Introduction
Endometrial cancer is the sixth most commonly
diagnosed cancer and the 14th leading cause of cancer death among
women worldwide (1). Moreover, the
incidence of endometrial cancer has been rising in recent years.
Treatments for endometrial cancer include surgery, chemotherapy,
radiotherapy, and/or hormone therapy, depending on the disease
stage and histologic type. When diagnosed at an early stage,
surgery generally entails hysterectomy with or without bilateral
salpingo-oophorectomy; at advanced stages, lymph node dissection is
also performed. In the past, these surgeries were performed
abdominally. In recent years, however, laparoscopic or vaginal
surgery, which are less invasive, is often selected for early stage
cancers (2). When diagnosed early,
endometrial cancer is treatable, but at more advanced stages, it is
often fatal. The 5-year survival rate is 95.3% if diagnosed at an
early stage, but it is 67.5% when diagnosed at stage III and 16.9%
when diagnosed at stage IV (3).
More than 80% of endometrial cancers are
estrogen-related (4). This suggests
the rising incidence in endometrial cancer may be related to the
increasing use of exogenous estrogen as well as to increased
exposure of the uterus to endogenous estrogen (nulliparity, fewer
pregnancies, earlier age at menarche, and obesity) (5). To exert is effects, estrogen binds to
estrogen receptors (ERs) in the nucleus. The ER is a
ligand-dependent transcription factor that regulates transcription
of target genes after binding estrogen. ERs are encoded by two
separate genes, the products of which are ERα and ERβ (6). ERα is known to be highly expressed in
certain endometrial and breast cancers, and is thought to play a
role in regulating the expression of genes involved in cell
proliferation, apoptosis, and differentiation. Activation of ERα
promotes cell growth and antagonizes the sensitivity of ovarian
cancer cells to chemotherapeutic agents (7).
BAG3 (hsp70 co-chaperone) is a stress-induced
anti-apoptotic protein that is reportedly involved in such cell
functions as proliferation, apoptosis, adhesion, and migration. We
previously showed that in endometrial cancer cell lines, BAG3
enhances cell migration and invasiveness through downregulation of
microRNA-29b (miR-29b) (8). Felzen
et al showed that in human neuroblastoma cell lines,
ERα-expressing cells exhibit higher levels of autophagy than cells
not expressing ERα, and that this receptor regulates a
non-canonical autophagy pathway involving BAG3 (9). In addition, Brendel et al showed
that ERα-expressing human neuroblastoma cells are more resistant to
apoptosis and express higher levels of BAG3 than human
neuroblastoma cells not expressing the receptor (10).
MicroRNAs (miRNAs) are small non-coding RNAs that
function as negative regulators of gene expression by targeting
mRNAs based on their complementarity to the mRNA 3′ untranslated
region (3′-UTR) (11). Through this
action, miRNAs play various roles during carcinogenesis,
functioning as tumor suppressors or oncogenes (12). As mentioned above, BAG3 enhances the
malignant behavior of endometrial cancer cells by suppressing
miR-29b expression (8). On the other
hand, in other cancer cells, miR-29b contributes to the acquisition
of resistance to anticancer drugs and apoptosis through
upregulation of Mcl-1, a survival-promoting protein with
anti-apoptotic activity (13,14).
In the context of the relationship between ERα and
BAG3 in endometrial cancer cell lines, here we also focused on the
relationship among ERα, BAG3, miR-29b and Mcl-1, which is situated
downstream of BAG3. Our findings provide further insight into the
relationship and function of ERα and BAG3 in endometrial cancer
cells.
Materials and methods
Cells and cell culture
Four established uterine cancer cell lines and one
breast cancer cell line were used in this study. All cells were
obtained from National Institutes of Biomedical Innovation, Health,
and Nutrition, JCRB cell bank (Tokyo, Japan). Mycoplasma testing
was done for all cell lines. The Ishikawa cell line was established
from a grade I endometrial carcinoma. The HEC-1-B cell line was
established from a grade II endometrial carcinoma, the SNG-II line
from an endometrial carcinoma, the EMTOKA line from a
carcinosarcoma, and the MCF-7 line from a human breast
adenocarcinoma. MCF-7 cells were used as a positive control in
western blot analyses. MCF-7, Ishikawa and HEC-1-B cells were
cultured in Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher
Scientific, Inc.), SNG-II cells in Ham's F12 medium (Thermo Fisher
Scientific, Inc.), and EMTOKA cells in Roswell Park Memorial
Institute (PRMI) medium (Thermo Fisher Scientific). All media were
supplemented with 10% fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc.) and 1% penicillin/streptomycin (Thermo Fisher
Scientific, Inc.). All cell lines were maintained in a
CO2 incubator (5% CO2) at 37°C. Cell culture
was performed according to Good Cell Culture Practice (GCCP),
paying sufficient attention to infection. This study focused mainly
on EMTOKA cells, which is a cell line established from uterine
tumors from a 64-year-old Japanese woman who underwent a simple
hysterectomy in 1989. Pathologic examination of the cultured
material showed papillary and tubular adenocarcinoma (carcinomatous
elements) and spindle shaped fiber cells and chondrosarcoma
(sarcomatous element). EMTOKA cells show at least five cell types,
which include columnar cells, small epithelial cells, moderately
sized or large epithelial like cells, malignant tumor giant cells,
and spindle cells (15).
ERα overexpression
pcDNA 3.1(+) was obtained from Addgene (Watertown,
MA, USA). After cleaving the plasmid with Kpn I (Takara Bio Inc.)
and Bam HI (Takara Bio Inc), ERα DNA was inserted using DNA
Ligation Kit Mighty Mix (Takara Bio Inc) according to the
manufacturer's protocol, yielding pcDNA-ERα. Ishikawa and EMTOKA
cells were transfected with the expression vector pcDNA-ERα or with
empty pcDNA vector (control) using Lipofectamine 3000 regent
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. After 24 h, the cells were split and allowed to adhere
overnight.
Reverse transcription-quantitative PCR
(RT-qPCR) for mRNA
Total RNA was extracted from cells using TRIzol
reagent (Thermo Fisher Scientific), after which cDNA was
synthesized from 1 µg of RNA using VILO master mix (Thermo Fisher
Scientific, Inc.). RT-qPCR was carried out using Fast SYBR Green
Master Mix (Thermo Fisher Scientific) in a StepOnePlus™ Real-Time
PCR system (Thermo Fisher Scientific, Inc.). mRNA levels were
standardized to the level of glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) mRNA. The PCR protocol entailed denaturation
at 95°C for 10 min followed by 40 cycles of 95°C for 15 sec and
60°C for 60 sec. The following primers were designed and used for
RT-qPCR: For BAG3, 5′-TGAGAAGTTTAACCCCGTTGCTTGT-3′ (forward) and
5′-CCCCATCTACCCCTCCAGTCCAG-3′ (reverse); for ERα,
5′-GTGCCAGGCTTTGTGGATTTG-3′ (forward) and
5′-GTTACTCATGTGCCTGATGTG-3′ (reverse); for GAPDH,
5′-TGAACGGGAAGCTCACTGG-3′ (forward) and 5′-TCCACCACCCTGTTGCTGTA-3′
(reverse). Gene expression was calculated using the
2−ΔΔCq method (16).
RT-qPCR for microRNA
Total RNA was extracted using TRIzol reagent, after
which reverse transcription was performed with 10 ng of total RNA
using a TaqMan® MicroRNA Reverse Transcription kit
(Thermo Fisher Scientific, Inc.) and sequence-specific RT primers
from the TaqMan MicroRNA assays (Thermo Fisher Scientific, Inc.).
Separate reverse transcription reactions were run for each TaqMan
MicroRNA assay with each RNA sample. RT-qPCR was performed with
cDNA using inventoried TaqMan MicroRNA assays and TaqMan Universal
Master Mix II (Thermo Fisher Scientific, Inc.). The assay was
performed in triplicate, and the PCR amplification was performed
using a StepOnePlus™ Real-Time PCR system. microRNA levels were
standardized to the level of RNU48 small-nucleolar RNA. Primer
sequences were as follows: miR-29b (assay ID:00413),
5′-UAGCACCAUUUGAAAUCAGUGUU-3′ and RNU48 (assay ID:001006),
5′-GATGACCCCAGGTAACTCTGAGTGTGTCGCTGATGCCATCACCGCAGCGCTCTGACC-3′.
Gene expression was calculated using the 2−ΔΔCq
method.
Lysate production
Cell lysates were produced from subconfluent cell
cultures. After scraping the cells from the dishes, they were lysed
by sonication in RIPA buffer (Nacalai Tesque) containing a protease
inhibitor cocktail (Thermo Fisher Scientific). The lysates were
then centrifuged at 17,000 × g for 15 min at 4°C to pellet the
nuclei, and the supernatant was collected as the cell lysate.
Western blotting
After measuring their protein content, lysates were
diluted in 2X sample buffer (Sigma-Aldrich,) and boiled for 5 min
at 100°C. Samples containing 30 µg of protein were then
electrophoresed (200 V for 35 min) on 12% SDS polyacrylamide gel,
after which the separated proteins were transferred onto PVDF
membranes. After blocking with 5% non-fat dry milk in TBS [10 mM
sodium phosphate (pH 7.8), 150 mM NaCl and 0.05% Tween-20], the
membranes were probed with the following primary antibodies: Rabbit
monoclonal anti-BAG3 (1:1,000 dilution; ab92309; Abcam), mouse
monoclonal anti-ERα (1:100 dilution; sc-8002; Santa Cruz
Biotechnology, Inc.), mouse monoclonal anti-Mcl-1 (1:1,000
dilution; ab32087; Abcam) and mouse monoclonal anti-β-actin
(1:5,000 dilution; A5441; Sigma-Aldritch). After washing with
PBS-T, the membranes were incubated with secondary horseradish
peroxidase-conjugated antibodies. Proteins were visualized using
ECL Prime Western Blotting Detection Reagent and an ImageQuant LAS
500 (GE Healthcare). Western blot bands were semi-quantified using
ImageJ (National Institutes of Health).
Cell viability assay
To test the sensitivity of cells to cisplatin under
various culture conditions, cells were plated in 96-well plates
(5,000 cells/well) in medium containing 5% serum and incubated at
37°C under a 5% CO2 atmosphere. After 24 h, the medium
was replaced with medium containing the indicated concentration of
cisplatin (Fujifilm Wako Chemical Corporation), and the cells were
incubated for an additional 48 h. Cell viability was then assessed
using a Cell Proliferation Kit II (XTT; Roche Diagnostics).
Following the incubation period, 50 µl of XTT labeling mixture was
added to each well, and the cells were incubated for 4 h, after
which the absorbance at 492 nm was recorded using an ELISA plate
reader.
Statistical analysis
Unpaired Student's t-tests were used for statistical
evaluation of the data. Values of P<0.05 were considered
significant. Two-way ANOVA was used for analysis of cell viability
assay results, and one-way ANOVA was used for other statistical
comparisons. As post hoc tests, Tukey's multiple comparisons test
was used for one-way ANOVA and Bonferroni's multiple comparisons
test was used for two-way ANOVA. SPSS 22.0 (IBM Corp.) and GraphPad
Prism version 8 (GraphPad Software Inc.) were used for
analyses.
Results
Expression of ERα and BAG3 in
endometrial cancer cell lines
Ishikawa, HEC-1-B, SNG-II, and EMTOKA cells were
used for western blot and RT-qPCR analyses. Among the four cell
lines, there was a significant difference in BAG3 mRNA expression
between Ishikawa and HEC-1-B (P=0.0058), Ishikawa and EMTOKA
(P=0.0006), and SNG-II and EMTOKA (P=0.0069), but no significant
difference in expression between other cells (Fig. 1A). On the other hand, expression of
BAG3 protein was detected more strongly in HEC-1-B and EMTOKA
cells, than in Ishikawa or SNG-II cells (Fig. 1C). Expression of ERα mRNA and protein
was detected only in Ishikawa cells (P<0.0001) (Fig. 1B). In subsequent experiments,
therefore, we used Ishikawa cells as representative of endometrial
cancer cells expressing ERα and EMTOKA cells as endometrial cancer
cells not expressing ERα.
 | Figure 1.Expression of BAG3 and ERα. RT-qPCR
analysis of (A) BAG3 and (B) ERα expression in Ishikawa, HEC-1-B,
SNG-2 and EMTOKA cells. Levels of BAG3 and ERα mRNA were determined
using real-time RT-qPCR. Bars depict the relative mRNA levels
normalized to the level of GAPDH mRNA. The results are presented as
means ± SD; **P<0.01. (C) Western blot analysis of BAG3, ERα and
actin expression in MCF-7, Ishikawa, HEC-1-B, SNG-2 and EMTOKA
cells. Blots were probed using a rabbit monoclonal anti-BAG3 or
mouse monoclonal anti ERα antibody. As a loading control, the blots
were probed using mouse monoclonal anti-actin antibody. MCF-7 cells
were used as a positive control. RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; ERα, estrogen
receptor α; BAG3, BCL-2-associated athanogene 3. |
Effect of ERα overexpression on BAG3
expression
To determine the effect of ERα overexpression,
Ishikawa and EMTOKA cells were transfected with pcDNA-ERα. In both
cell types, exogenous ERα expression led to upregulated expression
of BAG3 mRNA (Fig. 2A and B). ERα
overexpression also led to upregulated expression of BAG3 protein
in EMTOKA cells, but not in Ishikawa cells (Fig. 2C).
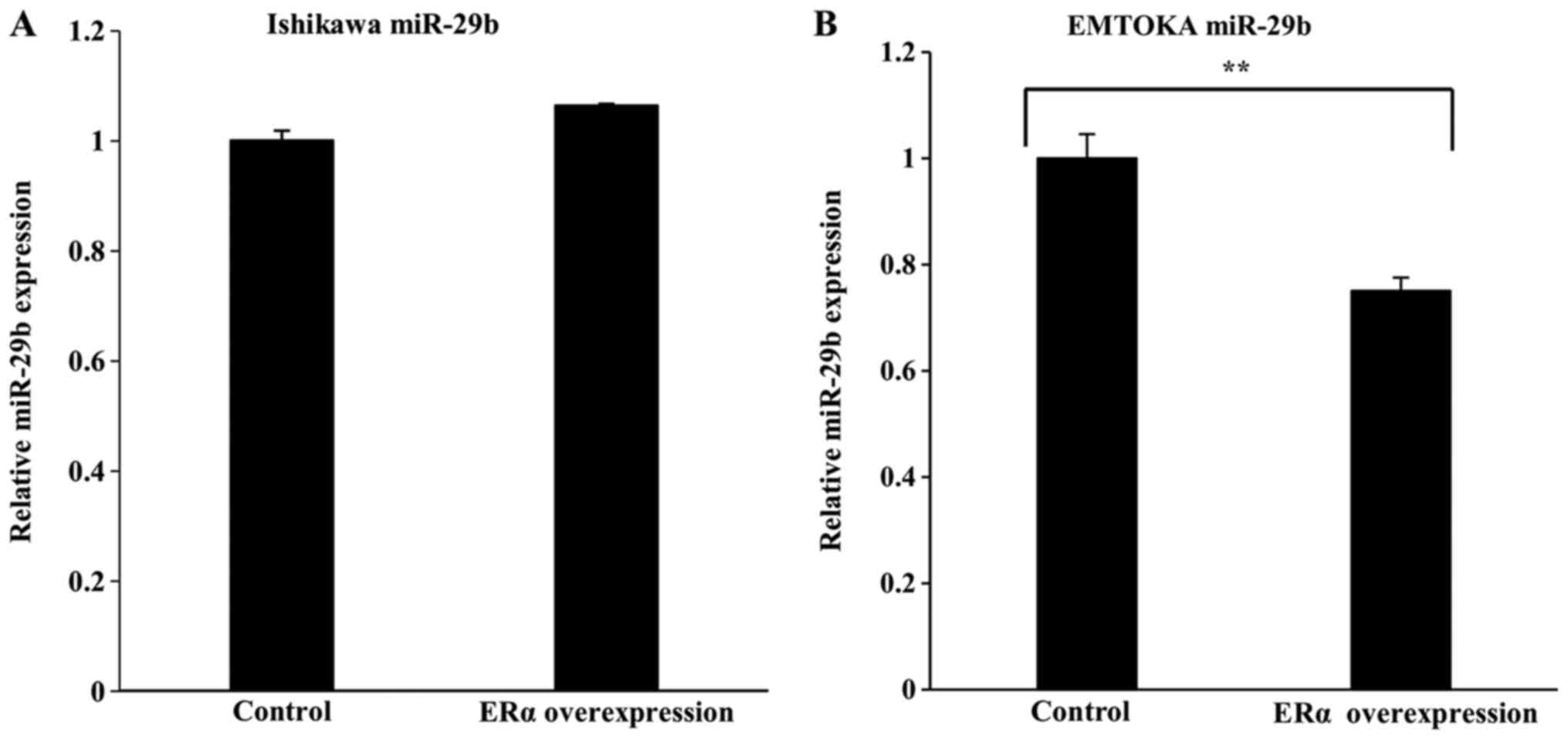
Effect of ERα overexpression on
miR-29b levels
RT-qPCR analysis revealed that in Ishikawa cells,
ERα overexpression had no effect on miR-29b expression (Fig. 3A). In EMTOKA cells, by contrast, ERα
overexpression led to downregulation of miR-29b (Fig. 3B).
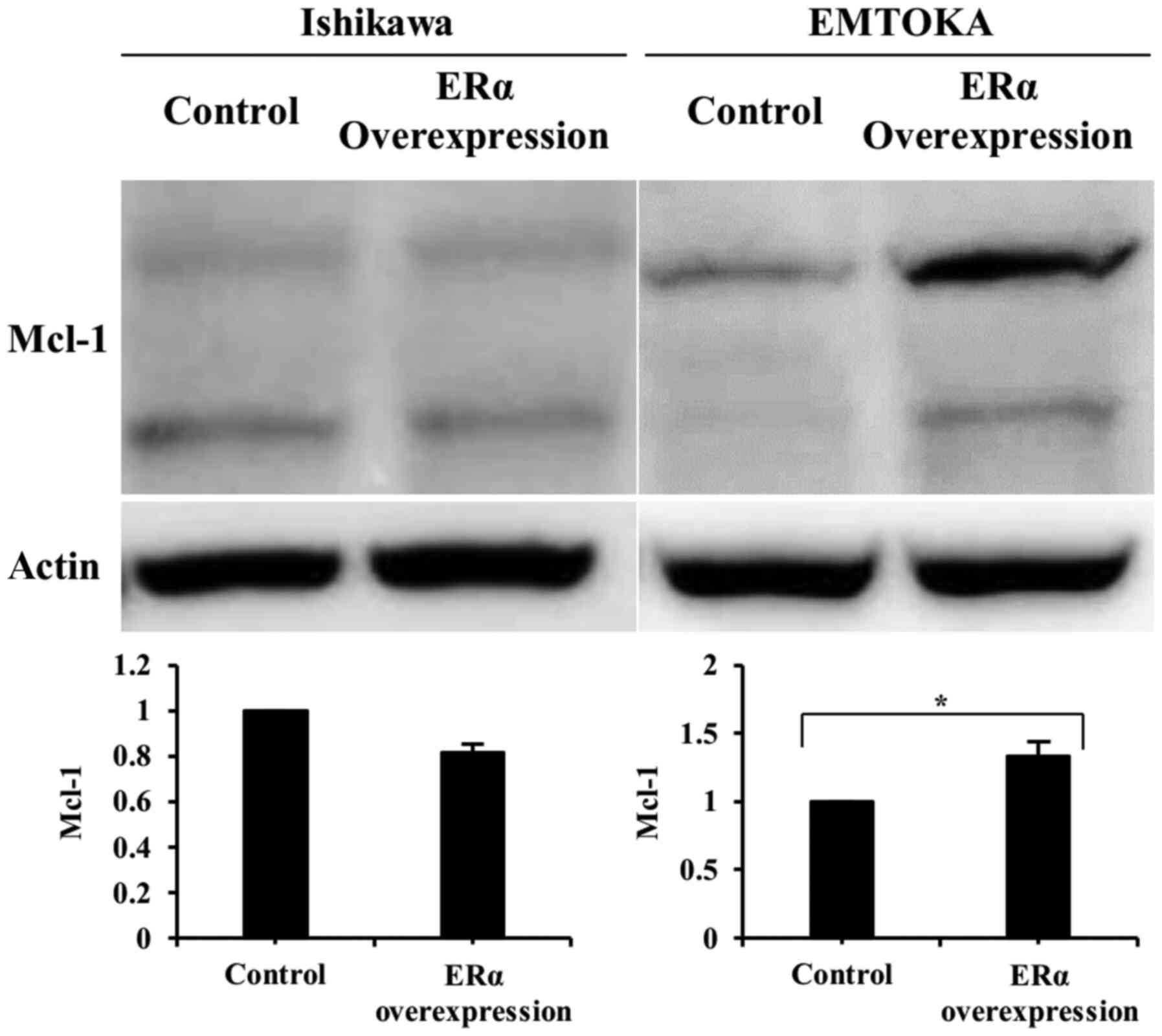
Effect of ERα overexpression on
expression Mcl-1 protein
In EMTOKA cells, overexpression of ERα led to
upregulation of Mcl-1, a mediator situated downstream of BAG3 and
miR-29b. In Ishikawa cells, however, overexpression of ERα had no
effect on Mcl-1 expression (Fig.
4).
Effect of ERα overexpression on
chemosensitivity to cisplatin
Finally, we investigated the effect of ERα
overexpression on the viability of cells exposed to cisplatin. We
found that after exposure to cisplatin for 48 h, the numbers of
viable ERα-overexpressing EMTOKA cells was significantly higher
than the number of control cells. On the other hand, ERα
overexpression had no effect on Ishikawa cell viability in the
presence of cisplatin (Fig. 5).
Discussion
Estrogen is known to be associated with
carcinogenesis and to promote the progression of endometrial cancer
(17). For example, ERα expression
on macrophages from endometrial cancer patients correlates
positively with cancer progression (18). In addition, in ovarian cancer cells,
activation of ERα by estrogen and cisplatin can induce
platinum-resistance by increasing expression of an anti-apoptotic
protein (7). Our results suggest
that ERα expression in EMTOKA human endometrial cancer cells
increases cell viability in the presence of cisplatin through
upregulation of BAG3, which plays important roles in the regulation
of apoptosis, autophagy, and cell differentiation. Notably, this
effect of exogenous ERα upregulation was not seen in Ishikawa
cells, which endogenously express ERα. The effect of exogenous ERα
upregulation was only seen in EMTOKA cells, which do not
endogenously express ERα. ERα is expressed in brain, mammary gland,
ovary (thecal cells), uterus, bone, and testis (19,20). The
ERα expression rates among endometrial cancer patients are 50–60%,
30–40%, and 5–15% in endometrioid cancer grades 1, 2, and 3,
respectively, but it is nearly absent in serous and clear cell
cancers (21,22). Felzen et al showed that in
human neuroblastoma cell lines, upregulation of ERα increased
autophagic activity by enhancing BAG3 expression, but in the MCF7
ERα-expressing human breast cancer cells line, ERα knockdown did
not alter BAG3 levels or autophagic activity (9). Our results also show that the level of
BAG3 expression is unaffected by ERα knockdown in the Ishikawa
ERα-expressing human endometrial cancer cell line. This suggests
that expression of a small amount of ERα is sufficient to enhance
expression downstream mediators (e.g., BAG3 and Mcl-1) in the ERα
signaling pathway, and that higher levels of ERα do not further
enhance expression of those proteins.
Previous studies indicate that miR-29b acts as a
tumor suppressor (23,24) and that it is associated with
differentiation, proliferation, invasiveness and metastasis of lung
cancer, breast cancer, cholangiocarcinoma, and leukemia cells
(25–28). miR-29b downregulates Mcl-1, thereby
promoting cell apoptosis. Correspondingly, downregulation of
miR-29b correlates with more aggressive forms of cancer and with
recurrence. In the present study, we demonstrated that ERα
overexpression leads to decreased miR-29b expression and thus
increased Mcl-1 expression.
Mcl-1 is an antiapoptotic Bcl-2 family member that
modulates apoptosis-related signaling pathways and promotes cell
survival. Mcl-1 also appears to be an important factor mediating
resistance to cancer chemotherapy, and its downregulation has
proved effective for inducing apoptosis (29–31).
Consistent with those findings, we observed here that suppression
of miR-29b through overexpression of ERα increased Mcl-1 levels and
induced resistance to cisplatin in EMTOKA endometrial cancer
cells.
In an earlier study, we found that upregulation of
BAG3 increased tumor cell motility and invasiveness through
downregulation of miR-29b and subsequent upregulation of MMP-2
(8). We also previously reported
that BAG3 upregulates Mcl-1 by suppressing miR-29b and induces
anticancer drug resistance in ovarian cancer cell lines (13). Consistent with those earlier
observations, our results in the present study show that ERα likely
contributes to the acquisition of resistance to anticancer drugs by
endometrial cancer cells via an ERα-BAG3-miR-29b-Mcl-1 pathway.
However, several issues remain to be addressed by future research.
First, the relationship between ERα and the Bcl-2 family does not
indicate a direct relationship between ERα and apoptosis. To
investigate the direct relationship, it will be necessary to
examine the relationship between ERα and caspase activity. Second,
because this report describes a basic study using endometrial
cancer cell lines, our findings will need to be verified and
extended through investigation of protein expression in human
endometrial cancer tissue. We anticipate the results of those
studies will deepen our understanding of the relationship between
ERα and chemoresistance and apoptosis, and shed light on whether
ERα can serve as an effective therapeutic target.
Although there are some challenges, these results
suggest that ERα is a key determinant of the responsiveness of some
endometrial cancer cells to cisplatin, and that ERα is a
potentially useful therapeutic target for the treatment of some
types of endometrial cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SA and MI designed and completed the experiments
together. SH provided guidance on the overall experimental
technique and also performed RT-qPCR along with SA. TM performed
statistical analysis. MT and MM revised the article and also
performed western blotting along with SA. SS performed cell culture
and cell viability assays along with MI and SA. TS oversaw the
composition of the manuscript and the overall experiments, and also
performed RT-qPCR. All authors read and approved the final
manuscript, and each author believes that the manuscript represents
honest work.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Berretta R, Merisio C, Melpignano M, Rolla
M, Ceccaroni M, De Ioris A, Patrelli TS and Nardelli GB: Vaginal
versus abdominal hysterectomy in endometrial cancer: A
retrospective study in a selective population. Int J Gynecol
Cancer. 18:797–802. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
International Agency for Research on
Cancer (IARC). World Cancer Report. 2014, Steward BW and Wild CP:
IARC; Lyon: pp. 465–481. 2014
|
|
5
|
Lortet-Tieulent J, Ferlay J, Bray F and
Jemal A: International patterns and trends in endometrial cancer
incidence, 1978-2013. J Natl Cancer Inst. 110:354–361. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matthews J and Gustafsson JA: Estrogen
signaling: A subtle balance between ER alpha and ER beta. Mol
Interv. 3:281–292. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsumura S, Ohta T, Yamanouchi K, Liu Z,
Sudo T, Kojimahara T, Seino M, Narumi M, Tsutsumi S, Takahashi T,
et al: Activation of estrogen receptor α by estradiol and cisplatin
induces platinum-resistance in ovarian cancer cells. Cancer Biol
Ther. 18:730–739. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Habata S, Iwasaki M, Sugio A, Suzuki M,
Tamate M, Satohisa S, Tanaka R and Saito T: BAG3 increases the
invasiveness of uterine corpus carcinoma cells by suppressing
miR-29b and enhancing MMP2 expression. Oncol Rep. 33:2613–2621.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Felzen V, Hiebel C, Koziollek-Drechsler I,
Reißig S, Wolfrum U, Kögel D, Brandts C, Behl C and Morawe T:
Estrogen receptor α regulates non-canonical autophagy that provides
stress resistance to neuroblastoma and breast cancer cells and
involves BAG3 function. Cell Death Dis. 6:e18122015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brendel A, Felzen V, Morawe T, Manthey D
and Behl C: Differential regulation of apoptosis-associated genes
by estrogen receptor alpha in human neuroblastoma cells. Restor
Neurol Neurosci. 31:199–211. 2013.PubMed/NCBI
|
|
11
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nelson KM and Weiss GJ: MicroRNAs and
cancer: Past, present, and potential future. Mol Cancer Ther.
7:3655–3660. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sugio A, Iwasaki M, Habata S, Mariya T,
Suzuki M, Osogami H, Tamate M, Tanaka R and Saito T: BAG3
upregulates Mcl-1 through downregulation of miR-29b to induce
anticancer drug resistance in ovarian cancer. Gynecol Oncol.
134:615–623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mott JL, Kobayashi S, Bronk SF and Gores
GJ: mir-29 regulates Mcl-1 protein expression and apoptosis.
Oncogene. 26:6133–6140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gorai I, Doi C and Minaguchi H:
Establishment and characterization of carcinosarcoma cell line of
the human uterus. Cancer. 71:775–786. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matsumoto M, Yamaguchi Y, Seino Y,
Hatakeyama A, Takei H, Niikura H, Ito K, Suzuki T, Sasano H,
Yaegashi N and Hayashi SI: Estrogen signaling ability in human
endometrial cancer through the cancer-stromal interaction. Endocr
Relat Cancer. 15:451–463. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jing X, Peng J, Dou Y, Sun J, Ma C, Wang
Q, Zhang L, Luo X, Kong B, Zhang Y, et al: Macrophage ERα promoted
invasion of endometrial cancer cell by mTOR/KIF5B-mediated
epithelial to mesenchymal transition. Immunol Cell Biol.
97:563–576. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Heldring N, Pike A, Andersson S, Matthews
J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M and
Gustafsson JA: Estrogen receptors: How do they signal and what are
their targets. Physiol Rev. 87:905–931. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dahlman-Wright K, Cavailles V, Fuqua SA,
Jordan VC, Katzenellenbogen JA, Korach KS, Maggi A, Muramatsu M,
Parker MG and Gustafsson JA: International union of pharmacology.
LXIV. Estrogen receptors. Pharmacol Rev. 58:773–781. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li SF, Shiozawa T, Nakayama K, Nikaido T
and Fujii S: Stepwise abnormality of sex steroid hormone receptors,
tumor suppressor gene products (p53 and Rb), and cyclin E in
uterine endometrioid carcinoma. Cancer. 77:321–329. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shih HC, Shiozawa T, Kato K, Imai T,
Miyamoto T, Uchikawa J, Nikaido T and Konishi I:
Immunohistochemical expression of cyclins, cyclin-dependent
kinases, tumor-suppressor gene products, Ki-67, and sex steroid
receptors in endometrial carcinoma: Positive staining for cyclin A
as a poor prognostic indicator. Hum Pathol. 34:471–478. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Steele R, Mott JL and Ray RB: MBP-1
upregulates miR-29b that represses Mcl-1, collagens, and
matrix-metalloproteinase-2 in prostate cancer cells. Genes Cancer.
1:381–387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Garzon R, Heaphy CE, Havelange V, Fabbri
M, Volinia S, Tsao T, Zanesi N, Kornblau SM, Marcucci G, Calin GA,
et al: MicroRNA 29b functions in acute myeloid leukemia. Blood.
114:5331–5341. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Y, Wang H, Tao K, Xiao Q, Huang Z,
Zhong L, Cao W, Wen J and Feng W: miR-29b suppresses CML cell
proliferation and induces apoptosis via regulation of BCR/ABL1
protein. Exp Cell Res. 319:1094–1101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao JJ, Lin J, Lwin T, Yang H, Guo J,
Kong W, Dessureault S, Moscinski LC, Rezania D, Dalton WS, et al:
microRNA expression profile and identification of miR-29 as a
prognostic marker and pathogenetic factor by targeting CDK6 in
mantle cell lymphoma. Blood. 115:2630–2639. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu Y, Crawford M, Mao Y, Lee RJ, Davis IC,
Elton TS, Lee LJ and Nana-Sinkam SP: Therapeutic delivery of
MicroRNA-29b by cationic lipoplexes for lung cancer. Mol Ther
Nucleic Acids. 2:e842013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang X, Schwind S, Yu B, Santhanam R,
Wang H, Hoellerbauer P, Mims A, Klisovic R, Walker AR, Chan KK, et
al: Targeted delivery of microRNA-29b by transferrin-conjugated
anionic lipopolyplex nanoparticles: A novel therapeutic strategy in
acute myeloid leukemia. Clin Cancer Res. 19:2355–2367.
2013.PubMed/NCBI
|
|
29
|
Sieghart W, Losert D, Strommer S, Cejka D,
Schmid K, Rasoul-Rockenschaub S, Bodingbauer M, Crevenna R, Monia
BP, Peck-Radosavljevic M and Wacheck V: Mcl-1 overexpression in
hepatocellular carcinoma: A potential target for antisense therapy.
J Hepatol. 44:151–157. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Akgul C: Mcl-1 is a potential therapeutic
target in multiple types of cancer. Cell Mol Life Sci.
66:1326–1336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aichberger KJ, Mayerhofer M, Krauth MT,
Skvara H, Florian S, Sonneck K, Akgul C, Derdak S, Pickl WF,
Wacheck V, et al: Identification of mcl-1 as a BCR/ABL-dependent
target in chronic myeloid leukemia (CML): Evidence for cooperative
antileukemic effects of imatinib and mcl-1 antisense
oligonucleotides. Blood. 105:3303–3311. 2005. View Article : Google Scholar : PubMed/NCBI
|