Introduction
Colorectal carcinoma (CRC) is the second most
commonly diagnosed cancer in women and the third in men around the
world (1). Although the initial
events of CRC development are relatively well understood and
therapeutic approaches for early-stage disease have significantly
improved (2), some aggressive types
of CRC have not received enough attention from pathologists. These
types of CRC may have higher rates of recurrence and metastasis,
such as micropapillary cancer (3,4).
Therefore, it is important to identify specific markers to diagnose
these forms of CRC.
Colorectal micropapillary carcinoma (MPC) is related
to a poor prognosis (5). The tumour
is characterized histologically by small papillary clusters of
cells lacking a central fibrous vascular core located in lacunar
spaces, with eosinophilic cytoplasm and pleomorphic nuclei
(6–11). This pattern was described first in
the breast (12) and ovarian cancer
(13) and subsequently in other
locations, including the urothelial (14), gastrointestinal (15), lung (16) and salivary gland cancer (17). An increasing number of studies have
reported that MPC is an aggressive variant of colorectal
adenocarcinoma (4,11,18,19).
However, there are only a few preliminary studies with small
samples of CRC with micropapillary pattern (MPP) and a few case
reports of clinicopathological studies.
MPC cells display reverse polarity and with the
characteristics of an inside-out (I/O) epithelial membrane antigen
(20,21). I/O pattern (I/OP) staining of
EMA/mucin (MUC)1 has also been confirmed in CRC with micropapillary
components (4,20,22,23).
However, some studies have demonstrated that the sensitivity of
EMA/MUC-1 staining is not high, and >50% of cases have no EMA
staining (4,23–25).
Like EMA/MUC-1, villin is a surface-related glycoprotein and is
also expressed in the normal colorectal mucosa (26). Using bioinformatics methods, the
expression of villin, E-cadherin and EMA in colorectal cancer and
normal mucosa was analysed. Immunohistochemical staining was used
to compare the expression of EMA, E-cadherin and villin in
colorectal MPC and common adenocarcinoma, aiming to identify
improved markers of MPC.
The present study collected 453 colorectal cancer
tissue samples between 2013 and 2018. The structure of colorectal
cancer tissue was observed using microscopy. In total, 90 cases
were accompanied by MPP and 85 cases of CRC with MPP and 201 cases
without MPP were analysed to compare their clinicopathological
parameters and the patient outcomes.
Materials and methods
Patients
The records of 453 patients who had undergone
surgical resection of CRC at The Third Affiliated Hospital of
Guangzhou Medical University (Guangzhou, China) between January
2013 and December 2018 were retrospectively analysed and followed
up by telephone. The inclusion criteria were a complete follow-up,
ranging from 1 to 58 months with a mean of 26 months. The patients
consisted of 162 men and 124 women. The age of patients ranged from
27 to 93 years with a mean age of 60 years. The patient had not
received any pre-operative chemotherapy or radiotherapy.
Clinicopathological characteristics, such as tumour
size, differentiation, vascular and lymphatic invasion, lymph node
metastasis and distant metastasis and pathological
Tumour-Node-Metastasis (pTNM) stages (27), were reviewed using medical charts,
pathological records and archived slides of tissue samples. All
patients provided written informed consent and the study was
approved by The Ethics Committee of The Third Affiliated Hospital
(Guangzhou, China).
Evaluation of MPP
To elucidate the clinicopathological significance of
MPCs, the presence of MPP was investigated in 453 CRCs by three
pathologists in the present group. MPP is characterized by: i) A
tumour cell nest, consisting of 3–20 tumour cells, with reverse
polarity with an outer common border (6); ii) tumour cell clusters without
fibrovascular cores (7); iii) tumour
cells with pleomorphic nuclei and eosinophilic cytoplasm (8); and iv) a clear lacunar space around the
tumour nest (11). In some cases,
MPP in the mucin pools was determined. Comparisons between CRCs
with MPP and without MPP were conducted.
GEPIA and the Human Protein Atlas
(HPA) database analysis
Villin, EMA and E-cadherin expression in colorectal
adenocarcinoma (COAD) and normal tissues were obtained from the
GEPIA database (http://gepia.cancer-pku.cn/). Immunohistochemistry
results of COAD with three different antibodies against villin,
five different antibodies against EMA/MUC-1 and E-cadherin, were
obtained from the HPA database (https://www.proteinatlas.org/), which has been
generated from RNA-sequencing analysis and immunohistochemistry
analysis (28). The HPA database
analyzes the expression of villin, E-cadherin and EMA/MUC-1
antibodies in colorectal cancer to compare the differences between
different clone antibodies, which is helpful for us to select more
effective antibodies.
Tissue microarray construction
The hematoxylin and eosin (H&E)-stained slides
and corresponding paraffin-embedded tissue (29) blocks of COAD with MPP were obtained
from the Department of Pathology (The Third Affiliated Hospital of
Guangzhou Medical University). Representative areas of MPC and
conventional CRC were marked with a marker pen on H&E slides.
Then these corresponding areas of paraffin-embedded tissue blocks
were marked by the same method. Tissue microarrays (TAMs) were
constructed using a tissue-arraying instrument (QuickRay; Unitma
Co., Ltd.). Briefly, tissue cores with a 1.5-mm diameter of MPC and
conventional CRC were obtained from donor tissue blocks, and were
transferred to two recipient paraffining blocks, respectively. The
tissues used for the TAMs were the same as those
aforementioned.
Immunohistochemistry
Consecutive 4-µm thick unstained sections were cut
from TAM blocks for immunohistochemical staining, which was
performed using the Leica automatic immunostaining device (Leica
Microsystems, Inc.). Primary antibodies against villin (1:100; cat.
no. PA0554, Leica Microsystems, Inc.), E-cadherin (1:100; cat. no.
GT210701) and EMA (1:100; cat. no. GM061301) (both Gene Tech
Biotechnology Co., Ltd.). The immunohistochemistry profile included
the expression of villin, E-cadherin and EMA.
Immunohistochemistry were conducted according to
previously described methods (24).
All slides were reviewed and scored independently by three
pathologists. The pathologists were blinded to the experiment. The
scoring method based on both the intensity (0, no staining; 1, weak
staining; 2, medium staining; 3, strong staining) and proportion of
positive cells (0, 0%; 1, 1–25%; 2, 26–75%; 3, 76–100%). The final
staining score was calculated by multiplying the staining intensity
score by extent of staining score. A final staining score of ≥3 was
considered positive, and others were classified as low expression.
The cases of positive expression were further classified into two
groups based on the location of the three markers. For MUC-1 and
villin, these cases with positive expression on the outer borders
of tumor cell clusters were regarded as having reversion of cell
polarity (I/OP staining group), and staining in the apical membrane
of adenocarcinoma were regarded as non-reversion of cell polarity
(no I/OP staining group). For E-cadherin, the cases with cup-shaped
expression patterns around cell nests were regarded as having
reversion of cell polarity (I/OP staining group) and complete cell
membrane expression were regarded as non-reversion of cell polarity
(no I/OP staining).
Statistical analysis
Statistical analyses were performed using SPSS
version 19.0 software (IMB Corp.). Data are presented as value (%),
unless otherwise shown. The significance of the association between
the presence of MPP and the clinicopathological characteristics was
determined by either the χ2 test or Fisher's exact test
(two-sided). Survival curves were estimated using the Kaplan-Meier
product-limit method, and differences between the survival curves
were determined using the log-rank test. The statistical method
used in the GEPIA database was one-way ANOVA with Least
Significance Difference post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Histological features of primary COAD
with MPP
A MPP with a proportion ≥5% of the tumour was
identified in 90/453 cases (19.87%) of CRC. The percentage of MPP
within the 90 cases ranged from 5–70%, as summarized in Table I. The morphology of MPP was different
from that of conventional adenocarcinoma (Fig. 1A). The glandular tube structure can
be seen in most conventional adenocarcinomas, while the MPP shows
nest-mass transformation. MPP was often present at the invasive
edge of the tumour. The main feature of the MPP was the presence of
clefts between the stroma, devoid of central fibrovascular cores
and with pleomorphic nuclei and eosinophilic cytoplasm (Fig. 1B). There were tumour cell nests of
MPP, consisting of 3–20 tumour cells with reverse polarity and with
an outer common border (Fig. 1C). In
some cases, adenocarcinoma with MPP in mucin pools was observed
(Fig. 1D). These were the
histological features of MPP.
 | Table I.Percentage of micropapillary pattern
within the 90 cases. |
Table I.
Percentage of micropapillary pattern
within the 90 cases.
| Percentage, % | Number of
cases |
|---|
| 5 | 11 |
| 10 | 37 |
| 15 | 10 |
| 20 | 11 |
| 25 | 7 |
| 30 | 5 |
| 40 | 3 |
| 50 | 4 |
| 60 | 1 |
| 70 | 1 |
COAD with the MPP predicts a poor
outcome and promotes disease progression
Follow-up data was collected for 286 patients. Their
age ranged from 27 to 93 years (mean 60 years), and 162 patients
were men and 124 patients were women. The tumour size ranged from
0.3 to 16.0 cm (mean 4.7 cm). The mean number of total lymph nodes
dissected was 11.4 (range, 0–29), and the mean number of positive
lymph nodes was 1.5 (range, 1–14). Of 286 cases, 201 carcinomas
were COAD, not otherwise specified, while 85 were MPC (all data not
shown).
A comparison of the clinicopathological features of
CRC with and without MPP is summarized in Table II. There was no significant
difference between age, sex, tumour size and tumour
differentiation. Meanwhile, carcinomas with MPP were significantly
associated with a more advanced T (P<0.001 T4 vs. T1-2), N
(P<0.001 N0 vs. N1-N2) and M stage (P=0.024), and higher levels
of lymphovascular invasion (P=0.009) and higher TNM stages
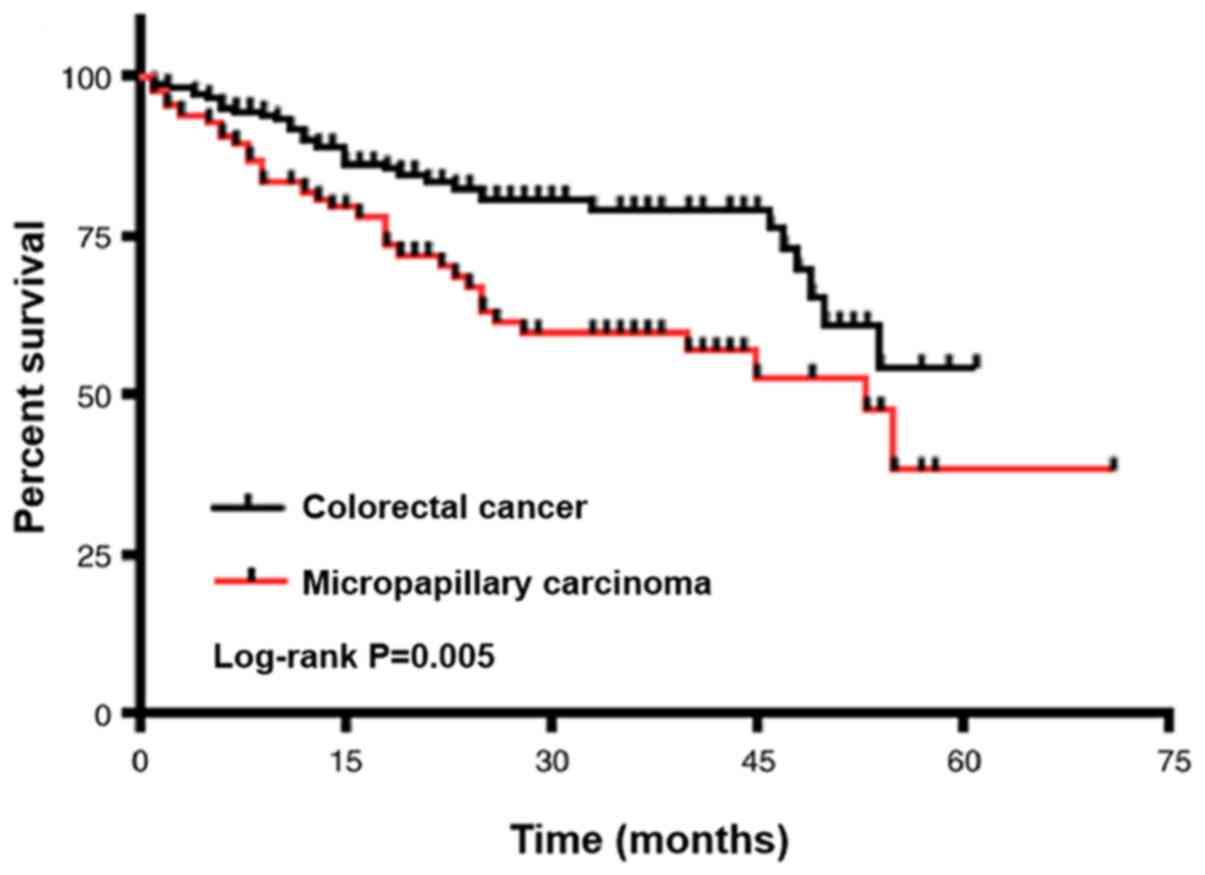
(P<0.001 I–II vs. IV). Consistent with these results,
Kaplan-Meier analysis also showed a positive association between
tumours with MPP and worse overall survival rate (P=0.005; Fig. 2). Kaplan Meier analysis showed no
significant association between depth of invasion (T stage) and
overall survival (Fig. S1A). Tumor
size was also not significantly associated with worse overall
survival (Fig. S1B). Although, Cox
regression analyses showed that CRC with MPP was not an independent
prognostic factor for the outcomes of patients, age was an
independent prognostic factor (Table
III).
 | Table II.Comparison of clinicopathological
data between 85 cases of MPC and 201 cases of non-MPC. |
Table II.
Comparison of clinicopathological
data between 85 cases of MPC and 201 cases of non-MPC.
| Clinicopathological
variables | Value, n | MPC, n (%) | Non-MPC, n (%) | χ2 | P-value |
|---|
| Age, years |
|
|
|
0.469 |
0.577 |
|
<60 | 88 | 23 (27.1) | 65
(32.3) |
|
|
|
≥60 | 198 | 62 (72.9) | 136 (67.7) |
|
|
| Sex |
|
|
|
0.807 |
0.434 |
|
Male | 162 | 53 (60.2) | 109 (54.2) |
|
|
|
Female | 124 | 32 (39.8) | 92
(45.8) |
|
|
| Tumor size, cm |
|
|
|
0.318 |
0.605 |
|
<5 | 149 | 43 (50.6) | 109 (54.2) |
|
|
| ≥5 | 137 | 42 (49.4) | 92
(45.8) |
|
|
| Tumor
differentiation |
|
|
|
0.298 |
0.862 |
|
Well | 59 | 17 (20.0) | 42
(20.9) |
|
|
|
Moderate | 217 | 66 (76.5) | 152 (75.6) |
|
|
|
Poor | 10 | 2
(3.5) | 7
(3.5) |
|
|
| T
classification |
|
|
|
4.942 |
0.031 |
|
T1-T2 | 36 | 5
(5.9) | 31
(15.4) |
|
|
|
T3-T4 | 250 | 80 (94.1) | 170 (84.6) |
|
|
| N
classification |
|
|
| 13.455 | <0.0001 |
| N0 | 116 | 20 (23.5) | 94
(46.8) |
|
|
|
N1-N2 | 170 | 65 (76.5) | 107 (53.2) |
|
|
| M
classification |
|
|
|
5.07 |
0.024 |
| M0 | 241 | 66 (77.6) | 177 (88.1) |
|
|
| M1 | 45 | 21 (22.4) | 24
(11.9) |
|
|
| Lymphovascular
invasion |
|
|
|
7.089 |
0.009 |
| No
invasion | 93 | 18 (21.2) | 75
(37.5) |
|
|
|
Invasion | 193 | 67 (78.8) | 126 (62.5) |
|
|
| Stage |
|
|
| 15.510 | <0.0001 |
|
I–II | 103 | 16 (18.8) | 87
(43.3) |
|
|
|
III–IV | 183 | 69 (81.2) | 114 (56.7) |
|
|
 | Table III.Summary of overall survival rate
analysis by univariate and multivariate Cox regression
analysis. |
Table III.
Summary of overall survival rate
analysis by univariate and multivariate Cox regression
analysis.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| Age, <60 vs. ≥60
years |
0.004 | 2.479 | 1.336–4.602 |
0.007 | 2.341 | 1.258–4.358 |
| T classification,
T1-T2 vs. T3-T4 | <0.001 | 2.144 | 1.458–3.154 |
|
|
|
| N classification,
N0 vs. N1-N2 | <0.001 | 3.535 | 1.975–6.329 |
|
|
|
| M classification,
M0 vs. M1 | <0.001 | 2.833 | 1.684–4.766 |
|
|
|
| Lymphovascular
invasion, yes vs. no |
0.002 | 2.361 | 1.358–4.104 |
|
|
|
| Stage, I–II vs.
III–IV | <0.001 | 2.303 | 1.693–3.235 | <0.001 | 2.314 | 1.663–3.222 |
| MPC vs.
non-MPC |
0.008 | 1.855 | 1.174–2.933 |
|
|
|
Bioinformatics analysis of villin
expression in CRC
I/OP staining of EMA/MUC-1 and E-cadherin has been
recognized as two hallmarks of MPCs (20–22),
especially EMA/MUC-1 (23,25). Villin is another surface-related
glycoprotein similar to EMA/MUC-1, which is also present in the
normal colonic mucosa (26).
To clarify the significance of these three
surface-related glycoproteins in CRC, the expression differences in
CRC tissues and normal tissues were analysed using bioinformatics.
Based on the GEPIA database, as shown in Fig. 3A, there was no difference between
COAD tissues and normal tissues for the transcription level of
EMA/MUC-1. The mRNA expression of E-cadherin and villin was higher
in COAD tissues compared with in normal tissues (Fig. 3B and C, respectively).
The HPA database is a repository of transcriptomics
and proteomics data generated from RNA sequencing and
immunohistochemistry analysis (28).
The present study analysed EMA/MUC-1, E-cadherin and villin
immunohistochemical results from patients with COAD using the HPA
database. In total, five different EMA/MUC-1 antibodies were
analysed and it was demonstrated that EMA/MUC-1 had the highest
positive expression rate with CAB000036, with high expression
accounting for 42%, middle expression accounting for 42% and low
expression accounting for 8%. For CAB001986, the positive
expression rate of EMA/MUC-1 was only 25% (Fig. 3D). E-cadherin had high expression in
>99% of CRC cases (Fig. 3E). In
total, three different villin antibodies were compared and the
positive expression rates were 100, 100 and 83.3%, and villin had
high expression in all cases with HPA006884 (Fig. 3F). These results showed that villin
and E-cadherin are more sensitive compared with EMA/MUC-1 in
COAD.
In addition, the subcellular locations of EMA,
E-cadherin and villin in CRCs and normal tissues were also
investigated using the HPA database (30). The results demonstrated that MUC-1
protein was expressed in the cytoplasm and on the apical surface of
glandular epithelial cells (Fig.
3G). E-cadherin was expressed on the cellular membrane, but
absent expression on the apical surface of glandular cells,
presenting as cup-like staining pattern (Fig. 3H). Similar to E-cadherin, villin had
high expression on the apical surface of glandular cells and weak
expression in the cytoplasm (Fig.
3I). Fig. S1 also demonstrates
the IHC expression patterns of EMA, E-cadherin and villin in normal
intestinal mucosa and conventional CRC, with similar results to
Fig. 3G-I.
Immunohistochemical evaluation
Among the 90 conventional CRCs, nine cases were
excluded because of the loss of tissue cores. Thus, 81 conventional
CRCs and 90 MPC cases were successfully examined using IHC. The IHC
results of EMA, E-cadherin and villin in the conventional CRCs and
MPCs are summarized in Table IV. In
the 81 conventional CRCs, EMA positive expression was detected in
43 (53.1%), E-cadherin positive expression in 76 (93.8%) and villin
positive expression in 77 (95.0%). Among the 90 MPC cases, EMA
positive expression was detected in 43 (47.8%), E-cadherin positive
expression in 84 (93.3%) and villin positive expression in 88
(97.8%). The IHC expression results (Table IV) showed that villin expression was
lost in six cases, all of which were poorly differentiated CRC. The
rates of positive expression for EMA, E-cadherin and villin were
not statistically different between conventional CRCs and MPCs.
However, the rate of positive expression of EMA was <60% in
conventional CRCs and MPCs. EMA, E-cadherin and villin in non-MPP
of CRCs generally displayed a similar profile of staining, namely
luminal staining (Fig. 4A-C).
 | Table IV.Immunohistochemical results for 81
cases of non-MMP and 90 cases of MPP. |
Table IV.
Immunohistochemical results for 81
cases of non-MMP and 90 cases of MPP.
| Protein | Non-MPP, n (%) | With MPP, n
(%) |
|---|
| Villin |
|
|
| Y | 3
(3.7) | 77 (85.6) |
| N | 74 (91.3) | 11 (12.2) |
| U | 4
(5.0) | 2
(2.2) |
| E-cadherin |
|
|
| Y | 2
(2.5) | 37 (41.1) |
| N | 74 (91.3) | 47 (52.2) |
| U | 5
(6.2) | 6
(6.7) |
| EMA |
|
|
| Y | 1
(1.2) | 26 (28.9) |
| N | 42 (51.9) | 17 (18.9) |
| U | 38 (46.9) | 47 (52.2) |
As shown in Table
IV, among the cases with positive expression for EMA, reverse
polarity of the tumour cells was detected in 26 (60.5%) MPCs
(Fig. 4D). E-cadherin was expressed
on the cellular membrane. E-cadherin expression showed that 37
(44%) MPCs had inverse tumour cell polarity (Fig. 4E).
Among most of the conventional CRCs, villin was
highly expressed on the apical surface of the glandular cells and
weakly expressed of absent in the cytoplasm. In the MPCs, there was
high villin expression on the outer borders of tumour cell clusters
and weak or absent expression in the centre of the tumour cell
clusters, displaying reversed cell polarity. According to villin
expression, 77 (87.5%) MPCs had inverse tumour cell polarity
(Fig. 4F). The results of
immunohistochemistry showed that compared with EMA and E-cadherin,
villin had an improved ability to recognize MPP.
Discussion
Cancer with MPP shows distinct histology
characterized by an eosinophilic cell cluster (6–8), a lack
of a fibrous blood vessel axis (9,10), voids
around the cell mass and cell polarity reversal (31). Pure MPC is rare and has only been
reported in the breast, pancreatic and colon cancer (6,32).
Previous research has reported that the incidence of CRC with MPP
is 9.4–27.8% (6,33,34), and
it was accompanied by aggressive histological features, including
higher levels of lymphovascular and perineural invasion, more
frequent lymph node and distant metastases and higher TNM stages
(6,18,34,35). The
present study, consistent with previous studies (3,4,6), identified 19.8% of CRCs with a MPC
component (90/453 cases). It was also reported that CRC with MPP
was significantly associated with more frequent lymph node
metastasis and higher TNM stages.
MPP should be distinguished from poorly
differentiated clusters (PDCs) and tumour budding (36). MPP generally consists of 3–20 cells
with cleft-like spaces and without fibrovascular cores at the
interface with the stroma (6–10). PDCs
are composed of no less than five cancer cells, generally located
on the invasive margin and without glandular formation (37). Tumour budding refers to single cells
or small clusters of dedifferentiated tumour cells (38). The epithelial-mesenchymal transition
can occur in aforementioned three pathological forms, leading to
decreased expression of E-cadherin in epithelial cells (37,38). The
peripheral space may be an important diagnostic clue to distinguish
MPP from PDCs. Hence, the morphological and immunohistochemical
expressions of MPP, PDCs and tumour budding are similar, so they
can be misdiagnosed. As CRC with MPP is more aggressive compared
with non-MPP CRC, pathologists need to carefully look for MPC in
colorectal biopsy materials (27,34,39).
I/OP staining of EMA was first detected in breast
cancer with micropapillary components and subsequently reported in
micropapillary carcinomas of the colorectum (40). E-cadherin is a calcium-dependent
cell-cell adhesion glycoprotein comprised of five extracellular
cadherin repeats, encoded by the gene cadherin 1 (41). E-cadherin is a classical member of
the cadherin superfamily of proteins and is often used as a useful
antibody in the diagnosis of micropapillary cancer (27). However, the expression of this
protein has not specifically analysed in colon cancer with MPP
(35).
Villin is also a member of a family of
calcium-regulated actin-binding proteins (26). This protein represents a dominant
part of the brush border cytoskeleton that functions in the
capping, severing and bundling of actin filaments (42). In normal intestinal epithelium and
colorectal cancer, villin can be seen on the apical/luminal surface
(26,42). The present bioinformatics analysis
demonstrated that EMA had the lowest sensitivity in COAD compared
with villin and E-cadherin, as reported by previous studies
(20,22,23,25). A
previous study reported that villin detection using IHC is useful
in the detection of reverse polarity of MPP (19).
The current IHC results were consistent with those
reported in the literature (19,20,22),
demonstrating that, when identifying the MPC of CRC, the
sensitivity and specificity of villin is higher compared with that
of E-cadherin and EMA. E-cadherin is one of the immunomarkers of
MPC (27,37,38), but
the current study and previous studies showed that it often had no
staining or only partial staining (35). In the present results, the I/OP
staining of EMA and E-cadherin showed poor sensitivity and was
often focal rather than the diffuse I/OP staining usually shown in
illustrations (20,23). However, villin expression was high on
the outer borders of tumour cell clusters and was weakly expressed
or absent in the centre of the tumour cell clusters, displaying
reversed cell polarity. The present study demonstrated that villin
was a useful marker of colorectal MPC and had greater sensitivity
for the detection of reverse polarity compared with EMA and
E-cadherin. Overall, the current data may help improve the clinical
diagnosis of MPC of the colorectum.
There are limitations to the present study. Western
blotting was not used to confirm the differential expression of
EMA, E-cadherin and villin in normal mucosa or in conventional CRC
and colorectal MPC. In addition, the molecular mechanisms
underlying the carcinogenesis and tumour progression of this unique
morphological pattern need to be elucidated in future research. For
example, second generation sequencing could be used to find
differentially expressed genes, screen regulatory molecules, find
relevant signalling pathways and clarify the relationship between
upstream and downstream regulation.
In summary, an MPP is not an infrequent finding in
CRC. CRC with a micropapillary component is significantly
associated with lymphovascular invasion and a higher TNM stage. MPC
is a histological prognostic factor of poor survival. Accurate
identification of micropapillary components is essential to
determine patient prognosis and to improve clinical management. The
I/OP staining with EMA and E-cadherin ranged from diffuse
circumferential through focal and partial to absent, and in most
cases, villin showed this pattern of staining more clearly compared
with EMA and E-cadherin.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article. Additional datasets generated
and/or analyzed during the current study are available in the GEPIA
(http://gepia.cancer-pku.cn/) repository
and the Human Protein Atlas (https://www.proteinatlas.org/) repository.
Authors' contributions
LZ, SYL, YML contributed to the reviewing the
literature, integrating and analyzing the literature results and
the collection of patient data. LZ, YML and ZTX designed the study
and analyzed the data. SYL performed the immunohistochemistry, and
LZ, ZTX and YML participated in the immunohistochemical scoring. LZ
contributed to the statistical analysis. ZTX supervised all the
work. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by The Ethics Committee of
The Third Affiliated Hospital (Guangzhou, China) and all patients
provided informed written consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
PDCs
|
poorly differentiated clusters
|
|
MPC
|
micropapillary carcinoma
|
|
I/OP
|
inside-out pattern
|
|
MPP
|
micropapillary pattern
|
|
EMA
|
epithelial membrane antigen
|
|
IHC
|
immunohistochemistry
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tauriello DV, Calon A, Lonardo E and
Batlle E: Determinants of metastatic competency in colorectal
cancer. Mol Oncol. 11:97–119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Remo A, Fassan M, Vanoli A, Bonetti LR,
Barresi V, Tatangelo F, Gafà R, Giordano G, Pancione M, Grillo F,
et al: Morphology and molecular features of rare colorectal
carcinoma histotypes. Cancers (Basel). 11:E10362019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo Z, Yang Z, Li D, Tang J, Xu J, Shen H
and Yuan Y: Colorectal cancer with invasive micropapillary
components (IMPCs) shows high lymph node metastasis and a poor
prognosis: A retrospective clinical study. Medicine (Baltimore).
99:e202382020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Muratsu A, Noura S, Matsumura T, Hirota M,
Yasuyama H, Koga C, Takata A, Kameda C, Murakami M, Kawabata R, et
al: A colorectal cancer with invasive micropapillary carcinoma. Gan
To Kagaku Ryoho. 44:1814–1816. 2017.(In Japanese). PubMed/NCBI
|
|
6
|
Haupt B, Ro JY, Schwartz MR and Shen SS:
Colorectal adenocarcinoma with micropapillary pattern and its
association with lymph node metastasis. Mod Pathol. 20:729–733.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Patankar M, Väyrynen S, Tuomisto A,
Mäkinen M, Eskelinen S and Karttunen TJ: Micropapillary structures
in colorectal cancer: An anoikis-resistant subpopulation.
Anticancer Res. 38:2915–2921. 2018.PubMed/NCBI
|
|
8
|
Fujita T, Konishi M, Gotohda N, Takahashi
S, Nakagohri T, Kojima M and Kinoshita T: Invasive micropapillary
carcinoma of the ampulla of Vater with extensive lymph node
metastasis: Report of a case. Surg Today. 40:1197–1200. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vardar E, Yardim BG, Vardar R and Ölmez M:
Primary gastric invasive micropapillary carcinoma: A case report.
Turk Patoloji Derg. 31:219–222. 2015.PubMed/NCBI
|
|
10
|
Ushiku T, Matsusaka K, Iwasaki Y, Tateishi
Y, Funata N, Seto Y and Fukayama M: Gastric carcinoma with invasive
micropapillary pattern and its association with lymph node
metastasis. Histopathology. 59:1081–1089. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wen P, Xu Y, Frankel WL and Shen R:
Invasive micropapillary carcinoma of the sigmoid colon: Distinct
morphology and aggressive behavior. Int J Clin Exp Pathol.
1:457–460. 2008.PubMed/NCBI
|
|
12
|
Collins K and Ricci A Jr: Micropapillary
variant of mucinous breast carcinoma: A distinct subtype. Breast J.
24:339–342. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Young RH: Ovarian tumors: A survey of
selected advances of note during the life of this journal. Hum
Pathol. 95:169–206. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shinagawa T, Hoshino H, Taga M, Sakai Y,
Imamura Y, Yokoyama O and Kobayashi M: Clinicopathological
implications to micropapillary bladder urothelial carcinoma of the
presence of sialyl Lewis X-decorated mucin 1 in stroma-facing
membranes. Urol Oncol. 35:606.e17–606.e23. 2017. View Article : Google Scholar
|
|
15
|
Guzińska-Ustymowicz K, Niewiarowska K and
Pryczynicz A: Invasive micropapillary carcinoma: A distinct type of
adenocarcinomas in the gastrointestinal tract. World J
Gastroenterol. 20:4597–4606. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Monroig-Bosque PDC, Morales-Rosado JA,
Roden AC, Churg A, Barrios R, Cagle P, Ge Y, Allen TC, Smith ML,
Larsen BT, et al: Micropapillary adenocarcinoma of lung:
Morphological criteria and diagnostic reproducibility among
pulmonary pathologists. Ann Diagn Pathol. 41:43–50. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Otsuru M, Aoki T, Kondo Y, Ota Y, Sasaki
M, Suzuki T, Ogura G and Kumaki N: Salivary duct carcinoma with
invasive micropapillary and rhabdoid feature arising in the
submandibular gland. Tokai J Exp Clin Med. 42:30–36.
2017.PubMed/NCBI
|
|
18
|
Kitagawa H, Yoshimitsu M, Kaneko M, Ibuki
Y, Emi M, Kohashi T, Mukaida H, Matsuura H, Ohge H, Ohdan H, et al:
Invasive micropapillary carcinoma component is an independent
prognosticator of poorer survival in Stage III colorectal cancer
patients. Jpn J Clin Oncol. 47:1129–1134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuroda N and Yorita K: Colon cancer with
micropapillary carcinoma component: A clinopathologic study of 9
cases. Pol J Pathol. 68:102–108. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cserni G: Reversed polarity of the
glandular epithelial cells in micropapillary carcinoma of the large
intestine and the EMA/MUC1 immunostain. Pathology.
46:527–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alsadoun N, MacGrogan G, Truntzer C,
Lacroix-Triki M, Bedgedjian I, Koeb MH, Alam EEl, Medioni D, Parent
M, Wuithier P, et al: Solid papillary carcinoma with reverse
polarity of the breast harbors specific morphologic,
immunohistochemical and molecular profile in comparison with other
benign or malignant papillary lesions of the breast: A comparative
study of 9 additional cases. Mod Pathol. 31:1367–1380. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lotan TL, Ye H, Melamed J, Wu XR, Shih IeM
and Epstein JI: Immunohistochemical panel to identify the primary
site of invasive micropapillary carcinoma. Am J Surg Pathol.
33:1037–1041. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Terada T: An immunohistochemical study of
primary signet-ring cell carcinoma of the stomach and colorectum:
II. Expression of MUC1, MUC2,
MUC5AC, and MUC6 in normal mucosa and in 42
cases. Int J Clin Exp Pathol. 6:613–621. 2013.PubMed/NCBI
|
|
24
|
Park SY, Lee HS, Choe G, Chung JH and Kim
WH: Clinicopathological characteristics, microsatellite
instability, and expression of mucin core proteins and p53 in
colorectal mucinous adenocarcinomas in relation to location.
Virchows Arch. 449:40–47. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Duncan TJ, Watson NF, AI-Attar AH,
Scholefield JH and Durrant LG: The role of MUC1 and
MUC3 in the biology and prognosis of colorectal cancer.
World J Surg Oncol. 5:312007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Y, Srinivasan K, Siddiqui MR, George
SP, Tomar A and Khurana S: A novel role for villin in intestinal
epithelial cell survival and homeostasis. J Biol Chem.
283:9454–9464. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gonzalez RS, Huh WJ, Cates JM, Washington
K, Beauchamp RD, Coffey RJ and Shi C: Micropapillary colorectal
carcinoma: Clinical, pathological and molecular properties,
including evidence of epithelial-mesenchymal transition.
Histopathology. 70:223–231. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Uhlén M, Fagerberg L, Hallström BM,
Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C,
Sjöstedt E, Asplund A, et al: Proteomics. Tissue-based map of the
human proteome. Science. 347:12604192015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wilson LA, Heraty L, Ashford BA, Coelho S,
Frangi AF, Pozo JM, Ince PG and Highley JR: Tissue microarray (TMA)
use in post mortem neuropathology. J Neurosci Methods.
347:1089632020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Uhlen M, Zhang C, Lee S, Sjöstedt E,
Fagerberg L, Bidkhori G, Benfeitas R, Arif M, Liu Z, Edfors F, et
al: A pathology atlas of the human cancer transcriptome. Science.
357:eaan25072017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eom DW, Kang GH, Han SH, Cheon GJ, Han KH,
Oh HS, Kim JH, Jang HJ and Hong SM: Gastric micropapillary
carcinoma: A distinct subtype with a significantly worse prognosis
in TNM stages I and II. Am J Surg Pathol. 35:84–91. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hattori T, Sentani K, Hattori Y, Oue N and
Yasui W: Pure invasive micropapillary carcinoma of the
esophagogastric junction with lymph nodes and liver metastasis.
Pathol Int. 66:583–586. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee HJ, Eom DW, Kang GH, Han SH, Cheon GJ,
Oh HS, Han KH, Ahn HJ, Jang HJ and Han MS: Colorectal
micropapillary carcinomas are associated with poor prognosis and
enriched in markers of stem cells. Mod Pathol. 26:1123–1131. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu F, Xu J, Lou Z, Di M, Wang F, Hu H and
Lai M: Micropapillary component in colorectal carcinoma is
associated with lymph node metastasis in T1 and
T2 stages and decreased survival time in TNM stages I
and II. Am J Surg Pathol. 33:1287–1292. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Verdú M, Román R, Calvo M, Rodón N, García
B, González M, Vidal A and Puig X: Clinicopathological and
molecular characterization of colorectal micropapillary carcinoma.
Mod Pathol. 24:729–738. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miyazaki M, Aoki M, Okado Y, Koga K,
Hamasaki M, Kiyomi F, Sakata T, Nakagawa T and Nabeshima K: Poorly
differentiated clusters predict a poor prognosis for external
auditory canal carcinoma. Head Neck Pathol. 13:198–207. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Barresi V, Branca G, Vitarelli E and
Tuccari G: Micropapillary pattern and poorly differentiated
clusters represent the same biological phenomenon in colorectal
cancer: A proposal for a change in terminology. Am J Clin Pathol.
142:375–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zlobec I and Lugli A: Epithelial
mesenchymal transition and tumor budding in aggressive colorectal
cancer: Tumor budding as oncotarget. Oncotarget. 1:651–661. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lino-Silva LS, Salcedo-Hernández RA and
Caro-Sánchez CH: Colonic micropapillary carcinoma, a recently
recognized subtype associated with histological adverse factors:
Clinicopathological analysis of 15 cases. Colorectal Dis.
14:e567–e572. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang YL, Liu BB, Zhang X and Fu L:
Invasive micropapillary carcinoma of the breast: An update. Arch
Pathol Lab Med. 140:799–805. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dalle Vedove A, Falchi F, Donini S, Dobric
A, Germain S, Di Martino GP, Prosdocimi T, Vettraino C, Torretta A,
Cavalli A, et al: Structure-based virtual screening allows the
identification of efficient modulators of e-cadherin-mediated
cell-cell adhesion. Int J Mol Sci. 20:E34042019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Revenu C, Courtois M, Michelot A, Sykes C,
Louvard D and Robine S: Villin severing activity enhances
actin-based motility in vivo. Mol Biol Cell. 18:827–838. 2007.
View Article : Google Scholar : PubMed/NCBI
|