Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most
common type of non-Hodgkin's lymphoma worldwide, representing
30-40% of all cases among different geographic regions (1). Although the use of anti-CD20 monoclonal
antibodies has significantly improved the survival of patients with
DLBCL, traditional chemotherapy is still indispensable for the
treatment of this lymphoma. Due to their high efficacy and broad
spectrum of activity, anthracyclines, such as Adriamycin and
epirubicin, play an important role in first-line chemotherapeutic
regimens for DLBCL (2).
Anthracyclines are commonly associated with
cardiotoxicity, occurring with an incidence of ~9% (3,4). The
prognosis of anthracycline-induced heart failure remains poor, with
a 2-year mortality rate of 60% (5).
Clinical guidelines suggest limiting the maximum cumulative dose to
reduce the incidence of cardiotoxicity while maintaining the
maximum antitumor effect (6).
Anthracycline-induced cardiotoxicity most likely
occurs at the time of exposure, while the symptoms of
cardiotoxicity associated with heart failure may be masked for
several years. Usually, anticongestive treatment is only given once
clinical manifestations show that the compensatory mechanism of the
heart is no longer adequate, leading to a seriously deteriorated
prognosis (7). With early
recognition and treatment initiation, the prognosis of patients can
be significantly improved (8). Acute
cardiotoxic side effects are rare and usually reversible.
Late-onset cardiomyopathy, which is difficult to treat and
associated with a poor prognosis, is more frequent. Therefore, it
is highly necessary to identify an appropriate and sensitive
approach for the early detection of myocardial injury to improve
the quality of life and prolong the survival of patients with
DLBCL.
The left ventricular ejection fraction (LVEF) refers
to the ratio of stroke volume divided by the left ventricular end
diastolic (LVED) volume (9).
Notably, LVEF remains used as a classic index to evaluate systolic
function, albeit being highly dependent on preload and afterload
conditions, and it is a late marker of cardiotoxicity (10). The activation of the sympathetic
nervous system in patients with heart failure maintains the normal
LVEF (7). It may be a late finding
to detect a decrease in LVEF after cancer treatment. Therefore,
early markers associated with myocardial dysfunction are highly
desired (11–13).
The risk of cardiotoxicity may be reduced if the
administration of an angiotensin-converting enzyme inhibitor or
angiotensin receptor blocker, β-blocker and/or statin starts early
(11). In order to reduce the risk
of cardiotoxicity and enhance the cardiac protection strategies,
researchers have made good efforts to find specific and sensitive
markers of early LV dysfunction, including cardiac biomarkers and
advanced imaging modalities (14,15).
Compared with other traditional methods such as
echocardiography, cardiac CT and cardiac MRI, gated myocardial
perfusion imaging (G-MPI) is a novel and sensitive method that can
identify the small myocardial injury caused by cancer therapeutics
(16,17). With regard to G-MPI, the majority of
studies focused on patients who underwent radiation therapy, and a
higher percentage of perfusion defects with associated wall motion
abnormalities after radiation therapy was found (18,19). The
summed rest score (SRS), summed motion score (SMS) and summed
thickening score (STS) are quantitative parameters obtained by
quantitative analysis of resting G-MPI. The SRS has been used to
evaluate the degree of damage to myocardial perfusion at rest, and
has higher diagnostic sensitivity and accuracy compared with visual
evaluation (20). Anthracycline
chemotherapeutics can damage myocardial cells, meaning that the
resultant myocardial perfusion can also be abnormal. However,
whether quantitative analysis of resting G-MPI compared with LVEF
can detect the chemotherapy-induced myocardial injury in lymphoma
patients at an earlier stage remains largely unexplored.
The present study aimed to investigate the
prognostic value of G-MPI parameters in the early detection of
impaired myocardial function in patients with DLBCL receiving
anthracycline chemotherapy.
Materials and methods
Study design
A total of 36 patients, who were newly diagnosed
with DLBCL in the Department of Hematology of the Third Affiliated
Hospital of Soochow University (Changzhou, China), were enrolled in
the present analysis between March 2016 and July 2019. Patients
with any heart disease at baseline examination were excluded. The
exclusion criteria included the following: Abnormalities on
electrocardiogram (ECG) or echocardiography, plus any abnormalities
in myocardial markers or brain natriuretic peptide (BNP). Patients
who met any of the four criteria were excluded from the study. All
DLBCL diagnoses were made based on the histological pathology
analysis (21). Histopathological
criteria for DLBCL are defined in the World Health Organization
2008 classification of lymphoid neoplasms (22). Clinical staging data were determined
according to the Ann Arbor staging classification (23). This study was designed to assess the
cardiac function of each patient by serum test, echocardiography
and G-MPI, at baseline and after the last cycle of chemotherapy
(mean time, 6 months).
All patients were administered with six cycles of an
R-CHOP regimen (375 mg/m2 rituximab, 750
mg/m2 cyclophosphamide, 1.4 mg/m2
vincristine, 70 mg/m2 epirubicin or 25 mg/m2
pegylated liposomal doxorubicin, and 100 mg prednisone for 5 days).
The R-CHOP regimen was administered every 3 or 4 weeks according to
the hematopoietic recovery of the patient. A series of serum
parameters were detected before and after chemotherapy, including
fast blood sugar (FBS; mmol/l), total cholesterol (TC; mmol/l),
high-density lipoprotein (mmol/l), low-density lipoprotein (LDL;
mmol/l), triacylglycerol (mmol/l), alanine aminotransferase (U/l),
creatinine (mmol/l), D-Dimer (mg/l), white blood cells
(×109/l), hemoglobin (g/l), platelets
(×109/l), red blood cell distribution width (%),
β2-microglobulin (mg/l), C-reactive protein (mg/l), procalcitonin
(ng/ml), BNP (pg/ml), aspartate transaminase (U/l), lactic
dehydrogenase (U/l), creatine kinase (CK; U/l), CK-MB (ng/ml),
troponin (µg/l) and myoglobin (ng/ml).
All procedures were in accordance with the ethical
standards of the responsible human experimentation committee
(institutional and national) and the Declaration of Helsinki
(1975), as amended in 2008. Patient samples and the clinical data
were obtained with approval from the Ethical Committee of The Third
Affiliated Hospital of Soochow University (approval number,
CL022-01), and written informed consent was obtained according to
the institutional guidelines.
Evaluation of cardiac function
The cardiac function of each patient was evaluated
using a series of serum indicators, ECG, echocardiography and
G-MPI. Treatment-related cardiotoxicity (TRC) was defined as when
the patient was identified to have one of the following clinical
manifestations: Symptomatic heart failure, cardiac death,
arrhythmia, infarction, a decrease in left ventricular ejection
fraction (LVEF) of >15% from baseline or a decrease in LVEF of
>10 to <50%. All other cases were defined as no-TRC (24,25).
The serum indicators for cardiac function included
brain natriuretic peptide, troponin, aspartate transaminase, lactic
dehydrogenase, creatine kinase (CK), CK-MB isoenzyme and myoglobin.
The levels of all these indicators were determined using an XE-2100
automated analyzer (Sysmex Co., Ltd.).
Heart rate, PR interval, QRS interval, QT interval
and QTc interval measurements were determined by a 12-lead ECG
((FX-8322; Fukuda Sangyo, Co., Ltd.). Echocardiographic
examinations were performed using the ultrasonic diagnostic
apparatus (Vivid E9; GE Healthcare) with a 3.5-MHz transducer.
Reports were made according to the American Society of
Echocardiography guidelines (26,27). The
indicators relevant to research included left atrial diameter, left
ventricular posterior wall thickness, left ventricular end systolic
diameter, interventricular septal thickness, LVED diameter and
LVEF.
Resting G-MPI was performed using a single-photon
emission CT/CT scanner (Symbia T16; Siemens Healthineers), which
was equipped with a low-energy high-resolution collimator. Briefly,
the patient was intravenously injected with 740-925 MBq for resting
studies, and the radiochemical purity was >95%. At 60 min after
the injection, the electrodes were placed upon the skin above the
heart of the patient with two detectors set at 90°. Images were
acquired every 35 sec and every 6° clockwise from 45° at the right
anterior oblique position to 45° at the left anterior oblique
position, with an acquisition matrix of 128×128 and a magnification
of 1.45. Subsequently, the gated acquisition was performed with
electrocardiographic R-wave as the acquisition trigger signal and
eight frames/R-R interval. The planar images were reconstructed by
a Flash 3D™ iterative method (28)
(16 iterations and two subsets) and reoriented to obtain LV
short-axis, horizontal long-axis and vertical long-axis images. The
left venticle was divided into 17 segments using QPS and QGS
quantitative analysis software (QPS & QGS 2009; Cedars-Sinai
Medical Center). The QPS was used to analyze myocardial perfusion.
The segmental radiotracer activity in the resting scans was scored
according to a standard 5-point scale (0, normal; 1, mild; 2,
moderate; 3, severe; and 4, absent activity) and summed to generate
an SRS (29). QGS was used to obtain
the LVEF, SMS and STS.
Statistical analysis
All statistical analyses in this study were
performed using SPSS version 24.0 statistical software (IBM Corp.)
and MedCalc software version 15.11.4 (MedCalc Software bvba).
Normally distributed data are expressed as the mean ± standard
error, while data without a normal distribution are presented as
the median (interquartile range). Paired t-tests, Wilcoxon's
matched pairs test, independent samples t-tests and Mann-Whitney U
tests were used to compare the differences between groups depending
on the type of data. Proportional differences were evaluated with
the χ2 test or Fisher's exact test in the supplementary
tables. The optimal cutoff value for the association of the SRS
level with TRC after anthracycline chemotherapy was determined
based on the result of receiver operating characteristic (ROC)
curve analysis. The initial univariate logistic regression analysis
was followed by a multivariate logistic regression model using
stepwise selection to identify univariate predictors of TRC.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Baseline patient characteristics
Between March 2016 and July 2019, 36 patients with
DLBCL were enrolled in the present study. Table I lists the baseline clinical
characteristics of these patients. The mean age of the cohort was
58 years (range, 27-72 years), and there were 20 males and 16
females. The body mass index (BMI) of these patients was
22.854±0.636. At baseline, all of the recruited patients had no
comorbidity of heart disease, while 8 patients had hypertension, 5
patients had diabetes, and 10 patients suffered from
hypercholesteremia and hypertriglyceridemia. Moreover, 10 patients
(28%) were found to be in the early stage of lymphoma according to
Ann Arbor classification (I–II), and 26 patients (72%) were found
to be in the late stage of lymphoma (III–IV). In addition, 10
patients (28%) were diagnosed with B symptoms.
 | Table I.Baseline of the clinical
characteristics of the 36 patients with diffuse large B-cell
lymphoma enrolled in the present study. |
Table I.
Baseline of the clinical
characteristics of the 36 patients with diffuse large B-cell
lymphoma enrolled in the present study.
|
Characteristics | Value |
|---|
| Median age (range),
years | 58 (27–82) |
| Sex, n (%) |
|
|
Male | 20 (56) |
|
Female | 16 (44) |
| BMIa | 22.854±0.636 |
| Comorbidity, n
(%) |
|
| Heart
disease | 0 (0) |
|
Hypertension | 8
(22) |
|
Diabetes | 5
(14) |
|
Hypercholesteremia | 10 (28) |
|
Hypertriglyceridemia | 10 (28) |
| Clinical stage, n
(%) |
|
|
I–II | 10 (28) |
|
III–IV | 26 (72) |
| B-symptoms, n
(%) | 10 (28) |
Comparison of serum, echocardiography,
ECG and G-MPI parameters at baseline and after chemotherapy
As listed in Table
II, by comparing the serum parameters of these patients before
and after six courses of R-CHOP chemotherapy, it was found that the
levels of FBS (P=0.002), TC (P=0.03) and LDL (P=0.001) were
significantly increased, and that the hemoglobin level was
significantly higher (P=0.034) after chemotherapy.
 | Table II.Comparison of plasma markers before
and after chemotherapy. |
Table II.
Comparison of plasma markers before
and after chemotherapy.
| Plasma markers | Before
chemotherapy | After
chemotherapy | P-value |
|---|
| FBS, mmol/l | 4.980 (0.870) | 5.300 (1.320) | 0.002 |
| TC, mmol/l | 4.287±0.156 | 4.716±0.159 | 0.030 |
| HDL, mmol/l | 1.076±0.057 | 1.166±0.053 | 0.073 |
| LDL, mmol/l | 2.521±0.108 | 2.847±0.134 | 0.001 |
| TG, mmol/l | 1.036 (0.600) | 1.475 (0.985) | 0.155 |
| ALT, U/l | 18
(19.500) | 18 (15) | 0.665 |
| Creatinine,
mmol/l | 65.667±1.709 | 68.273±2.029 | 0.164 |
| D-Dimer, mg/l | 0.505 (0.740) | 0.395 (0.398) | 0.275 |
| WBC
(×109/l) | 5.060 (2.318) | 4.51 (2.495) | 0.139 |
| Hemoglobin,
g/l | 121.500
(24.250) | 125.500
(25.500) | 0.034 |
| Platelets
(×109/l) | 194.794±11.824 | 187.147±10.871 | 0.464 |
| RDW, % | 13.100 (1.775) | 13.500 (1.875) | 0.174 |
| β2-MG, mg/l | 1.798±0.125 | 1.854±0.091 | 0.706 |
| CRP, mg/l | 7.250 (42.250) | 4.950 (5.300) | 0.177 |
| PCT, ng/ml | 0.053 (0.071) | 0.069 (0.085) | 0.655 |
| BNP, pg/ml | 67.500
(64.250) | 36.000
(57.388) | 0.060 |
| AST, U/l | 24 (11) | 26 (11) | 0.820 |
| LDH, U/l | 587.136±51.855 | 557.136±28.157 | 0.527 |
| CK, U/l | 49 (24.500) | 55 (38) | 0.509 |
| CK-MB, ng/ml | 0.700 (0.700) | 0.9 (0.800) | 0.149 |
| Troponin, µg/l | 0.001 (0.003) | 0.003 (0.002) | 0.142 |
| Myoglobin,
ng/ml | 15.100 (8.800) | 15.800
(10.050) | 0.765 |
Considering the cumulative cardiotoxicity of
anthracyclines, cardiac function-related serum indicators of these
patients were compared at the time of initial treatment and after
chemotherapy. There were no significant differences, as listed in
Table II (P>0.05).
As listed in Table
III, echocardiographic and ECG parameters of these patients
were compared at baseline and after chemotherapy. No significant
differences in these parameters were found by echocardiography,
including LVEF (P>0.05). Notably, it was found that the QTc
interval (P=0.015) was significantly increased after chemotherapy.
By using the novel G-MPI method, it was found that SRS, the
indicator representing myocardial perfusion, was significantly
higher after chemotherapy (P=0.012) (Table IV; Fig.
1).
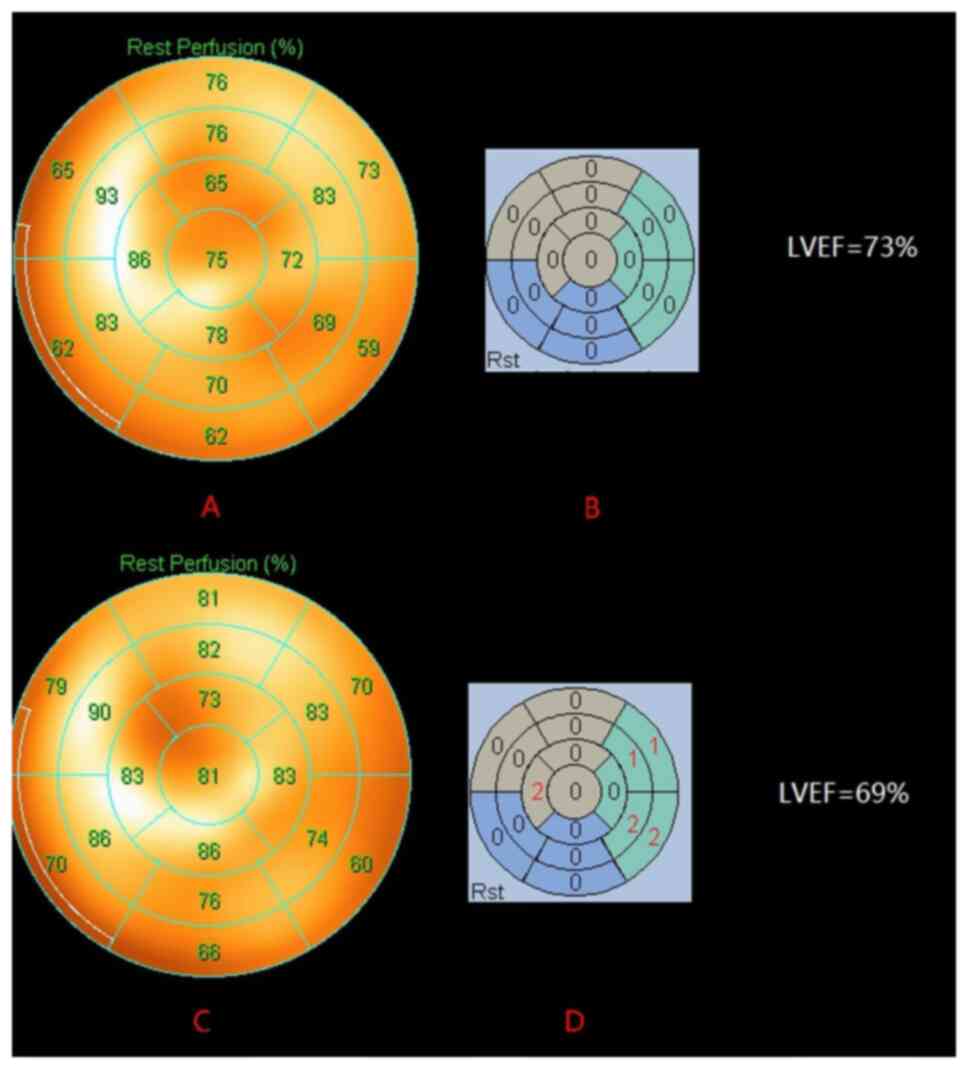 | Figure 1.A representative case showing a
significant change in G-MPI SRS in a DLBCL patient before and after
R-CHOP chemotherapy. The patient was a 69-year-old male. The LV
systolic function was normal before chemotherapy (LVEF, 73%), (A)
the MPI resting myocardial perfusion bull's eye chart showed an
even distribution of imaging agent in each wall of the LV, and (B)
SRS was 0, indicating that the LV myocardial perfusion before
chemotherapy was good. The LV systolic function was not
significantly damaged after six courses of R-CHOP chemotherapy
regimens (LVEF, 69%), (C) while the MPI resting myocardial
perfusion bull's eye chart showed uneven distribution compared with
that before chemotherapy, and (D) the SRS was 8, revealing that
despite no obvious changes in LVEF after anthracycline chemotherapy
in patients with DLBCL, LV myocardial perfusion was already
abnormal. LV, left ventricular; G-MPI, gated myocardial perfusion
imaging; SRS, summed rest score; DLBCL, diffuse large B-cell
lymphoma; LVEF, LV ejection fraction. |
 | Table III.Comparison of markers in
echocardiogram and electrocardiogram before and after
chemotherapy. |
Table III.
Comparison of markers in
echocardiogram and electrocardiogram before and after
chemotherapy.
| Marker | Before
chemotherapy | After
chemotherapy | P-value |
|---|
| Echocardiogram |
| LAD,
mm | 35.389±1.462 | 34.500±0.883 | 0.371 |
| LVEDD,
mm | 46.000±0.957 | 45.667±0.886 | 0.674 |
| LVESD,
mm | 31 (5) | 30.500 (2.500) | 0.754 |
| IVST,
mm | 9
(1) | 9 (1) | 0.604 |
| LVPWT,
mm | 9 (0.500) | 9 (1.250) | 0.964 |
| LVEF,
% | 62.889±0.593 | 62.500±0.445 | 0.369 |
|
Electrocardiogram |
| Heart
rate, bpm | 73 (18) | 79
(10.500) | 0.102 |
| PR
interval, msc | 153.500 (28) | 174 (19.500) | 0.475 |
| QRS
interval, msec | 100 (10.500) | 100 (13.000) | 0.283 |
| QT
interval, msec | 375.970±5.607 | 372.636±4.482 | 0.549 |
| QTc
interval, msec | 421.250±3.775 | 429.344±3.349 | 0.015 |
 | Table IV.Comparison of markers associated with
myocardial perfusion in gated myocardial perfusion imaging before
and after chemotherapy. |
Table IV.
Comparison of markers associated with
myocardial perfusion in gated myocardial perfusion imaging before
and after chemotherapy.
| Markers | Before
chemotherapy | After
chemotherapy | P-value |
|---|
| SRS | 2 | 3 | 0.012 |
| SMS rest | 0 | 0 | 0.900 |
| STS rest | 0 | 0 | 0.858 |
| EF rest | 65.514±1.608 | 64.714±1.746 | 0.582 |
Association between SRS and TRC
TRC was defined using similar criteria as previously
described (20,23), including a decrease in LVEF of
>15% from baseline, a decrease in LVEF of >10 to <50%,
symptomatic heart failure, arrhythmia, infarction or cardiac death.
According to these criteria and the characteristics of patients
during observation, all the patients were divided into the TRC
group (n=22) and the no-TRC group (n=14). In the TRC group, 1
patient had a myocardial infarction, 1 patient suffered cardiac
death, 20 patients had other types of arrhythmia and 4 patients had
a decrease in LVEF of >15% from baseline or a decrease in LVEF
of >10 to <50%. No patients had symptomatic heart
failure.
The baseline characteristics of patients in these
two groups were compared. Moreover, the plasma markers,
echocardiography, ECG and G-MPI parameters of patients after six
courses of chemotherapy were also assessed between these two groups
(Tables SI–III and V).
There was no significant difference in any of aforementioned
parameters after chemotherapy, with the exception of SRS from
G-MPI. The median SRS level of the TRC and no-TRC groups was 4 and
0, respectively (P<0.0001).
 | Table V.Comparison of markers in gated
myocardial perfusion imaging in the no-TRC and TRC groups after
chemotherapy. |
Table V.
Comparison of markers in gated
myocardial perfusion imaging in the no-TRC and TRC groups after
chemotherapy.
| Markers | No TRC | TRC | P-value |
|---|
| SRS | 0 (1) | 4 (3.25) | <0.0001 |
| SMS rest | 0 (0.25) | 0 (1.5) | 0.375 |
| STS rest | 0 (0) | 0 (0.5) | 0.455 |
| EF rest | 61.357±2.122 | 66.952±2.463 | 0.118 |
After adjustment for LVEF in echocardiography and
QTc interval in ECG, multivariate logistic regression analysis
showed that the SRS level was the only independent predictor for
TRC (OR, 6.503); 95% CI, 1.364-26.869; P=0.018; Table VI).
 | Table VI.Univariate and multivariate logistic
regression analyses. |
Table VI.
Univariate and multivariate logistic
regression analyses.
|
|
| Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | Univariate analysis
P-value | OR | 95% CI | P-value |
|---|
| Clinical
characteristics |
|
SRS | <0.0001 | 6.503 | 1.709-24.738 | 0.006 |
|
QTc | 0.953 | 0.982 | 0.888-1.086 | 0.721 |
| EF
rest | 0.118 | 1.031 | 0.894-1.189 | 0.673 |
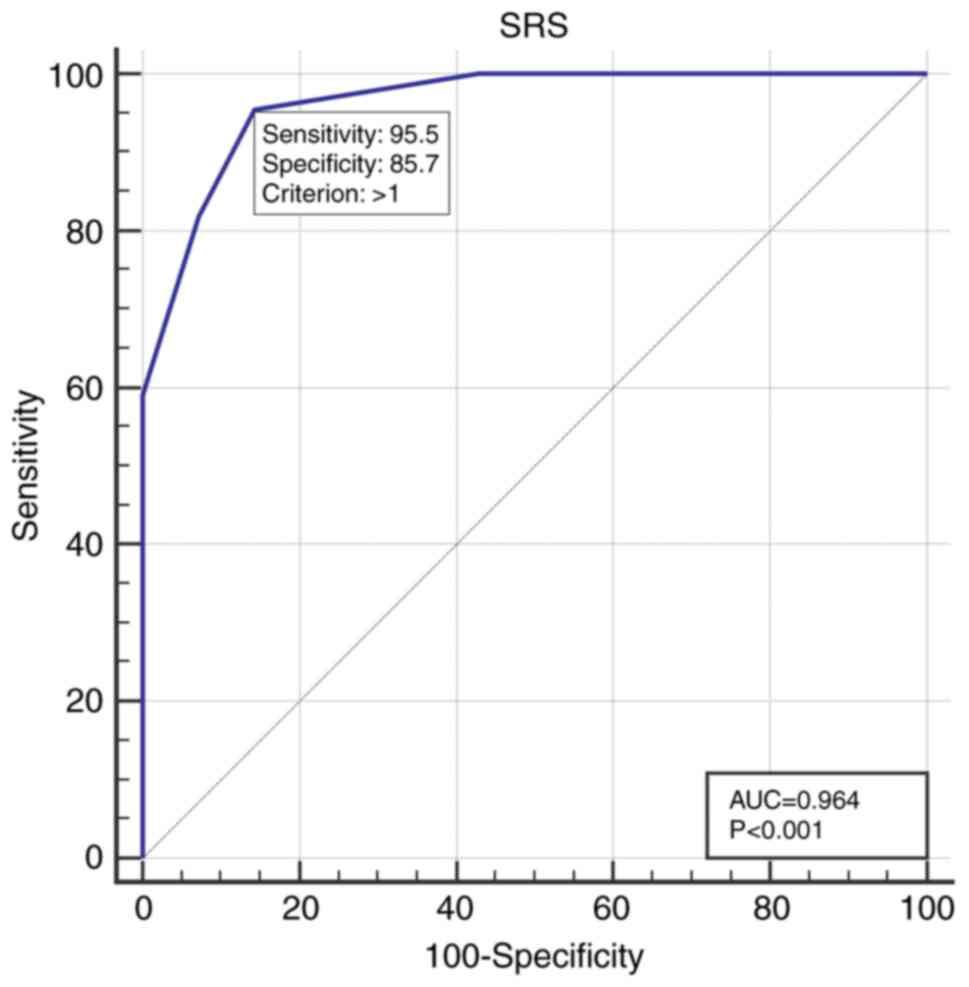
Using ROC curve analysis, an optimal SRS cutoff of
>1 was identified for predicting TRC, with a sensitivity of
95.5%, a specificity of 85.7% and an area under the curve (AUC) of
0.964 (P<0.001; Fig. 2).
Discussion
To the best of our knowledge, the present study is
the first to use the resting G-MPI to evaluate the abnormal changes
in LV myocardial perfusion in DLBCL patients before and after
chemotherapy. The results showed that the SRS value detected after
chemotherapy was significantly higher than that before
chemotherapy, indicating that there was abnormal LV perfusion after
chemotherapy. Logistic multivariate analysis showed that SRS was an
independent risk factor for TRC after chemotherapy in patients with
DLBCL. ROC curve analysis showed that when SRS was >1, the
possibility of TRC was high.
The prognosis of patients with DLBCL has been
greatly improved due to earlier detection and new, targeted
therapeutic drugs. In terms of longer survivorship, a large number
of patients with DLBCL have to face the risk of cardiovascular
morbidity and mortality, which are caused by the long-term toxicity
of anthracycline chemotherap (30–33).
Cardiotoxicity caused by anthracycline is often progressive and
irreversible, which severely affects the prognosis of patients with
DLBCL (14,34,35).
Serial echocardiographic evaluation of resting LVEF is still a
classic index to monitor the anthracycline-induced cardiac side
effects from chemotherapy. However, LVEF tests are limited by their
low sensitivity. A normal LVEF level does not mean that there is no
cardiotoxicity. The irreversible cardiomyopathy often happens
before the observation of a decreased LVEF (4,7). It has
been reported that at the early stage of cardiac function
impairment, most patients present with subclinical myocardial
damage (36). The early detection
and treatment of cardiotoxicity, even when asymptomatic, seem to be
critical for cardiac function recovery and for reduction in
associated adverse cardiac events. There is no significant change
in LV function, ECG results or myocardial markers in the early
stage of myocardial damage. Only when the myocardial function is
severely damaged or the overall heart function is impaired, do
abnormal changes become detectable in indicators, such as LV
systolic function. In the present study, there was no significant
difference in LVEF values before and after chemotherapy in the
patients with DLBCL. According to the TRC criteria of the LVEF
value, only 4 patients with DLBCL developed LVEF-related TRC after
chemotherapy. Univariate and multivariate analyses of TRC showed
that LVEF was not an independent risk factor for TRC. The results
further confirmed that LVEF could not evaluate the cardiotoxicity
caused by anthracycline drugs in the early stage.
The QTc interval on the ECG refers to the QT
interval corrected according to heart rate or R-R interval
(37). Antineoplastic drugs open
sodium and calcium channels by blocking potassium channels,
resulting in cardiac repolarization abnormality, and a prolonged
QTc interval would be demonstrated on the ECG (38). Drug-induced prolongation of QTc
interval has been considered to be a critical risk factor for
cardiac toxicity, and it likely leads to palpitations, syncope and
sudden cardiac death (SCD) caused by severe ventricular arrhythmias
(35,38,39). In
a follow-up study consisting of 147 adult survivors who previously
suffered from childhood tumors and received anthracycline
chemotherapy, Markman et al (14) found that the prolonged QTc interval
is associated with the subsequent development of LV dysfunction,
suggesting that the QTc interval is an earlier indicator for
evaluating the impairment of cardiac function compared with LVEF.
The normal range of the QTc interval is 350-450 msec in adult males
and 360-460 msec in adult femal (38). Puppe et al (38) highlighted the tripled risk of SCD
when the QT interval is >470 msec. The study by Porta-Sanchez
(36) indicated that the patients
should be carefully evaluated to determine whether they should
discontinue the usage of all offending drugs when a prolonged QTc
interval is detected (>500 msec or an increase of >60 msec
greater than baseline) (36). When
the QTc interval of a patient fluctuates within the normal range,
clinicians are prone to lacking the same vigilance in checking for
myocardial injury. In addition, considerable variation may be
caused by differences in the technical circumstances and
non-pathological biological variability. All the aforementioned
factors have contributed to the uncertainty of using the ECG QTc
interval as an indicator of cardiac toxicity monitoring. In the
present study, the mean QTc interval of the patients after
anthracycline chemotherapy was 429.344 msec. Although there was a
significant difference before and after treatment, the mean QTc
interval after anthracycline chemotherapy did not reach the upper
limit of the normal QTc interval of 450/460 msec. Moreover, the
results of this study showed that the QTc interval was not an
independent risk factor for TRC. Therefore, there were still
limitations of using the QTc interval as an early indicator of
cardiac toxicity.
The mechanisms of anthracycline-induced
cardiomyopathies include endoplasmic reticulum stress, calcium
dysregulation, activation of the immune system and impairment of
progenitor cells, among others (40,41). To
date, the most widely cited and accepted mechanism is the formation
of reactive oxygen species (ROS), which leads to oxidative stress
(42–45). Koleini and Kardami (46) indicated that cardiomyocytes need a
large number of healthy functioning mitochondria to product
sufficient ATP to maintain contractile function and cell survival.
Autophagic cell death and mitophagy have been proposed to be
associated with the cardiotoxicity of anthracycline (46). Ichikawa et al (47) found that anthracycline-dependent
cardiotoxicity occurs through ROS production and possibly
mitochondrial iron accumulation. Mitochondria play a pivotal role
in the metabolism and apoptosis of cardiomyocytes. Technetium 99m
sestamibi (99mTc-MIBI) is a lipophilic cation imaging
tracer mainly localized in the mitochondria; it is mainly
sequestered within the mitochondria by a huge negative
transmembrane potential (48). The
distribution of 99mTc-MIBI in the myocardium is directly
proportional to myocardial blood flow. When the mitochondrial
function of cardiomyocytes is impaired, collapse of the
mitochondrial membrane potential will lead to quick release of
extracted 99mTc-MIBI. Therefore, G-MPI can show abnormal
local myocardial perfusion, which can be used for early
non-invasive evaluation of abnormal changes in the mitochondrial
function of cardiomyocytes (48–50).
SRS is an indicator of G-MPI for the quantitative
evaluation of resting myocardial perfusion abnormalities and can
directly reflect changes in cardiomyocyte function (51,52). To
the best of our knowledge, no studies have reported the
quantitative analysis of G-MPI in the early evaluation of
myocardial damage caused by chemotherapy. In the present study,
99mTc-MIBI G-MPI was used to quantitatively analyze the
changes in myocardial perfusion before and after chemotherapy. The
results showed that the resting perfusion SRS score in DLBCL
patients after chemotherapy was significantly higher than that
before chemotherapy. In addition, logistic multivariate analysis we
applied and SRS was found to be an independent risk factor for
predicting TRC in patients with DLBCL after chemotherapy. ROC curve
analysis indicated that an SRS >1 had the highest efficiency in
predicting TRC (AUC, 0.964; sensitivity, 95.5%; specificity,
85.7%). In other words, when the SRS was >1, prompt intervention
was required to prevent TRC. In the present study, after six
courses of anthracycline-containing chemotherapy, all of the DLBCL
patients with TRC developed arrhythmia (22/22), and only a small
portion of patients showed a significant decrease in LVEF (4/22).
According to the results, after anthracycline chemotherapy, the
patients with DLBCL had abnormal regional myocardial perfusion,
which was characterized by an increased SRS. As a much earlier
indicator compared with LVEF and QTc interval, SRS could be used to
evaluate the cardiac toxicity associated with LV dysfunction not
only caused by chemotherapy (decreased LVEF) at an early stage, but
also caused by chemotherapy-induced arrhythmia at an early stage.
Such a conclusion might be attributed to abnormal perfusion being
the pathological basis for the impairment of LV myocardial
function. The severity of perfusion abnormality is closely
associated with the decline in LV function. The detected value of
LVEF might still be within the normal range when slight myocardial
perfusion abnormalities occur. Therefore, compared with LVEF, SRS
could evaluate cardiotoxicity at an earlier stage. Cardiomyocyte
damage could cause abnormalities in myocardial electrical
conduction and myocardial perfusion at the same time, while the
sequence and mechanisms underlying these two types of abnormalities
remain unclear. In the present study, the SRS abnormalities
occurred earlier than QTc interval prolongation, indicating that
myocardial mitochondrial damage after chemotherapy might first
manifest as abnormal regional myocardial perfusion, which could be
one of the factors causing arrhythmia. Studies have reported that
patients with abnormal LV myocardial perfusion have a significantly
higher incidence of arrhythmia (53). Therefore, compared with the QTc
interval, SRS could predict the cardiac toxicity-associated
arrhythmia caused by chemotherapy at an earlier stage.
There are several limitations to the present study.
First, the sample size of this study was relatively small, and only
36 DLBCL patients were included. In the future, expansion of the
sample size could continue, and a larger sample and more convincing
data should be obtained. Second, the follow-up time was not long
enough. In this study, the cardiac function of patients with DLBCL
was monitored and compared only at the initial diagnosis and after
six courses of R-CHOP chemotherapy. There may be some patients with
delayed cardiac function. Therefore, the myocardial function of
these patients should be followed up for longer and the follow-up
results should be improved by rechecking G-MPI, color Doppler
echocardiography, ECG and serum indices associated with myocardial
function every 6 months. Third, the present study did not compare
cardiac function among subgroups of patients with DLBCL with
different prognostic stratifications due to the small sample size.
DLBCL is a heterogeneous disease. Patients of different subtypes
have very different prognoses. In the future, the number of
patients with DLBCL should be expanded and the research refined
further. In this way, cardiac function could be compared among
different subgroups of patients with DLBCL.
In addition, complete and accurate data of
event-free curves could not be provided for all cardiac events in
these patients. Some patients were not able to visit the hospital
for accurate cardiovascular event screening, and a small number of
other patients were lost to follow-up for communication reasons.
Missing data due to these objective reasons may affect the accuracy
of the results of event-free curve analysis. We are currently in
the process of expanding the number of patients enrolled and hope
to complete an effective follow-up of all patients soon and analyze
the relevant data.
In conclusion, the present study found that
anthracycline chemotherapy was closely associated with the
cardiotoxicity that occurred in the patients with DLBCL, especially
the abnormal electrical conduction of the myocardium. The G-MPI SRS
measurement was a highly sensitive method for detecting early
subclinical cardiac damage to myocardial electrical conduction in
patients with DLBCL treated with anthracyclines, which may be
helpful for hematologists to monitor and treat the cardiac
dysfunction of patients with DLBCL in a much more timely and
convenient manner.
To the best of our knowledge, the present study was
the first to use resting G-MPI to evaluate abnormal changes in LV
myocardial perfusion in patients with DLBCL before and after
chemotherapy. The G-MPI SRS level was an early indicator for TRC
surveillance in patients with DLBCL after anthracycline
chemotherapy, thus contributing to early treatment and a subsequent
decrease in mortality caused by cardiovascular complications.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Changqing Lu and
Dr Tongbin Chen (The Third Affiliated Hospital of Soochow
University, Changzhou, China) for providing technological
assistance.
Funding
This study was supported by the Youth Science Fund
Project of the National Natural Science Foundation of China (grant
nos. 81800100 and 81701737), the National Natural Science
Foundation of China (grant no. 81871381), the Youth Science Fund
Project of the National Natural Science Foundation of Jiangsu,
China (grant no. BK20150253), the Fund of 333 Project of Jiangsu,
China (grant no. BRA2015088) and the Social Development Foundation
of Changzhou Science and Technology Bureau, Jiangsu, China (grant
no. CE20175029).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL and JW conceived and designed the study. MX, CQ,
PX, LS, BH, FW, YY, YG, FL and WD performed all the clinical
diagnoses and treatments. YL and PX analyzed the data. YL, JW and
PX wrote the manuscript. XX contributed to statistical analysis and
revised the content of manuscript. YW and WG collected the
clinicopathological data, made the tables and figures, and
supervised the research group. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
All procedures were in accordance with the ethical
standards of the responsible human experimentation committee
(institutional and national) and the Declaration of Helsinki
(1975), as amended in 2008. Patient samples were obtained with
approval from the Ethical Committee at the First People's Hospital
of Changzhou (Changzhou, China; approval number, CL022-01), and
written informed consent was obtained from all patients according
to the institutional guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li S, Young KH and Medeiros LJ: Diffuse
large B-cell lymphoma. Pathology. 50:74–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miyazaki K: Treatment of diffuse large
B-cell lymphoma. J Clin Exp Hematop. 56:79–88. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kubuschok B, Held G and Pfreundschuh M:
Management of diffuse large B-cell lymphoma (DLBCL). Cancer Treat
Res. 165:271–288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cardinale D, Colombo A, Bacchiani G,
Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N,
Curigliano G, et al: Early detection of anthracycline
cardiotoxicity and improvement with heart failure therapy.
Circulation. 131:1981–1988. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Linschoten M, Teske AJ, Baas AF, Vink A,
Dooijes D, Baars HF and Asselbergs FW: Truncating Titin (TTN)
variants in chemotherapy-induced cardiomyopathy. J Card Fail.
23:476–479. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ewer MS and Ewer SM: Cardiotoxicity of
anticancer treatments. J Nat Rev Cardiol. 12:547–558. 2015.
View Article : Google Scholar
|
|
7
|
Laursen AH, Thune JJ, Hutchings M, Hasbak
P, Kjaer A, Elming MB and Ripa RS: 123I-MIBG imaging for
detection of anthracycline-induced cardiomyopathy. Clin Physiol
Funct Imaging. 38:176–185. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mcmurray JJ, Adamopoulos S, Anker SD,
Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C,
Gomez-Sanchez MA, et al: ESC Guidelines for the diagnosis and
treatment of acute and chronic heart failure 2012: The Task Force
for the diagnosis and treatment of acute and chronic heart failure
2012 of the European Society of Cardiology. Developed in
collaboration with the Heart Failure Association (HFA) of the ESC.
Eur J Heart Fail. 14:803–869. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dutta T, Spevack DM and Aronow WS: The
left ventricular ejection fraction: New insights into an old
parameter. Hosp Pract (1995). 47:221–230. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ong G, Brezden-Masley C, Dhir V, Deva DP,
Chan KKW, Chow CM, Thavendiranathan D, Haq R, Barfett JJ, Petrella
TM, et al: Myocardial strain imaging by cardiac magnetic resonance
for detection of subclinical myocardial dysfunction in breast
cancer patients receiving trastuzumab and chemotherapy. Int J
Cardiol. 261:228–233. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Virani SA, Dent S, Brezden-Masley C,
Clarke B, Davis MK, Jassal DS, Johnson C, Lemieux J, Paterson I,
Sebag IA, et al: Canadian cardiovascular society guidelines for
evaluation and management of cardiovascular complications of cancer
therapy. Can J Cardiol. 32:831–841. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zamorano JL, Lancellotti P, Muñoz DR,
Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GY,
Lyon AR, et al: 2016 ESC Position Paper on cancer treatments and
cardiovascular toxicity developed under the auspices of the ESC
Committee for Practice Guidelines. Kardiol Pol. 74:1193–1233.
2016.(In Polish). View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seraphim A, Westwood M, Bhuva AN, Crake T,
Moon JC, Menezes LJ, Lloyd G, Ghosh AK, Slater S, Oakervee H and
Manisty CH: Advanced imaging modalities to monitor for
cardiotoxicity. Curr Treat Options Oncol. 20:732019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Markman TM, Ruble K, Loeb D, Chen A, Zhang
Y, Beasley GS, Thompson WR and Nazarian S: Electrophysiological
effects of anthracyclines in adult survivors of pediatric
malignancy. Pediatric Blood Cancer. 642017.doi: 10.1002/pbc.26556.
PubMed/NCBI
|
|
15
|
Yu AF, Manrique C, Pun S, Liu JE, Mara E,
Fleisher M, Patil S, Jones LW, Steingart RM, Hudis CA and Dang CT:
Cardiac safety of paclitaxel plus trastuzumab and pertuzumab in
patients with HER2-positive metastatic breast cancer. Oncologist.
21:418–424. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Soufer A, Liu C, Henry ML and Baldassarre
LA: Nuclear cardiology in the context of multimodality imaging to
detect cardiac toxicity from cancer therapeutics: Established and
emerging methods. J Nucl Cardiol. 27:1210–1224. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mahabadi AA and Rischpler C:
Cardiovascular imaging in cardio-oncology. J Thorac Dis. 10 (Suppl
35):S4351–S4366. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gayed IW, Liu HH, Yusuf SW, Komaki R, Wei
X, Wang X, Chang JY, Swafford J, Broemeling L and Liao Z: The
prevalence of myocardial ischemia after concurrent chemoradiation
therapy as detected by gated myocardial perfusion imaging in
patients with esophageal cancer. J Nucl Med. 47:1756–1762.
2006.PubMed/NCBI
|
|
19
|
Zellars R, Bravo PE, Tryggestad E, Hopfer
K, Myers L, Tahari A, Asrari F, Ziessman H and Garrett-Mayer E:
SPECT analysis of cardiac perfusion changes after
whole-breast/chest wall radiation therapy with or without active
breathing coordinator: Results of a randomized phase 3 trial. Int J
Radiat Oncol Biol Phys. 88:778–785. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Germano G, Kavanagh PB, Slomka PJ, Van
Kriekinge SD, Pollard G and Berman DS: Quantitation in gated
perfusion SPECT imaging: The Cedars-Sinai approach. J Nucl Cardiol.
14:433–454. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
King JF and Lam JT: A practical approach
to diagnosis of B-cell lymphomas with diffuse large cell
morphology. Arch Pathol Lab Med. 144:160–167. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sabattini E, Bacci F, Sagramoso C and
Pileri SA: WHO classification of tumours of haematopoietic and
lymphoid tissues in 2008: An overview. Pathologica. 102:83–87.
2010.PubMed/NCBI
|
|
23
|
Rosenberg SA: Validity of the Ann Arbor
staging classification for the non-Hodgkin's lymphomas. J Cancer
Treat Rep. 61:1023–1027. 1977.
|
|
24
|
Perez EA, Suman VJ, Davidson NE, Sledge
GW, Kaufman PA, Hudis CA, Martino S, Gralow JR, Dakhil SR, Ingle
JN, et al: Cardiac safety analysis of doxorubicin and
cyclophosphamide followed by paclitaxel with or without trastuzumab
in the North Central Cancer Treatment Group N9831 adjuvant breast
cancer trial. J Clin Oncol. 26:1231–1238. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goel S, Liu J, Guo H, Barry W, Bell R,
Murray B, Lynch J, Bastick P, Chantrill L, Kiely BE, et al: Decline
in left ventricular ejection fraction following anthracyclines
predicts trastuzumab cardiotoxicity. JACC. Heart Fail. 7:795–804.
2019.
|
|
26
|
Ho E, Brown A, Barrett P, Morgan RB, King
G, Kennedy MJ and Murphy RT: Subclinical anthracycline- and
trastuzumab-induced cardiotoxicity in the long-term follow-up of
asymptomatic breast cancer survivors: A speckle tracking
echocardiographic study. Heart. 96:701–707. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu W, Li SN, Chan GC, Ha SY, Wong SJ and
Cheung YF: Transmural strain and rotation gradient in survivors of
childhood cancers. Eur Heart J Cardiovasc Imaging. 14:175–182.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Armstrong IS, Arumugam P, James JM, Tonge
CM and Lawson RS: Reduced-count myocardial perfusion SPECT with
resolution recovery. Nucl Med Commun. 33:121–129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cerqueira MD, Weissman NJ, Dilsizian V,
Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T and
Verani MS; American Heart Association Writing Group on Myocardial
Segmentation and Registration for Cardiac Imaging, : Standardized
myocardial segmentation and nomenclature for tomographic imaging of
the heart. A statement for healthcare professionals from the
cardiac imaging committee of the council on clinical cardiology of
the american heart association. J Nucl Cardiol. 9:240–245. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
McGowan JV, Chung R, Maulik A, Piotrowska
I, Walker JM and Yellon DM: Anthracycline chemotherapy and
cardiotoxicity. Cardiovasc Drugs Ther. 31:63–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ferraro MP, Gimeno-Vazquez E, Subirana I,
Gómez M, Díaz J, Sánchez-González B, García-Pallarols F, Martínez
L, Ble M, Molina L, et al: Anthracycline-induced cardiotoxicity in
diffuse large B-cell lymphoma: NT-proBNP and cardiovascular score
for risk stratification. Eur J Haematol. 102:509–515.
2019.PubMed/NCBI
|
|
32
|
Zelenetz AD, Gordon LI, Abramson JS,
Advani RH, Bartlett NL, Caimi PF, Chang JE, Chavez JC, Christian B,
Fayad LE, et al: NCCN guidelines insights: B-cell lymphomas,
version 3.2019. J Natl Compr Canc Netw. 17:650–661. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thieblemont C, Bernard S, Meignan M and
Molina T: Optimizing initial therapy in DLBCL. Best Pract Res Clin
Haematol. 31:199–208. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bansal N, Amdani S, Lipshultz ER and
Lipshultz SE: Chemotherapy-induced cardiotoxicity in children.
Expert Opin Drug Metab Toxicol. 13:817–832. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Veronese P, Hachul DT, Scanavacca MI,
Hajjar LA, Wu TC, Sacilotto L, Veronese C and Darrieux FCDC:
Effects of anthracycline, cyclophosphamide and taxane chemotherapy
on QTc measurements in patients with breast cancer. PLoS One.
13:e01967632018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Porta-Sanchez A, Gilbert C, Spears D, Amir
E, Chan J, Nanthakumar K and Thavendiranathan P: Incidence,
diagnosis, and management of qt prolongation induced by cancer
therapies: A systematic review. J Am Heart Assoc. 6:e0077242017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Heemskerk CPM, Pereboom M, van Stralen K,
Berger FA, van den Bemt PMLA, Kuijper AFM, van der Hoeven RTM,
Mantel-Teeuwisse AK and Becker ML: Risk factors for QTc interval
prolongation. Eur J Clin Pharmacol. 74:183–191. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Puppe J, van Ooyen D, Neise J, Thangarajah
F, Eichler C, Krämer S, Pfister R, Mallmann P, Wirtz M and Michels
G: Evaluation of QTc Interval prolongation in breast cancer
patients after treatment with epirubicin, cyclophosphamide, and
docetaxel and the influence of interobserver variation. Breast Care
(Basel). 12:40–44. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Etchegoyen CV, Keller GA, Mrad S, Cheng S
and Di Girolamo G: Drug-induced QT interval prolongation in the
intensive care unit. Curr Clin Pharmacol. 12:210–222. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Renu K, Abilash VG, Tirupathi PB and
Arunachalam S: Molecular mechanism of Doxorubicin-induced
cardiomyopathy-An update. Eur J Pharmacol. 818:241–253. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Henninger C and Fritz G: Statins in
Anthracycline-induced cardiotoxicity: Rac and Rho, and the
heartbreakers. Cell Death Dis. 8:e25642017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hahn VS, Lenihan DJ and Ky B: Cancer
Therapy-induced cardiotoxicity: Basic mechanisms and potential
cardioprotective therapies. J Am Heart Assoc. 3:e0006652014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rochette L, Guenancia C, Gudjoncik A,
Hachet O, Zeller M, Cottin Y and Vergely C:
Anthracyclines/trastuzumab: New aspects of cardiotoxicity and
molecular mechanisms. Trends Pharmacol Sci. 36:326–348. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mukai M, Komori K and Oka T: Mechanism and
management of cancer Chemotherapy-induced atherosclerosis. J
Atheroscler Thromb. 25:994–1002. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yin J, Guo J, Zhang Q, Cui L, Zhang L,
Zhang T, Zhao J, Li J, Middleton A, Carmichael PL and Peng S:
Doxorubicin-induced mitophagy and mitochondrial damage is
associated with dysregulation of the PINK1/parkin pathway. Toxicol
In Vitro. 51:1–10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Koleini N and Kardami E: Autophagy and
mitophagy in the context of Doxorubicin-induced cardiotoxicity.
Oncotarget. 8:46663–46680. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ichikawa Y, Ghanefar M, Bayeva M, Wu R,
Khechaduri A, Naga Prasad SV, Mutharasan RK, Naik TJ and Ardehali
H: Cardiotoxicity of doxorubicin is mediated through mitochondrial
iron accumulation. J Clin Invest. 124:617–630. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Beller GA and Watson DD: Physiological
basis of myocardial perfusion imaging with the technetium 99m
agents. Semin Nucl Med. 21:173–181. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li J, Lu J and Zhou Y:
Mitochondrial-targeted molecular imaging in cardiac disease. Biomed
Res Int. 2017:52468532017.PubMed/NCBI
|
|
50
|
Bartholoma MD, Zhang S, Akurathi V, Pacak
CA, Dunning P, Fahey FH, Cowan DB, Treves ST and Packard AB:
(18)F-labeled rhodamines as potential myocardial perfusion agents:
Comparison of pharmacokinetic properties of several rhodamines.
Nucl Med Biol. 42:796–803. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang L, Liu Z, Hu KY, Tian QB, Wei LG,
Zhao Z, Shen HR and Hu J: Early myocardial damage assessment in
dystrophinopathies using (99)Tc(m)-MIBI gated myocardial perfusion
imaging. Ther Clin Risk Manag. 11:1819–1827. 2015.PubMed/NCBI
|
|
52
|
Arruda-Olson AM, Roger VL, Jaffe AS, Hodge
DO, Gibbons RJ and Miller TD: Troponin T levels and infarct size by
SPECT myocardial perfusion imaging. JACC Cardiovasc Imaging.
4:523–533. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sood N, Al Badarin F, Parker M, Pullatt R,
Jacobson AF, Bateman TM and Heller GV: Resting perfusion MPI-SPECT
combined with cardiac 123I-mIBG sympathetic innervation imaging
improves prediction of arrhythmic events in Non-ischemic
cardiomyopathy Patients: Sub-study from the ADMIRE-HF trial. J Nucl
Cardiol. 20:813–820. 2013. View Article : Google Scholar : PubMed/NCBI
|