaş LA, Dezmirean D, Drâglă F and
Bobiş O: Caffeic acid phenethyl ester (CAPE) in Romanian propolis.
Bull UASVM Anim Sci Biotechnol. 71:111–114. 2014.
|
|
10
|
Portokalakis I, Mohd Yusof HI, Ghanotakis
DF, Nigam PS and Owusu-Apenten RK: Manuka honey-induced
cytotoxicity against MCF7 breast cancer cells is correlated to
total phenol content and antioxidant power. J Adv Biol Biotechnol.
8:1–10. 2016. View Article : Google Scholar
|
|
11
|
Mahani, Nurhadi B, Subroto E and
Herudiyanto M: Bee propolis Trigona spp. potential and
uniqueness in Indonesia. Proc Univ Malaysia Teren Annu Sci.
2011:2011.
|
|
12
|
Riswahyuli Y, Rohman A, Setyabudi FMCS and
Raharjo S: Indonesian wild honey authenticity analysis using
attenuated total reflectance-fourier transform infrared (ATR-FTIR)
spectroscopy combined with multivariate statistical techniques.
Heliyon. 6:e036622020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kitamura H: Effects of propolis extract
and propolis-derived compounds on obesity and diabetes: Knowledge
from cellular and animal models. Molecules. 24:43942019. View Article : Google Scholar
|
|
14
|
Murtaza G, Karim S, Akram MR, Khan SA,
Azhar S, Mumtaz A and Bin Asad MH: Caffeic acid phenethyl ester and
therapeutic potentials. Biomed Res Int. 2014:1453422014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
American Type Culture Collection: SOP:
Thawing, Propagating and Cryopreserving of NCI-PBCF-HTB22 (MCF-7).
Version 1.6. 2012.
|
|
16
|
García-Viguera C, Ferreres F and
Tomás-Barberán FA: Study of canadian propolis by GC-MS and HPLC. Z
Natforsc C. 48c:731–735. 1993. View Article : Google Scholar
|
|
17
|
Ji LI, Larregieu CA and Benet LZ:
Classification of natural products as sources of drugs according to
the biopharmaceutics drug disposition classification system
(BDDCS). Chin J Nat Med. 14:888–897. 2016.PubMed/NCBI
|
|
18
|
Tsume Y, Mudie DM, Langguth P, Amidon GE
and Amidon GL: The biopharmaceutics classification system:
Subclasses for in vivo predictive dissolution (IPD) methodology and
IVIVC. Eur J Pharm Sci. 57:152–163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Irnawati, Purba M, Mujadilah R and
Sarmayani: Penetapan kadar vitamin C DAN UJI aktifitas antioksidan
sari buah songi (Dillenia serrata Thunb.) terhadap radikal
DPPH (Diphenylpicrylhydrazyl). Pharmacon. 6:40–44. 2017.(In
Indonesian).
|
|
20
|
Bankovaa VS, de Castro SL and Marcucci MC:
Propolis: Recent advances in chemistry and plant origin.
Apidologie. 31:3–15. 2000. View Article : Google Scholar
|
|
21
|
Ahmed S and Othman NH: The anti-cancer
effects of Tualang honey in modulating breast carcinogenesis: An
experimental animal study. BMC Complement Altern Med. 17:2082017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wlodkowic D, Skommer J and Darzynkiewicz
Z: Flow cytometery-based apoptosis detection. Methods Mol Biol.
559:19–32. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hingorani R, Deng J, Elia J, McIntyre C
and Mittar D: Detection of apoptosis using the BD Annexin V FITC
assay on the BD FACSVerse™ system. BD Bioscience application note.
2011:1–12. 2011.
|
|
24
|
Comşa Ş, Cîmpean AM and Raica M: The story
of MCF-7 breast cancer cell line: 40 tears of experience in
research. Anticancer Res. 35:3147–3154. 2015.PubMed/NCBI
|
|
25
|
Camarillo IG, Xiao F, Madhivanan S,
Salameh T, Nichols M, Reece LM, Leary JF, Otto KJ, Natarajan A,
Ramesh A and Sundararajan R: Low and high voltage
electrochemotherapy for breast cancer: An in vitro model study.
Electroporation Based Therapies Cancer. 55–102. 2014. View Article : Google Scholar
|
|
26
|
Kiosses WB, Hahn KM, Giannelly G and
Quaranta V: Characterization of morphological and cytoskeletal
changes in MCF10A breast epithelial cells plated on Laminin-5:
Comparison with breast cancer cell line MCF7. Cell Commun Adhes.
8:29–44. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reed JC: Mechanisms of apoptosis. Am J
Pathol. 157:1415–1430. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Niknafs B: Induction of apoptosis and
non-apoptosis in human breast cancer cell line (MCF-7) by cisplatin
and caffeine. Iran Biomed J. 15:130–133. 2011.PubMed/NCBI
|