Introduction
Despite advancements in the prevention, detection
and treatment of diseases during the past decade, cervical cancer
(CC) remains the fourth most common malignancy in women worldwide
(1). In 2018, the incidence of
cervical cancer was 6.6% worldwide (2). Radiotherapy is an effective treatment,
particularly for patients with advanced cancer (3). However, patients with advanced stages
of CC still suffer from treatment failure due to the development of
resistance to radiotherapy (4).
Increasing the sensitivity to radiotherapy in patients resistant to
it can improve tumor control and decrease the side effects of
conventional treatment (5).
Technological advancements in radiotherapy, such as
intensity-modulated radiotherapy, have contributed to decreased
treatment-related toxicity for patients with locally-advanced
cancer (6); however, the molecular
mechanisms underlying the development of radioresistance, and the
associated biomarkers remain unknown. Currently, several gene
variations (7), tumor
microenvironmental changes (8),
hypoxia (9) and specific signaling
pathways (10,11) contribute to cellular resistance
against radiotherapy. These factors enhance DNA repair, decrease
apoptosis and increase genetic instability (7,12). Thus,
it remains critical to investigate the molecular mechanism and
prognostic markers of radioresistance, in order to increase
effectiveness of radiotherapy.
Recent studies have demonstrated an association
between the radiosensitivity of cancer cells and methylation levels
(13–15). Epigenetic modifications, particularly
promoter hypermethylation, which results in silencing of the
expression of tumor suppressor genes, contribute to the regulation
of cellular events associated with cancer development and
progression, such as apoptosis (16), cell cycle (17), proliferation (18) and DNA repair (19); and the mechanisms above have been
identified to have an effect on radiosensitivity (20,21).
Previous studies have reported that hypermethylation
of DNA is observed in CC (22–25).
Currently, few reports have investigated the association between
DNA methylation and radioresistance of CC (26,27).
Notably, it has been suggested that the GADD45 gene family act as
DNA damage-inducing and growth-inhibiting genes, which function as
tumor suppressors for targeted therapy (28). Furthermore, it has been demonstrated
that GADD45 induces epigenetic inactivation in different types of
cancer and cancer cell lines, including anaplastic thyroid cancer,
hepatocellular carcinomas, non-Hodgkin, Hodgkin lymphoma,
nasopharyngeal, cervical, esophageal and lung carcinoma (28,29).
However, whether aberrant GADD45A methylation is
associated with radiosensitivity in CC remains to be determined.
Thus, the present study aimed to investigate the function of
GADD45A methylation in CC radiotherapy, and determine its
underlying molecular mechanism. Taken together, the results of the
present study suggest that aberrant GADD45A methylation is
associated with decreased radiosensitivity in CC, which is mediated
via the PI3K/AKT signaling pathway.
Materials and methods
Cell culture and treatment
The human SiHa, CaSki and HeLa CC cell lines were
purchased from The Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences. CC cells were plated into 60 mm
culture dishes at a density of 6×105 cells/well and
maintained in RPMI-1640 supplemented with 10% fetal bovine serum
(FBS) and 1% ampicillin and streptomycin (all purchased from Gibco;
Thermo Fisher Scientific, Inc.), at 37°C with 5%
CO2.
Cells were subsequently treated with 5-azacytidine
(5-azaC; Sigma-Aldrich; Merck KGaA) and/or irradiated with 3.6
Gy/min at room temperature using an X-Rad 225 X-ray generator
(Precision X-ray Inc., http://precisionxray.com/x-rad/small-animal-irradiator/).
The experimental groups were as follows: 5-azaC group (0, 1, 3, 5
or 10 µmol/l for 24, 48 and 72 h); ionizing radiation (IR) group
(0, 2, 4, 6, 8, 10 Gy given in a single fraction); PBS group and
combined treatment group (IR after pretreatment with 5-azaC for 72
h).
Cell Counting Kit-8 (CCK-8) assay
SiHa CC cells were seeded into 96-well plates at a
density of 3×103 and treated with 5-azaC (1, 3, 5 or 10
µmol/l) after 24 h. The CCK-8 assay (Dojindo Molecular
Technologies, Inc.) was performed after 24, 48 and 72 h of 5-azaC
treatment, and viability was subsequently analyzed at a wavelength
of 450 nm using a microplate reader (Thermo Fisher Scientific,
Inc.). Cell viability was assessed using the following formula:
Cell viability (%) = [(As-Ab)/(Ac-Ab)]x100; where As = absorbance
of the experimental well, Ab = blank well absorbance and Ac =
control well absorbance.
Clonogenic assay
Cells were seeded into 60 mm dishes at a density of
6×105 cells/well and treated with 5-azaC alone (0 or 5
µmol/l) for 72 h or MK-2206 (0 or 0.5 µmol/l) for 48 h, IR alone
(0, 2, 4, 6, 8 or 10 Gy) or IR and 5-azaC/MK-2206. Following
treatment, cells were digested, collected via centrifugation (1,000
× g for 5 min at room temperature), diluted and seeded into 6-well
plates at different cell densities (100 cells for control, 200
cells for 2 Gy, 800 cells for 4 Gy, 1,800 cells for 6 Gy, 3,000
cells for 8 Gy and 5,000 cells for 10 Gy). After 10–15 days, the
cells were fixed in methanol for 15 min and stained with trypan
blue solution for 20 min at room temperature, and the number of
cell colonies per dish were counted with the naked eye. Colonies
containing at least 50 cells were counted. Cell survival curves
were constructed using a multi-target single-hit model:
S(D)=1-(1-e−D/D0)n; where D is the single
dose fraction, and D0 is defined as the given average hit dose per
target. The number of sensitive targets in a cell was denoted by n
(30). The survival fraction (SF)
was determined from the number of colonies formed after treatment
relative to the colony counts with the plating efficiency of the
non-irradiated cells.
Methylation-specific PCR (MSP)
Genomic DNA was extracted from CC cells
(5×106) using a DNA Extraction kit (cat. no. K180001;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. To assess the DNA methylation patterns, an
EpiTect® Bisulfite kit (cat. no. 59110; Qiagen, Inc.)
was used to perform the sodium bisulfite modification. The primer
sequences and annealing temperatures were used as previously
described (31). The amplified
products were analyzed on 3% agarose gels, stained with ethidium
bromide (Thermo Fisher Scientific, Inc.) for 15 min at room
temperature and visualized under a UV light.
Reverse transcription-quantitative
(RT-q)PCR analysis
Total cellular RNA was extracted using RNAiso Plus
(Takara Bio, Inc.). Agarose gel analysis was performed to assess
the quality of RNA. cDNA was produced from 1 µg total RNA using the
RT Reagent kit (cat. no. RR037A, Takara Bio, Inc.), according to
the manufacturer's protocol. qPCR was subsequently performed using
the SuperScript III Platinum SYBR Green One-Step qPCR kit (cat. no.
11736059, Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol, and a Bio-Rad CFX96 sequence detection
system (Bio-Rad Laboratories Inc.) was used to perform the
amplification. The following thermocycling conditions were used:
Initial denaturation at 95°C for 5 min, followed by 40 cycles of
denaturation at 95°C for 15 sec, and annealing and extension at
60°C for 30 sec. The following primer sequences were used for qPCR:
GADD45A forward, 5′-GAGAGCAGAAGACCGAAAGGA-3′ and reverse,
5′-CACAACACCACGTTATCGGG-3′; and GAPDH forward,
5′-ACAACTTTGGTATCGTGGAAGG-3′ and reverse,
5′-GCCATCACGCCACAGTTTC-3′. Relative expression levels were
quantified using the 2−ΔΔCq method (32) and normalized to the internal
reference gene GAPDH.
Western blotting
SiHa CC cells were homogenized on ice using RIPA
lysis buffer (Beyotime Institute of Biotechnology), and centrifuged
at 1,200 × g for 5 min at 4°C. The supernatant was collected and
stored at −80°C until subsequent experimentation.
Protein concentrations were determined using a BCA
Protein assay kit (cat. no. 7780, Cell Signaling Technology, Inc.)
and ~40 µg protein/lane was separated via SDS-PAGE on a 10% gel at
120 V constant voltage. The separated proteins were subsequently
transferred onto PVDF membranes (EMD Millipore) and blocked with 5%
non-fat dry milk (cat. no. 9999, Cell Signaling Technology, Inc.)
in PBS with 0.1% Tween-20 for 2 h at room temperature. The
membranes were incubated with primary antibodies against GADD45A
(1:1,000; cat. no. 4632), AKT (1:1,000; cat. no. 4685), p-AKT (Ser
473) (1:2,000; cat. no. 4060) and GAPDH (1:1,000; cat. no. 5174),
overnight at 4°C. All primary antibodies were purchased from Cell
Signaling Technology, Inc. Membranes were washed three times with
0.1% TBST, and subsequently incubated with goat anti-rabbit
secondary antibody (1:2,000; cat. no. 7074; Cell Signaling
Technology, Inc.) at room temperature for 2 h. Protein bands were
visualized using SignalFire™ ECL reagent (cat. no. 6883; Cell
Signaling Technology, Inc.).
Cell transfection
GFP-GADD45A-expression plasmid (GFP-GADD45A),
negative control (GFP-NC) and a constitutively active
Akt1-expression plasmid (pT3-myr-AKT-HA) were purchased from
Shanghai GenePharma Co., Ltd. and Zhongyuan (https://www.tianyancha.com/brand/b44b6550128),
respectively. Briefly, 1 day prior to transfection, SiHa cells were
seeded into 60 mm culture dishes at a density of 3×105
until they reached 80% confluence. A total of 4 µg plasmid and 10
µl Lipofectamine® 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) were diluted in 200 µl RPMI-1640 without
FBS, and set aside for 5 min at room temperature. The solution was
subsequently mixed, gently swayed and placed into an incubator for
20 min at room temperature. The transfection complex was directly
added to the culture dish, and the transfected cells were obtained
after 48 h.
Cell cycle analysis
SiHa cells were directly harvested or treated with
MK-2206 (0 or 0.5 µmol/l) for 48 h at room temperature, where the
drug was added every 24 h. Cells were washed three times with PBS,
and flow cytometric analysis was performed to assess cell cycle
distribution. Briefly, when cells reached 70% confluence, they were
fixed with 70% ethanol overnight at 4°C. Cells were rehydrated in 5
ml PBS at room temperature for 15 min, collected via centrifugation
(at 400 × g for 5 min at room temperature) and resuspended in
staining buffer supplemented with 3 µmol/l propidium iodide (cat.
no. P1304MP, Thermo Fisher Scientific, Inc.) at room temperature
for 30 min. Cells were subsequently incubated with staining buffer
for 15 min at room temperature, and cell cycle analysis was
performed using a FACScan flow cytometer (Beckman Coulter, Inc.)
and CellQuest software version 4.0 (Becton Dickinson).
Tissue samples and grouping
criteria
A total of 59 patients with International Federation
of Gynecology and Obstetrics 2009 (33) stages IIB-IIIB CC, histologically
diagnosed with squamous CC and treated at the Affiliated Tumor
Hospital of Guangxi Medical University between September 2015 and
June 2016 were enrolled in the present study. The median age of the
patients was 45.8 years (age range, 27–74 years). CC tissues were
collected from patients who had not received chemotherapy prior to
radiation therapy. Patients with standard radiotherapy received
concurrent weekly cisplatin (40 mg/m2).
Radioresistant and radiosensitive patients were
assessed 6 months after completion of radiation therapy via
colposcopically directed biopsy. Patients were considered
radiosensitive if the lesions completely subsided at the end of
radiotherapy and the tumor could not be observed in the cervix at
the 6-month follow-up. Patients were considered radioresistant if
there was still a residual tumor visible to the naked eye following
completion of radiotherapy, which was confirmed by biopsy in the
cervix or the presence of tumor tissue confirmed by cervical biopsy
at the 6-month follow-up. The present study was approved by the
Research Ethics Committee of Guangxi Medical University (Guangxi,
China; approval no. LW2020063), and written informed consent was
provided by all participants prior to the study start.
Statistical analysis
Statistical analysis was performed using SPSS
version 24.0 (SPSS Inc.). In vitro experiments were
performed in triplicate and data are presented as the mean ±
standard deviation. Unpaired Student's t-test was used to compare
differences between two independent groups, while and one-way
analysis of variance, followed by Tukey's post hoc test were
performed to compare differences between multiple groups. The
χ2 test was used to compare the effect of GADD45A
hypermethylation on radiosensitivity in patients with CC. P<0.05
was considered to indicate a statistically significant
difference.
Results
Promoter methylation of GADD45A in
primary CCs and their association with radioresistance
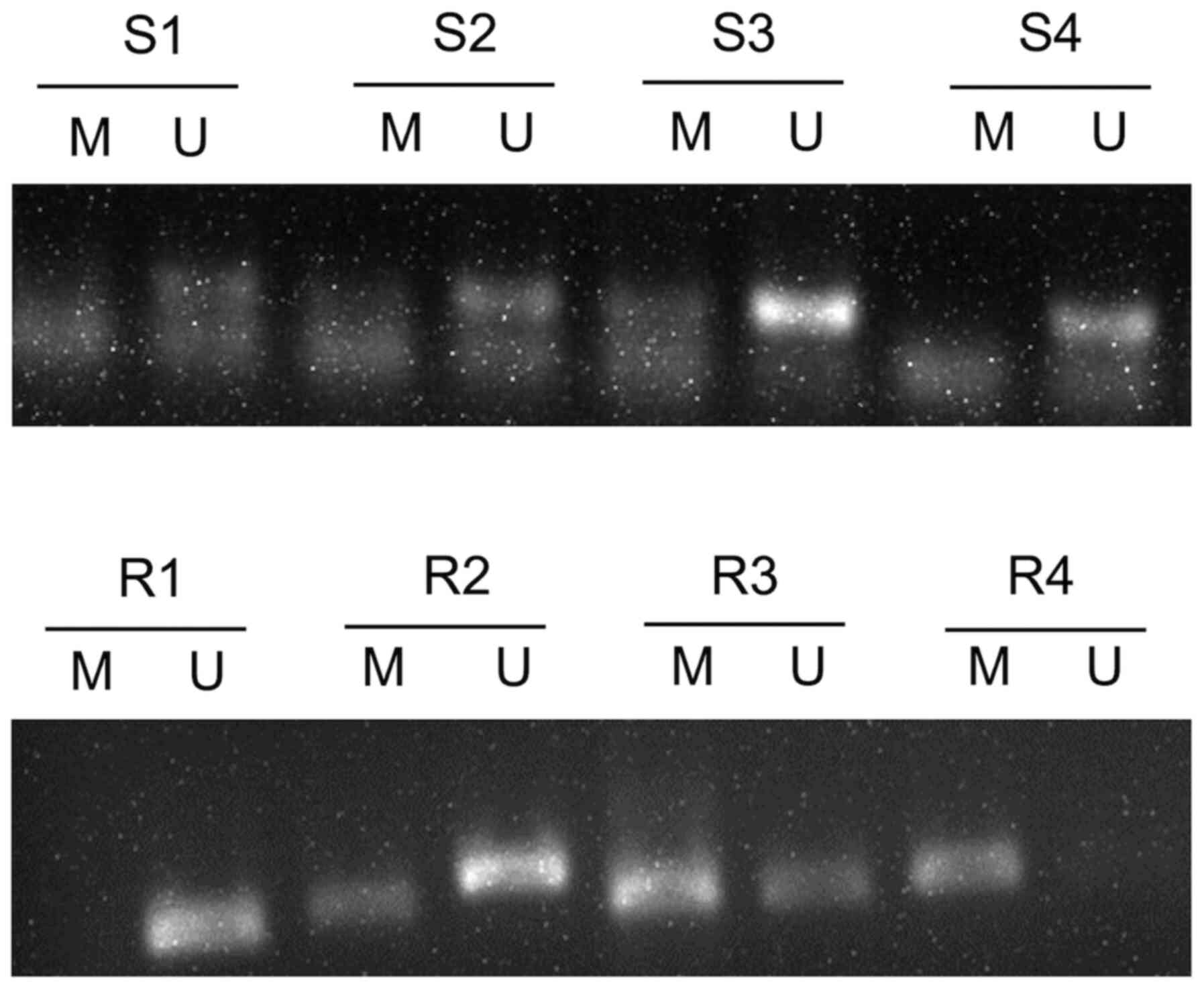
All 59 CC tissue samples were assessed via MSP, and
the results demonstrated the possibility of three states:
Methylation, incomplete methylation and no methylation (Fig. 1). When incomplete methylation was
included into the methylation status for statistical analysis, the
results demonstrated that methylation of GADD45A was significantly
higher in the radioresistant tissues (63.16%) compared with the
radiosensitive samples (33.33%) (P<0.05; Table I). GADD45A promoter methylation in
the remaining patients with CC is presented in Fig. S1.
 | Table I.Association between GADD45A
hypermethylation and radiosensitivity in cervical cancer
tissues. |
Table I.
Association between GADD45A
hypermethylation and radiosensitivity in cervical cancer
tissues.
| Tumor status | GADD45A
hypermethylation | P-value |
|---|
| Radiosensitive | 33.33% (7/21) | <0.05 |
| Radioresistant | 63.16% (24/38) |
|
Differential GADD45A CpG methylation
status, and protein and mRNA levels in the three CC cell lines with
different tolerances to radiotherapy
Radiosensitivity was assessed by comparing the SF
values in cells treated with 2 Gy irradiation (SF2). As presented
in Fig. 2A, the SF2 value of SiHa
cells was the highest amongst the three CC cells (CaSki, 57±9.5%;
HeLa, 70±4% and SiHa, 75±10%). Consistent with previous findings,
the results of the present study demonstrated that SiHa cells
exhibited low sensitivity to irradiation, as they had significantly
higher SF2 values compared with the other two CC cell lines
(P<0.05).
RT-qPCR and western blot analyses were performed to
detect GADD45A mRNA (Fig. 2B) and
protein expression levels (Fig. 2C),
respectively. Notably, GADD45A mRNA and protein expression levels
were lower in SiHa cells compared with the other two CC cell
lines.
MSP analysis was performed to determine whether
there was an association between the methylation status of GADD45A
and downregulation of GADD45A (Fig.
2D). Methylation of GADD45A was evident in SiHa cells; however,
a negative association was observed between the methylation status
and expression of GADD45A.
Effects of the DNA methylation
inhibitor, 5-azaC, on the viability of SiHa cells and the role of
GADD45A
Based on the aforementioned results, SiHa cells were
selected for subsequent experimentation. SiHa cells were incubated
with 0, 1, 3, 5, or 10 µmol/l 5-azaC to assess the cytotoxic
effects of 5-azaC. Cell viability was assessed via the CCK-8 assay
following treatment with different concentrations of 5-azaC
treatment for 24, 48 and 72 h. As presented in Fig. 3A, no statistically significant
differences were observed between treatment with the different
concentrations of 5-azaC (P>0.05).
 | Figure 3.Effects of the DNA methylation
inhibitor, 5-azaC, on the viability of SiHa cells and the role of
GADD45A. (A) Effect of 5-azaC treatment on cell viability. No
significant change in cell viability was observed following
treatment with 1, 3, 5 or 10 µmol/l 5-azaC for 24, 48 and 72 h.
GADD45A (B) mRNA and (C) protein expression levels following
treatment with 1, 3, 5 or 10 µmol/l 5-azaC for 72 h. PBS was used
as the negative control. *P<0.05, **P<0.01, ***P<0.001.
5-azaC, 5-azacytidine; NS, not significant. |
Notably, GADD45A mRNA and protein expression levels
significantly increased in SiHa cells following treatment with 5 or
10 µmol/l 5-azaC for 72 h (Fig. 3B and
C).
5-azaC decreases radioresistance of
SiHa cells in vitro
To determine whether the demethylating agent
decreased the radioresistance of SiHa cells, the survival fraction
of SiHa cells was assessed via the colony formation assay following
treatment with 5 µmol/l 5-azaC. The survival fraction of SiHa cells
treated with 5-azaC and IR significantly decreased in a
dose-dependent manner compared with IR treatment alone (P<0.05;
Fig. 4).
Overexpression of GADD45A increases
the radiosensitivity of SiHa cells in vitro
To assess the effect of GADD45A on the
radiosensitivity of CC cells, GADD45A was overexpressed in SiHa
cells using a GFP-GADD45 expression plasmid. RT-qPCR analysis was
performed to detect GADD45A mRNA expression levels (Fig. 5A). The clonogenic assay was performed
to assess the effect of GADD45A on the sensitivity of SiHa cells to
irradiation. As presented in Fig.
5B, overexpression of GADD45A or treatment with the
demethylating agent significantly increased the radiosensitivity of
SiHa cells compared with the Vec and PBS treated groups,
respectively (P<0.05).
PI3K/AKT signaling is required for
radioresistance, which is mediated by downregulation of
GADD45A
To determine the underlying molecular mechanism of
GADD45A-related regulation on the radiosensitivity of CC cells,
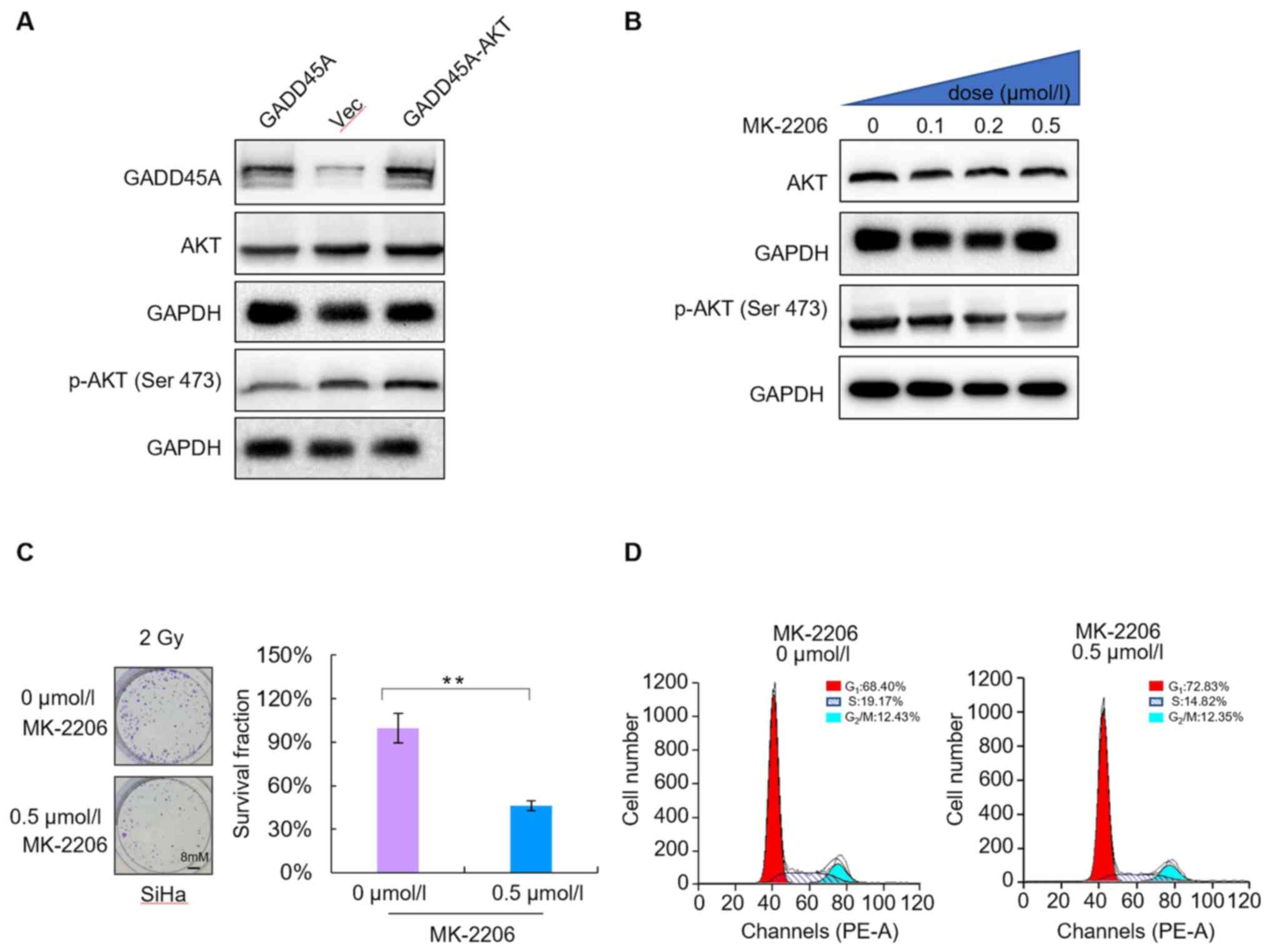
SiHa cells were transfected with GADD45A and/or AKT DNA. Western
blot analysis was performed to detect GADD45A and AKT protein
expression levels in SiHa cells. The results demonstrated that
co-transfection with GADD45A and AKT reversed the effects of
GADD45A overexpression (Fig. 5A).
The levels of phosphorylated AKT significantly decreased in a
dose-dependent manner following treatment with different
concentrations of MK-2206 (0, 0.1, 0.2 and 0.5 µmol/l) for 48 h. In
addition, phosphorylation of AKT protein at Ser 473 decreased,
whereas total AKT protein levels were not significantly altered
(Fig. 6B). The colony formation
assay was performed to assess the radiosensitivity of SiHa cells
treated with 0.5 µmol/l MK-2206. The results demonstrated that
MK-2206 enhanced the radiosensitivity of SiHa CC cells compared
with the untreated cells (Fig. 6C).
In addition, flow cytometric analysis demonstrated that MK-2206 did
not significantly alter the proportion of cells in the different
phases of the cell cycle (P>0.05; Fig. 6D). Taken together, these results
suggest that the PI3K/AKT signaling pathway is essential for
radioresistance, which is mediated by GADD45A downregulation;
however, it is not associated with cell cycle distribution.
Discussion
5-azaC is a well-known DNA methyltransferase
inhibitor, which induces DNA hypomethylation and epigenetic
silencing of gene expression (34).
The results of the present study demonstrated that application of a
demethylation reagent (5 µmol/l 5-azaC) regulated GADD45A
expression. Notably, 5 µmol/l 5-azaC did not exhibit substantial
cytotoxic effects in the three CC lines. Furthermore, in
vitro experiments indicated that the colony formation ability
of SiHa cells was further inhibited by a combination of 5-azaC and
IR, compared with IR alone.
Based on current literature, GADD45 family members
appear to be infrequently mutated in cancer; however, decreased
expression levels of GADD45 family members, due to DNA methylation,
have been frequently reported in several types of cancer, including
gastric, colorectal and pancreatic cancers (35). The results of the present study
demonstrated that GADD45A hypermethylation was significantly higher
in the radiotherapy resistance tissues (63.16%) compared with the
radiotherapy sensitive tissues (33.33%). Similar results were
observed at the cellular level. The GADD45A promoter was completely
methylated in SiHa cells, but unmethylated in CaSki cells. Notably,
the majority of epigenetic studies in cancer have focused on the
methylation of tumor suppressor gene promoters in cell lines,
demonstrating different sensitivities (36–38). For
example, Kim et al (39)
reported that aberrant methylation of ataxia telangiectasia mutated
(ATM) is associated with decreased radioresistance in a colorectal
cell line, HCT-116.
Based on the effective radiosensitization of 5-azaC,
the underlying molecular mechanism was further assessed in SiHa
cells. Apoptosis is one of the most common causes of
radiosensitization (40). In the
present study, 10 µmol/l 5-azaC failed to induce substantial
cytotoxicity in unirradiated SiHa cells. However, 5-azaC induced a
significant increase in apoptosis during irradiation. In addition,
re-expression of GADD45A following treatment with 5-azaC increased
the radiosensitivity of SiHa cells. The results demonstrated that
the AKT inhibitor, MK-2206, enhanced the radiosensitivity of SiHa
cells. It has been reported that the AKT signaling pathway promotes
resistance to chemotherapy, radiotherapy and targeted therapy in
different types of cancer, including colorectal cancer (41), non-small cell lung cancer (42), ovarian cancer (43) and glioma cell lines (44). Thus, it was hypothesized that
downregulation of GADD45A decreases inactivation of the PI3K/AKT
signaling pathway, and contributes to cellular resistance against
radiotherapy.
Cell cycle arrest is a common cause of increased
radiosensitivity (45). In the
present study, pretreatment with 5 µmol/l 5-azaC did not impact the
cell cycle of SiHa cells. These results are consistent with studies
on colorectal cancer (46),
nasopharyngeal carcinoma (47), lung
cancer (48) and glioblastoma cell
lines (49). However, SiHa cells
treated with 10 µmol/l 5-azaC remained in the G2/M phase
in CC cells (unpublished data). Similar results have been reported
following treatment with demethylating agents in breast cancer
(50) and endometrial carcinoma
(51). The reason for this
discrepancy may be due to the different types of cell lines or the
different concentrations of demethylation agents used.
In conclusion, the results of the present study
demonstrated that hypermethylation of GADD45A was observed in
patients with CC, with decreased radiosensitivity, and the PI3K/AKT
signaling pathway was required for radioresistance due to
downregulation of GADD45A in CC. Taken together, these results
suggest that clinical application of epigenetic regulators may be a
promising avenue to increase the radiosensitivity of CC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
ML, QZ, and LL designed the present study. ML and
LYZ performed the experiments. ML, RL, and TYL analyzed the data.
ML and RL drafted the initial manuscript. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Research
Ethics Committee of Guangxi Medical University (Guangxi, China;
approval no. LW2020063), and written informed consent was provided
by all participants prior to the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cohen PA, Jhingran A, Oaknin A and Denny
L: Cervical cancer. Lancet. 393:169–182. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dickinson JA: Age of initiation of
cervical cancer screening. JAMA. 321:611–612. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Petrelli F, De Stefani A, Raspagliesi F,
Lorusso D and Barni S: Radiotherapy with concurrent cisplatin-based
doublet or weekly cisplatin for cervical cancer: A systematic
review and meta-analysis. Gynecol Oncol. 134:166–171. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Global Burden of Disease Cancer
Collaboration, ; Fitzmaurice C, Dicker D, Pain A, Hamavid H,
Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, et
al: The Global Burden of Cancer 2013. JAMA Oncol. 1:505–527. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Begg AC, Stewart FA and Vens C: Strategies
to improve radiotherapy with targeted drugs. Nat Rev Cancer.
11:239–253. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang W, Zhang F, Hu K and Hou X:
Image-guided, intensity-modulated radiation therapy in definitive
radiotherapy for 1,433 patients with cervical cancer. Gynecol
Oncol. 151:444–448. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishikawa K, Koyama-Saegusa K, Otsuka Y,
Ishikawa A, Kawai S, Yasuda K, Suga T, Michikawa Y, Suzuki M,
Iwakawa M and Imai T: Gene expression profile changes correlating
with radioresistance in human cell lines. Int J Radiat Oncol Biol
Phys. 65:234–245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Theys J, Jutten B, Habets R, Paesmans K,
Groot AJ, Lambin P, Wouters BG, Lammering G and Vooijs M:
E-Cadherin loss associated with EMT promotes radioresistance in
human tumor cells. Radiother Oncol. 99:392–397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carlson DJ, Yenice KM and Orton CG: Tumor
hypoxia is an important mechanism of radioresistance in
hypofractionated radiotherapy and must be considered in the
treatment planning process. Med Phys. 38:6347–6350. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grana TM, Rusyn EV, Zhou H, Sartor CI and
Cox AD: Ras mediates radioresistance through both
phosphatidylinositol 3-kinase-dependent and Raf-dependent but
mitogen-activated protein kinase/extracellular signal-regulated
kinase kinase-independent signaling pathways. Cancer Res.
62:4142–4150. 2002.PubMed/NCBI
|
|
11
|
Skvortsova I, Skvortsov S, Stasyk T, Raju
U, Popper BA, Schiestl B, von Guggenberg E, Neher A, Bonn GK, Huber
LA and Lukas P: Intracellular signaling pathways regulating
radioresistance of human prostate carcinoma cells. Proteomics.
8:4521–4533. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bristow RG and Hill RP: Hypoxia and
metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev
Cancer. 8:180–192. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fabbrizi MR, Warshowsky KE, Zobel CL,
Hallahan DE and Sharma GG: Molecular and epigenetic regulatory
mechanisms of normal stem cell radiosensitivity. Cell Death Discov.
18:4:1172018. View Article : Google Scholar
|
|
14
|
Wei W, Dong Z, Gao H, Zhang YY, Shao LH,
Jin LL, Lv YH, Zhao G, Shen YN and Jin SZ: MicroRNA-9 enhanced
radiosensitivity and its mechanism of DNA methylation in non-small
cell lung cancer. Gene. 710:178–185. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sutton LP, Jeffreys SA, Phillips JL,
Taberlay PC, Holloway AF, Ambrose M, Joo JE, Young A, Berry R,
Skala M and Brettingham-Moore KH: DNA methylation changes following
DNA damage in prostate cancer cells. Epigenetics. 14:989–1002.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hervouet E, Cheray M, Vallette FM and
Cartron PF: DNA methylation and apoptosis resistance in cancer
cells. Cells. 2:545–573. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Desjobert C, El Maï M, Gérard-Hirne T,
Guianvarc'h D, Carrier A, Pottier C, Arimondo PB and Riond J:
Combined analysis of DNA methylation and cell cycle in cancer
cells. Epigenetics. 10:82–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pfeifer GP: Defining driver DNA
methylation changes in human cancer. Int J Mol Sci. 19:11662018.
View Article : Google Scholar
|
|
19
|
Russo G, Landi R, Pezone A, Morano A,
Zuchegna C, Romano A, Muller MT, Gottesman ME, Porcellini A and
Avvedimento EV: DNA damage and repair modify DNA methylation and
chromatin domain of the targeted locus: Mechanism of allele
methylation polymorphism. Sci Rep. 6:332222016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Borràs-Fresneda M, Barquinero JF, Gomolka
M, Hornhardt S, Rössler U, Armengol G and Barrios L: Differences in
DNA repair capacity, cell death and transcriptional response after
irradiation between a radiosensitive and a radioresistant cell
line. Sci Rep. 6:270432016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dukaew N, Konishi T, Chairatvit K,
Autsavapromporn N, Soonthornchareonnon N and Wongnoppavich A:
Enhancement of radiosensitivity by eurycomalactone in human NSCLC
cells through G2/M cell cycle arrest and delayed DNA
double-strand break repair. Oncol Res. 28:161–175. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng Q, Hawes SE, Stern JE, Dem A, Sow PS,
Dembele B, Toure P, Sova P, Laird PW and Kiviat NB: Promoter
hypermethylation of tumor suppressor genes in urine from patients
with cervical neoplasia. Cancer Epidemiol Biomarkers Prev.
16:1178–1184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Snoek BC, Splunter APV, Bleeker MCG,
Ruiten MCV, Heideman DAM, Rurup WF, Verlaat W, Schotman H, Gent MV,
Trommel NEV and Steenbergen RDM: Cervical cancer detection by DNA
methylation analysis in urine. Sci Rep. 9:30882019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kazim N, Adhikari A, Oh TJ and Davie J:
The transcription elongation factor TCEA3 induces apoptosis in
rhabdomyosarcoma. Cell Death Dis. 11:672020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chi HC, Tsai CY, Tsai MM and Lin KH:
Impact of DNA and RNA methylation on radiobiology and cancer
progression. Int J Mol Sci. 19:5552018. View Article : Google Scholar
|
|
26
|
Jiao X, Zhang S, Jiao J, Zhang T, Qu W,
Muloye GM, Kong B, Zhang Q and Cui B: Promoter methylation of SEPT9
as a potential biomarker for early detection of cervical cancer and
its overexpression predicts radioresistance. Clin Epigenetics.
11:1202019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guerrero-Setas D, Pérez-Janices N,
Blanco-Fernandez L, Ojer A, Cambra K, Berdasco M, Esteller M,
Maria-Ruiz S, Torrea N and Guarch R: LRASSF hypermethylation is
present and related to shorter survival in squamous cervical
cancer. Mod Pathol. 26:1111–1122. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zerbini LF and Libermann TA: GADD45
deregulation in cancer: Frequently methylated tumor suppressors and
potential therapeutic targets. Clin Cancer Res. 11:6409–6413. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ying J, Srivastava G, Hsieh WS, Gao Z,
Murray P, Liao SK, Ambinder R and Tao Q: The stress-responsive gene
GADD45G is a functional tumor suppressor, with its response to
environmental stresses frequently disrupted epigenetically in
multiple tumors. Clin Cancer Res. 11:6442–6449. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zackrisson B: Radiobiological cell
survival models. A methodological overview. Acta Oncol. 31:433–441.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Na YK, Lee SM, Hong HS, Kim JB, Park JY
and Kim DS: Hypermethylation of growth arrest DNA-damage-inducible
gene 45 in non-small cell lung cancer and its relationship with
clinicopathologic features. Mol Cells. 30:89–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tsikouras P, Zervoudis S, Manav B, Tomara
E, Iatrakis G, Romanidis C, Bothou A and Galazios G: Cervical
cancer: Screening, diagnosis and staging. J BUON. 21:320–325.
2016.PubMed/NCBI
|
|
34
|
Egger G, Liang G, Aparicio A and Jones PA:
Epigenetics in human disease and prospects for epigenetic therapy.
Nature. 429:457–463. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang W, Li T, Shao Y, Zhang C, Wu Q, Yang
H, Zhang J, Guan M, Yu B and Wan J: Semi-quantitative detection of
GADD45-gamma methylation levels in gastric, colorectal and
pancreatic cancers using methylation-sensitive high-resolution
melting analysis. J Cancer Res Clin Oncol. 136:1267–1273. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim JS, Kim SY, Lee M, Kim SH, Kim SM and
Kim EJ: Radioresistance in a human laryngeal squamous cell
carcinoma cell line is associated with DNA methylation changes and
topoisomerase II α. Cancer Biol Ther. 16:558–566. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Smits KM, Melotte V, Niessen HE, Dubois L,
Oberije C, Troost EG, Starmans MH, Boutros PC, Vooijs M, van
Engeland M and Lambin P: Epigenetics in radiotherapy: Where are we
heading? Radiother Oncol. 111:168–177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dote H, Cerna D, Burgan WE, Carter DJ,
Cerra MA, Hollingshead MG, Camphausen K and Tofilon PJ: Enhancement
of in vitro and in vivo tumor cell radiosensitivity by the DNA
methylation inhibitor zebularine. Clin Cancer Res. 11:4571–4579.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim EH, Park AK, Dong SM, Ahn JH and Park
WY: Global analysis of CpG methylation reveals epigenetic control
of the radiosensitivity in lung cancer cell lines. Oncogene.
29:4725–4731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Muschel RJ, Soto DE, McKenna WG and
Bernhard EJ: Radiosensitization and apoptosis. Oncogene.
17:3359–3363. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yuan YH, Wang HY, Lai Y, Zhong W, Liang
WL, Yan FD, Yu Z, Chen JK and Lin Y: Epigenetic inactivation of
HOXD10 is associated with human colon cancer via inhibiting the
RHOC/AKT/MAPK signaling pathway. Cell Commun Signal. 17:92019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Schuurbiers OC, Kaanders JH, van der
Heijden HF, Dekhuijzen RP, Oyen WJ and Bussink J: The
PI3-K/AKT-pathway and radiation resistance mechanisms in non-small
cell lung cancer. J Thorac Oncol. 4:761–767. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhou HM, Sun QX and Cheng Y: Paeonol
enhances the sensitivity of human ovarian cancer cells to
radiotherapy-induced apoptosis due to downregulation of the
phosphatidylinositol-3-kinase/Akt/phosphatase and tensin homolog
pathway and inhibition of vascular endothelial growth factor. Exp
Ther Med. 14:3213–3220. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Man J, Shoemake JD, Ma T, Rizzo AE, Godley
AR, Wu Q, Mohammadi AM, Bao S, Rich JN and Yu JS: Hyperthermia
sensitizes glioma stem-like cells to radiation by inhibiting AKT
signaling. Cancer Res. 75:1760–1769. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Otani K, Naito Y, Sakaguchi Y, Seo Y,
Takahashi Y, Kikuta J, Ogawa K and Ishii M: Cell-cycle-controlled
radiation therapy was effective for treating a murine malignant
melanoma cell line in vitro and in vivo. Sci Rep. 6:306892016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim JG, Bae JH, Kim JA, Heo K, Yang K and
Yi JM: Combination effect of epigenetic regulation and ionizing
radiation in colorectal cancer cells. PLoS One. 9:e1054052014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jiang W, Li YQ, Liu N, Sun Y, He QM, Jiang
N, Xu YF, Chen L and Ma J: 5-Azacytidine enhances the
radiosensitivity of CNE2 and SUNE1 cells in vitro and in vivo
possibly by altering DNA methylation. PLoS One. 9:e932732014.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Momparler RL: Epigenetic therapy of
non-small cell lung cancer using decitabine
(5-aza-2′-deoxycytidine). Front Oncol. 3:1882013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim HJ, Kim JH, Chie EK, Young PD, Kim IA
and Kim IH: DNMT (DNA methyltransferase) inhibitors radiosensitize
human cancer cells by suppressing DNA repair activity. Radiat
Oncol. 7:392012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang L, Zhang Y, Li R, Chen Y, Pan X, Li
G, Dai F and Yang J: 5-aza-2′-Deoxycytidine enhances the
radiosensitivity of breast cancer cells. Cancer Biother Radiopharm.
28:34–44. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Steiner M, Clark B, Tang JZ, Zhu T and
Lobie PE: 14-3-3σ mediates G2-M arrest produced by
5-aza-2′-deoxycytidine and possesses a tumor suppressor role in
endometrial carcinoma cells. Gynecol Oncol. 127:231–240. 2012.
View Article : Google Scholar : PubMed/NCBI
|