Introduction
Ovarian cancer is one of the most refractory and
fatal cancers among women worldwide, with approximately 300,000 new
cases and 200,000 associated deaths annually (1,2). The
majority of patients with advanced disease respond to
platinum/taxane therapy; however, most will ultimately experience
recurrence and eventually die as a result of disease progression
(3,4). Although the biological mechanisms of
ovarian cancer invasion, metastasis, and drug resistance remain
relatively poorly understood, recent studies revealed HER3
(erbB3) signaling plays an important role in development and
chemoresistance in ovarian cancer (5,6). The
HER3 is a member of the erythroblastic leukemia viral oncogene
homolog (ERBB) receptor family, which is often aberrantly expressed
and related to poor prognosis in several solid tumors (7–10). HER3
promotes tumor initiation, progression, and treatment resistance
mainly through heterodimerization with other ERBB family receptor
tyrosine kinases, which activate oncogenic signaling via the
PI3K/AKT, MAPK/ERK, and JAK/STAT pathways (11,12).
Although HER3 overexpression has been reported to account for
41.3–67.5% of ovarian cancer patients in several studies, it
remains unknown what determines whether a patient has high or low
HER3 expression (5,13,14).
Simpson et al (13) suggested
early-stage ovarian cancers were more likely than late-stage
disease to display intense tumor HER3 staining; however, other
studies failed to support the correlation between HER3 expression
and disease characteristics, including the International Federation
of Gynecology and Obstetrics (FIGO) stage, histologic grade and
type, residual disease, age, p53, progesterone and estrogen
receptors, EGF receptor, c-MYC, or MDM-2 (5,15–17).
However, those studies did not account for the type of previous
treatment; therefore, the effect of neoadjuvant chemotherapy on
HER3 expression status remains unclear.
Bezler et al showed in vitro
chemotherapeutic drugs such as doxorubicin activated the
erbB3/PI3K/AKT signaling in ovarian cancer cells, which could be
overcome by blocking the HER2 with trastuzumab (18). We hypothesized that HER3 expression
in ovarian cancer patients would be upregulated by chemotherapy and
involve chemotherapeutic resistance. In the present study, we
investigated how chemotherapy and other clinical factors affect the
expression of HER3 in surgically resected ovarian cancer patients
and how those factors affect patient prognosis.
Materials and methods
Patients
We retrospectively reviewed medical records of
patients who received surgery and were diagnosed with primary
ovarian cancer between January 2011 and December 2018 at the
National Cancer Center Hospital, Japan. Patients with benign and
nonepithelial tumors, borderline tumors, and recurrent disease were
excluded from this study. Patients were included in this study if
they met the following criteria: i) pathologically diagnosed
primary ovarian cancer, ii) with sufficient paraffin blocks of
formalin-fixed surgical specimens for evaluation of HER3
immunohistochemistry (IHC). Core needle biopsy specimens obtained
before neoadjuvant chemotherapy administration were also retrieved
and evaluated for HER3 expression of the representative section
which correspond to the histology od the surgically resected
specimen if available. Chart reviews were performed and the
following information was extracted: Age; performance status at
diagnosis; FIGO stage; chemotherapy regimen; dates of surgery,
chemotherapy initiation, recurrence confirmation, chemotherapy
progression, last follow-up; and survival status. In patients with
FIGO stages III or IV, which could not be completely removed, a
debulking surgery was performed, if applicable, to reduce the
remaining tumor to a diameter not exceeding 2 cm. This study was
conducted with the approval of the Ethics Committee of the National
Cancer Center Hospital, Japan (2014-393). Written informed consent
was obtained from all participants.
Perioperative chemotherapy
Patients diagnosed with clinical FIGO stages III or
IV high-grade serous carcinoma (HGSC), where optimal surgery
appeared impossible (leaving a residual tumor up to 2 cm in maximal
diameter), received neoadjuvant chemotherapy with carboplatin (area
under the curve, 6 mg/ml per min) and paclitaxel (180
mg/m2 on day 1 or 80 mg/m2 on days 1, 8, and
15) for up to 4 courses prior to surgery. Pathological diagnosis of
HGSC was confirmed by needle biopsy or cell block specimens
obtained before the induction of neoadjuvant chemotherapy.
Patients, except for pathological FIGO stages Ia or Ib with
low-grade histology, received postoperative chemotherapy with
carboplatin and paclitaxel in line with neoadjuvant chemotherapy
within 3 to 4 weeks after primary surgery.
Immunohistochemical staining
For all patients, hematoxylin and eosin-stained
slides of surgical and presurgical biopsy specimens obtained before
neoadjuvant chemotherapy were reviewed to select representative
sections. New 4-µm-thick sections were prepared from paraffin
blocks of 10% neutral buffered formalin-fixed surgical specimens
and were immunohistochemically stained. Sections were dewaxed,
rehydrated, and moistened with phosphate-buffered saline (pH 7.4).
After deparaffinization, the expression of HER3 was evaluated by
using a rabbit monoclonal antibody against HER3/ErbB3 (1:59
dilution; clone D22C5, Cell Signaling Technology Inc.). Antigen
retrieval was achieved by using a PT Link machine (Dako; Agilent
Technologies, Inc.) at high pH. IHC staining was performed using
the Dako autostainer Link48 (Dako; Agilent Technologies, Inc.) and
EnVision Flex Mini Kit (Dako; Agilent Technologies, Inc.),
according to the manufacturer's instructions. The slides were
counterstained with hematoxylin.
Evaluation of HER3 expression was performed by an
experienced gynecological pathologist and two additional observers
that independently evaluated HER3 scores in accordance with the
HER2 testing guidelines for gastroesophageal cancer from the
College of American Pathologists, American Society for Clinical
Pathology, and American Society of Clinical Oncology (19). High HER3 expression (HER3-high) was
defined as a score of 2+ or 3+, and low HER3 expression (HER3-low)
was defined as a score of 0 or 1+; discrepancies were resolved by
discussion and consensus. Those pathologist and observers were
blinded to clinical data when evaluating the slides.
Statistical analysis
Progression-free survival (PFS) was defined as the
time from the date of initial treatment, such as surgery or
neoadjuvant chemotherapy, to the detection of any recurrence or
death due to any cause. The absence of recurrence was treated as a
censored observation.
We used the Mann-Whitney U test to compare
continuous variables and the χ2 or Fisher's exact tests
to compare categorical variables between the groups. To identify
independent prognostic factors for HER3 expression, a multivariate
logistic regression model was applied with forced entry method
after adjustment for candidate predictive factors for HER3
expression. PFS was estimated using the Kaplan-Meier method; the
corresponding 95% confidence intervals (CIs) were calculated and
comparisons between groups were performed by the log-rank test. A
statistically significant difference was considered for two-sided
P<0.05, or <0.05/n (n, number of comparisons) for multiple
comparisons according to Bonferroni methodology. All analyses were
performed using the Statistical Package for the Social Sciences
(SPSS v.26; IBM Corp.).
Results
Patient characteristics
In total, data from 111 patients with sufficient
surgical resected tumor samples were extracted from medical
records. HER3-high was observed in 64 patients (58%), while
HER3-low was observed in 47 patients (42%). Characteristics of
patients in each group are summarized in Table I. Most of the patients had FIGO stage
I–III disease. Around half of the patients had HGSC histology
(56.8%), followed by clear cell carcinoma (26.1%), endometrioid
adenocarcinoma (9.0%), and mucinous adenocarcinoma (8.1%).
Thirty-four patients (30.6%) received neoadjuvant chemotherapy and
subsequent surgery, while 63 patients (56.8%) underwent surgery and
thereafter adjuvant chemotherapy. HER3-high was relatively
frequently observed in patients with clear cell carcinoma histology
(21/29, 72.4%), those with stage IV disease (12/15, 80.0%), or
those who had received neoadjuvant chemotherapy (25/34, 73.5%). The
HER3-high rate was 50.8% for HGSC, 72.4% for clear cell carcinoma,
50.0% for endometrioid adenocarcinoma, and 66.7% for mucinous
adenocarcinoma.
 | Table I.Patient characteristics at the
initiation of treatment. |
Table I.
Patient characteristics at the
initiation of treatment.
| Characteristics | HER3-high (n=64) | HER3-low (n=47) | P-value |
|---|
| Age, median (range),
years | 56.5 (36–81) | 53 (28–79) | 0.140 |
| FIGO stage 2014, n
(%) |
|
| 0.048 |
| I | 25 (39.1) | 13 (27.7) |
|
| II | 6
(9.3) | 4
(8.5) |
|
|
III | 21 (32.8) | 27 (57.4) |
|
| IV | 12 (18.8) | 3
(6.4) |
|
| Histology, n
(%) |
|
| <0.001 |
|
High-grade serous
carcinoma | 32 (50.0) | 31 (66.0) |
|
| Clear
cell carcinoma | 21 (32.8) | 8
(17.0) |
|
|
Endometrioid
adenocarcinoma | 5
(7.8) | 5
(10.6) |
|
|
Mucinous adenocarcinoma | 6
(9.3) | 3
(6.4) |
|
| ECOG-PS, n (%) |
|
| 0.422 |
|
0-1 | 36 (56.3) | 30 (63.8) |
|
|
2-3 | 28 (43.8) | 17 (36.2) |
|
| Treatment, n
(%) |
|
| 0.069 |
| Surgery
alone | 8
(12.5) | 6
(12.8) |
|
| Surgery
plus neoadjuvant chemotherapy | 25 (39.1) | 9
(19.1) |
|
| Surgery
plus adjuvant chemotherapy | 31 (48.4) | 32 (68.1) |
|
Predictive factors for high HER3
expression
We conducted univariate and multivariate logistic
analyses to investigate factors associated with HER3-high, and
identified neoadjuvant chemotherapy prior to surgery [odds ratio
(OR), 7.49; 95% CI, 2.48–22.64; P<0.001] and non-HGSC histology
(OR, 5.42; 95% CI, 1.99–14.78; P<0.001) as significant
predictive factors for HER3-high (Table
II).
 | Table II.Multivariate logistic regression of
predictive factors for overexpression of HER3. |
Table II.
Multivariate logistic regression of
predictive factors for overexpression of HER3.
|
| Univariate | Multivariate |
|---|
| Factor | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Age ≥55 years (vs.
<55) | 1.46 | 0.69–3.11 | 0.326 | 1.60 | 0.70–3.70 | 0.267 |
| FIGO stage ≥III
(vs. ≤II) | 0.60 | 0.28–1.30 | 0.199 | 0.46 | 0.12–1.74 | 0.255 |
| Non-HGSC histology
(vs. HGSC histology) | 1.94 | 0.89–4.21 | 0.095 | 5.42 | 1.99–14.78 | <0.001 |
| Neoadjuvant
chemotherapy yes (vs. no) | 2.71 | 1.12–6.55 | 0.027 | 7.49 | 2.48–22.64 | <0.001 |
Among patients who received neoadjuvant chemotherapy
before surgery, pre-chemotherapy biopsy specimens were available in
28 patients who had HGSC histology. The specimens were obtained
from the peritoneum (11), omentum
(9), ascitic fluid (cell block, 4),
and other sites (cervix, vagina, cervical lymph node, and ovary).
Changes between pre- and post-chemotherapy HER3 expression status
are presented in Fig. 1. In
pre-chemotherapy biopsy specimens, 15 and 13 patients showed
HER3-high and HER3-low, respectively. After chemotherapy, 8 of 13
patients with HER3-low changed their tumor HER3 expression to
HER3-high, resulting in 22 patients in total being classified as
HER3-high, with a significant difference from pre-chemotherapy,
whereas only 1 of 15 patients changed from HER3-high to -low
(P=0.042, χ2 test). Fig.
2 shows a representative case where HER3 expression was
upregulated through the course of neoadjuvant chemotherapy.
Survival analysis
At the point of data cut-off, the median follow-up
period was 32.6 months (range, 1.4–89.9 months). To evaluate the
impact of HER3 expression on PFS, Kaplan Meier analysis was
performed to estimate PFS among HGSC patients. Patients with
HER3-high showed a non-significant but consistent trend toward poor
PFS (Fig. 3).
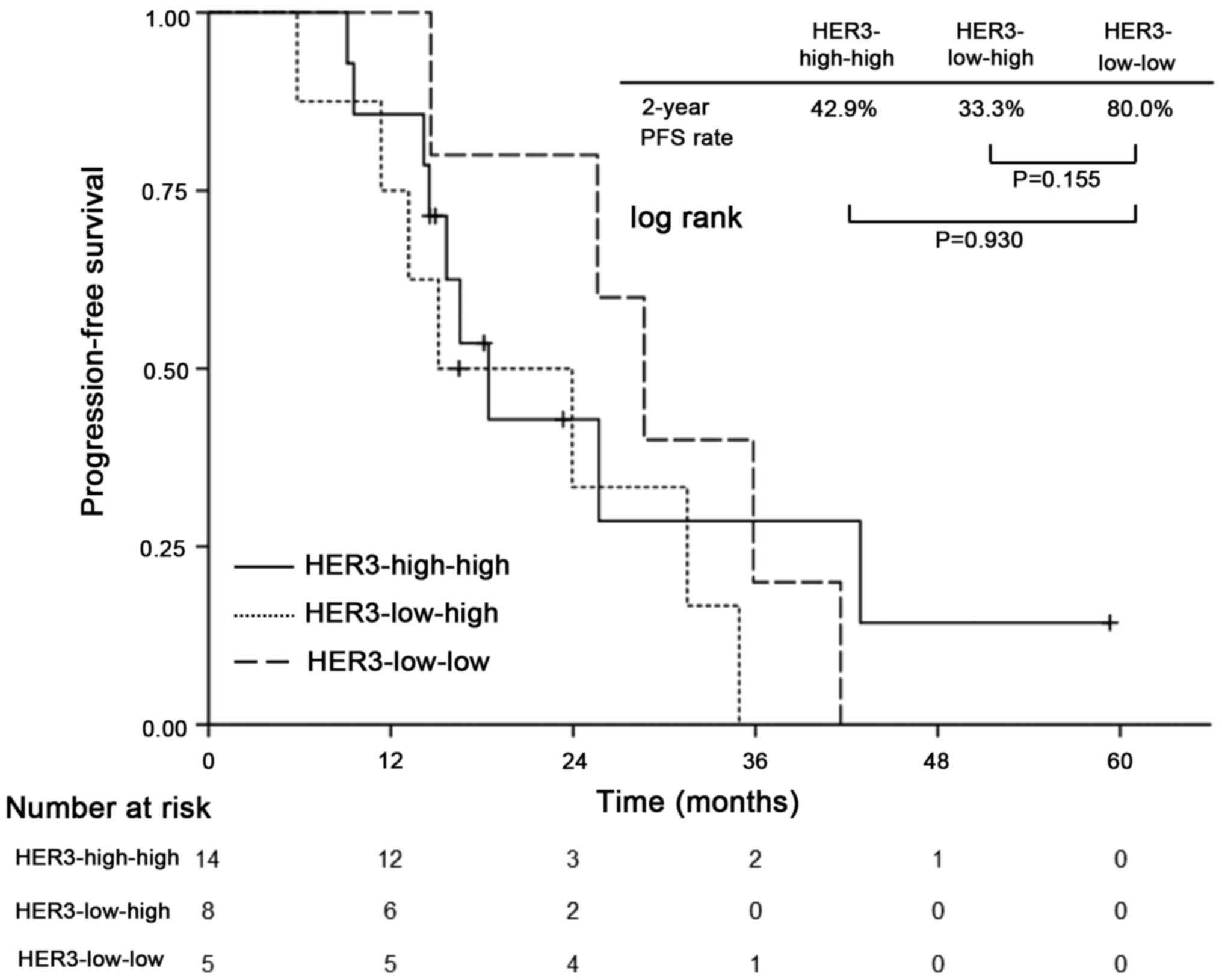
In exploratory analysis of patients who received
neoadjuvant chemotherapy, patients were divided into four
subgroups: Patients who changed their HER3 status from HER3-low to
HER3-high (HER3-low-high, n=8) and vice versa (HER3-high-low, n=1);
and those who maintained their HER3 status as either HER3-high-high
(n=14) or HER3-low-low (n=5). The Kaplan-Meier analysis revealed a
trend toward impaired PFS among patients with HER3-high-high and
HER3-low-high compared with those with HER3-low-low, but without
statistically significant differences (2-year PFS: 42.9, 33.3,
80.0%, respectively; P=0.155 for HER3-low-high with HER3-low-low
and P=0.930 for HER3-high-high with HER3-low-low) (Fig. 4). HER3-high-low was omitted because
of the small number of cases.
Discussion
This is the first study to demonstrate upregulation
of HER3 overexpression through neoadjuvant chemotherapy in
surgically resected ovarian cancer patients. In patients with HGSC
histology, the frequency of HER3-high was significantly increased
between pre- and post-chemotherapy paired samples. HER3-low
patients often upregulated their HER3 status to HER3-high with a
trend toward impaired PFS, while HER3-high patients rarely
attenuated their expression through the course of chemotherapy.
Additionally, multivariate analysis revealed that non-HGSC
histology and neoadjuvant chemotherapy independently affect
overexpression of HER3. In the era of precision medicine, these
perspectives would be a step toward a biomarker-based treatment
strategy to overcome chemoresistance in ovarian cancer
patients.
Over the past two decades, a growing body of
evidence has shown that HER3 plays an important role in
carcinogenesis, progression, and chemoresistance in solid tumors,
including ovarian cancer (7–10,18).
Several therapeutic strategies targeting HER3 in human cancers are
under development, including monoclonal antibodies blocking ligand
binding, promoting HER3 destruction, or locking HER3 in a tethered
conformation (20,21). Seribantumab, a fully human
immunoglobulin G2 monoclonal antibody targeting HER3 by blocking
heregulin (HRG)-mediated HER3 signaling and inducing HER3
downregulation, was evaluated in a randomized phase II study in
combination with paclitaxel compared with paclitaxel alone
(22). Although the study did not
reach its primary endpoint, showing no difference in PFS between
two arms, patients with high HRG and low HER2 expression seemed to
benefit from the combination of seribantumab with paclitaxel.
Recently, manageable safety profiles and antitumor activities of
U3-1402, an anti-ERBB3 antibody conjugate with a novel
topoisomerase I inhibitor, were reported in two phase I studies in
which patients with HER3-positive breast cancer and EGFR mutated
non-small cell lung cancer were enrolled (23,24). A
phase II/III study (NCT02980341) of U3-1402 targeting HER3-positive
breast cancer is ongoing. The high frequency of HER3 overexpression
across all histology types (50.0–72.4%) and upregulation of HER3
expression after chemotherapy observed in the current study suggest
that ovarian cancer patients are good candidates for HER3-targeting
therapies, in particular, patients with clear cell carcinoma, which
is often resistant to conventional chemotherapies (25–27).
Our findings raise several questions, including how
long the upregulation of HER3 is likely to last and what mechanisms
underlie HER3 upregulation. We usually perform surgery 1–2 months
after the last administration of neoadjuvant chemotherapy;
therefore, HER3 expression seems to be upregulated for at least
several months in vivo, despite the short half-life of HER3
after activation in vitro (28,29)
Additionally, Bezler et al suggested that metalloprotease
ADAM17-mediated long-term upregulation of HER3 ligands in
previously treated ovarian cancer cell lines results in activation
of HER3 and subsequent AKT phosphorylation (18). Therapeutic strategies that involve
combinations of such molecules could be used in targeted therapy
for HER3. Concurrently, there is a possibility that HER3-negative
clones responding to chemotherapy are expelled by neoadjuvant
chemotherapy, and HER3-positive chemoresistant clones are then
selected and retained in surgical samples. Further studies are
needed to clarify the underlying biological mechanism of HER3
upregulation.
HER3 overexpression has been reported as a factor
predictive of worse prognosis in patients with ovarian cancer
(5,30). This is expected because HER3
activation is related to one of the mechanisms of chemo-resistance
among ovarian cancer patients. We investigated the relationship
between HER3-high and PFS among HGSC patients and showed a trend
toward worse PFS than that of HER3-low in the present study,
although the difference was not significant due to a small sample
size. Additionally, patients who changed their HER3 status from
HER3-low to -high through the course of neoadjuvant chemotherapy
showed a trend toward relatively poorer PFS, as did patients who
maintained their HER3-high status. While these findings did not
conclusively demonstrate differences between these groups due to
small sample sizes, these observations are noteworthy and warrant
further exploration.
Our study has several limitations. First, this was a
retrospective study conducted at a single institution and included
a small number of patients with ovarian cancer. By their nature,
such studies are prone to bias, which cannot be adjusted for in
multivariate analyses. Second, small biopsy samples acquired from
mainly metastatic sites might not reflect the overall primary tumor
expression of HER3; therefore, the reported HER3 expression in
pre-chemotherapy samples might be an underestimate. In a previous
examination of HER2 expression, which compared core needle biopsy
and surgical specimens among breast cancer patients, the
concordance of HER2 scores between these types of specimens
examined by IHC was 90% for 2×2 categories (0–2+ vs. 3+) (31). Although validation of HER3 expression
assessment remains required, this previous finding might be
applicable to the present study. Third, our study population
consisted of relatively few stage IV patients (13.5%), as we only
included patients with sufficient surgical specimens. It is
uncertain whether our study results generalize to patients with
stage IV disease. In a report from the United States, the
prevalence of stage IV disease was estimated as 28% of all
epithelial ovarian cancer cases. Given the fact that some patients
with stage IV disease undergo neoadjuvant chemotherapy and surgery,
our results might be relevant to most of ovarian cancer patients.
Fourth, the median follow-up period in our study was relatively
short. Further examination is needed to evaluate the long-term
prognosis in our study population. Moreover, it remains to be
elucidated whether the upregulation of HER3 occurs also in
platinum-resistant ovarian cancer, whether the upregulated HER3 is
also targetable by HER3-targeting compounds such as U3-1402, and
whether a sequential or combination strategy is the most
efficacious in HER3-targeted therapies for ovarian cancer.
This is the first study to demonstrate that
chemotherapy may upregulate HER3 expression in ovarian cancer
patients. In this study, non-HGSC histology was a factor predictive
of high HER3 expression. In the era of precision medicine, these
perspectives contribute toward development of a biomarker-based
treatment strategy for this disease.
Acknowledgements
The authors would like to thank Ms. Nao Nakamura and
Ms. Kotone Shoji (Department of Breast and Medical Oncology,
National Cancer Center Hospital) for secretarial assistance.
Funding
The National Cancer Center Biobank is supported by
the National Cancer Center Research and Development Fund,
Japan.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TM, YK, KY, HY, YS, YoO, HSO, TN, MT, KS, AS, EN,
TK, TS, MU, MI, YF, YuO and KT were responsible for the conception
and design of the present study, drafted the manuscript, were
responsible for the collection and assembly of the data, performed
the data analysis and interpretation, and read, revised and
approved the final manuscript. All authors read and approved the
final manuscript, are in agreement to be accountable for all
aspects of the work in ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the National Cancer Center Hospital (Tokyo, Japan;
approval no. 2014-393). Written informed consent was obtained from
all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cancer Registry and Statistics, . Cancer
Statistics in Japan - 2018. Cancer Information Service NCC.
(Japan). simplehttps://ganjoho.jp/en/professional/statistics/brochure/2018_en.htmlJune
26–2020
|
|
3
|
Ozols RF, Bundy BN, Greer BE, Fowler JM,
Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM
and Baergen R; Gynecologic Oncology Group, : Phase III Trial of
carboplatin and paclitaxel compared with cisplatin and paclitaxel
in patients with optimally resected stage III ovarian cancer: A
Gynecologic Oncology Group Study. J Clin Oncol. 21:3194–3200. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
du Bois A, Lück HJ, Meier W, Adams HP,
Möbus V, Costa S, Bauknecht T, Richter B, Warm M, Schröder W, et
al: A randomized clinical trial of Cisplatin/paclitaxel versus
Carboplatin/paclitaxel as First-line treatment of ovarian cancer. J
Natl Cancer Inst. 95:1320–1329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanner B, Hasenclever D, Stern K,
Schormann W, Bezler M, Hermes M, Brulport M, Bauer A, Schiffer IB,
Gebhard S, et al: ErbB-3 predicts survival in ovarian cancer. J
Clin Oncol. 24:4317–4323. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sheng Q, Liu X, Fleming E, Yuan K, Piao H,
Chen J, Moustafa Z, Thomas RK, Greulich H, Schinzel A, et al: An
activated ErbB3/NRG1 autocrine loop supports in vivo proliferation
in ovarian cancer cells. Cancer Cell. 17:298–310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xue C, Liang F, Mahmood R, Vuolo M,
Wyckoff J, Qian H, Tsai KL, Kim M, Locker J, Zhang ZY and Segall
JE: ErbB3-dependent motility and intravasation in breast cancer
metastasis. Cancer Res. 66:1418–1426. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reschke M, Mihic-Probst D, van der Horst
EH, Knyazev P, Wild PJ, Hutterer M, Meyer S, Dummer R, Moch H and
Ullrich A: HER3 is a determinant for poor prognosis in melanoma.
Clin Cancer Res. 14:5188–5197. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ledel F, Hallstrom M, Ragnhammar P,
Ohrling K and Edler D: HER3 expression in patients with primary
colorectal cancer and corresponding lymph node metastases related
to clinical outcome. Eur J Cancer. 50:656–662. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Q, Zhang R, Yan H, Zhao P, Wu L, Wang
H, Li T and Cao B: Prognostic significance of HER3 in patients with
malignant solid tumors. Oncotarget. 8:67140–67151. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Amin DN, Campbell MR and Moasser MM: The
role of HER3, the unpretentious member of the HER family, in cancer
biology and cancer therapeutics. Semin Cell Dev Biol. 21:944–950.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baselga J and Swain SM: Novel anticancer
targets: Revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer.
9:463–475. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Simpson BJ, Weatherill J, Miller EP,
Lessells AM, Langdon SP and Miller WR: c-erbB-3 protein expression
in ovarian tumours. Br J Cancer. 71:758–762. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cirstea AE, Stepan AE, Margaritescu C,
Zavoi RE, Olimid DA and Simionescu CE: The immunoexpression of
EGFR, HER2 and HER3 in malignant serous ovarian tumors. Rom J
Morphol Embyol. 58:1269–1273. 2017.
|
|
15
|
Théret N, Musso O, Campion JP, Turlin B,
Loréal O, L'Helgoualc'h A and Clément B: Overexpression of matrix
metalloproteinase-2 and tissue inhibitor of matrix
metalloproteinase-2 in liver from patients with gastrointestinal
adenocarcinoma and no detectable metastasis. Int J Cancer.
74:426–432. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tanner B, Hengstler JG, Luch A, Meinert R,
Kreutz E, Arand M, Wilkens C, Hofmann M, Oesch F, Knapstein PG and
Becker R: C-myc mRNA expression in epithelial ovarian carcinomas in
relation to estrogen receptor status, metastatic spread, survival
time, FIGO stage, and histologic grade and type. Int J Gynecol
Pathol. 17:66–74. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hengstler JG, Pilch H, Schmidt M,
Dahlenburg H, Sagemüller J, Schiffer I, Oesch F, Knapstein PG,
Kaina B and Tanner B: Metallothionein expression in ovarian cancer
in relation to histopathological parameters and molecular markers
of prognosis. Int J Cancer. 95:121–127. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bezler M, Hengstler JG and Ullrich A:
Inhibition of doxorubicin-induced HER3-PI3K-AKT signalling enhances
apoptosis of ovarian cancer cells. Mol Oncol. 6:516–529. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bartley AN, Washington MK, Ventura CB,
Ismaila N, Colasacco C, Benson AB III, Carrato A, Gulley ML, Jain
D, Kakar S, et al: HER2 Testing and clinical decision making in
gastroesophageal adenocarcinoma: Guideline from the college of
american pathologists, american society for clinical pathology, and
american society of clinical oncology. Arch Pathol Lab Med.
140:1345–1363. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mishra R, Patel H, Alanazi S, Yuan L and
Garrett JT: HER3 signaling and targeted therapy in cancer. Oncol
Rev. 12:3552018.PubMed/NCBI
|
|
21
|
Black LE, Longo JF and Carroll SL:
Mechanisms of receptor Tyrosine-protein kinase ErbB-3 (ERBB3)
action in human neoplasia. Am J Pathol. 189:1898–1912. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu JF, Ray-Coquard I, Selle F, Poveda AM,
Cibula D, Hirte H, Hilpert F, Raspagliesi F, Gladieff L, Harter P,
et al: Randomized phase II trial of seribantumab in combination
with paclitaxel in patients with advanced platinum-resistant or
-refractory ovarian cancer. J Clin Oncol. 34:4345–4353. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kogawa T, Yonemori K, Masuda N, Takahashi
S, Takahashi M, Iwase H, Nakayama T, Saeki T, Toyama T, Takano T,
et al: Single agent activity of U3-1402, a HER3-targeting
antibody-drug conjugate, in breast cancer patients: Phase 1 dose
escalation study. J Clin Oncol. 36 (Suppl 15):S25122018. View Article : Google Scholar
|
|
24
|
Janne PA, Yu HA, Johnson ML, Steuer CE,
Vigliotti M, Iacobucci C, Chen S, Yu C and Sellami DB: Safety and
preliminary antitumor activity of U3-1402: A HER3-targeted antibody
drug conjugate in EGFR TKI-resistant, EGFRm NSCLC. J Clin Oncol. 37
(Suppl 15):S90102019. View Article : Google Scholar
|
|
25
|
Mizuno M, Kikkawa F, Shibata K, Kajiyama
H, Ino K, Kawai M, Nagasaka T and Nomura S: Long-term follow-up and
prognostic factor analysis in clear cell adenocarcinoma of the
ovary. J Surg Oncol. 94:138–143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Crotzer DR, Sun CC, Coleman RL, Wolf JK,
Levenback CF and Gershenson DM: Lack of effective systemic therapy
for recurrent clear cell carcinoma of the ovary. Gynecol Oncol.
105:404–408. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takano M, Sugiyama T, Yaegashi N, Sakuma
M, Suzuki M, Saga Y, Kuzuya K, Kigawa J, Shimada M, Tsuda H, et al:
Low response rate of second-line chemotherapy for recurrent or
refractory clear cell carcinoma of the ovary: A retrospective Japan
clear cell carcinoma study. Int J Gynecol Cancer. 18:937–942. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cao Z, Wu X, Yen L, Sweeney C and Carraway
KL III: Neuregulin-induced ErbB3 downregulation is mediated by a
protein stability cascade involving the E3 ubiquitin ligase Nrdp1.
Mol Cell Biol. 27:2180–2188. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sak MM, Breen K, Ronning SB, Pedersen NM,
Bertelsen V, Stang E and Madshus IH: The oncoprotein ErbB3 is
endocytosed in the absence of added ligand in a clathrin-dependent
manner. Carcinogenesis. 33:1031–1039. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ocana A, Vera-Badillo F, Seruga B,
Templeton A, Pandiella A and Amir E: HER3 overexpression and
survival in solid tumors: A meta-analysis. J Natl Cancer Inst.
105:266–273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsuda H, Kurosumi M, Umemura S, Yamamoto
S, Kobayashi T and Osamura RY: HER2 testing on core needle biopsy
specimens from primary breast cancers: Interobserver
reproducibility and concordance with surgically resected specimens.
BMC Cancer. 10:5342010. View Article : Google Scholar : PubMed/NCBI
|