Introduction
Bladder cancer (BLCA) is the fourth most common
malignancy in males and it has high incidence and mortality rates
despite improvements in its management over the past decade
(1,2). Non-muscle invasive bladder cancer is
the most common type of BLCA because its low progression rates lead
to longer patient survival times (3). However, 25–30% of bladder tumors are
found to be muscle invasive at the time of diagnosis and these
patients often have a poor prognosis despite optimal treatment
(4,5). The prognosis of BLCA is determined by
the initial tumor stage, but patients do not show specific symptoms
in the early stages of BLCA. Therefore, identifying promising early
molecular markers has an enormous application potential for
monitoring the onset of malignant tumors and improving the clinical
strategies for managing BLCA.
P4HB, also known as protein disulfide-isomerase; is
a multifunctional protein that catalyzes the formation, breakage
and rearrangement of disulfide bonds. It acts as a molecular
chaperone that aids in ameliorating misfolded proteins in response
to ER stress (6). In addition,
upregulation of P4HB has been reported in certain cancer types and
overexpression of P4HB may promote the progression of malignant
tumors, including gastric cancer, clear cell renal cell carcinoma
and colon cancer (7–9). P4HB inhibition has been associated with
chemosensitivity in glioblastoma multiforme via the endoplasmic
reticulum stress response pathways (10,11). In
addition, P4HB-knockdown sensitized glioblastoma to radiotherapy by
leading to ER stress and downregulating RAD51 gene expression
(12). However, little is known
regarding the association between P4HB and BLCA. The present study
therefore analyzed the prognostic value of P4HB and the association
between clinicopathological characteristics and P4HB in BLCA.
Materials and methods
Tissue samples
A total of 69 BLCA tissues and adjacent tissues were
obtained from patients (age range, 37–91 years; mean age, 64.1
years) with BLCA at the Department of Urology, Peking University
First Hospital who underwent radical cystectomy between January
2007 and November 2012. Patients who were lost to follow-up and had
missing data were excluded. The database included a population of
44 males and 25 females and the pathological diagnosis of these
patients was transitional cell carcinoma. The histological
characteristics of these samples were evaluated by
hematoxylin-eosin staining and confirmed by experienced urological
pathologists. Fresh samples were fixed with 4% paraformaldehyde for
12–24 h at room temperature and then paraffin-embedded for
immunohistochemistry (IHC). The present study was approved by the
Biomedical Research Ethics Committee of Peking University First
Hospital and written informed consent was obtained from all
patients.
Cell culture
The cell lines (SV-HUC-1, T24, SW780 and 5637) were
obtained from the American Type Culture Collection and cultured
according to the manufacturer's protocols. The normal human urinary
tract epithelial SV-HUC-1 cell line was cultured in F-12K medium
(Gibco; Thermo Fisher Scientific, Inc.), while the T24 and SW780
cell lines were cultured in Dulbecco's modified Eagle's medium
(Gibco; Thermo Fisher Scientific, Inc.) and the 5637 cells were
cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.). All
media contained 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin G-streptomycin (Sigma-Aldrich;
Merck KGaA). The cell cultures were maintained as a monolayer
culture in a humidified atmosphere containing 5% CO2 at
37°C.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissue samples or cell
lines using TRIzol reagent (Invitrogen; Thermo Fisher Scientific
Inc.) and used according to the manufacturer's protocol. cDNA was
generated using reverse transcription with the following
conditions: 42°C for 15 min and 95°C for 3 min (TansGen). RT-qPCR
was performed using the 7500 Fluorescent Quantitative PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.), and GAPDH was
used for normalization. The primer sequences used in the present
study were as follows: P4HB forward, 5′-GGCTATCCCACC-ATCAAGTTC-3′
and reverse, 5′-TCACGATGTCATCAGCCTCTC-3′; GAPDH forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTC-ATACTTCTCATGG-3′. The PCR reaction was performed as
follows: 94°C for 30 sec, then 40 cycles at 94°C for 5 sec and at
60°C for 30 sec. All experiments were repeated at least three
times. The relative expression level was calculated by using
2−ΔΔCq method (13).
IHC
The 5-µm sections were cut from paraffin-embedded
tissue samples, deparaffinized in xylene and rehydrated in a
descending alcohol series. Next, the sections were heated (120°C)
for 20 min in citrate buffer (10 mmol/l; pH 6.0). Following
treatment with 3% hydrogen peroxide to block the endogenous
peroxidase activity, 10% normal goat serum was applied to reduce
non-specific binding for 1 h at room temperature. Subsequently, the
sections were incubated with primary rabbit anti-human P4HB
polyclonal antibody (1:10,000; cat. no. ab137110; Abcam) at 4°C
overnight. The sections were then incubated with
peroxidase-conjugated secondary antibodies (1:1,000; PV-9000;
OriGene Technologies, Inc.) for 20 min at room temperature. All
slides were washed three times and then the PowerVision™ two-step
histostaining reagent and the 3,3-diaminobenzidine
tetrahydrochloride substrate kit (Zhongshan Golden Bridge
Biotechnology) were used to visualize the localization of the
antigen according to the manufacturer's protocol. Two experienced
independent pathologists examined all tumor slides by examining
three random fields of view using a light microscope at ×400
magnification (Olympus Corporation). The intensity of cellular
staining was assigned a score of 0 (negative), 1 (weak), 2
(moderate) and 3 (strong). The proportion of stained tumor cells
was scored as 0 (0–5%), 1 (6–25%), 2 (26–50%), 3 (51–75%) or 4
(>75%). The multiplication of these two variables was calculated
as the final score.
Western blotting
Total proteins were extracted from cell lines using
NP-40 lysis buffer, quantified using BCA protein assay reagent
(Pierce; Thermo Fisher Scientific, Inc.), and loaded onto 10% SDS
gels (20 µg/lane). Proteins in the gel were transferred onto PVDF
membranes (EMD Millipore) following electrophoresis. Following
blocking at room temperature for 1 h with 5% skimmed milk, the
membranes were incubated overnight at 4°C with an antibody against
P4HB (1:1,000; cat. no. ab137110; Abcam), followed by horseradish
peroxidase-labeled anti-rabbit secondary antibody (1:5,000; cat.
no. sc-2004/sc-2005; Santa Cruz Biotechnology, Inc.). β-actin
(1:1,000; cat. no. sc-8432; Santa Cruz Biotechnology, Inc.) and
GAPDH (1:1,000; cat. no. sc-47724; Santa Cruz Biotechnology, Inc.)
were used as the internal reference. Signals were detected by
chemiluminescence using the ECL Western Blotting Detection Reagents
(Beyotime Institute of Biotechnology).
In silico analysis of P4HB using
online datasets
Transcriptome and clinical data from the TCGA-BLCA
datasets (including 410 patients with bladder carcinoma) were
downloaded from UCSC XENA (https://xena.ucsc.edu/) (14). Affymetrix gene expression profiles
were performed using Affymetrix Human Genome U133 Plus 2.0 (HG-U133
Plus_2.0) for two datasets (GSE42089 and GSE31189) and using
Affymetrix Human Genome U133A Array (HG-U133A) for one dataset
(GSE3167). The prognostic analyses were performed by gene
expression profiling interactive analysis (GEPIA; http://gepia.cancer-pku.cn/) (15). To investigate the mechanism of P4HB
in BLCA, the co-expression genes of P4HB were obtained using the
limma R package (http://bioinf.wehi.edu.au/limma). The Pearson
correlation coefficients between P4HB and protein-coding genes were
calculated to determine the co-expression correlations (|Pearson
correlation coefficient|>0.40 and P<0.01). Gene Ontology (GO)
analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG)
analysis were performed and visualized using the R package
‘clusterProfiler’ (16). The PPI
network was constructed by Search Tool for the Retrieval of
Interacting Genes (STRING; http://string-db.org/) (17) and modeled using Cytoscape software
(Cytoscape_v3.6.1) (18). Gene set
enrichment analysis (GSEA) was conducted to assess whether a
predefined set of genes exhibited statistically significant,
concordant differences according to P4HB expression by GSEA
software (version 4.0.1; downloaded from http://software.broadinstitute.org/gsea/index.jsp)
(19). The high expression group and
the low expression group were defined according to the median mRNA
level of P4HB. |Normalized enrichment score (NES)|>1, false
discovery rate (FDR) <25% and nominal P<0.05 were regarded as
the cut-off criteria.
Statistical analysis
All experimental data were obtained from three
independent experiments. SPSS version 24.0 (IBM Corp.) was used for
statistical analyses. The difference between two sets of data was
analyzed by Student's t-test or Mann-Whitney U test. One-way
analysis of variance, followed by Dunnett's post hoc test, was
applied to investigate the differences in P4HB expression between
the normal cell line (SV-HUC-1) and the BLCA cell lines. Survival
analysis was performed using the Kaplan-Meier estimator (log-rank
test). Cox regression was conducted for univariate and multivariate
analyses. The receiver operating characteristic curve (ROC) was
plotted to evaluate the value of P4HB expression as a predictor for
early detection of BLCA. P<0.05 was considered statistically
significant.
Results
P4HB is upregulated in BLCA and
associated with tumor stage
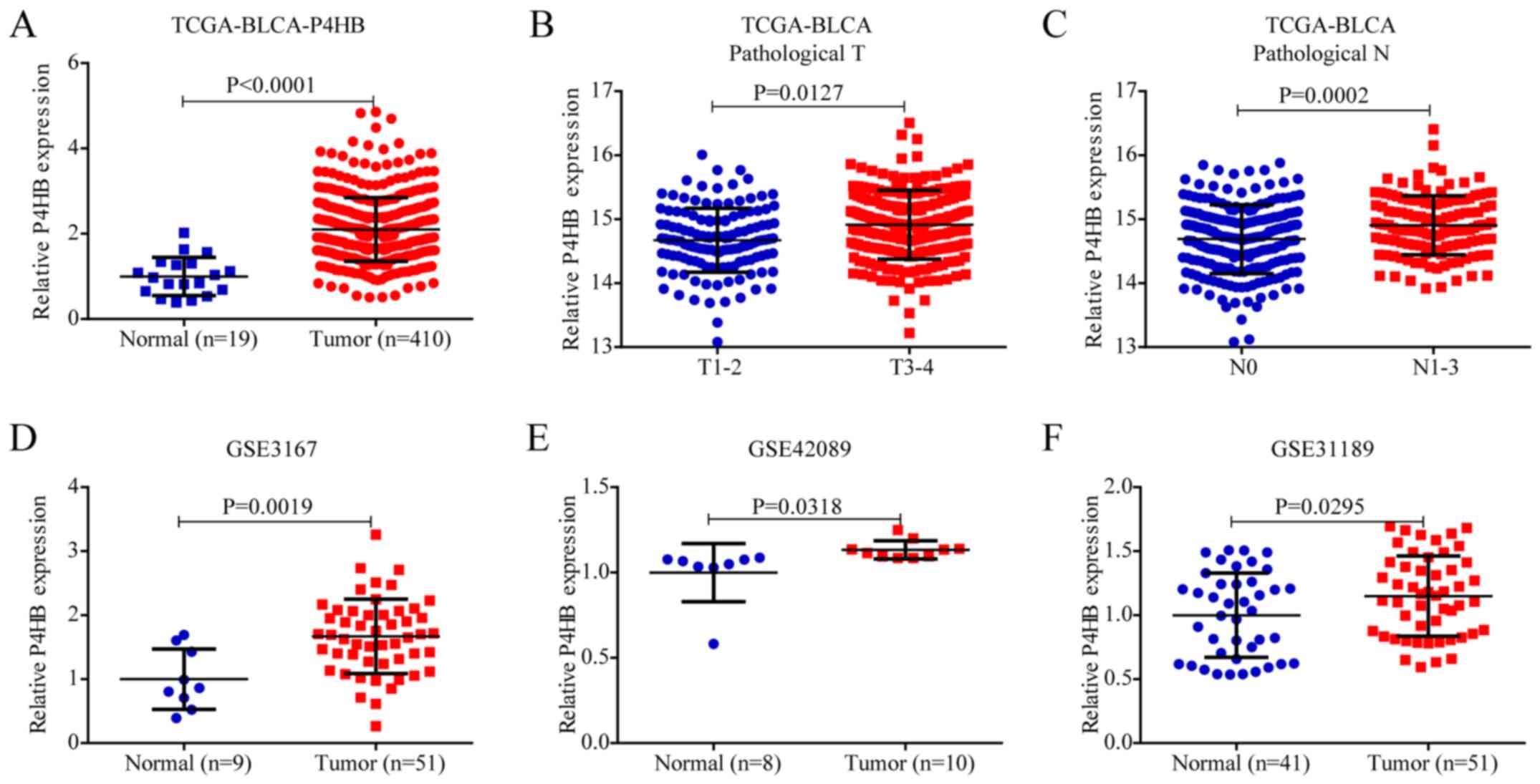
To investigate the expression of P4HB in BLCA, the
mRNA expression of P4HB was analyzed in TCGA database. As shown in
Fig. 1A, P4HB expression was higher
in BLCA tissues than in normal tissues in TCGA (P<0.0001).
Notably, the mRNA expression of P4HB was significantly higher at
advanced pathological T stage (P<0.05) (Fig. 1B) and pathological N stage
(P<0.001) (Fig. 1C). These data
suggested that P4HB may be a promising biomarker for diagnosis and
early detection in BLCA. The mRNA expression of P4HB was also
verified in the GEO database, and analysis of the results of the
GSE3167, GSE42089 and GSE31189 microarray datasets demonstrated
that P4HB was highly expressed in tumor tissues compared with
normal tissues (Fig. 1D-F).
P4HB is highly expressed in bladder
cancer cell lines and tissues
RT-qPCR was performed to investigate the mRNA
expression of P4HB in BLCA cell lines and tissues. The results
demonstrated that the mRNA expression level of P4HB was higher in
tumor cell lines than in SV-HUC-1 cells (Fig. 2A). The mRNA expression of P4HB was
increased in BLCA tissues, compared with paired normal mucosa. The
average log2-fold-change of T/N was 1.67 (Fig. 2B). High mRNA expression of P4HB was
associated with a high pathological T stage (P<0.05; Fig. 2C) and N stage (P<0.05; Fig. 2D). In addition, protein expression of
P4HB was upregulated in BLCA cell lines (Fig. 2E) and tissues (Fig. 2F). To better understand the
expression of P4HB in BLCA tissues, an IHC assay was performed to
investigate P4HB protein expression levels in 69 paired BLCA
tissues and their adjacent normal tissues. The results demonstrated
that P4HB was highly expressed in BLCA tissues compared with paired
normal mucosa (Fig. 2G). The average
IHC score of T/N was 2.43 (Fig.
2H).
P4HB is an early detection biomarker
for BLCA
Considering the differential expression of P4HB
between BLCA and normal mucosa tissues, ROC curves were generated
to determine the diagnostic value of P4HB in TCGA database and our
database. The ROC curve suggested that the AUC value for P4HB
expression to discriminate between tumor and normal was up to 0.888
(Fig. 3A; 95% CI: 0.801–0.975;
P<0.001) and 0.881 (Fig. 3B; 95%
CI: 0.825–0.937; P<0.001) in TCGA and our database,
respectively. In addition, the average IHC score of P4HB expression
was significantly higher in advanced-stage and high-grade BLCA than
in early-stage (P<0.01; Fig. 3C)
and low-grade BLCA (P<0.05; Fig.
3D). Taken together, these results suggest that P4HB may be an
early detection biomarker for BLCA.
High P4HB expression predicts overall
survival of patients with BLCA
A prospective study was performed to investigate the
correlation between the OS time of BLCA and P4HB expression. In
addition to the tumor pathological N stage, P4HB expression was
negatively correlated with OS in univariate analysis (95% CI:
1.011–6.296; P=0.047) in our database (Table I). Higher expression of P4HB
exhibited a poorer OS based on Kaplan-Meier survival analysis
(P<0.001; Fig. 4A). In
multivariate analysis, N stage (95% CI: 1.552–23.254; P=0.009) and
P4HB protein expression (95% CI: 1.240–8.798; P=0.017) were still
correlated with a poorer OS, indicating that P4HB may be an
independent prognostic factor for patients with BLCA. Furthermore,
the impact of clinicopathological parameters on patient survival in
TCGA database was investigated. The mRNA expression of P4HB
remained an independent prognostic factor (Table SI), and high mRNA expression of P4HB
was associated with a short OS time (P<0.01; Fig. 4B). Considering that the pathological
stage has a significant impact on patient survival, a nomogram
containing IHC score, pathological T stage and N stage was
constructed to predict the 5-year survival rate of patients with
BLCA (Fig. 4C). The calibration
curves for 5-year survival demonstrated that the predicted OS was
close to the actual OS (Fig.
4D).
 | Table I.Univariate analysis and multivariate
analysis of overall survival of bladder cancer in our database. |
Table I.
Univariate analysis and multivariate
analysis of overall survival of bladder cancer in our database.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Characteristic | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| P4HB level, high
vs. low | 2.523
(1.011–6.296) | 0.047a | 3.303
(1.240–8.798) | 0.017a |
| Sex, male vs.
female | 0.855
(0.198–3.697) | 0.834 |
|
|
| Age at initial
diagnosis, ≥70 vs. <70 years | 0.829
(0.247–2.785) | 0.762 |
|
|
| Pathological T, T3
+ T4 vs. T1 + T2 | 2.438
(0.991–5.999) | 0.052 |
|
|
| Pathological N,
N1-3 vs. N0 | 3.802
(1.084–13.338) | 0.037a | 6.008
(1.552–23.254) | 0.009a |
| Histological grade,
high vs. low | 1.958
(0.884–4.333) | 0.098 |
|
|
High P4HB expression predicted
recurrence-free survival in patients with BLCA
The correlation between the RFS of BLCA and P4HB
expression was further investigated. The results of IHC
demonstrated that high pathological N stage and P4HB protein levels
were significantly associated with a poorer RFS by univariate and
multivariate analyses (Table II). A
similar result was obtained in TCGA database (Table SII). To determine whether P4HB was a
promising target for predicting RFS, Kaplan-Meier survival analysis
was conducted in our database and TCGA database. The results
demonstrated that high expression of P4HB was associated with low
RFS (Fig. 4E and F).
 | Table II.Univariate analysis and multivariate
analysis of recurrence-free survival of bladder cancer in our
database. |
Table II.
Univariate analysis and multivariate
analysis of recurrence-free survival of bladder cancer in our
database.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Characteristic | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| P4HB level, high
vs. low | 2.265
(1.001–5.696) | 0.042a | 2.936
(1.104–7.805) | 0.031a |
| Sex, male vs.
female | 0.932
(0.216–4.031) | 0.925 |
|
|
| Age at initial
diagnosis, ≥70 vs. <70 years | 0.735
(0.218–2.479) | 0.620 |
|
|
| Pathological T, T3
+ T4 vs. T1 + T2 | 2.187
(0.883–5.417) | 0.091 |
|
|
| Pathological N,
N1-3 vs. N0 | 4.199
(1.181–14.922) | 0.027a | 6.318
(1.629–24.504) | 0.008a |
| Histological grade,
high vs. low | 2.007
(0.907–4.438) | 0.085 |
|
|
Functional prediction of P4HB in BLCA
by enrichment analysis
To investigate the biological function of P4HB in
BLCA, GO term enrichment analysis and KEGG pathway analysis were
performed using the co-expression genes of P4HB. A total of 233
genes co-expressed with P4HB were selected from TCGA-BLCA database
(Table SIII). Terms related to
transferase activity, ribonucleoprotein complex binding, signal
sequence binding and relevant member terms in ER were enriched
(Fig. 5A and Table SIV). These terms were associated
with carbohydrate metabolism and protein metabolism. Genes involved
in protein processing in the ER, N-glycan biosynthesis, various
types of N-glycan biosynthesis and other relevant pathways were
also enriched (Fig. 5B and Table SV). In addition, GSEA in BLCA was
performed by ranking the genes based on the expression of P4HB.
High P4HB expression was associated with several significant
processes that promote tumorigenesis, including N-glycan
biosynthesis, pathways in cancer and primary immunodeficiency
(Fig. 5C). These results indicated
that P4HB may promote bladder tumorigenesis. PPI analysis revealed
that the potential targets of P4HB were mainly genes associated
with posttranslational protein modification and response to ER
stress (Fig. 5D and Table III). Taken together, these results
implied that P4HB may promote tumorigenesis by interfering with
protein processing in the ER, including N-glycan modification and
protein metabolic processes responding to ER stress.
 | Table III.Functional enrichments in the
protein-protein interactions network of prolyl 4-hydroxylase, beta
polypeptide. |
Table III.
Functional enrichments in the
protein-protein interactions network of prolyl 4-hydroxylase, beta
polypeptide.
| Term
description | FDR | Matching proteins
in the network (labels) |
|---|
| Post-translational
protein modification | 1.52E-09 | ALB, APOB, CALU,
DNAJC3, HSP90B1, NUCB1, P4HB, PDIA6, PRKCSH, RCN1 |
| Response to
endoplasmic reticulum stress | 1.03E-08 | CALR, DNAJC3,
ERO1L, HSP90B1, HSPA5, OS9, P4HB, PDIA6 |
Discussion
P4HB, encoded by the P4HB gene on human chromosome
17q25, is the β-subunit of prolyl-4-hydroxylase involved in
antioxidant and detoxification reactions (20). P4HB may catalyze the oxidation,
reduction and isomerization of disulfide bonds in the ER, which is
essential to maintain homeostasis of the ER (20,21).
Furthermore, P4HB acts as a chaperone and binds to misfolded
proteins to prevent their excessive aggregation (22). The ER is an important organelle in
eukaryotic cells that participates in a variety of intracellular
metabolic and signal transduction pathways. Once ER homeostasis is
disturbed, misfolded proteins accumulate, eventually leading to ER
stress. The ER has formed self-protecting signal transduction
pathways during evolution, including the unfolded protein response
(UPR) of the ER, ER-associated degradation and autophagy to restore
proteostasis. Furthermore, the ability to tolerate persistent ER
stress enhances cancer cell survival, angiogenesis, aggressiveness,
drug resistance and immunosuppression (23,24).
As a potential therapeutic target, P4HB has been
found to be a novel diagnostic and prognostic marker in various
cancer types, including colon, gastric and clear cell renal cell
carcinoma (7,8,25). Lovat
et al (26) demonstrated that
overexpression of wild-type P4HB in melanoma cells decreased the
level of apoptosis in response to ER stress, which was beneficial
to the survival of tumor cells. Furthermore, recent studies have
demonstrated that P4HB is overexpressed in gliomas and that P4HB
suppression sensitizes glioblastoma to chemotherapy and
radiotherapy by abrogating ER stress-induced UPR signaling
(10,12,27).
These results suggested the clinical importance of P4HB in
tumorigenesis and progression.
The present study investigated and confirmed the
expression and clinical significance of P4HB in BLCA by analyses in
TCGA, GEO and our clinical database. The results demonstrated that
P4HB expression was increased in BLCA tissues and cells. The
expression of P4HB was significantly correlated with tumor stage.
Furthermore, based on immunohistochemical staining analysis, it was
revealed that the expression of P4HB was an independent risk factor
for OS and RFS. Furthermore, high expression of P4HB was associated
with low OS and RFS rates by Kaplan-Meier survival analysis in TCGA
database and our database. These data indicated that P4HB may be a
promising biomarker for predicting survival and tumor progression
in patients with BLCA.
To better understand the oncogenic role of P4HB in
BLCA, functional prediction was performed using bioinformatics
analysis. As indicated by the enrichment analyses, P4HB may be
involved in protein processes responding to ER stress and N-glycan
biosynthesis. It was reported that N-glycans of certain proteins
have a vital role in metastatic process, including
epithelial-mesenchymal transition (EMT), migration,
invasion/intravasation and extravasation of tumor cells (28). Inhibiting N-glycans markedly
decreased receptor tyrosine kinase signaling in cancer cells and
blocking N-glycan precursor biosynthesis may be a novel target for
cancer therapy (29,30). The target genes predicted by PPI
analysis were mainly involved in posttranslational protein
modification and protein processing in response to ER stress. The
results of GSEA suggested that P4HB may enhance the development of
BLCA by multiple significant processes, including N-glycan
biosynthesis, pathways in cancer and primary immunodeficiency.
Although the expression profile of P4HB in BLCA has
been identified in the present study, the oncogenic functions of
P4HB in BLCA need to be clarified in vitro and in
vivo. Furthermore, the molecular mechanisms by which P4HB
functions need to be demonstrated in subsequent studies.
In summary, the results of the present study
demonstrated that P4HB may be a promising biomarker as it was
overexpressed in BLCA and high expression of P4HB was associated
with a poor prognosis. Bioinformatics analysis revealed that P4HB
may be involved in N-glycan biosynthesis and ER stress responses to
promote BLCA tumorigenesis and progression. The detailed mechanisms
of P4HB in regulating BLCA and the potential association between
P4HB, N-glycans and ER stress require further investigation.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by The National Key
R&D Program of China (grant no. 2019YFA09006001) and The
National Natural Science Foundation of China (grant nos. 81772703
and 81972380).
Availability of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request. Publicly available datasets can be found in TCGA
(https://portal.gdc.cancer.gov/) and GEO
(https://www.ncbi.nlm.nih.gov/geo/)
databases.
Author contributions
YW and YP designed the study, analyzed and
interpreted the data, and drafted and critically revised the
manuscript; AH, KY and BG acquired the data and performed the
statistical analysis; SH directed the research and performed the
statistcal analysis; YG, XL and LZ interpreted the data and
critically revised the manuscript for important intellectual
content, provided administrative support, obtained funding and
supervised the study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Biomedical
Research Ethics Committee of Peking University First Hospital
(Beijing, China), and written informed consent was obtained from
all patients.
Patient consent for publication
Written consent for publication was obtained from
all participants.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BLCA
|
bladder cancer
|
|
P4HB
|
prolyl 4-hydroxylase, beta
polypeptide
|
|
TCGA
|
The Cancer Genome Atlas
|
|
GEO
|
Gene Expression Omnibus
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
IHC
|
immunohistochemistry
|
|
OS
|
overall survival
|
|
RFS
|
recurrence-free survival
|
|
ER
|
endoplasmic reticulum
|
|
GEPIA
|
gene expression profiling interactive
analysis
|
|
GO
|
gene ontology
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
GSEA
|
gene set enrichment analysis
|
|
PPI
|
protein-protein interaction
|
|
NES
|
normalized enrichment score
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Antoni S, Ferlay J, Soerjomataram I, Znaor
A, Jemal A and Bray F: Bladder cancer incidence and mortality: A
global overview and recent trends. Eur Urol. 71:96–108. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Babjuk M, Burger M, Compérat EM, Gontero
P, Mostafid AH, Palou J, van Rhijn BW, Rouprêt M, Shariat SF,
Sylvester R, et al: European association of urology guidelines on
non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ) -
2019 Update. Eur Urol. 76:639–657. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burger M, Catto JW, Dalbagni G, Grossman
HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C,
Shariat S, et al: Epidemiology and risk factors of urothelial
bladder cancer. Eur Urol. 63:234–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alfred Witjes J, Lebret T, Compérat EM,
Cowan NC, De Santis M, Bruins HM, Hernández V, Espinós EL, Dunn J,
Rouanne M, et al: Updated 2016 EAU guidelines on muscle-invasive
and metastatic bladder cancer. Eur Urol. 71:462–475. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Parakh S and Atkin JD: Novel roles for
protein disulphide isomerase in disease states: A double edged
sword? Front Cell Dev Biol. 3:302015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang J, Guo S, Wu Y, Zheng ZC, Wang Y and
Zhao Y: P4HB, a novel hypoxia target gene related to gastric cancer
invasion and metastasis. BioMed Res Int.
2019:97497512019.PubMed/NCBI
|
|
8
|
Zhou Y, Yang J, Zhang Q, Xu Q, Lu L, Wang
J and Xia W: P4HB knockdown induces human HT29 colon cancer cell
apoptosis through the generation of reactive oxygen species and
inactivation of STAT3 signaling. Mol Med Rep. 19:231–237.
2019.PubMed/NCBI
|
|
9
|
Zhu Z, He A, Lv T, Xu C, Lin L and Lin J:
Overexpression of P4HB is correlated with poor prognosis in human
clear cell renal cell carcinoma. Cancer Biomark. 26:431–439. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun S, Lee D, Ho AS, Pu JK, Zhang XQ, Lee
NP, Day PJ, Lui WM, Fung CF and Leung GK: Inhibition of prolyl
4-hydroxylase, beta polypeptide (P4HB) attenuates temozolomide
resistance in malignant glioma via the endoplasmic reticulum stress
response (ERSR) pathways. Neuro Oncol. 15:562–577. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee D, Sun S, Zhang XQ, Zhang PD, Ho AS,
Kiang KM, Fung CF, Lui WM and Leung GK: MicroRNA-210 and
endoplasmic reticulum chaperones in the regulation of
chemoresistance in glioblastoma. J Cancer. 6:227–232. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Y, Ji W, Shergalis A, Xu J, Delaney
AM, Calcaterra A, Pal A, Ljungman M, Neamati N and Rehemtulla A:
Activation of the unfolded protein response via inhibition of
protein disulfide isomerase decreases the capacity for DNA repair
to sensitize glioblastoma to radiotherapy. Cancer Res.
79:2923–2932. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goldman MJ, Craft B, Hastie M, Repečka K,
McDade F, Kamath A, Banerjee A, Luo Y, Rogers D, Brooks AN, et al:
Visualizing and interpreting cancer genomics data via the Xena
platform. Nat Biotechnol. 38:675–678. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu G, Wang LG, Han Y and He QY:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43:D447–D452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Subramanian A, Kuehn H, Gould J, Tamayo P
and Mesirov JP: GSEA-P: A desktop application for Gene Set
Enrichment Analysis. Bioinformatics. 23:3251–3253. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hatahet F and Ruddock LW: Substrate
recognition by the protein disulfide isomerases. FEBS J.
274:5223–5234. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Freedman RB, Hirst TR and Tuite MF:
Protein disulphide isomerase: Building bridges in protein folding.
Trends Biochem Sci. 19:331–336. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wilson R, Lees JF and Bulleid NJ: Protein
disulfide isomerase acts as a molecular chaperone during the
assembly of procollagen. J Biol Chem. 273:9637–9643. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moon HW, Han HG and Jeon YJ: Protein
quality control in the endoplasmic reticulum and cancer. Int J Mol
Sci. 19:30202018. View Article : Google Scholar
|
|
24
|
Cubillos-Ruiz JR, Bettigole SE and
Glimcher LH: Tumorigenic and immunosuppressive effects of
endoplasmic reticulum stress in cancer. Cell. 168:692–706. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xie L, Li H, Zhang L, Ma X, Dang Y, Guo J,
Liu J, Ge L, Nan F, Dong H, et al: Autophagy-related gene P4HB: A
novel diagnosis and prognosis marker for kidney renal clear cell
carcinoma. Aging (Albany NY). 12:1828–1842. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lovat PE, Corazzari M, Armstrong JL,
Martin S, Pagliarini V, Hill D, Brown AM, Piacentini M,
Birch-Machin MA and Redfern CP: Increasing melanoma cell death
using inhibitors of protein disulfide isomerases to abrogate
survival responses to endoplasmic reticulum stress. Cancer Res.
68:5363–5369. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peng Z, Chen Y, Cao H, Zou H, Wan X, Zeng
W, Liu Y, Hu J, Zhang N, Xia Z, et al: Protein disulfide isomerases
are promising targets for predicting the survival and tumor
progression in glioma patients. Aging (Albany NY). 12:2347–2372.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oliveira-Ferrer L, Legler K and
Milde-Langosch K: Role of protein glycosylation in cancer
metastasis. Semin Cancer Biol. 44:141–152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Contessa JN, Bhojani MS, Freeze HH, Ross
BD, Rehemtulla A and Lawrence TS: Molecular imaging of N-linked
glycosylation suggests glycan biosynthesis is a novel target for
cancer therapy. Clin Cancer Res. 16:3205–3214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Contessa JN, Bhojani MS, Freeze HH,
Rehemtulla A and Lawrence TS: Inhibition of N-linked glycosylation
disrupts receptor tyrosine kinase signaling in tumor cells. Cancer
Res. 68:3803–3809. 2008. View Article : Google Scholar : PubMed/NCBI
|