Introduction
Colorectal cancer (CRC) was the third most common
cancer among men and women in 2020 South Korea, and it is one of
the deadliest types of cancer worldwide (1,2). In the
United States, there are 147,950 new cases and 53,200 deaths
annually associated with CRC (3).
The development of CRC is very complex and is associated with
genetic and epigenetic alterations (4). In addition, the causes of CRC can be
heterogenic, and there are multiple underlying molecular pathways,
such as the suppressor pathway, the serrated pathway and the Lynch
syndrome (5). These various
molecular pathways result in difficulties in CRC treatment;
therefore it is important identify effective biomarkers and
efficient methods to predict the prognosis of CRC.
Methylation results in the silencing of cancer
suppressor or base repair genes and occurs through the binding of
methylated complexes (6). Oxidation
of 5-methylcytosine (5-mC) into 5-hydroxymethylcytosine (5-hmC), as
well as 5-hmC-induced 5-formylcytosine (5-fC) and
5-carboxylcytosine (5-caC) are epigenetic modifications (7,8). 5-mC
can be actively removed by oxidative demethylation by the
ten-eleven translocation enzyme family (TET1, TET2, and TET3) or by
passive demethylation through replication (9). 5-hmC is a hallmark of DNA
demethylation, and its levels have been reported to be
downregulated in various types of cancer, including colon cancer
(10,11). The loss of 5-hmC is caused by the
inhibition of TET enzyme activity along with an increase of the
onco-metabolite 2-hydroxyglutarate due to a mutation in the
isocitrate dehydrogenases (IDH1/2) and by a TET mutation, reducing
the TET stability (10).
AMP-activated protein kinase (AMPK) is a member of
the serine/threonine kinase family and forms a heterotrimeric
complex with one catalytic subunit (α1 and α2), and two regulatory
β (β1 or β2) and γ (γ1, γ2 or γ3) subunits (12). AMPK is a direct intracellular sensor
that responds to ATP depletion and restores energy homeostasis by
inhibiting ATP-consuming fatty acid and cholesterol synthesis and
promoting the generation of ATP (13). The activation of AMPK serves an
important role in the survival of tumor cells under stressful
conditions such as energy stress, hypoxia and hypoglycemia, which
are common in the tumor microenvironment (13,14). In
addition, previous studies have demonstrated that the metabolic
state regulates the epigenome directly through the AMPK-dependent
phosphorylation of the epigenetic modifying enzyme (14,15). Wu
et al (15) have reported
that AMPK increases the activity of TET2 and stabilizes it by
phosphorylation in normal compared with high glucose conditions,
which converts 5-mC bases to 5-hmC, thus altering the
epigenome.
However, it is still unclear how AMPK/TET2 protein
expression is affected in patients with CRC. The aim of the present
study was to investigate the association between AMPK/TET2
expression levels and other clinicopathological factors in patients
with CRC and to investigate its role as a prognostic factor.
Materials and methods
Patients and samples
Between January 2010 and December 2014, 360 patients
who were diagnosed with CRC underwent surgical resection at the
Department of Surgery, Soonchunhyang University Cheonan Hospital
(Cheonan, South Korea) were included in the present study. Only
patients with stage I–IV CRC were included. The specimens used in
the study were fixed in 10% formalin for 24–48 h at room
temperature, embedded in paraffin and stored at the Department of
Pathology. The exclusion criteria were as follows: i) Patients who
underwent preoperative chemotherapy and radiotherapy; ii) those who
died within 30 days of the surgery; iii) those under 18 years old;
iv) those with Lynch syndrome or familial adenomatous polyposis.
Subsequently, a total of 343 patients were enrolled in the present
study. The patient clinicopathological data were retrospectively
collected through medical records. For the survival analysis, the
patients' medical records were assessed, or they were contacted by
a direct telephone call; the duration of disease-free survival
(DFS) was analyzed by imaging (CT or MRI) and an endoscopic
follow-up. The median follow-up time for all patients was 2.4 years
(range, 0–7.5 years); 17 patients were lost to the follow-up. Tumor
stage was defined according to the Tumor-Node-Metastasis (TNM)
classification of the American Joint Committee on International
Union against Cancer 7th Edition (16). This study was approved by the
Institutional Review Board of the Soonchunhyang University Cheonan
Hospital (approval no. SCHCA 2019-08-018).
Immunohistochemical staining of TET2
and AMPK
Immunohistochemical staining of the CRC tissues was
performed using a tissue microarray (TMA) block of 343 patient
tissues. The tissue core punched by a 2-mm puncher (Unitech Korea
Co., Ltd.) was embedded in a recipient paraffin block (Unitech
Korea Co., Ltd.). The TMA blocks were cut into 4-µm sections,
dewaxed in xylene and rehydrated through a gradient concentration
of ethanol (100, 95, 90, 80 and 70%) for 5 min per concentration.
Subsequently, the blocks were incubated in 0.01 M citrate buffer
(pH 6.0) at 95°C for 30 min in a microwave. Endogenous peroxidase
activity was inactivated using 0.3% H2O2 for
1 h at room temperature. The sections were subsequently incubated
with antibodies against TET2 (1:100; cat. no. ab245287; Abcam) and
AMPK (1:100; cat. no. GTX52341; GeneTex, Inc.) overnight at 4°C,
followed by incubation with an anti-rabbit EnVision secondary
antibody (cat. no. K4002; Dako; Agilent Technologies, Inc.) for 1 h
at 37°C. For visualization, the sections were treated with µl
3,3′-diaminobenzidine solution (Dako; Agilent Technologies, Inc.)
and counterstained with Harris' hematoxylin (EMD Millipore). The
sections were mounted using Canada Balsam (Sigma-Aldrich; Merck
KGaA).
Semiquantitative analysis of AMPK and
TET2
Protein expression was analyzed by two independent
groups of researchers who were blinded to patient clinical data;
they reached consensus scores for each specimen by evaluating the
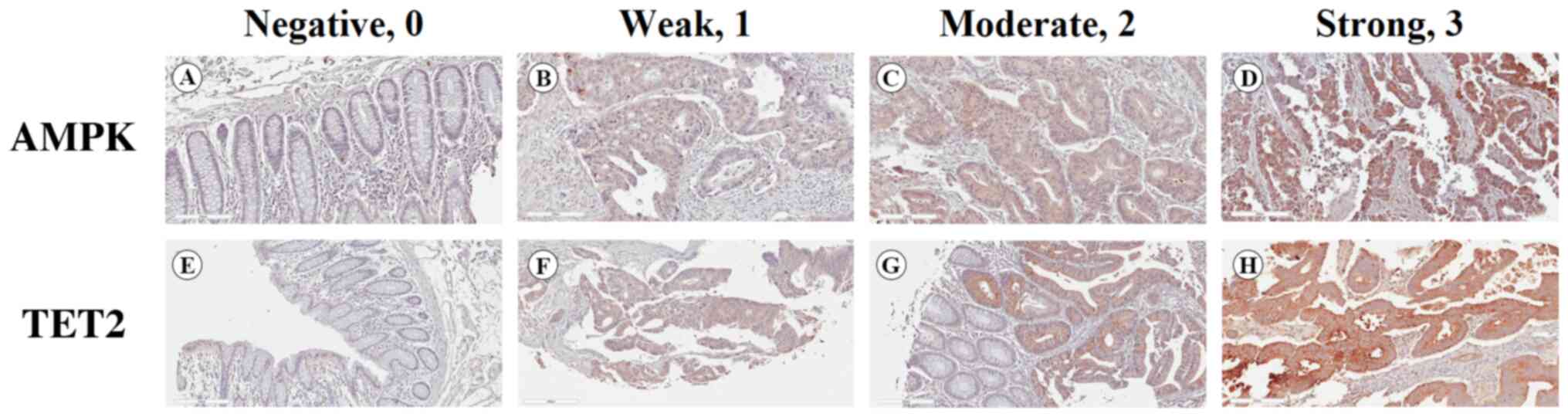
percentage of positive cells and the intensity of staining. The
proportion of stained cells was classified on a scale of 0 to 3 as
follows: 0, 0%; 1, 1–33%; 2, 34–66%; and 3, 67–100%. The staining
intensity was classified into four grades: 0, negative; 1, weak; 2,
moderate; and 3, strong (Fig. 1).
The two scores were multiplied to obtain final protein expression
scores, which were classified as follows: 0, negative; 1–3, weak;
4–6, moderate; and 7–9, strong. ‘Negative’ and ‘weak’ denoted low
expression, whereas ‘moderate’ and ‘strong’ denoted high
expression.
Statistical analysis
Statistical analyses were performed using PASW
Statistics v.18.0 (SPSS, Inc.). The χ2 or Fisher's exact
test were used to analyze the associations between categorical
clinicopathological variables and the expression levels of AMPK and
TET2. Phi correlation analysis was used to determine the
relationship between AMPK and TET2 expression levels. Survival
curves for overall survival (OS) and DFS rates were calculated
using the Kaplan-Meier method and compared by the log-rank test,
and the Bonferroni correction was used to adjust for multiple
comparisons. Univariate and multivariate analyses of patient
prognosis were performed using Cox proportional hazards modeling.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Baseline clinicopathological data
The baseline clinicopathological characteristics of
the patients are presented in Table
I. Among them, 145 were female and 198 were male. The median
age was 64.2 (range, 29–89) years, male patients (57.7%) were more
common than female patients, and patients with diabetes comprised
19.8% of the study cohort. The predominant primary tumor location
was on the left side (66.2%). The percentages of patients at each
pathological stage were 16.6% at stage I, 44.3% at stage II, 32.9%
at stage III and 6.1% at stage IV. In addition, there were more
patients without lymph node metastasis compared with those with
lymph node metastasis, and 7% of patients presented with distant
metastasis.
 | Table I.Clinicopathological characteristics
of patients with colorectal cancer. |
Table I.
Clinicopathological characteristics
of patients with colorectal cancer.
|
Characteristics | Frequency, n
(%) |
|---|
| Total | 343 (100%) |
| Age, mean (range)
years | 64
(29–89) |
| Sex |
|
|
Male | 198 (57.7%) |
|
Female | 145 (42.3%) |
| Diabetes
mellitus |
|
|
Yes | 68
(19.8%) |
| No | 275 (80.2%) |
| pT stage |
|
| T1 | 26
(7.6%) |
| T2 | 45
(13.1%) |
| T3 | 216 (63.0%) |
| T4 | 56
(16.3%) |
| pN stage |
|
| N0 | 218 (63.5%) |
| N1 | 81
(23.6%) |
| N2 | 44
(12.9%) |
| pM stage |
|
| M0 | 322 (93.8%) |
| M1 | 21
(6.2%) |
| Tumor location |
|
|
Right | 116 (33.8%) |
|
Left | 227 (66.2%) |
| Tumor size |
|
| <5
cm | 226 (65.9%) |
| ≥5
cm | 117 (34.1%) |
| Vascular
invasion |
|
|
Yes | 54
(15.7%) |
| No | 289 (84.3%) |
| Lymphatic
invasion |
|
|
Yes | 96
(28.0%) |
| No | 247 (72.0%) |
| Perineural
invasion |
|
|
Yes | 116 (33.8%) |
| No | 227 (66.2%) |
|
Differentiation |
|
|
Well/moderately
differentiated | 322 (93.9%) |
| Poorly
differentiated | 21
(6.1%) |
| AMPK levels |
|
|
High | 89 (25.9%) |
|
Low | 254 (74.1%) |
| TET2 levels |
|
|
High | 93
(27.1%) |
|
Low | 250 (72.9%) |
Expression of AMPK and TET2 in CRC
tissue
Expression of AMPK and TET2 was assessed in tissues
from 343 patients with CRC by immunohistochemical staining. AMPK
and TET2 were stained in the cytoplasm and membrane of tumor cells
and confirmed by microscopy. According to the semiquantitative
analysis, the percentages of patients with high expression levels
of AMPK and TET2 were 25.9 and 27.1%, respectively (Table I).
Associations between the expression
levels of AMPK and TET2 and the clinicopathological characteristics
of patients with CRC
Age, sex, diabetes mellitus status, fasting glucose
and glycated hemoglobin (HbA1c) levels, tumor size, tumor location,
vascular, lymphatic and perineural invasion, pTNM status, tumor
differentiation and overall stage were included to evaluate the
clinical relevance of AMPK and TET2 expression levels. Patients
with high expression levels of AMPK more frequently presented with
lymphatic invasion (P<0.001), lymph node metastasis
(P<0.001), distant metastasis (P=0.019) and an advanced stage
(P<0.001) compared with those in the low AMPK expression group.
However, distant metastasis was more common among patients with low
expression levels of TET2 compared with those in the high TET2
expression group (P=0.017) (Table
II). No associations were observed between the expression
levels of TET2 and the following clinicopathological variables:
Age, sex, diabetes mellitus status, fasting glucose or HbA1c
levels, tumor size, location, vascular or lymphatic invasion and
overall stage.
 | Table II.Associations between AMPK and TET2
levels and the clinicopathological factors of patients with
colorectal cancer. |
Table II.
Associations between AMPK and TET2
levels and the clinicopathological factors of patients with
colorectal cancer.
|
|
| AMPK levels, n
(%) | TET2 levels, n
(%) |
|---|
|
|
|
|
|
|---|
|
Characteristics | n (%) | Low (%) | High (%) | P-value | Low (%) | High (%) | P-value |
|---|
| Total | 343 (100) | 254 (74.1) | 89 (25.9) |
| 250 (72.9) | 93 (27.1) |
|
| Age, years |
|
|
| 0.729 |
|
| 0.459 |
|
<60 | 122 (35.6) | 89
(35.0) | 33 (37.1) |
| 86
(34.4) | 36 (38.7) |
|
|
≥60 | 221 (64.4) | 165 (65.0) | 56 (62.9) |
| 164 (65.6) | 57 (61.3) |
|
| Sex |
|
|
| 0.180 |
|
| 0.746 |
|
Male | 198 (57.7) | 152 (59.8) | 46 (51.7) |
| 143 (57.2) | 55 (59.1) |
|
|
Female | 145 (42.3) | 102 (40.2) | 43 (48.3) |
| 107 (42.8) | 38 (40.9) |
|
| Diabetes
mellitus |
|
|
| 0.611 |
|
| 0.894 |
|
Yes | 68
(19.8) | 52
(20.5) | 16 (18.0) |
| 200 (80.0) | 75 (80.6) |
|
| No | 275 (80.2) | 202 (79.5) | 73 (82.0) |
| 50
(20.0) | 18 (19.4) |
|
| Fasting glucose
level, mg/dl |
|
|
| 0.406 |
|
| 0.713 |
|
>126 | 114 (32.7) | 84
(33.1) | 30 (33.7) |
| 82
(32.8) | 32 (34.4) |
|
|
100-125 | 117 (34.1) | 82
(32.3) | 35 (39.3) |
| 85
(34.0) | 32 (34.4) |
|
|
<100 | 112 (33.2) | 88
(34.6) | 24 (27.0) |
| 83
(33.2) | 29 (31.2) |
|
| Glycated
hemoglobin, % |
|
|
| 0.524 |
|
| 0.175 |
|
<6.5 | 126 (68.9) | 87
(67.4) | 39 (72.2) |
| 100 (71.4) | 26 (60.5) |
|
|
≥6.5 | 57
(31.1) | 42
(32.6) | 15 (27.8) |
| 40
(28.6) | 17 (39.5) |
|
| Tumor size, cm |
|
|
| 0.257 |
|
| 0.340 |
|
<5 | 226 (65.9) | 163 (64.2) | 63 (70.8) |
| 161 (64.4) | 65 (69.9) |
|
| ≥5 | 117 (34.1) | 91
(35.8) | 26 (29.2) |
| 89
(35.6) | 28 (30.1) |
|
| Tumor location |
|
|
| 0.815 |
|
| 0.154 |
|
Right | 116 (33.8) | 85
(33.5) | 31 (34.8) |
| 79
(31.6) | 37 (39.8) |
|
|
Left | 227 (66.2) | 169 (66.5) | 58 (65.2) |
| 171 (68.4) | 56 (60.2) |
|
| Vascular
invasion |
|
|
| 0.043 |
|
| 0.378 |
|
Yes | 54
(15.7) | 34
(13.4) | 20 (22.5) |
| 42
(16.8) | 12 (12.9) |
|
| No | 289 (84.3) | 220 (86.6) | 69 (77.5) |
| 208 (83.2) | 81 (87.1) |
|
| Lymphatic
invasion |
|
|
| <0.001 |
|
| 0.994 |
|
Yes | 96
(28.0) | 56
(22.0) | 40 (44.9) |
| 70
(28.0) | 67 (72.0) |
|
| No | 247 (72.0) | 198 (78.0) | 49 (55.1) |
| 180 (72.0) | 26 (28.0) |
|
| Perineural
invasion |
|
|
| 0.310 |
|
| 0.375 |
|
Yes | 116 (33.8) | 82
(32.3) | 34 (38.2) |
| 88
(35.2) | 28 (30.1) |
|
| No | 227 (66.2) | 172 (67.7) | 55 (61.8) |
| 162 (64.8) | 65 (69.9) |
|
| pT stage |
|
|
| 0.766 |
|
| 0.155 |
| T1 | 26
(7.6) | 20
(7.9) | 6
(6.7) |
| 22
(8.8) | 4
(4.3) |
|
| T2 | 45
(13.1) | 32
(12.6) | 13 (14.6) |
| 29
(11.6) | 16 (17.2) |
|
| T3 | 216 (63.0) | 163 (64.2) | 53 (59.6) |
| 162 (64.8) | 54 (58.1) |
|
| T4 | 56
(16.3) | 39
(15.4) | 17 (19.1) |
| 37
(14.8) | 19 (20.4) |
|
| pN stage |
|
|
| <0.001 |
|
| 0.978 |
| N0 | 218 (63.6) | 178 (70.1) | 40 (44.9) |
| 159 (63.6) | 59 (63.4) |
|
| N1 +
N2 | 125 (36.4) | 76
(29.9) | 49 (55.1) |
| 91
(36.4) | 34 (36.6) |
|
| Distant
metastasis |
|
|
| 0.019 |
|
| 0.017 |
|
Absent | 322 (93.9) | 243 (95.7) | 79 (88.8) |
| 230 (92.0) | 92 (98.9) |
|
|
Present | 21
(6.1) | 11
(4.3) | 10 (11.2) |
| 20
(8.0) | 1
(1.1) |
|
|
Differentiation |
|
|
| 0.457 |
|
| 0.877 |
|
Well/moderately
differentiated | 322 (93.9) | 237 (93.3) | 85 (95.5) |
| 235 (94.0) | 87 (93.5) |
|
| Poorly
differentiated | 21
(6.1) | 17
(6.7) | 4
(4.5) |
| 15
(6.0) | 6
(6.5) |
|
| Overall stage |
|
|
| <0.001 |
|
| 0.615 |
|
I–II | 199 (58.0) | 164 (64.6) | 35 (39.3) |
| 143 (57.2) | 56 (60.2) |
|
|
III–IV | 144 (42.0) | 90
(35.4) | 54 (60.7) |
| 107 (42.8) | 37 (39.8) |
|
Association between the expression
levels of AMPK and TET
The expression levels of AMPK and TET2 were both low
in 194 (56.5%) cases, and both were high in 33 (9.6%) cases. In
addition, high expression levels of TET2 were more frequently
observed in patients with high AMPK expression levels (P=0.014;
Table III). There was also a
statistically significant correlation between AMPK and TET2
expression levels (P=0.014).
 | Table III.Correlation between the expression
levels of AMPK and TET2. |
Table III.
Correlation between the expression
levels of AMPK and TET2.
|
|
| TET2, n (%) |
|---|
|
|
|
|
|---|
| Protein
markers | n, (%) | Low (%) | High (%) | P-value |
|---|
| AMPK, n (%) |
|
|
| 0.014 a |
|
Low | 254 (74.1%) | 194 (77.6) | 60 (64.5) |
|
|
High | 89
(25.9%) | 56
(22.4) | 33 (35.5) |
|
| Phi
coefficient | 0.341 |
|
| 0.014b |
Univariate and multivariate survival
analysis
The Cox proportional hazard model was used to
determine whether the independent factors affected the OS and DFS
rates in patients with CRC. Regarding the DFS rates, vascular
invasion (HR, 2.020; 95% CI, 1.241-3.288; P=0.005), lymphatic
invasion (HR, 2.075; 95% CI, 1.364-3.156; P=0.001), perineural
invasion (HR, 2.723; 95% CI, 1.807-4.105; P<0.001), AJCC stage
(HR, 4.588; 95% CI, 2.923-7.200; P<0.001), high expression
levels of TET2 (HR, 0.582; 95% CI, 0.348-0.975; P=0.040) and AMPK
(HR, 1.586; 95% CI, 1.029-2.444; P=0.037) were significant
prognostic factors according to the results of the univariate
analysis. The multivariate analysis demonstrated that perineural
invasion (HR, 1.812; 95% CI, 1.153-2.847; P=0.010) and AJCC stage
(HR, 4.515; 95% CI, 2.706-7.532; P<0.001) were independent
prognostic factors associated with DFS. High expression levels of
TET2 (HR, 0.568; 95% CI, 0.339-0.952; P=0.032) was identified to be
an independent predictor of a favorable prognosis; however, the
expression levels of AMPK (HR, 1.359; 95% CI, 0.865-2.137; P=0.183)
were not significantly associated with DFS in the multivariate
analysis (Table IV).
 | Table IV.Univariate and multivariate analysis
using Cox's proportional hazard regression model for disease-free
survival. |
Table IV.
Univariate and multivariate analysis
using Cox's proportional hazard regression model for disease-free
survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Clinicopathological
parameters | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (<60 vs. ≥60
years) | 1.332
(0.861-2.060) | 0.198 |
|
|
| Sex (male vs.
female) | 1.178
(0.782-1.775) | 0.433 |
|
|
| DM (yes vs.
no) | 0.794
(0.457-1.381) | 0.415 |
|
|
| Tumor location
(right vs. left) | 0.976
(0.634-1.504) | 0.914 |
|
|
| Vascular invasion
(yes vs. no) | 2.020
(1.241-3.288) | 0.005 | 1.629
(0.951-2.791) | 0.076 |
| Lymphatic invasion
(yes vs. no) | 2.075
(1.364-3.156) | 0.001 | 0.643
(0.386-1.071) | 0.090 |
| Perineural invasion
(yes vs. no) | 2.723
(1.807-4.105) | <0.001 | 1.812
(1.153-2.847) | 0.010 |
| AJCC stage (I, II
vs. III, IV) | 4.588
(2.923-7.200) | <0.001 | 4.515
(2.706-7.532) | <0.001 |
| TET2 (high vs.
low) | 0.582
(0.348-0.975) | 0.040 | 0.568
(0.339-0.952) | 0.032 |
| AMPK (high vs.
low) | 1.586
(1.029-2.444) | 0.037 | 1.359
(0.865-2.137) | 0.183 |
| AMPK high/TET2 low
vs. AMPK low/TET2 high | 3.569
(1.579-8.066) | 0.002 | 2.840
(1.241-6.498) | 0.013 |
In the OS rate univariate analysis, vascular (HR,
2.467; 95% CI, 1.416-4.299; P=0.001), lymphatic (HR, 2.976; 95% CI,
1.822-4.862; P<0.001) and perineural (HR, 2.869; 95% CI,
1.754-4.692; P<0.001) invasion, AJCC stage (HR, 7.681; 95% CI,
4.097-14.401; P<0.001), and high expression levels of TET2 (HR,
0.369; 95% CI, 0.182-0.748; P=0.006) and AMPK (HR, 1.761; 95% CI,
1.061-2.921; P=0.028) were significant prognostic factors. By
contrast, the results of the multivariate analysis demonstrated
that the expression levels of TET2 (HR, 0.369; 95% CI, 0.182-0.748;
P=0.006) were an independent predictor of a favorable prognosis;
however, the expression levels of AMPK were not significantly
associated with OS (Table V).
 | Table V.Univariate and multivariate analysis
using Cox's proportional hazard regression model for overall
survival. |
Table V.
Univariate and multivariate analysis
using Cox's proportional hazard regression model for overall
survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Clinicopathological
parameters | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (<60 vs. ≥60
years) | 1.422
(0.843-2.397) | 0.187 |
|
|
| Sex (male vs.
female) | 1.332
(0.816-2.175) | 1.332 |
|
|
| Diabetes mellitus
(yes vs. no) | 0.945
(0.504-1.771) | 0.860 |
|
|
| Tumor location
(right vs. left) | 0.853
(0.512-1.421) | 0.540 |
|
|
| Vascular invasion
(yes vs. no) | 2.467
(1.416-4.299) | 0.001 | 2.029
(1.156-3.562) | 0.014 |
| Lymphatic invasion
(yes vs. no) | 2.976
(1.822-4.862) | <0.001 | 0.849
(0.476-1.515) | 0.580 |
| Perineural invasion
(yes vs. no) | 2.869
(1.754-4.692) | <0.001 | 1.496
(0.863-2.594) | 0.151 |
| AJCC stage (I, II
vs. III, IV) | 7.681
(4.097-14.401) | <0.001 | 7.211
(3.811-13.641) | <0.001 |
| TET2 (high vs.
low) | 0.369
(0.182-0.748) | 0.006 | 0.369
(0.182-0.748) | 0.006 |
| AMPK (high vs.
low) | 1.761
(1.061-2.921) | 0.028 | 1.288
(0.763-2.176) | 0.344 |
| AMPK high/TET2 low
vs. AMPK low/TET2 high | 5.449
(2.043-14.533) | 0.001 | 3.304
(1.232-8.861) | 0.018 |
The results of Kaplan-Meier analysis with the
log-rank test demonstrated that the 5-year DFS rate of patients
with high expression levels of TET2 was significantly higher
compared with that of patients with low TET2 levels (P=0.037).
However, patients with high expression levels of AMPK exhibited
lower survival rates compared with those in the low AMPK expression
group (P=0.035; Fig. 2). The 5-year
OS rate of patients with high expression levels of TET2 was higher
compared with that of patients in the low TET2 expression group
(P=0.004). By contrast, high expression levels of AMPK were
significantly associated with a lower survival rates compared with
low expression levels of AMPK (P=0.026; Fig. 3). The associations between the
combined expression of AMPK and TET2 and the clinical outcome,
including OS and tumor recurrence, were also investigated. The
samples were divided into groups based on the expression levels of
both AMPK and TET2. Kaplan-Meier curve analysis results
demonstrated that the DFS and OS prognosis was poorer in the AMPK
high/TET2 low expression group compared with that in the AMPK
low/TET2 high group (P=0.017 and P<0.001, respectively, with
Bonferroni correction; Fig. 4). The
results of the multivariate analysis revealed that the AMPK
high/TET2 low expression pattern was an independent prognostic
factor for DFS and OS (P=0.013 and P=0.018, respectively; Tables IV and V).
Discussion
The present study investigated the clinical
relevance of AMPK and TET2 expression levels in a large cohort of
patients with CRC. The results of the present study demonstrated
that high expression levels of TET2, but not AMPK, predicted a
favorable prognosis for patients with CRC, despite the significant
correlation between AMPK and TET2 expression levels. The first
group to establish a relationship between cellular metabolism and
tumorigenesis was Warburg et al (17); their study demonstrated continued
aerobic glycolysis of tumor cells despite oxygen-rich conditions.
AMPK is an enzyme associated with cancer and cell metabolism
(18,19). A number of studies, including those
in CRC, have studied the prognostic roles of AMPK; in the majority
of cases, the expression levels of AMPK have been associated with a
favorable prognosis (20–22). Based on preclinical data, these
results indicate that AMPK generally serves a role as a tumor
suppressor, regulates cell proliferation and induces apoptosis by
upregulating p53 (23). In addition,
AMPK-dependent activation of the tuberous sclerosis complex
inhibits the mTOR pathway, further inhibiting cell proliferation
and protein synthesis (12). By
contrast, in the present study, the expression levels of AMPK were
associated with lymphatic invasion and an advanced cancer stage,
indicating a poor prognosis. These results suggested that AMPK may
serve different roles depending on the tumor microenvironment.
In an in vivo study, Jang et al
(24) reported that the development
of glioma was associated with high levels of AMPK activation in the
early tumor microenvironment. In addition, AMPK has been reported
to serve a role in inducing tumor cell proliferation, and the
deletion of oncogenic RAS and PTEN has been demonstrated to
activate AMPK (18). The activation
of AMPK has also been observed in non-stressful conditions,
typically by hormones (25).
Hormones such as leptin and interleukin-6 are involved in the
progress and development of certain solid tumors, such as prostate,
colorectal and breast (26). In
hypoxic tumor cells, inducible AMPK may also serve a pro-survival
role analogous to that proposed for hypoxia-inducible factors
during tumorigenesis (27). In
clinical studies, tumor grade, prognosis and their association with
high expression levels of AMPK or phosphorylated AMPK have been
reported in various solid tumors, including colon, breast, ovarian,
prostate and cervical cancer (19,21,28). In
addition, AMPK has been demonstrated to be involved in the invasion
and migration of cancer cells through various other signaling
pathways, and novel kinase family 1, which are members of the AMPK
family, serve an important role in cell migration and invasion by
regulating the AKT/NF-κB signaling pathway (29,30).
A recent study has demonstrated that AMPK is a key
nutrient or energy sensor with high sensitivity for blood glucose
levels, and that TET2 expression is upregulated by AMPK (15). In addition, AMPK serves an important
role in protecting the stability of TET2 by phosphorylating TET2
S99 in high glucose conditions (15). In the present study, no statistical
associations were identified between the expression levels of AMPK
or TET2 and diabetes mellitus or high glucose levels. However,
those results were obtained as single data points measured during
the study; therefore, to overcome these limitations, further
studies are required to measure blood glucose levels at various
time points.
The results of the present study were consistent
with those of previous studies suggesting that low expression
levels of TET2 indicate a poor prognosis and demonstrating that
methylation serves an important role in CRC (31,32). The
inactivation of TET2 has been reported in 15% of hematopoietic
malignancies as well as CRC; in addition, IDH1/2 gene mutations
have also been identified in glioma, chondrosarcoma and thyroid
carcinoma (33–35). DNA methylation occurs throughout the
genome and is constantly maintained during the replication process
(36). Although DNA methylation is a
physiological process, a decrease in the levels of 5-hmC can affect
tumorigenesis (10). Various
mechanisms exist to protect CpG island promoters from DNA
methylation, such as the binding of TET1 and the exclusion of de
novo DNA methyltransferases by trimethylation of histone H3 at
lysine 4 (37,38). In addition, CpG islands and promoters
inhibit ectopic DNA methylation by the polycomb-associated F-box
and leucine-rich repeat protein 10 (39). Therefore, DNA methylation is
protected through various defensive mechanisms in addition to TET2,
which is why it is necessary to conduct further studies on AMPK, as
well as TET2.
The results of the present study demonstrated that
although a significant correlation existed between the expression
levels of AMPK and TET2, they were associated with conflicting
prognoses. This result may suggest that TET2 increases in response
to methylation as CRC progresses, and that the role of AMPK
increases during tumor cell migration and invasion according to the
metabolic needs of the tumor cells.
There were several limitations to the present study,
including its retrospective nature and selection biases. In
addition, inconsistent results may have been observed due to a lack
of an established methodology for evaluating the expression of AMPK
and TET2. Also, clinical in vivo and in vitro studies
such as functional tests and prospective studies are needed to
evaluate the AMPK/TET/5-hmC axis further. Although the association
between diabetes mellitus and AMPK/TET2 expression, which was one
of the hypotheses of the present study, was not determined, the
results demonstrated that the levels of AMPK/TET2 expression may be
a powerful prognostic predictor of diabetes-associated CRC.
However, it is difficult to determine the relevance of AMPK/TET2
expression levels in diabetes mellitus based solely on the results
of the present study.
In conclusion, the results of the present study
demonstrated that TET2 expression was an independent factor for
recurrence and survival of patients with CRC, and was a more
significant predictor of prognosis compared with AMPK. In addition,
the prognostic value of AMPK and TET2 levels combined was greater
compared that of the expression levels of each protein alone.
Further analyses are warranted to fully establish AMPK/TET2/5-hmC
as a predictive biomarker or a therapeutic target for CRC.
Acknowledgements
The authors would like to thank Mr. Tae Wan Kim and
Dr Son Myung Won at Soonchunhyang University Cheonan Hospital for
their help. The abstract of the present study was presented at the
39th Congress of the European Society of Surgical Oncology, Oct
9–11 (29), 2019 in Rotterdam, The
Netherlands, and published as abstract no. 477 in the European
Journal of Surgical Oncology 46.2 (2020): e171.
Funding
This research was supported by the Soonchunhyang
University Research Fund, the Korea Health Technology R&D
Project through the Korea Health Industry Development Institute and
the Ministry of Health & Welfare, Republic of Korea (grant no.
HI17C0031).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DHK developed the project, collected and analyzed
the data, and wrote the manuscript DJJ and TSA analyzed the data
and edited the manuscript. HYL collected the data. HaJK and SBB
collected the data and edited the manuscript. HyJK performed the
tissue experiments. HYK designed the study and analyzed the data.
MSL designed the study, performed statistical analysis and edited
the manuscript. MJB designed the study and revised the manuscript.
DHK, TSA and MJB confirm the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All participants provided written informed consent
and agreed to scientific use of their data. The Institutional
Review Board of the Soonchunhyang University Cheonan Hospital
approved the present study (approval no. SCHCA 2019-08-018;
Cheonan, South Korea).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jung KW, Won YJ, Hong S, Kong HJ and Lee
ES: Prediction of cancer incidence and mortality in Korea, 2020.
Cancer Res Treat. 52:351–358. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ogino S and Goel A: Molecular
classification and correlates in colorectal cancer. J Mol Diagn.
10:13–27. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li D: Recent advances in colorectal cancer
screening. Chronic Dis Transl Med. 4:139–147. 2018.PubMed/NCBI
|
|
6
|
Di Croce L, Raker VA, Corsaro M, Fazi F,
Fanelli M, Faretta M, Fuks F, Lo Coco F, Kouzarides T, Nervi C, et
al: Methyltransferase recruitment and DNA hypermethylation of
target promoters by an oncogenic transcription factor. Science.
295:1079–1082. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ko M, An J and Rao A: DNA methylation and
hydroxymethylation in hematologic differentiation and
transformation. Curr Opin Cell Biol. 37:91–101. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jones PA: Functions of DNA methylation:
Islands, start sites, gene bodies and beyond. Nat Rev Genet.
13:484–492. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tahiliani M, Koh KP, Shen Y, Pastor WA,
Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L and
Rao A: Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in
mammalian DNA by MLL partner TET1. Science. 324:930–935. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin SG, Jiang Y, Qiu R, Rauch TA, Wang Y,
Schackert G, Krex D, Lu Q and Pfeifer GP: 5-Hydroxymethylcytosine
is strongly depleted in human cancers but its levels do not
correlate with IDH1 mutations. Cancer Res. 71:7360–7365. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Haffner MC, Chaux A, Meeker AK, Esopi DM,
Gerber J, Pellakuru LG, Toubaji A, Argani P, Iacobuzio-Donahue C,
Nelson WG, et al: Global 5-hydroxymethylcytosine content is
significantly reduced in tissue stem/progenitor cell compartments
and in human cancers. Oncotarget. 2:627–637. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang W and Guan KL: AMP-activated protein
kinase and cancer. Acta Physiol (Oxf). 196:55–63. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hardie DG: AMP-activated/SNF1 protein
kinases: Conserved guardians of cellular energy. Nat Rev Mol Cell
Biol. 8:774–785. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marin TL, Gongol B, Zhang F, Martin M,
Johnson DA, Xiao H, Wang Y, Subramaniam S, Chien S and Shyy JY:
AMPK promotes mitochondrial biogenesis and function by
phosphorylating the epigenetic factors DNMT1, RBBP7, and HAT1. Sci
Signal. 10:eaaf74782017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu D, Hu D, Chen H, Shi G, Fetahu IS, Wu
F, Rabidou K, Fang R, Tan L, Xu S, et al: Glucose-regulated
phosphorylation of TET2 by AMPK reveals a pathway linking diabetes
to cancer. Nature. 559:637–641. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Warburg O, Wind F and Negelein E: The
metabolism of tumors in the body. J Gen Physiol. 8:519–530. 1927.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ríos M, Foretz M, Viollet B, Prieto A,
Fraga M, Costoya JA and Señarís R: AMPK activation by oncogenesis
is required to maintain cancer cell proliferation in astrocytic
tumors. Cancer Res. 73:2628–2638. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park HU, Suy S, Danner M, Dailey V, Zhang
Y, Li H, Hyduke DR, Collins BT, Gagnon G, Kallakury B, et al:
AMP-activated protein kinase promotes human prostate cancer cell
growth and survival. Mol Cancer Ther. 8:733–741. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baba Y, Nosho K, Shima K, Meyerhardt JA,
Chan AT, Engelman JA, Cantley LC, Loda M, Giovannucci E, Fuchs CS
and Ogino S: Prognostic significance of AMP-activated protein
kinase expression and modifying effect of MAPK3/1 in colorectal
cancer. Br J Cancer. 103:1025–1033. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Buckendahl AC, Budczies J, Fiehn O,
Darb-Esfahani S, Kind T, Noske A, Weichert W, Sehouli J, Braicu E,
Dietel M and Denkert C: Prognostic impact of AMP-activated protein
kinase expression in ovarian carcinoma: Correlation of protein
expression and GC/TOF-MS-based metabolomics. Oncol Rep.
25:1005–1012. 2011.PubMed/NCBI
|
|
22
|
Tsavachidou-Fenner D, Tannir N, Tamboli P,
Liu W, Petillo D, The B, Mills GB and Jonasch E: Gene and protein
expression markers of response to combined antiangiogenic and
epidermal growth factor targeted therapy in renal cell carcinoma.
Ann Oncol. 21:1599–1606. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Okoshi R, Ozaki T, Yamamoto H, Ando K,
Koida N, Ono S, Koda T, Kamijo T, Nakagawara A and Kizaki H:
Activation of AMP-activated protein kinase induces p53-dependent
apoptotic cell death in response to energetic stress. J Biol Chem.
283:3979–3987. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jang T, Calaoagan JM, Kwon E, Samuelsson
S, Recht L and Laderoute KR: 5′-AMP-activated protein kinase
activity is elevated early during primary brain tumor development
in the rat. Int J Cancer. 128:2230–2239. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kola B, Boscaro M, Rutter GA, Grossman AB
and Korbonits M: Expanding role of AMPK in endocrinology. Trends
Endocrinol Metab. 17:205–215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mistry T, Digby JE, Desai KM and Randeva
HS: Obesity and prostate cancer: A role for adipokines. Eur Urol.
52:46–53. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rankin EB and Giaccia AJ: The role of
hypoxia-inducible factors in tumorigenesis. Cell Death Differ.
15:678–685. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hadad SM, Baker L, Quinlan PR, Robertson
KE, Bray SE, Thomson G, Kellock D, Jordan LB, Purdie CA, Hardie DG,
et al: Histological evaluation of AMPK signalling in primary breast
cancer. BMC Cancer. 9:3072009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen P, Li K, Liang Y, Li L and Zhu X:
High NUAK1 expression correlates with poor prognosis and involved
in NSCLC cells migration and invasion. Exp Lung Res. 39:9–17. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Suzuki A, Lu J, Kusakai G, Kishimoto A,
Ogura T and Esumi H: ARK5 is a tumor invasion-associated factor
downstream of Akt signaling. Mol Cell Biol. 24:3526–3535. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang H, Liu Y, Bai F, Zhang JY, Ma SH, Liu
J, Xu ZD, Zhu HG, Ling ZQ, Ye D, et al: Tumor development is
associated with decrease of TET gene expression and
5-methylcytosine hydroxylation. Oncogene. 32:663–669. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang LY, Li PL, Wang TZ and Zhang XC:
Prognostic values of 5-hmC, 5-mC and TET2 in epithelial ovarian
cancer. Arch Gynecol Obstet. 292:891–897. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hemerly JP, Bastos AU and Cerutti JM:
Identification of several novel non-p.R132 IDH1 variants in thyroid
carcinomas. Eur J Endocrinol. 163:747–755. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pansuriya TC, van Eijk R, d'Adamo P, van
Ruler MA, Kuijjer ML, Oosting J, Cleton-Jansen AM, van Oosterwijk
JG, Verbeke SL, Meijer D, et al: Somatic mosaic IDH1 and IDH2
mutations are associated with enchondroma and spindle cell
hemangioma in Ollier disease and Maffucci syndrome. Nat Genet.
43:1256–1261. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Parsons DW, Jones S, Zhang X, Lin JC,
Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, et
al: An integrated genomic analysis of human glioblastoma
multiforme. Science. 321:1807–1812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rasmussen KD and Helin K: Role of TET
enzymes in DNA methylation, development, and cancer. Genes Dev.
30:733–750. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Guo X, Wang L, Li J, Ding Z, Xiao J, Yin
X, He S, Shi P, Dong L, Li G, et al: Structural insight into
autoinhibition and histone H3-induced activation of DNMT3A. Nature.
517:640–644. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Otani J, Nankumo T, Arita K, Inamoto S,
Ariyoshi M and Shirakawa M: Structural basis for recognition of
H3K4 methylation status by the DNA methyltransferase 3A
ATRX-DNMT3-DNMT3L domain. EMBO Rep. 10:1235–1241. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Boulard M, Edwards JR and Bestor TH:
FBXL10 protects Polycomb-bound genes from hypermethylation. Nat
Genet. 47:479–485. 2015. View Article : Google Scholar : PubMed/NCBI
|