Introduction
Colorectal cancer (CRC) was the third most common
cause of cancer-associated mortality worldwide, and caused
approximately 900,000 deaths in 2013 (1). Due to technological advances in
colonoscopy and other screening measures, the health condition of
patients with CRC has improved in recent years. However, it has
been estimated that the overall incidence of CRC worldwide will
increase by 60% to >2.2 million cases and 1.1 million deaths by
2030 (2). Notably, CRC incidence and
mortality rates are increasing rapidly in developing countries
(3). Furthermore, the incidence of
CRC in the young generation is rising, as epidemiological studies
have noted the rising numbers of adolescents and adults <50
years old who are diagnosed with this disease (4,5). Genetic
and lifestyle factors are the major contributors to the disease;
however, the exact pathogenesis of CRC is unknown. Therefore, it is
important to identify a novel target for the diagnosis and clinical
treatment of CRC.
Long non-coding RNAs (lncRNAs) represent a class of
transcripts with a length >200 nucleotides, and do not exhibit
any capacity to encode proteins (6).
lncRNAs are indispensable regulators in the process of gene
expression, and emerging evidence has revealed the involvement of
lncRNAs in cancer development and progression, since several
studies have identified that the aberrant expression of lncRNAs is
closely associated with biological behaviors of malignant carcinoma
cells, such as proliferation, invasion and metastasis (7,8). lncRNA
ovarian tumor domain containing 6B antisense RNA1 (OTUD6B-AS1) is
oriented in an antisense direction relative to the protein-coding
gene OTUD6B on the opposite DNA strand (9). OTUD6BAS1 is located on chromosome
8q21.3 and has 2,179 bp (NR_110439, ENST00000524003.1) (10). A previous study has demonstrated that
OTUD6B-AS1 expression is downregulated in clear cell renal cell
carcinoma tissue samples, while overexpression of OTUD6B-AS1
inhibits cell proliferation, migration and invasion of clear cell
renal cell carcinoma (11). However,
to the best of our knowledge, the role of OTUD6B-AS1 in CRC has not
yet been determined.
MicroRNAs (miRNAs/miRs) are highly conserved
endogenous non-coding RNAs that are ~22 nucleotides long. They have
been demonstrated to serve important roles in a variety of
biological processes, including development, differentiation and
signaling (12–15). Dysregulated miRNAs may function as
either tumor suppressors or oncogenes in carcinoma by targeting
each one of these features (16).
Using Starbase, it was predicted that OTUD6B-AS1 can bind to
miR-3171. Studies have demonstrated that miR-3171 expression is
abnormally increased in bladder cancer and hepatocellular carcinoma
tissues (17,18). Therefore, it was hypothesized that
OTUD6B-AS1 could exert certain effects on the proliferation,
invasion and migration of CRC cells by regulating miR-3171.
To the best of our knowledge, the present study was
the first to investigate the role of OTUD6B-AS1 in CRC, and to
examine whether OTUD6B-AS1 could affect the proliferation, invasion
and migration of CRC cells by regulating miR-3171.
Materials and methods
The Cancer Genome Atlas (TCGA)
database analysis
Human RNA-sequencing data from colorectal cancer
projects, which included 482 patients with CRC and 155 normal
tissues (project no. TCGA-COAD) were obtained by TCGA (https://portal.gdc.cancer.gov/) analysis in the
UALCAN database (ualcan.path.uab.edu/) (19). A Mann-Whitney test was used to
determine the statistical significance of the difference in
OTUD6B-AS1 expression between normal and tumor samples.
Cell culture
CRC cell lines (Caco2, HCT116, LoVo, SW480 and
SNU-C1) and the normal intestinal epithelial cell line (HIEC) were
purchased from American Type Culture Collection. All cell lines
were routinely maintained in RPMI-1640 medium (HyClone; Cytiva)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.),
100 U/ml penicillin and 100 µg/ml streptomycin (Invitrogen; Thermo
Fisher Scientific, Inc.). Cells were cultured in a humidified
incubator at 37°C with 5% CO2. The culture medium was
replaced every 3 days. Cells were passaged when 80% confluence was
reached.
Cell transfection
The OTUD6B-AS1 overexpression plasmid
(Oe-OTUD6B-AS1; 1 µg) and empty vector (Oe-NC; 1 µg) were obtained
from Shanghai GenePharma Co., Ltd. miR-3171 mimics (40 nM; cat. no.
miR10015046-1-5) and corresponding scrambled mimic negative control
(mimic-NC; 40 nM; cat. no. miR1N0000001-1-5) were purchased from
Guangzhou RiboBio Co., Ltd. Cells (1×106 cells/well) were
transfected with the aforementioned oligonucleotides using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocols at 37°C
for 48 h. At 48 h after transfection, HCT116 cells were harvested
for further experiments, and successful transfection was verified
using reverse transcription-quantitative PCR (RT-qPCR).
RT-qPCR
Total RNA was extracted from HCT116 cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Total RNA was then reverse transcribed into cDNA at 42°C for
30 min using a reverse transcription kit (PrimeScript™ RT Reagent
Kit; Takara Bio, Inc.). qPCR was performed using iTaq™ Universal
SYBR®-Green Supermix (Bio-Rad Laboratories, Inc.) on an
ABI 7500 instrument (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The following thermocycling conditions were used:
Pre-denaturation at 95°C for 10 min, denaturation at 95°C for 15
sec and annealing at 60°C for 1 min (40 cycles). The sequences of
the gene-specific primers used in the present study were as
follows: lncRNA OTUD6B-AS1 forward, 5′-AGCACACCCAGTCAGAAACCAG-3′
and reverse, 5′-TCTACAAACGGGAATGTCG-3′; miR-3171 forward,
5′-AGATGTATGGAATCTGTATATA-3′ and reverse,
5′-GAACATGTCTGCGTATCTC-3′; GAPDH forward,
5′-TGTGGGCATCAATGGATTTGG-3′ and reverse,
5′-ACACCATGTATTCCGGGTCAAT-3′; and U6 forward,
5′-TCTGCTCCTATCCCAATTACCTG-3′ and reverse,
5′-ACTCCCGGATCTCTTCTAAGTTG-3′. GAPDH and U6 were used as internal
controls for OTUD6B-AS1 and miR-3171, respectively, and relative
expression was calculated based on the 2−ΔΔCq method
(20).
Cell Counting Kit-8 (CCK-8) assay
HCT116 cells were seeded into a 96-well plate at a
density of 2×104 cells/well and cultured at 37°C with 5%
CO2. Following transfection for 24, 48 and 72 h, 10 µl
CCK-8 solution [OBiO Technology (Shanghai) Corp., Ltd.] was added
to each well. Following incubation at 37°C for 4 h, the absorbance
at 450 nm was detected using a spectrophotometer (Thermo Fisher
Scientific, Inc.).
Colony formation assay
The HCT116 cells (0.5×103 cells/well) were seeded in
a six-well plate and cultured for 10 days after treatment.
Subsequently, colonies were fixed with 10% formaldehyde for 10 min
at room temperature and stained with 0.5% crystal violet for 5 min
at room temperature. The number of colonies was counted using
ImageJ software (version 1.52r; National Institutes of Health) and
images were captured under a fluorescence inversion microscope
(Olympus Corporation).
Immunofluorescence staining
Treated HCT116 cells were fixed with 4%
paraformaldehyde for 30 min at 37°C, and then 0.5% Triton X-100 was
used to permeabilize the cells at room temperature for 20 min.
After blocking with 5% BSA (Beyotime Institute of Biotechnology)
for 1 h at room temperature, slides were incubated overnight at 4°C
with a primary antibody against Ki67 (cat. no. ab15580; dilution,
1:1,000; Abcam). Subsequently, the slides were incubated with a
fluorescent secondary antibody (cat. no. BA1105; dilution,
1:10,000; Boster Biological Technology) for 1 h in a wet box at
room temperature in the dark. Finally, DAPI was used to
counterstain the nuclei at room temperature for 15 min. Images were
captured under a fluorescence inversion Olympus microscope (Olympus
Corporation).
Luciferase reporter assay
Potential target genes of lncRNA OTUD6B-AS1 were
predicted using an online bioinformatics software Starbase 2.0
(http://starbase.sysu.edu.cn/) (21). HCT116 cells were reseeded into
24-well plates and cultured for 24 h. The fragments of OTUD6B-AS1
containing predicted wild-type (WT) and mutant (MUT) miR-3171
binding sequences were amplified by Shanghai GenePharma Co., Ltd.,
and inserted into the luciferase reporter gene of the pmirGLO
vector (Promega Corporation) to produce the reporter plasmids
OTUD6B-AS1-WT and OTUD6B-AS1-MUT, respectively. Subsequently, the
cells (1×104 cells/well) were co-transfected with plasmids and
miR-3171 mimic (40 nM; cat. no. miR10015046-1-5; Guangzhou RiboBio
Co., Ltd.) or mimic-NC vector (40 nM; cat. no. miR1N0000001-1-5;
Guangzhou RiboBio Co., Ltd.) using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) for 24 h. At 48 h
after transfection, the culture medium was removed and the cells
were rinsed twice with PBS. The HCT116 cells were lysed to obtain
cell lysates, which were swirled for 10 min and centrifuged at
12,000 × g for 10 min at 4°C, and the supernatant was transferred
to a new Eppendorf tube. According to the instructions of the
dual-luciferase reporter assay kit (Beijing Solarbio Science &
Technology Co., Ltd.), the fluorescence value was used as an
internal reference, and the fluorescence value was detected using
an enzyme marker. The results were normalized to Renilla
luciferase activity.
Transwell assay
The invasion ability of HCT116 cells was evaluated
using Transwell assay (pore size, 8.0 µm; Corning Inc.) coated with
Matrigel (BD Biosciences) overnight at 37°C. A total of 2×104
HCT116 cells in serum-free medium were seeded into each upper
chamber. DMEM containing 10% FBS was added to the lower chamber as
a chemoattractant. After 48 h of incubation at 37°C, 4%
paraformaldehyde was used to fix invasive cells for 30 min at room
temperature and 0.1% crystal violet was subsequently applied to
stain cells for 30 min at room temperature. Images were obtained
under an inverted light microscope and the numbers of invasive
HCT116 cells were counted using ImageJ software (version 1.52r;
National Institutes of Health).
Wound healing assay
HCT116 cells from each group were collected and
seeded into 6-well plates at a density of 1×106 cells/ml. When the
cells completely covered the bottom of the well, a vertical line
was drawn across the well with a 10-µl pipette tip. HCT116 cells
were washed with PBS three times to remove cell debris, and
subsequently visualized under an inverted microscope. The images
were labeled as 0 h. Subsequently, the medium was replaced with
serum-free medium and cells were continuously cultured for 24 h.
Images were captured under an inverted light microscope and labeled
as 24 h. The 0 h images were used as a reference. The relative cell
migration was analyzed using ImageJ software (version 1.52r;
National Institutes of Health).
Western blot analysis
The samples were collected from HCT116 cells using
RIPA buffer (Beyotime Institute of Biotechnology). Afterwards, the
concentration of proteins was measured using the BCA method
(Beyotime Institute of Biotechnology). Subsequently, the proteins
(40 µg/lane) were separated on a 10% SDS-PAGE gel (Beyotime
Institute of Biotechnology). The proteins were transferred to PVDF
membranes (EMD Millipore). The PVDF membranes were blocked with 5%
non-fat milk powder for 1.5 h at room temperature. Then, the
membranes were incubated with primary antibodies at 4°C overnight.
Primary antibodies, including anti-MMP2 (cat. no. 40994S; dilution,
1:1,000), anti-MMP9 (cat. no. 13667T; dilution, 1:1,000) and the
loading control anti-GAPDH (cat. no. 5174T; dilution, 1:1,000),
were purchased from Cell Signaling Technology, Inc. On the next
day, the membranes were washed with PBS with 0.2% Tween-20 (PBST)
three times and probed with horseradish peroxidase-conjugated
secondary antibodies (cat. no. sc-2004; dilution, 1:3,000; Santa
Cruz Biotechnology, Inc.) for 1.5 h at room temperature.
Afterwards, the immunoreactive bands were washed with PBST again.
Finally, the signal was monitored and visualized using an enhanced
chemiluminescence assay (EMD Millipore) and the Odyssey Infrared
Imaging system (LI-COR Biosciences). GAPDH was used as an internal
control. The intensity of the bands was semi-quantified using
ImageJ software (version 1.52r; National Institutes of Health).
Statistical analysis
All experiments were repeated three times
independently. Data are presented as the mean ± standard deviation
and were analyzed using GraphPad Prism software (version 6.0;
GraphPad Software, Inc.). Comparisons between groups were performed
using an unpaired t-test, and multiple comparisons were performed
using one-way ANOVA followed by Tukey's post hoc test. P<0.05
was considered to indicate a statistically significant
difference.
Results
OTUD6B-AS1 expression is downregulated
in HCT116 cells and CRC tissues
As predicted by TCGA, OTUD6B-AS1 expression was
markedly downregulated in CRC tissues compared with in normal
tissues (Fig. 1A). Furthermore, the
expression levels of OTUD6B-AS1 were assessed in different CRC cell
lines and in normal HIEC cells. Compared with those in normal HIEC
cells, the expression levels of OTUD6B-AS1 in CRC cell lines
(Caco2, HCT116, LoVo, SW480 and SNU-C1) were markedly
downregulated, particularly in HCT116 cells (Fig. 1B). Therefore, HCT116 cells were
selected for subsequent experiments.
Overexpression of OTUD6B-AS1 inhibits
the proliferation, invasion and migration of HCT116 cells
After constructing the overexpression plasmid of
OTUD6B-AS1, transfection efficiency was verified by RT-qPCR, and it
was demonstrated that OTUD6B-AS1 expression was significantly
upregulated in the Oe-OTUD6B-AS1 group compared with the Oe-NC
group (Fig. 2A). The results of
CCK-8 and colony formation assays revealed that the proliferation
and colony formation abilities of cells in the Oe-OTUD6B-AS1 group
were inhibited compared with those in the Oe-NC group (Fig. 2B and C). Additionally, an
immunofluorescence assay revealed a decrease in the expression
levels of proliferation-related protein Ki67 following OTUD6B-AS1
overexpression (Fig. 2D).
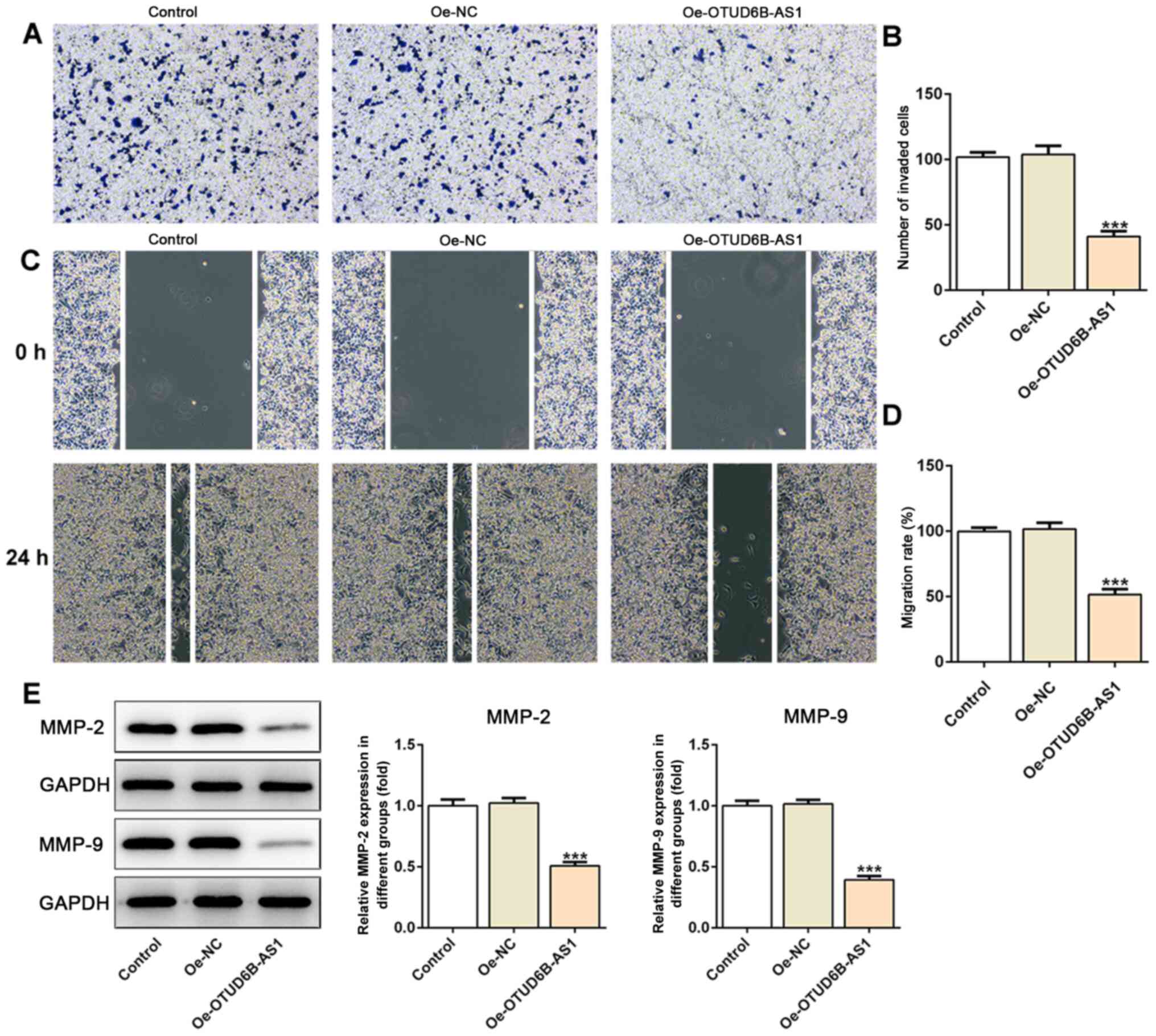
Furthermore, as shown in Fig. 3A-D,
OTUD6B-AS1 overexpression notably suppressed the invasion and
migration of HCT116 cells compared with those of cells in the empty
vector (Oe-NC) group. Additionally, the expression levels of
migration-associated proteins, including MMP2 and MMP9, were
decreased in the overexpression group (Fig. 3E). Therefore, it could be concluded
that the overexpression of OTUD6B-AS1 suppressed the proliferation,
invasion and migration of CRC cells.
miR-3171 is a direct target of
OTUD6B-AS1
The binding site between OTUD6B-AS1 and miR-3171 was
predicted using Starbase 2.0 (Fig.
4A). Subsequently, RT-qPCR was utilized to detect the
expression levels of miR-3171 in CRC cell lines and HIEC cells. It
was identified that miR-3171 expression was markedly upregulated in
CRC cell lines compared with in the HIEC cell line, particularly in
HCT116 cells (Fig. 4B). The miR-3171
level was markedly elevated following transfection with miR-3171
mimic (Fig. 4C). The dual-luciferase
reporter assay demonstrated the binding of miR-3171 and OTUD6B-AS1,
since the miR-3171 mimic + OTUD6B-AS1 WT group exhibited lower
luciferase activity compared with the mimic-NC + OTUD6B-AS1 WT
group (Fig. 4D). As expected, the
results of the RT-qPCR assay (Fig.
4E) indicated that miR-3171 expression was markedly reduced
following OTUD6B-AS1 overexpression compared with that in the OE-NC
group. These results suggested that miR-3171 is a direct target of
OTUD6B-AS1.
 | Figure 4.miR-3171 is a direct target of
OTUD6B-AS1. (A) Binding region between OTUD6B-AS1 and miR-3171. (B)
Expression levels of miR-3171 in colorectal cancer cell lines
(Caco2, HCT116, LoVo, SW480 and SNU-C1) were detected by RT-qPCR.
*P<0.05, ***P<0.001 vs. HIEC. (C) RT-qPCR was used to
evaluate the expression levels of miR-3171 after transfection.
***P<0.001 vs. mimic-NC. (D) A luciferase reporter assay was
performed to detect the relative luciferase activity. **P<0.01
vs. mimic-NC. (E) RT-qPCR was used to assess the expression levels
of miR-3171 following OTUD6B overexpression. ***P<0.001 vs.
Oe-NC. OTUD6B-AS1, ovarian tumor domain containing 6B antisense
RNA1; NC, negative control; Oe, overexpression; WT, wild-type; MUT,
mutant; miR-3171, microRNA-3171; RT-qPCR, reverse
transcription-quantitative PCR; luc/R-Luc, luciferase
activity/Renilla luciferase activity. |
OTUD6B-AS1 suppresses the
proliferation, invasion and migration of HCT116 cells by targeting
miR-3171
To determine whether OTUD6B-AS1 can exert its
effects on proliferation, invasion and migration of HCT116 cells by
targeting miR-3171, a series of functional experiments was
performed. As shown in Fig. 5A-C,
co-transfection of OTUD6B-AS1 overexpression plasmid and miR-3171
mimics reversed the inhibitory effects of OTUD6B-AS1 overexpression
alone on the proliferation, colony formation abilities and the Ki67
expression of HCT116 cells. Furthermore, the addition of miR-3171
mimics attenuated the effects of OTUD6B-AS1 overexpression on the
invasive and migratory abilities of HCT116 cells (Fig. 6A-D). Additionally, the expression
levels of migration-associated proteins were elevated when the
HCT116 cells with overexpression of OTUD6B-AS1 were co-transfected
with miR-3171 mimic (Fig. 6E). These
results indicated that OTUD6B-AS1 inhibited the proliferation,
invasion and migration of HCT116 cells by targeting miR-3171.
Discussion
CRC is the third most common type of cancer
worldwide, and is a leading cause of mortality that threatens tens
of thousands of lives (22,23). During the initiation and development
of CRC, various germline and somatic mutations assemble (24). With the help of advanced technology
for the screening and treatment of CRC, the number of individuals
>50 years old who are suffering from CRC is lower than several
decades ago; however, the incidence is rising in young patients
(25,26). Due to the low survival rate and
complex molecular mechanism underlying CRC tumorigenesis, it is
necessary to identify novel and effective biomarkers for the
treatment of the disease.
lncRNAs serve as important regulators in diverse
physiological and pathological processes (27). Previous studies have revealed that
lncRNAs are involved in all stages of carcinogenesis and tumor
progression, such as tumor growth, tumor metastasis and tumor
angiogenesis (6,28). Several lncRNAs have been demonstrated
to be associated with the entire process of CRC development. For
example, glycolysis-associated lncRNA of colorectal cancer serves
as an oncogene in colorectal carcinogenesis, and it can stabilize
c-Myc to exert its promoting effect on CRC and glucose metabolism
(26). Colorectal cancer-associated
lncRNA could stimulate CRC progression via activation of the
Wnt/β-catenin signaling pathway by suppressing the activator
protein 2α (29). HOX transcript
antisense RNA, which is highly expressed in a large number of
cancer cells, can regulate the methylation levels of histone H3K27,
thus contributing to CRC development (30). These lncRNAs with abnormal expression
in different phases of tumors could be potential diagnostic and
prognostic markers (31). A previous
study has demonstrated that overexpression of OTUD6B-AS1 could
inhibit the proliferation, invasion and migration of renal clear
cell carcinoma cells (11). When
analyzing data from TCGA, it was revealed that the levels of
OTUD6B-AS1 in CRC tissues were notably decreased compared with
those in the adjacent non-tumor tissues, suggesting a potential
antitumor effect of OTUD6B-AS1 in CRC progression. It is well-known
that abnormal and uncontrolled proliferation is a characteristic of
cancer cells (32). Furthermore,
invasion and migration are considered to be two dominant processes
for tumor metastasis, which leads to cancer-associated mortality
(33). Thus, interruption of the
aforementioned processes is an effective method for the inhibition
of cancer metastasis. Therefore, the present study aimed to
investigate whether OTUD6B-AS1 can have an effect on the
proliferation, invasion and migration of CRC cells. The present
study demonstrated that OTUD6B-AS1 expression was decreased in CRC
cells compared with in a normal intestinal epithelial cell line,
which was in accordance with the results of TCGA database analysis.
Furthermore, overexpression of OTUD6B-AS1 inhibited the
proliferation, migration and invasion of CRC cells.
Evolutionarily conserved miRNAs can suppress gene
expression at the posttranscriptional level (34). Furthermore, they are considered to
mediate the expression levels of ≥30% of all protein-coding genes,
and are involved in most cellular processes (35). Over the past few decades, the
aberrant expression profile of miRNAs has been frequently
recognized in the peripheral blood and tumor tissue specimens from
patients with CRC, hinting at the oncogenic effects of miRNAs in
various types of cancer (36,37). The
upregulation of miR-135b has been commonly detected in patients
with CRC by functional screening, and is associated with the
clinical stage and cancer-specific survival of patients (38). Additionally, increased expression
levels of miR-31 in patients with CRC are associated with poor
prognosis (39). It has been
hypothesized that the inhibition of dysregulated miRNAs could
decrease the activity of CRC cells (40). In the present study, a binding site
between OTUD6B-AS1 and miR-3171 was predicted using the Starbase
database, and miR-3171 expression was upregulated in HCT116 cells.
Therefore, it was hypothesized that OTUD6B-AS1 could inhibit CRC
progression via inhibition of miR-3171 expression. To further
verify this hypothesis, another RT-qPCR assay was performed and
revealed that miR-3171 expression was markedly reduced following
OTUD6B-AS1 overexpression. In a series of assays, it was observed
that the additional treatment with miR-3171 mimics following
OTUD6B-AS1 overexpression in HCT116 cells promoted the
proliferation, invasion and migration of HCT116 cells, suggesting
that OTUD6B-AS1 overexpression inhibited the proliferation,
invasion and migration of HCT116 cells via downregulation of
miR-3171 expression.
In conclusion, to the best of our knowledge, the
present study was the first to investigate the role of OTUD6B-AS1
in CRC cells, and to reveal that lncRNA OTUD6B-AS1 overexpression
inhibited the proliferation, invasion and migration of HCT116
cells, at least partially, by targeting miR-3171. Therefore,
OTUD6B-AS1 may serve as a potential novel biomarker and target for
the diagnosis and treatment of CRC. However, the use of only one
CRC cell line in the cell function experiments and mechanism
experiments, and lack of data obtained from clinical samples and
investigations in animal models were limitations of the present
study. Therefore, a comprehensive analysis is required in the
future. Additionally, the use of only one TCGA dataset to
investigate OTUD6B-AS1 expression is another limitation of the
present study, and future studies should improve upon this and the
other limitations.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WW and XC searched the literature, designed the
experiments and performed the experiments. WW and JZ analyzed the
data and wrote the manuscript. JZ revised the manuscript. All
authors read and approval the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kuipers EJ, Grady WM, Lieberman D,
Seufferlein T, Sung JJ, Boelens PG, van de Velde CJ and Watanabe T:
Colorectal cancer. Nat Rev Dis Primers. 1:15065. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou LL, Zou MD and Li WM: Recent advances
in colorectal cancer-specific nucleic acid aptamers for diagnostic
and therapeutic applications. Sci Adv Mater. 12:38–43. 2020.
View Article : Google Scholar
|
|
4
|
The Lancet Oncology: Colorectal cancer: A
disease of the young? Lancet Oncol. 18:4132017. View Article : Google Scholar
|
|
5
|
Kasi PM, Shahjehan F, Cochuyt JJ, Li Z,
Colibaseanu DT and Merchea A: Rising proportion of young
individuals with rectal and colon cancer. Clin Colorectal Cancer.
18:e87–e95. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu M, Xu X, Pan B, Chen X, Lin K, Zeng K,
Liu X, Xu T, Sun L, Qin J, et al: LncRNA SATB2-AS1 inhibits tumor
metastasis and affects the tumor immune cell microenvironment in
colorectal cancer by regulating SATB2. Mol Cancer. 18:1352019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quinn JJ and Chang HY: Unique features of
long non-coding RNA biogenesis and function. Nat Rev Genet.
17:47–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rathinasamy B and Velmurugan BK: Role of
lncRNAs in the cancer development and progression and their
regulation by various phytochemicals. Biomed Pharmacother.
102:242–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takata M, Pachera E, Frank-Bertoncelj M,
Kozlova A, Jüngel A, Whitfield ML, Assassi S, Calcagni M, de
Vries-Bouwstra J, Huizinga TW, et al: OTUD6B-AS1 might be a novel
regulator of apoptosis in systemic sclerosis. Front Immunol.
10:11002019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Z, Xia F, Feng T, Jiang B, Wang W and
Li X: OTUD6B-AS1 inhibits viability, migration, and invasion of
thyroid carcinoma by targeting miR-183-5p and miR-21. Front
Endocrinol (Lausanne). 11:1362020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang G, Zhang ZJ, Jian WG, Liu PH, Xue W,
Wang TD, Meng YY, Yuan C, Li HM, Yu YP, et al: Novel long noncoding
RNA OTUD6B-AS1 indicates poor prognosis and inhibits clear cell
renal cell carcinoma proliferation via the Wnt/β-catenin signaling
pathway. Mol Cancer. 18:152019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mahesh G and Biswas R: MicroRNA-155: A
master regulator of inflammation. J Interferon Cytokine Res.
39:321–330. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu Y, Yang L, Yu M and Wang J:
Identification and expression analysis of microRNAs during ovule
development in rice (Oryza sativa) by deep sequencing. Plant Cell
Rep. 36:1815–1827. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rane S, Sayed D and Abdellatif M: MicroRNA
with a MacroFunction. Cell Cycle. 6:1850–1855. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iorio MV and Croce CM: MicroRNA
dysregulation in cancer: Diagnostics, monitoring and therapeutics.
A comprehensive review. EMBO Mol Med. 9:8522017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei Y, He R, Wu Y, Gan B, Wu P, Qiu X, Lan
A, Chen G, Wang Q, Lin X, et al: Comprehensive investigation of
aberrant microRNA profiling in bladder cancer tissues. Tumour Biol.
37:12555–12569. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Z, Zhao Y, Wang Y and Jin C: Circular
RNA circHIAT1 inhibits cell growth in hepatocellular carcinoma by
regulating miR-3171/PTEN axis. Biomed Pharmacother. 116:1089322019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo S, Zhu KX, Yu WH, Wang T, Li S, Wang
YX, Zhang CC, Guo JQ, et al: SH3PXD2A-AS1/miR-330-5p/UBA2
ceRNAnetwork mediates the progression of colorectal cancer through
regulating the activity of the Wnt/beta-catenin signaling pathway.
Environ Toxicol. Oct 19–2020.(Epub ahead of print). doi:
10.1002/tox.23038. View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42:D92–D97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hua RX, Zhuo ZJ, Zhu J, Zhang SD, Xue WQ,
Zhang JB, Xu HM, Li XZ, Zhang PF, He J, et al: XPG Gene
Polymorphisms contribute to colorectal cancer susceptibility: A
two-stage case-control study. J Cancer. 7:1731–1739. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He D, Ma L, Feng R, Zhang L, Jiang Y,
Zhang Y and Liu G: Analyzing large-scale samples highlights
significant association between rs10411210 polymorphism and
colorectal cancer. Biomed Pharmacother. 74:164–168. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Global Burden of Disease Cancer
Collaboration, ; Fitzmaurice C, Allen C, Barregard L, Bhutta ZA,
Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, Dandona L,
Fleming T, et al: Global, regional, and national cancer incidence,
mortality, years of life lost, years lived with disability, and
disability-adjusted life-years for 32 cancer groups, 1990 to 2015:
A Systematic Analysis for the Global Burden of Disease Study. JAMA
Oncol. 3:524–548. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tang J, Yan T, Bao Y, Shen C, Yu C, Zhu X,
Tian X, Guo F, Liang Q, Liu Q, et al: LncRNA GLCC1 promotes
colorectal carcinogenesis and glucose metabolism by stabilizing
c-Myc. Nat Commun. 10:34992019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Heo JB, Lee YS and Sung S: Epigenetic
regulation by long noncoding RNAs in plants. Chromosome Res.
21:685–693. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang H, Chen Z, Wang X, Huang Z, He Z and
Chen Y: Long non-coding RNA: A new player in cancer. J Hematol
Oncol. 6:372013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma Y, Yang Y, Wang F, Moyer MP, Wei Q,
Zhang P, Yang Z, Liu W, Zhang H, Chen N, et al: Long non-coding RNA
CCAL regulates colorectal cancer progression by activating
Wnt/β-catenin signalling pathway via suppression of activator
protein 2α. Gut. 65:1494–1504. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cai B, Song XQ, Cai JP and Zhang S:
HOTAIR: A cancer-related long non-coding RNA. Neoplasma.
61:379–391. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Galamb O, Barták BK, Kalmár A, Nagy ZB,
Szigeti KA, Tulassay Z, Igaz P and Molnár B: Diagnostic and
prognostic potential of tissue and circulating long non-coding RNAs
in colorectal tumors. World J Gastroenterol. 25:5026–5048. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang AH, Fan WJ, Fu L and Wang XT: LncRNA
PCAT-1 regulated cell proliferation, invasion, migration and
apoptosis in colorectal cancer through targeting miR-149-5p. Eur
Rev Med Pharmacol Sci. 23:8310–8320. 2019.PubMed/NCBI
|
|
33
|
Zhang H, Song Y, Yang C and Wu X:
Overexpression of lncRNA TUSC7 reduces cell migration and invasion
in colorectal cancer. Oncol Rep. 41:3386–3392. 2019.PubMed/NCBI
|
|
34
|
Lin J, Chuang CC and Zuo L: Potential
roles of microRNAs and ROS in colorectal cancer: Diagnostic
biomarkers and therapeutic targets. Oncotarget. 8:17328–17346.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Z, Wang Y, Borlak J and Tong W:
Mechanistically linked serum miRNAs distinguish between drug
induced and fatty liver disease of different grades. Sci Rep.
6:237092016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gmerek L, Martyniak K, Horbacka K,
Krokowicz P, Scierski W, Golusinski P, Golusinski W, Schneider A
and Masternak MM: MicroRNA regulation in colorectal cancer tissue
and serum. PLoS One. 14:e02220132019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nagy ZB, Barták BK, Kalmár A, Galamb O,
Wichmann B, Dank M, Igaz P, Tulassay Z and Molnár B: Comparison of
circulating miRNAs expression alterations in matched tissue and
plasma samples during colorectal cancer progression. Pathol Oncol
Res. 25:97–105. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Valeri N, Braconi C, Gasparini P, Murgia
C, Lampis A, Paulus-Hock V, Hart JR, Ueno L, Grivennikov SI, Lovat
F, et al: MicroRNA-135b promotes cancer progression by acting as a
downstream effector of oncogenic pathways in colon cancer. Cancer
Cell. 25:469–483. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sun D, Yu F, Ma Y, Zhao R, Chen X, Zhu J,
Zhang CY, Chen J and Zhang J: MicroRNA-31 activates the RAS pathway
and functions as an oncogenic MicroRNA in human colorectal cancer
by repressing RAS p21 GTPase activating protein 1 (RASA1). J Biol
Chem. 288:9508–9518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yin Y, Yan ZP, Lu NN, Xu Q, He J, Qian X,
Yu J, Guan X, Jiang BH and Liu LZ: Downregulation of miR-145
associated with cancer progression and VEGF transcriptional
activation by targeting N-RAS and IRS1. Biochim Biophys Acta.
1829:239–247. 2013. View Article : Google Scholar : PubMed/NCBI
|