Introduction
Breast cancer is the most common cancer in women
worldwide (1–3) and is classified by subtype based on the
expression of estrogen receptor (ER), progesterone receptor (PgR)
and HER2. Luminal A and B breast cancer is hormone
receptor-positive (ER+ and/or PgR+) and
accounts for ~70% of all patients with breast cancer (4). However, triple-negative breast cancer
(TNBC), which lacks expression of all receptors (ER−,
PgR− and HER2−), is the most difficult
malignancy to treat because there are no specific targets (5). The treatment of TNBC generally requires
a highly toxic chemotherapeutic regimen that includes
anthracyclines or taxanes (6,7).
Although a number of patients may respond well during the initial
stages of chemotherapy, there are numerous cases where patients
develop resistance to these chemotherapeutic agents over time
(8).
Molecules derived from natural products have been
explored as novel chemotherapeutic agents because of their high
affinity and selectiveness to physiologically relevant receptors
(9,10). Tetrandrine (TET) is a
bis-benzylisoquinoline alkaloid that has been extracted from the
traditional Chinese herb Stephania tetrandra (Fig. 1) (11). TET possesses a notable antitumor
activity in numerous types of cancer, including gastric, lung,
liver and colorectal cancer, both in vitro and in
vivo (12,13). The mechanisms of action of TET are
associated with multiple factors, such as modulating molecular
signaling pathways (14), inducing
cancer cell apoptosis (15),
promoting cell cycle arrest (16)
and increasing cell autophagy (17).
Although TET has been approved in China for some types of cancer,
such as acute myelogenous leukemia and advanced non-small cell lung
cancer (18,19), it has not been yet approved in Japan
as an antitumor drug for patients with cancer. Cepharanthine (CEP),
a TET analog extracted from traditional Japanese herbs,
Stephania cephalantha, has been approved in Japan for the
treatment of alopecia areata and leukopenia caused by radiation
therapy (Fig. 1) (20). However, less research on CEP as an
anticancer agent has been conducted (21,22).
Therefore, it is imperative to evaluate the effectiveness of CEP
for cancer treatment as an alternative drug in Japan compared with
TET in China.
In 2D cell culture, cells are grown on a flat
surface as a monolayer, and this culture method is widely used in
numerous biological studies. However, all cells in the human body
are both morphologically and functionally different from flat,
planar cells (23,24). To apply CEP towards clinical use, it
is important to consider the cell culture techniques that best
mimic the intricate in vivo cell environment. The 3D cell
culture method has been demonstrated to mimic the in vivo
environment more appropriately compared with 2D cell cultures
(25,26). There are a variety of 3D methods that
require special reagents and culture techniques, such as the
spinner cultivation technique (27),
alginate (28), agarose (29), soft agarose, Matrigel®
(30) and collagen matrix gel
(31) containing cytotoxic enzymes.
However, the ultra-low adherence (ULA) method, which is a type of
scaffold-free 3D cell culture method, is very simple and convenient
to use (32). ULA plates are
specially precoated with a hydrogel to enable globose spheroids
that are structured by cells (33).
The hydrogel coating is stable, displays no cytotoxicity, has no
biological activity and does not dissolve (33). In addition, the cells seeded in these
plates float in the medium and start forming spheroids
independently (34). The shape of
the well is round and promotes the formation of a single spheroid
per well, located centrally; additionally, cells can be imaged
easily since there is only one spheroid per well (35).
The aim of the present study was to evaluate and
compare the antitumor activities of TET and CEP on the
ER+ breast cancer MCF-7 cell line and the TNBC
MDA-MB-231 cell line, using a 3D culture system compared with a 2D
culture system.
Materials and methods
Materials and drug preparation
TET and CEP were purchased from Cayman Chemical and
Kakenshoyaku Co., Ltd., respectively. DMSO and Triton X-100 were
obtained from FUJIFILM Wako Pure Chemical Corporation. FBS was
purchased from Sigma-Aldrich (Merck KGaA). Minimum essential medium
(MEM)-α, penicillin-streptomycin (10,000 U/ml penicillin and 10,000
µg/ml streptomycin), 10X PBS, trypan blue and TrypLE Express were
purchased from Gibco (Thermo Fisher Scientific, Inc.). The Cell
Counting Kit-8 (CCK-8), calcein-AM, Hoechst 33342 and propidium
iodide (PI) were obtained from Dojindo Molecular Technologies, Inc.
TET and CEP solutions were prepared by first dissolving them in
DMSO and then diluting them into the media to a final concentration
of 0.1%. They were stored at −20°C until further use.
Cell lines
The human breast cancer MDA-MB-231 and MCF-7 cell
lines were purchased from RIKEN BioResource Center and the American
Type Culture Collection, respectively. MCF-7 and MDA-MB-231 cells
were maintained in MEM-α supplemented with 10% FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin at 37°C in a humidified
atmosphere with 5% CO2.
Cell culture and drug treatment
2D culture
MDA-MB-231 and MCF-7 cells were seeded in 96-well
plates (IWAKI & Co., Ltd.) or OptiPlates (PerkinElmer, Inc.) at
a density of 1×105, 2.5×105 or
5×105 cells/ml, and in 24-well plates at a density of
2×105 cells/ml depending on the experiments and
cultivated for 24 h at 37°C in a humidified atmosphere with 5%
CO2.
3D culture
MDA-MB-231 and MCF-7 cells were seeded in 96-well
CellCarrier Spheroid ULA plates (PerkinElmer, Inc.) at a density of
1×105 or 2×105 cells/ml depending on the
experiments and cultivated for 24 h at 37°C in a humidified
atmosphere containing 5% CO2. The seeding density had to
be below complete confluency and was determined based on the
manufacturer's maximum suggested number of 4×104
cells/well for 100% confluency.
Drug treatment
Vehicle for controls (cell culture medium) and
different concentrations of TET or CEP were subsequently added into
the corresponding wells and adjusted to final drug concentrations
of 0, 0.1, 0.3, 1, 3, 4, 6, 8, 10, 30 and 100 µg/ml. These
concentrations were selected based on a previous report
demonstrating that CEP and TET caused apoptosis in
glucocorticoid-resistant human leukemia Jurkat T cells after 72 h
of treatment (36). MDA-MB-231 and
MCF-7 cells were allowed to grow for 72 h at 37°C in the presence
of the drug. Long-term treatment times are traditionally used for
determining the efficacy of anticancer drugs, and therefore the
72-h time point was selected to ensure sufficient exposure to CEP
and TET as previously described (35,37).
Cell treatment was followed by a cell viability assay, cytotoxicity
assay and apoptosis detection.
Determination of cell viability
inhibition
MDA-MB-231 and MCF-7 cells (1×105,
2.5×105 or 5×105 cells/ml) were seeded into
96-well flat bottom plates, and then TET or CEP were added at
various concentrations as aforementioned. The plates were
subsequently incubated at 37°C with 5% CO2 for 72 h.
After incubation, 10 µl CCK-8 reagent was added into each well
according to the manufacturer's protocol, followed by an additional
incubation for 3 h at 37°C. The optical density (OD) of each well
was measured using a Corona MT P-32 micro-plate reader (Corona
Electric Co., Ltd.) at 450 nm. Cell viability was calculated
according to the following equation: Cell viability rate = (OD
sample value-OD blank value)/(OD control value-OD blank value) ×
100. The half-maximal inhibitory concentration (IC50)
was calculated using GraphPad Prism 8.0 (GraphPad Software, Inc.),
based on the cell viability of the untreated cells (control).
Spheroid area and roundness
evaluation
The microscopic images of the 2D- and 3D-cultured
cells or spheroids seeded as 1×105 cells/ml were taken
using an Operetta CLS high-content analysis system (PerkinElmer,
Inc.) and the cell morphological structure of MDA-MB-231 and MCF-7
cells in both 2D and 3D cultures were analyzed. The spheroid area
and roundness were observed 72 h after drug treatment for 3D
cultures using the same system. A series of images were recorded
and examined using ×5 magnification. Spheroid area and roundness
were measured and analyzed using the Harmony 4.5 software
(PerkinElmer, Inc.).
Cell cytotoxicity
The cytoplasm and nuclei of the dead cells were
evaluated in order to determine the cause of cell toxicity. The
supernatant of each well was removed, and the cells were washed
twice with sterile PBS after 72 h of drug treatment. MDA-MB-231 and
MCF-7 2D-cultured cells (1×105 cells/ml) were stained
with 4 µM calcein-AM, 10 µM of Hoechst 33342 and 4 µM PI for 15 min
at room temperature in the dark. The spheroids were only stained
with calcein-AM and PI in order to detect cell viability. The
Harmony 4.5 software was used to detect and determine the
fluorescence intensity using the following wavelengths: Calcein-AM
excitation, 496 nm and emission, 517 nm; Hoechst 33342 excitation,
343 nm and emission, 483 nm; and PI excitation, 560 nm and
emission, 595 nm.
Assessment of apoptosis
Apoptotic cell rates in MDA-MB-231 and MCF-7 cells
were analyzed using an Annexin V-FITC apoptosis detection kit (BD
Biosciences). Both cell lines adjusted to 2×105 cells/ml
were seeded in 24-well plates for the 2D culture or in 96-well
CellCarrier Spheroid ULA plates for the 3D culture, and treated by
serial dilution with TET or CEP (final concentrations: 0, 1, 3, 10
and 30 µg/ml) followed by an additional 72 h of incubation at 37°C.
Subsequently, the staining procedure was performed according to the
manufacturer's protocol. The duration of staining with Annexin
V-FITC (excitation and emission wavelengths at 485 and 535 nm,
respectively) and PI (excitation and emission wavelengths at 560
and 595 nm, respectively) was 15 min at room temperature in the
dark. Dissociation of the spheroids into single cells was performed
by resuspending the spheroids in TrypLE Express. A total of
1×104 cells was analyzed via flow cytometry (FACSCanto
and FACSDiva software v6.0; both BD Biosciences). The cells were
subsequently assessed for viable (Annexin
V−/PI−), early apoptotic (Annexin
V+/PI−), late apoptotic (Annexin
V+/PI+) and necrotic (Annexin
V−/PI+) cells. Triton-X100 was used for
detecting PI+ control cells.
Statistical analysis
All experiments were repeated ≥3 times and the data
are presented as the mean ± SD. Cell viability of 2D MDA-MB-231 or
MCF-7 monolayer cells treated with TET or CEP cultured at different
cell densities was analyzed using one-way ANOVA followed by Tukey's
multiple comparisons test. The comparison of the cell viability
between 2D-cultured MCF-7 cells treated with TET and CEP versus
MDA-MB-231 cells was analyzed using the Mann-Whitney U test, while
the comparison of IC50 values of CEP or TET between 2D
monolayer MDA-MB-231 and MCF-7 cells was analyzed via one-way ANOVA
with a post-hoc Sidak's multiple comparisons test. A one-way ANOVA
followed by Dunnett's multiple comparisons test was used for the
comparisons of the intensity of calcein, Hoechst 33342 and PI, the
spheroid area and roundness, and the percentages of viable, early
apoptotic and late apoptotic cells of 2D- and 3D-cultured
MDA-MB-231 and MCF-7 cells treated with CEP or TET compared with
control cells. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using GraphPad Prism 8.0 (GraphPad Software, Inc.).
Results
Cell viability and IC50
values of TET and CEP
The cell viability and IC50 values of TET
and CEP were determined in 2D monolayers of MCF-7 and MDA-MB-231
cells using the CCK-8 assay. Cell viability decreased with TET or
CEP treatment in a dose-dependent manner (Fig. 2). MDA-MB-231 cells (Fig. 2C and D) were more affected by the
cytotoxic effects of TET and CEP compared with MCF-7 cells
(Fig. 2A and B). Using a
concentration of >3 µg/ml CEP suppressed the viability of
MDA-MB-231 cells significantly compared with the control
(P<0.05; Fig. 2D). The viability
of MDA-MB-231 cells was significantly suppressed using 0.1 µg/ml
and high doses of CEP compared with that of MCF-7 cells (Fig. 3A and B). Furthermore, the viability
of MDA-MB-231 cells was significantly suppressed compared with that
of MCF-7 cells when high doses of TET were used (Fig. 3C and D).
The IC50 values of TET and CEP required
to suppress the cell viability of each cell line were calculated
(Table I). MDA-MB-231 cells were
more sensitive to CEP and TET compared with MCF-7 cells. The
IC50 value of CEP for MDA-MB-231 cells was 7 times lower
than that for MCF-7 cells at 5×105 cells/ml.
Additionally, the IC50 value of TET for MDA-MB-231 cells
was >8 times lower than that for MCF-7 cells at 5×105
cells/ml. Therefore, CEP affected the cell viability of MDA-MB-231
cells at lower concentrations than that of MCF-7 cells. There were
significant differences in the IC50 values of CEP
between MDA-MB-231 and MCF-7 cells at densities of
2.5×105 and 5×105 cells/ml, and in the
IC50 values of TET between MDA-MB-231 and MCF-7 cells at
a density of 5×105 cells/ml (P<0.05; Fig. 4).
 | Table I.Mean IC50 values of CEP
and TET on the cell viability of 2D monolayer MDA-MB-231 and MCF-7
cells. |
Table I.
Mean IC50 values of CEP
and TET on the cell viability of 2D monolayer MDA-MB-231 and MCF-7
cells.
| Drug | Cell line | 1×105
cells/ml | 2.5×105
cells/ml | 5×105
cells/ml |
|---|
| CEP | MDA-MB-231 | 3.8 | 5.4a | 8.6a |
|
| MCF-7 | 17.6 | 35.6 | 60.4 |
| TET | MDA-MB-231 | 6.4 | 7.6 | 9.6a |
|
| MCF-7 | 13.5 | 26 | 84.7 |
Microscopic analyses on cell
cytotoxicity
To further understand cell cytotoxicity of CEP and
TET on breast cancer cells or spheroids, 2D monolayer and 3D
spheroids were stained with calcein, Hoechst and PI, and the
fluorescence intensity was measured to identify changes in the
cytoplasm, nuclei and dead cells, respectively.
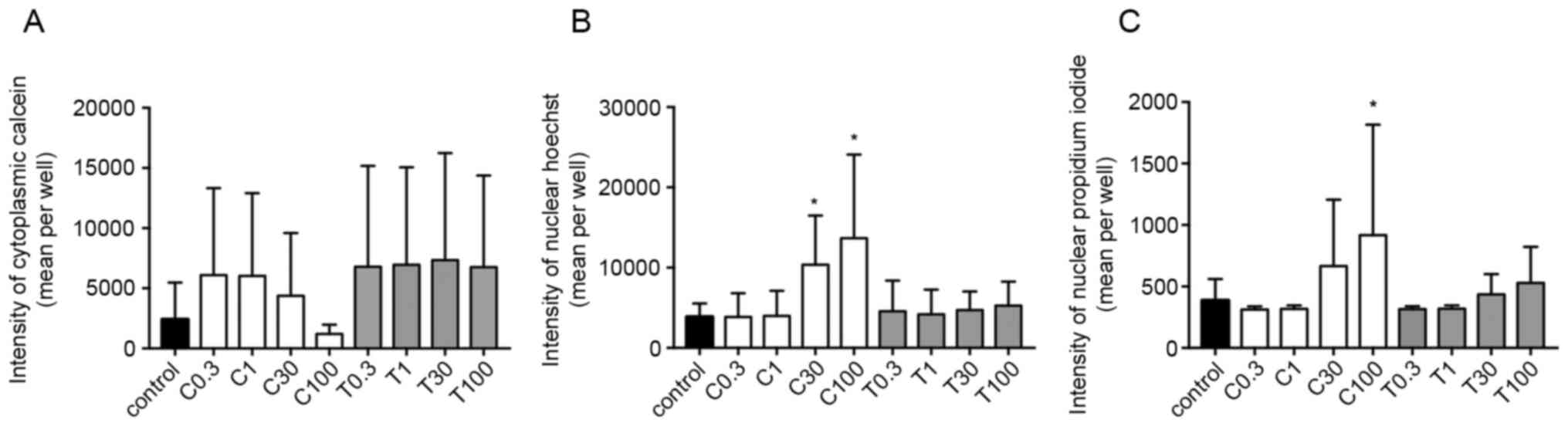
The calcein fluorescence intensity in the cytoplasm
of 2D-cultured MDA-MB-231 cells was decreased in a
concentration-dependent manner by CEP, but not by TET (Fig. 5A). Additionally, the fluorescence
intensities of Hoechst and PI were significantly increased using
100 µg/ml CEP, but not by TET (P<0.05; Fig. 5B and C). These effects were more
strongly observed in 2D monolayer cultures of MCF-7 cells compared
with those in MDA-MB-231 cells. The fluorescence intensity in the
cytoplasm of MCF-7 cells was significantly decreased using 100
µg/ml of CEP compared with that in the control group (P<0.05;
Fig. 6A). Conversely, the intensity
in the nucleus of Hoechst and PI in MCF-7 cells was significantly
increased when using >30 µg/ml of CEP compared with that in the
control group, indicating the induction of stronger cell death
compared with the control group in these cells (P<0.05; Fig. 6B and C). However, TET did not have
any significant effects on the three intensities (Fig. 6). Microscopic images of 2D-cultured
MCF-7 cells treated with 100 µg/ml of CEP revealed no cytoplasm
intensity and increased intensity of the nucleus and dead cells
compared with in the control group (Fig.
7). The fluorescence images of all cells with all treatment
concentrations are shown in Fig.
S1.
In MDA-MB-231 (Fig.
8) and MCF-7 (Fig. 9) spheroids,
CEP significantly decreased the calcein intensity in a
dose-dependent manner (P<0.05; Figs.
8A and 9A). Although the
cytoplasm intensity in MDA-MB-231 cells was significantly decreased
using 100 µg/ml TET, TET had a lower effect on the cytoplasm
intensity in spheroids of both cell lines. Conversely, TET
significantly increased the PI level in both cell lines (P<0.05;
Figs. 8B and 9B) when used at 30 and 100 µg/ml, compared
with in the control group. Additionally, CEP significantly
increased the number of dead cells only in 3D MCF-7 spheroids
(P<0.05; Fig. 9B) at 30 and 100
µg/ml, but not in 3D MDA-MB-231 spheroids (Fig. 8B). The microscopic images of the
3D-cultured MCF-7 spheroids revealed that 100 µg/ml of CEP
decreased the intensity of calcein and increased the intensity of
PI compared with that in control spheroids (Fig. 10). The fluorescence images of all
cells with all treatment concentrations are shown in Fig. S2.
In addition to the calcein and PI staining, the
spheroid area and roundness of 3D spheroids were calculated. The
‘roundness’ parameter was defined as a measure of how close the
shape of the 2D spheroid was to a circle (the gross feature of the
shape). The spheroid areas in both MDA-MB-231 and MCF-7 cells did
not significantly change from the control depending on treatment,
except when using 30 µg/ml TET, which significantly decreased the
spheroid area of MCF-7 cells (Figs.
8C and 9C). The roundness tended
to decrease with higher concentrations of CEP and TET (Figs. 8D and 9D), although the roundness of MDA-MB-231
spheroids treated with 1 µg/ml TET was increased compared with the
control. Using 30 and 100 µg/ml of CEP significantly decreased the
roundness of MDA-MB-231 spheroids (P<0.05; Fig. 8D).
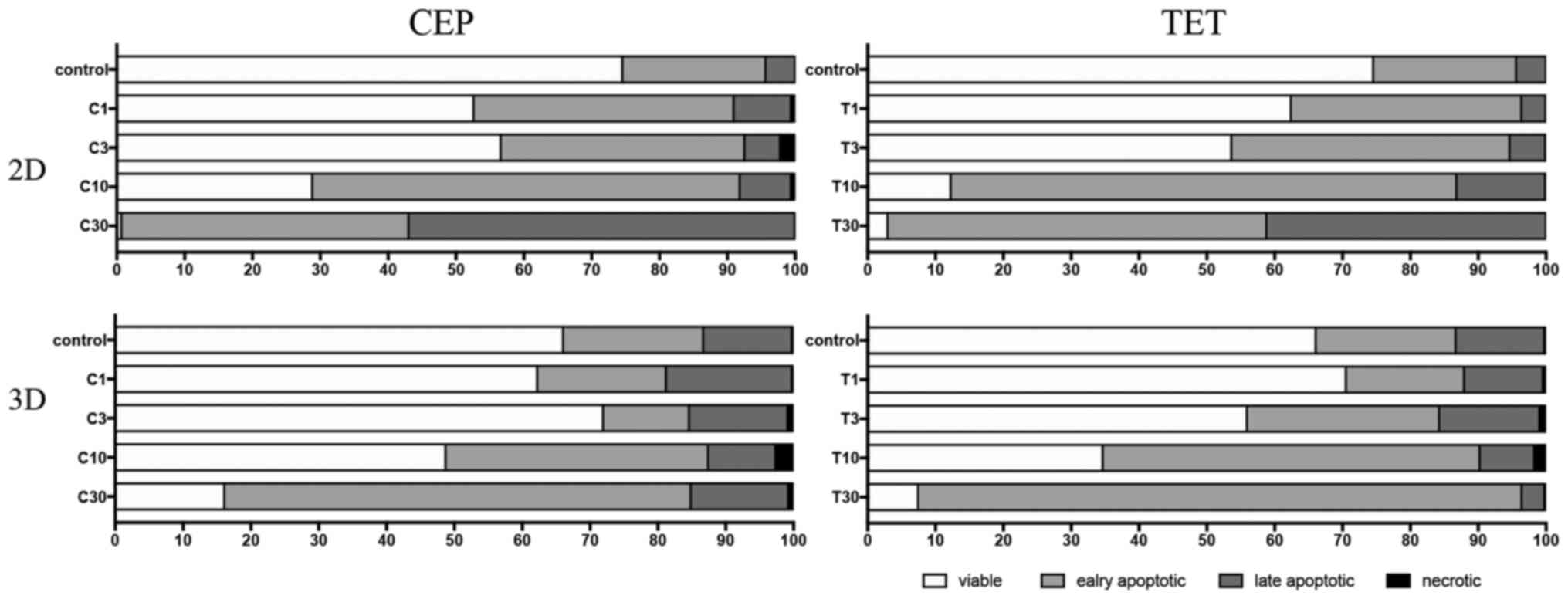
Apoptosis
Cell apoptosis was examined by measuring the
percentages of viable, early apoptotic and late apoptotic cells in
both 2D-cultured cells and 3D-cultured spheroids. The flow
cytometry plots for all cells with all treatment concentrations are
shown in Figs. S3 and S4. For 2D-cultured MDA-MB-231 cells, there
was a statistically significant greater difference in cell survival
when >10 µg/ml CEP was used compared with in the control
(P<0.05; Fig. 11A). Early
apoptotic cells were significantly increased at 10 µg/ml CEP
(P<0.05; Fig. 11B), and CEP at
30 µg/ml significantly induced late apoptosis compared with in the
control (P<0.05; Fig. 11C).
Compared with TET, CEP induced apoptosis of MDA-MB-231 cells at a
high rate (Fig. 11A-C). By
contrast, MCF-7 cells required high concentrations of CEP and TET
to induce apoptosis (Fig. 11E-G).
Using 30 µg/ml CEP or TET significantly decreased the number of
viable cells compared with in the control (P<0.05; Fig. 11E), but it did not influence the
rate of early apoptotic cells in MCF-7 cells (Fig. 11F). Additionally, there were no
significant differences compared with in the control group for the
rate of late apoptotic cells, except with 30 µg/ml TET (P<0.05;
Fig. 11G).
 | Figure 11.Effects of CEP and TET on percentages
of viable, early apoptotic, late apoptotic and necrotic cells of
MDA-MB-231 and MCF-7 monolayer cells. After treatment with
different concentrations of CEP or TET, 2D monolayer MDA-MB-231
were stained with Annexin V-FITC and PI to identify (A) viable, (B)
early apoptotic, (C) late apoptotic and (D) necrotic cells.
Similarly, MCF-7 cells were stained with Annexin V-FITC and PI to
identify (E) viable, (F) early apoptotic, (G) late apoptotic and
(H) necrotic cells. Data are shown as the mean + SD of 4
independent experiments. (I) Cells treated with 30 µg/ml CEP or TET
were classified into four groups based on different quadrants:
Viable cells (bottom left), early apoptotic cells (bottom right),
late apoptotic cells (upper right) and necrotic cells (upper left)
using flow cytometry. *P<0.05 vs. control analyzed via one-way
ANOVA and Dunnett's multiple comparisons test. C/CEP,
cepharanthine; T/TET, tetrandrine; PI, propidium iodide. |
In MDA-MB-231 spheroids, the rate of early apoptotic
cells increased after treatment with 10 µg/ml CEP and TET compared
with in the control (Fig. 12A and
B). However, the drugs exhibited no significant effect on the
rate of late apoptotic cells in MDA-MB-231 spheroids (Fig. 12C). Consequently, the viability of
the MCF-7 spheroids seemed to be suppressed (Fig. 12E), but the percentage of early
apoptotic cells among groups did not significantly change (Fig. 12F). Only 30 µg/ml TET significantly
increased the rate of late apoptotic cells in MCF-7 spheroids
(P<0.05; Fig. 12G).
 | Figure 12.Effects of CEP and TET on percentages
of viable, early apoptotic, late apoptotic and necrotic cells of
MDA-MB-231 and MCF-7 spheroids. After the treatment with different
concentrations of CEP or TET, 3D MDA-MB-231 spheroids were stained
with Annexin V-FITC and PI to identify (A) viable, (B) early
apoptotic, (C) late apoptotic and (D) necrotic cells. Similarly,
MCF-7 spheroids were stained with Annexin V-FITC and PI to identify
(E) viable, (F) early apoptotic, (G) late apoptotic and (H)
necrotic cells. The data are shown as the mean + SD of 4
independent experiments. (I) Cells treated with 30 µg/ml CEP or TET
were classified into four groups based on different quadrants:
Viable cells (bottom left), early apoptotic cells (bottom right),
late apoptotic cells (upper right) and necrotic cells (upper left)
using flow cytometry. *P<0.05 vs. control analyzed via one-way
ANOVA and Dunnett's multiple comparisons test. C/CEP,
cepharanthine; T/TET, tetrandrine; PI, propidium iodide. |
The percentages of viable, early apoptotic, late
apoptotic and necrotic cells after treatment with CEP or TET in 2D
or 3D cultures of MDA-MB-231 and MCF-7 cells are comparatively
shown in Figs. 13 and 14, respectively. According to these
summarized models, MDA-MB-231 2D cell cultures, compared with the
3D spheroids, appeared to be more sensitive to the apoptotic
inducing effects of CEP and TET (Fig.
13). CEP and TET did not cause major necrosis in 2D- and
3D-cultured MDA-MB-231 and MCF-7 cells (Figs. 13 and 14).
Discussion
The present study demonstrated the antitumor effects
of CEP and TET in human breast cancer monolayer cells and
spheroids. Both drugs suppressed cell viability and induced
apoptosis, and MDA-MB-231 cells exhibited a higher sensitivity to
the suppressive effects of TET and CEP.
In the current study, the cell density in 2D and 3D
cell culture systems was chosen as 10,000 and 20,000 cells/well,
respectively, to compare the differences of CEP and TET. These
numbers were selected based on the protocol for the CellCarrier
Spheroid ULA 96-well microplates, which stated the maximum seeding
density should be 40,000 cells/well. As shown in Table I, the IC50 values of CEP
and TET on the viability of cells cultured in high cell numbers
with the 2D method were higher compared with those in low cell
numbers, and it was estimated that 3D spheroids require higher
concentrations of each drug to decrease cell viability.
Alternatively, if a high cell density was chosen for the 2D method,
the control breast cancer cells would be confluent after
incubation. Therefore, the cell densities in the present study used
for the 2D and 3D cell culture systems were chosen as 10,000 and
20,000 cells/well, respectively, to compare the differences of the
effect of CEP and TET between cells cultured in 2D and those
cultured in 3D.
The drug concentrations used in the present study
were chosen based on a previous report demonstrating that CEP and
TET caused apoptosis in glucocorticoid resistant human leukemia
Jurkat T cells after 72 h of drug treatment, in which the
IC50 values were 3.66±0.22 µM for CEP and 3.98±0.05 µM
for TET (36). The units were
converted to ‘µg/ml’ based on the molecular weights of the test
compounds, giving a value of ~2.5 µg/ml. It was hypothesized that
spheroids would require a higher concentration compared with
2D-cultured cells to induce apoptosis. Based on the aforementioned
calculation and hypothesis, it was decided to use a drug
concentration range between 0 and 100 µg/ml. The present results
indicated that 3D spheroids required higher concentrations to
induce cell death compared with 2D monolayer cells.
Additionally, it was considered that the longer
treatment period may improve the exposure to the drugs, since drugs
require longer to penetrate enough into spheroids (35). Previous studies have evaluated the
effects of drugs on the growth of MDA-MB-231 spheroids using a
treatment period of 72 h (35,37).
According to these studies, it was speculated that a treatment time
of 72 h would also be appropriate for the present study. However,
studying the effects on 3D spheroids may require a longer treatment
period in order to understand drug metabolism or drug distribution,
which should be investigated in future studies.
Since there are no available targeted therapy
options for TNBC, the standard treatment regimen remains
chemotherapy (38). Notably, TNBC
generally has improved responses to chemotherapy compared with
other subtypes (38). However,
patients without complete response account for ~80% of TNBC cases
(38), and therefore, the
development of novel strategies or drugs is essential for the
treatment of TNBC. The present study revealed that the TNBC
MDA-MB-231 cells were more sensitive compared with MCF-7 cells to
the cytotoxic effects of TET and CEP in the 3D culture system.
Other types of breast cancer cells, such as HER2+ breast
cancer cells, or cancer cells modeling other diseases were not used
in the current study to evaluate the efficacy of TET and CEP, and
therefore, the present results may only reflect MCF-7 and
MDA-MB-231 cell lines. However, these results demonstrated the
potential for CEP and TET to be used as novel therapeutic
strategies for breast cancer.
TET has been demonstrated to have positive
therapeutic effects on cardiovascular disease, hypertension,
silicosis and autoimmune diseases (39). In addition, TET is already used
clinically for the treatment of some types of cancer, such as acute
myelogenous leukemia and advanced non-small cell lung cancer, in
China (18,19). Notably, the present study
demonstrated that CEP had stronger effects on MDA-MB-231 and MCF-7
cells compared with TET, in a concentration-dependent manner. This
suggested that CEP may potentially be used as an anticancer drug
for breast cancer and may be an alternative treatment of TET.
The present study evaluated the morphological
changes of the cytoplasm, nucleus and nucleic acid of dead cells in
order to examine the cell toxicity caused by CEP and TET.
Non-fluorescent calcein crosses the membrane of living cells easily
and is hydrolyzed in the cytoplasm by intracellular esterases to a
membrane-impermeable green-fluorescent calcein (40). Generally, PI does not penetrate the
cell membrane of living cells, but it only enters into dead cells
and then intercalates with DNA in the nucleus to emit red
fluorescence (41). The strength of
the fluorescence indicates more chromatin aggregation, which is a
degree of increased apoptosis (41).
In the present study, both CEP and TET induced apoptosis in
MDA-MB-231 and MCF-7 cell lines in a dose-dependent manner.
Additionally, CEP decreased the cytoplasmic space, increased
chromatin condensation and increased the number of dead cells in a
dose-dependent manner. The current results suggested that CEP may
be more potent at inducing cell death compared with TET, which is
already used for cancer treatment (18,19).
Notably, a previous report demonstrated that TET and CEP induced
apoptosis of leukemia Jurkat T cells in vitro, and the
important markers of apoptosis, p53 and Bax, were both upregulated
via treatment with TET and CEP (36). While the mechanism remains unclear,
this apoptotic effect may be due to the methoxy group of TET, which
is likely involved with the blockade of voltage-operated
Ca2+ entry, and since CEP has the same methoxy group, it
may behave similarly (42).
Therefore, further studies are required to understand the detailed
underlying apoptotic mechanism.
The present study evaluated the effects of TET and
CEP on breast cancer cell lines using models of 2D monolayer and 3D
spheroids. Previously, numerous types of in vitro 3D culture
systems have been developed to mimic the in vivo growth
environment of cancer (43). These
cancer 3D culture systems aim to preserve and improve the
biological characteristics of the original tumor microenvironment
compared with conventional 2D monolayer culture systems (25). Some 3D culture systems have been
successful for screening out the chemotherapeutic drug
sensitivities of cancer cells (44).
However, 2D culture systems have been used to compare the results
conducted by 2D and 3D systems (45). The present experiments demonstrated
that 3D spheroids had a higher sensitivity to CEP compared with
TET. Furthermore, CEP decreased the volume of the cytoplasm and
increased apoptosis. As demonstrated by the apoptosis assay using
flow cytometry, the viability of MDA-MB-231 spheroids was markedly
suppressed by CEP and TET compared with that of MCF-7 spheroids.
However, compared with 2D monolayer cells, 3D spheroids exhibited a
lower response to the suppressive effects of CEP and TET,
especially in MCF-7 cells. Imamura et al (26) reported that the central part of
spheroids of breast cancer cells formed in 3D culture becomes
hypoxic, which may cause dormancy of tumor cells in the
G0 phase, downregulating the expression levels of
caspase-3. This suggests that higher concentrations of CEP are
required to have an effect on cancer spheroids. Furthermore, a
previous study reported that a conventional anticancer medicine,
cisplatin, in combination with CEP increased the efficacy of the
conventional drug in esophageal squamous cell carcinoma and
decreased its side effects on gut microorganisms and intestinal
mucosal immunity (46). Detailed
analysis of the effect of CEP combined with cisplatin against
breast cancer cells is warranted in future studies.
CEP was approved in 1969 and has been used for a
long period of time in Japan; it has been used for patients with
alopecia areata and leukopenia caused by radiation therapy, and it
has been demonstrated to be safe and effective in Japan (20). Therefore, CEP would be practical,
safe and easier to be approved for use in patients with cancer
after additional clinical research and trials. Additionally, there
are several formulations of CEP, including tablets, powder and
injection (20).
In conclusion, in the present study CEP and TET
demonstrated toxicity in MDA-MB-231 and MCF-7 monolayer cells, as
well as in spheroids of these cell lines, observed via decreases in
the cytoplasm and chromatin aggregation. Although the current
results suggested that the cytotoxicity of CEP and TET may be
associated with the induction of apoptosis, the exact mechanism is
not completely understood and requires further investigation. CEP
and TET caused cytotoxicity in spheroids of MDA-MB-231 and MCF-7
cells, with MDA-MB-231 cells exhibiting higher sensitivity to these
compounds. CEP induced stronger apoptosis and spheroid cytotoxicity
than TET. Therefore, the present results suggested that CEP may
have a stronger antitumor activity against TNBC spheroids compared
with TET.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Anh Tan Truong
(University of Southern California, Los Angeles, CA, USA) for the
constructive comments and proofreading of the manuscript, and Mrs.
Atsuko Kiyomi for figure formatting and editing.
Funding
Funding was provided by the Tokyo University of
Pharmacy and Life Sciences.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
AK, BY, TH and MS designed the study. AK, RM, JM and
KY conducted the experiments and analyzed the data. AK, SI, BY, TH
and MS interpreted the data. AK wrote the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CEP
|
cepharanthine
|
|
TET
|
tetrandrine
|
|
ER
|
estrogen receptor
|
|
PgR
|
progesterone receptor
|
|
TNBC
|
triple-negative breast cancer
|
|
ULA
|
ultra-low adherence
|
|
CCK-8
|
Cell Counting Kit-8
|
References
|
1
|
Tao Z, Shi A, Lu C, Song T, Zhang Z and
Zhao J: Breast cancer: Epidemiology and etiology. Cell Biochem
Biophys. 72:333–338. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coughlin S: Epidemiology of breast cancer
in women. Adv Exp Med Biol. 1152:9–29. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Winters S, Martin C, Murphy D and Shokar
N: Breast cancer epidemiology, prevention, and screening. Prog Mol
Biol Transl Sci. 151:1–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Harbeck N and Gnant M: Breast cancer.
Lancet. 389:1134–1150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carey L, Winer E, Viale G, Cameron D and
Gianni L: Triple-negative breast cancer: Disease entity or title of
convenience? Nat Rev Clin Oncol. 7:683–692. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liedtke C, Mazouni C, Hess K, André F,
Tordai A, Mejia J, Symmans WF, Gonzalez-Angulo A, Hennessy B, Green
M, et al: Response to neoadjuvant therapy and long-term survival in
patients with triple-negative breast cancer. J Clin Oncol.
26:1275–1281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rakha EA, El-Sayed ME, Green AR, Lee AH,
Robertson JF and Ellis IO: Prognostic markers in triple-negative
breast cancer. Cancer. 109:25–32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Denkert C, Liedtke C, Tutt A and von
Minckwitz G: Molecular alterations in triple-negative breast
cancer-the road to new treatment strategies. Lancet. 389:2430–2442.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thomford NE, Senthebane DA, Rowe A, Munro
D, Seele P, Maroyi A and Dzobo K: Natural products for drug
discovery in the 21st century: Innovations for novel drug
discovery. Int J Mol Sci. 19:15782018. View Article : Google Scholar
|
|
10
|
Harvey AL: Natural products in drug
discovery. Drug Discov Today. 13:894–901. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen Y: Potential role of tetrandrine in
cancer therapy. Acta Pharmacol Sin. 23:1102–1106. 2002.PubMed/NCBI
|
|
12
|
Liu T, Liu X and Li W: Tetrandrine, a
Chinese plant-derived alkaloid, is a potential candidate for cancer
chemotherapy. Oncotarget. 7:40800–40815. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu B, Wang T, Qian X, Liu G, Yu L and
Ding Y: Anticancer effect of tetrandrine on primary cancer cells
isolated from ascites and pleural fluids. Cancer Lett. 268:166–175.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu Y, Li F, Xu T and Sun J: Tetrandrine
prevents multidrug resistance in the osteosarcoma cell line, U-2OS,
by preventing Pgp overexpression through the inhibition of NF-κB
signaling. Int J Mol Med. 39:993–1000. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu R, Liu T, Tan Z, Wu X, Li M, Jiang L,
Bao R, Shu Y, Lu A and Liu Y: Tetrandrine induces apoptosis in
gallbladder carcinoma in vitro. Int J Clin Pharmacol Ther.
52:900–905. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun XC, Cheng HY, Deng YX, Shao RG and Ma
J: Tetrandrine: A potent abrogator of G2 checkpoint function in
tumor cells and its mechanism. Biomed Environ Sci. 20:495–501.
2007.PubMed/NCBI
|
|
17
|
Qiu W, Zhang AL and Tian Y: Tetrandrine
triggers an alternative autophagy in DU145 cells. Oncol Lett.
13:3734–3738. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu WL, Shen HL, Ao ZF, Chen BA, Xia W, Gao
F and Zhang YN: Combination of tetrandrine as a potential-reversing
agent with daunorubicin, etoposide and cytarabine for the treatment
of refractory and relapsed acute myelogenous leukemia. Leuk Res.
30:407–413. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu W, Zhang J, Ying C, Wang Q, Yan C,
Jingyue Y, Zhaocai Y, Yan X, Heng-jun S and Lin J: Tetrandrine
combined with gemcitabine and cisplatin for patients with advanced
non-small cell lung cancer improve efficacy. Int J Biomed Sci.
8:28–35. 2012.PubMed/NCBI
|
|
20
|
Bailly C: Cepharanthine: An update of its
mode of action, pharmacological properties and medical
applications. Phytomedicine. 62:1529562019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Asaumi J, Nishikawa K, Matsuoka H, Iwata
M, Kawasaki S, Hiraki Y and Nishijima K: Direct antitumor effect of
cepharanthin and combined effect with adriamycin against Ehrlich
ascites tumor in mice. Anticancer Res. 15:67–70. 1995.PubMed/NCBI
|
|
22
|
Ono M and Tanaka N: Positive interaction
of bisbenzylisoquinoline alkaloid, cepharanthin, with vinca
alkaloid agents against human tumors. In Vivo. 11:233–241.
1997.PubMed/NCBI
|
|
23
|
Rosso F, Giordano A, Barbarisi M and
Barbarisi A: From cell-ECM interactions to tissue engineering. J
Cell Physiol. 199:174–180. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ryu N, Lee S and Park H: Spheroid culture
system methods and applications for mesenchymal stem cells. Cells.
8:16202019. View Article : Google Scholar
|
|
25
|
Breslin S and O'Driscoll L: The relevance
of using 3D cell cultures, in addition to 2D monolayer cultures,
when evaluating breast cancer drug sensitivity and resistance.
Oncotarget. 7:45745–45756. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Imamura Y, Mukohara T, Shimono Y,
Funakoshi Y, Chayahara N, Toyoda M, Kiyota N, Takao S, Kono S,
Nakatsura T and Minami H: Comparison of 2D- and 3D-culture models
as drug-testing platforms in breast cancer. Oncol Rep.
33:1837–1843. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moscona A: Rotation-mediated histogenetic
aggregation of dissociated cells. A quantifiable approach to cell
interactions in vitro. Exp Cell Res. 22:455–475. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
O'Keane JC, Kupchik HZ, Schroy PC, Andry
CD, Collins E and O'Brien MJ: A three-dimensional system for
long-term culture of human colorectal adenomas. Am J Pathol.
137:1539–1547. 1990.PubMed/NCBI
|
|
29
|
Carlsson J, Nilsson K, Westermark B,
Pontén J, Sundström C, Larsson E, Bergh J, Påhlman S, Busch C and
Collins VP: Formation and growth of multicellular spheroids of
human origin. Int J Cancer. 31:523–533. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kleinman HK, McGarvey ML, Hassell JR, Star
VL, Cannon FB, Laurie GW and Martin GR: Basement membrane complexes
with biological activity. Biochemistry. 25:312–318. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lawler EM, Miller FR and Heppner GH:
Significance of three-dimensional growth patterns of mammary
tissues in collagen gels. In Vitro. 19:600–610. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bresciani G, Hofland LJ, Dogan F, Giamas
G, Gagliano T and Zatelli MC: Evaluation of spheroid 3D culture
methods to study a pancreatic neuroendocrine neoplasm cell line.
Front Endocrinol (Lausanne). 10:6822019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nath S and Devi G: Three-dimensional
culture systems in cancer research: Focus on tumor spheroid model.
Pharmacol Ther. 163:94–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kelm JM, Timmins NE, Brown CJ, Fussenegger
M and Nielsen LK: Method for generation of homogeneous
multicellular tumor spheroids applicable to a wide variety of cell
types. Biotechnol Bioeng. 83:173–180. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vinci M, Gowan S, Boxall F, Patterson L,
Zimmermann M, Court W, Lomas C, Mendiola M, Hardisson D and Eccles
SA: Advances in establishment and analysis of three-dimensional
tumor spheroid-based functional assays for target validation and
drug evaluation. BMC Biol. 10:292012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu W, Wang X, Tu Y, Masaki H, Tanaka S,
Onda K, Sugiyama K, Yamada H and Hirano T: Tetrandrine and
cepharanthine induce apoptosis through caspase cascade regulation,
cell cycle arrest, MAPK activation and PI3K/Akt/mTOR signal
modification in glucocorticoid resistant human leukemia Jurkat T
cells. Chem Biol Interact. 130:1087262019. View Article : Google Scholar
|
|
37
|
Nigjeh SE, Yeap SK, Nordin N, Kamalideghan
B, Ky H and Rosli R: Citral induced apoptosis in MDA-MB-231
spheroid cells. BMC Complement Altern Med. 18:562018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jhan JR and Andrechek ER: Triple-negative
breast cancer and the potential for targeted therapy.
Pharmacogenomics. 18:1595–1609. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bhagya N and Chandrashekar KR:
Tetrandrine-A molecule of wide bioactivity. Phytochemistry.
125:5–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gatti R, Belletti S, Orlandini G,
Bussolati O, Dall'Asta V and Gazzola GC: Comparison of annexin V
and calcein-AM as early vital markers of apoptosis in adherent
cells by confocal laser microscopy. J Histochem Cytochem.
46:895–900. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Basmaciyan L, Azas N and Casanova M:
Calcein+/PI-as an early apoptotic feature in Leishmania. PLoS One.
12:e01877562017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Leung YM, Berdik M, Kwan CY and Loh TT:
Effects of tetrandrine and closely related bis-benzylisoquinoline
derivatives on cytosolic Ca2+ in human leukemic HL-60
cells: A structure-activity relationship study. Clin Exp Pharmacol
Physiol. 23:653–659. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kunz-Schughart L, Freyer J, Hofstaedter F
and Ebner R: The use of 3-D cultures for high-throughput screening:
The multicellular spheroid model. J Biomol Screen. 9:273–285. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kiyomi A, Makita M, Ozeki T, Li N,
Satomura A, Tanaka S, Onda K, Sugiyama K, Iwase T and Hirano T:
Characterization and clinical implication of Th1/Th2/Th17 cytokines
produced from three-dimensionally cultured tumor tissues resected
from breast cancer patients. Transl Oncol. 8:318–326. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jo Y, Choi N, Kim K, Koo HJ, Choi J and
Kim HN: Chemoresistance of cancer cells: Requirements of tumor
microenvironment-mimicking in vitro models in anti-cancer drug
development. Theranostics. 8:5259–5275. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhou P, Li Z, Xu D, Wang Y, Bai Q, Feng Y,
Su G, Chen P, Wang Y, Liu H, et al: Cepharanthine hydrochloride
improves cisplatin chemotherapy and enhances immunity by regulating
intestinal microbes in mice. Front Cell Infect Microbiol.
9:2252019. View Article : Google Scholar : PubMed/NCBI
|