Introduction
Primary hepatocellular carcinoma (HCC) is the second
most common cause of cancer-associated mortality worldwide
(1), with exceedingly high rates in
South Asia and sub-Saharan Africa (2). Infection with hepatitis B and C virus
(3,4), long-term alcoholism, non-alcoholic
fatty liver disease and certain hereditary metabolic diseases
contribute to the high incidence rates of HCC (5). The risk of metastasis, which leads to a
high incidence of early postoperative recurrence, makes HCC one of
the most lethal types of cancer. Despite the fact that several
advanced techniques have been successfully applied in the clinical
management of patients with HCC (6),
the long-term prognosis of patients with HCC remains
unsatisfactory. Therefore, it is essential to investigate novel
genes or molecules with the aim of developing new therapeutic
techniques, and improving the survival rate of patients with
HCC.
Phosphatidylinositol glycan anchor biosynthesis
class C (PIGC) encodes an endoplasmic reticulum (ER)-associated
protein that is vital for the biosynthesis of
glycosylphosphatidylinositol (GPI) (7). GPI is utilized to anchor a variety of
membrane proteins for expression on the surface of eukaryotic
cells. GPI anchoring is a process involved in post-translational
modification, which occurs in the ER (1). Mutations in the PIGC gene result in
aberrant GPI anchoring, which contributes to seizures, mental
retardation and obesity (8–10). Recent studies have demonstrated that
dysregulation of PIGC may be implicated in the pathogenesis of
several malignant tumor types. Yang et al (11) detected PIGC mutations in the
pancreatic ductal adenocarcinoma cell line AsPC-1 and observed that
the mutations of PIGC enhanced the motility of cancer cells.
Furthermore, Kim et al (12)
used Helicobacter pylori-released proteins (G27 strain) to
stimulate AGS gastric cancer cells. PIGC was found to be
upregulated and may be associated with the transferase activity of
cancer cells (12). However, to the
best of our knowledge, the expression profile of PIGC in HCC has
not yet been investigated and the mechanism underlying its
dysregulation in cancer remains unclear. The present study
investigated the associations between expression of PIGC and the
clinical features of HCC. Furthermore, the present study also aimed
to gain an improved understanding of the way in which PIGC is
associated with the clinical prognosis of patients with HCC.
Materials and methods
Bioinformatics analysis
PIGC mRNA expression and clinical information data
were downloaded from The Cancer Genome Atlas-Liver Hepatocellular
Carcinoma database (TCGA-LIHC, http://tcga-data.nci.nih.gov/tcga/) and Cbioportal
(http://www.cbioportal.org/). Combined
with the two databases, a total of 370 cases of HCC with
information on the clinical characteristics and PIGC expression
were included in the present study. The expression levels of PIGC
mRNA in liver cancer and normal tissues were investigated using
Oncomine gene expression array datasets (https://www.oncomine.org). Furthermore, the
methylation status of PIGC in HCC tissues and adjacent normal
tissues was evaluated using the methHC database (http://methHC.mbc.nctu.edu.tw/php/index.php). The
prognostic value of PIGC mRNA in HCC was further verified using
Gene Expression Profiling Interactive Analysis (GEPIA; http://gepia.cancer-pku.cn/). Additionally, in order
to investigate the potential roles of PIGC in the pathogenesis of
cancer, the present study performed a protein-protein interaction
(PPI) analysis of PIGC using the Search Tool for the Retrieval of
Interacting Genes/Proteins (STRING) database (https://string-db.org/cgi/input.pl) and Kyoto
Encyclopedia of Genes and Genomes (KEGG) analysis of PPI proteins
closely associated with PIGC.
Patients and follow-up
Liver cancer and matched adjacent tissue samples
were obtained from 97 patients with HCC who received surgical
resection at the Surgery Department of Renmin Hospital of Wuhan
University (Wuhan, China) between January 2013 and December 2015.
All patients provided written informed consent. Out of the 97
patients, three passed away within 1 month after surgery, two
patients underwent radiotherapy and four patients received
chemotherapy prior to surgery. Thus, these nine patients were
excluded from the study. Clinicopathological features, including
age, sex, Tumor-Node-Metastasis (TNM) stage, grade (G) stage and
American Joint Committee on Cancer stage, were obtained from the
electronic record system. The follow-up initiated on the date of
surgical resection and ended in March 2018. Follow-up was performed
every 3 months during the first 3 years, and twice per year after 3
years once cases became outpatients or received telephone
follow-up. Overall survival (OS) was defined as the period from
confirmed diagnosis to the end of follow-up or death. The present
study was approved by the Institutional Review Board of Renmin
Hospital of Wuhan University.
Construction of tissue microarray and
analysis of immunohistochemistry (IHC)
Paraffin-embedded HCC tissues and matched
non-cancerous tissues were obtained from the Department of
Pathology at Renmin Hospital, Wuhan University. The most
characteristic liver cancerous and non-cancerous tissue samples
were selected to construct a tissue microarray (TMA). The TMA was
constructed in line with a previous study (13); a 1.5-mm diameter core for each sample
was punched into the TMA. In total, the TMA consisted of 88 HCC and
corresponding adjacent tissue samples. The expression of PIGC was
assessed using a semi-quantitation method according to the
intensity of staining (IS) and the area of positivity (AP)
(14). IS was classified as follows:
0, negative; 1, weak; 2, moderate; and 3, strong. AP depended on
the percentage of positive-stained cells as follows: 0, 0%; 1,
<50% PIGC+ cells; 2, 50–75% PIGC+ cells;
and 3, >75% PIGC+ cells. The H-score was calculated
by multiplying the IS with the AP. X-tile 3.6.1 software (15) (Yale University, New Haven, CT, USA)
was used to determine the optimal cut-off values for the expression
of PIGC.
IHC assay
The process of IHC staining was performed as
previously described (16).
Deparaffinized TMA was washed in 3% H2O2,
then subjected to antigen retrieval with citric acid (pH, 6.0).
After an overnight incubation at 4°C with primary antibody PIGC
(1:50; cat. no. HPA036663; Sigma Aldrich; Merck KGaA), the TMA was
incubated for 15 min at room temperature with horseradish
peroxidase-labeled polymer conjugated goat anti-rabbit IgG (1:50;
cat. no. KIT-5005; Maxvision Biosciences, Inc.). After incubation
for 1 min with 3,3′-diaminobenzidine chromogen (Fuzhou Maixin
Biotech Co., Ltd.) the TMA was counterstained with hematoxylin. In
the negative control group, PBS was used as the primary
antibody.
Statistical analysis
SPSS (version 20.0; IBM Corp.) software was used to
perform all statistical analyses. Student's t-tests were used to
assess the differential expression levels of PIGC and the clinical
characteristics. Pearson's χ2 tests were utilized to
evaluate the difference between two groups stratified by median
PIGC mRNA expression. Kaplan-Meier estimates (log-rank test) were
applied to investigate the association between PIGC expression and
clinical outcomes in patients with HCC using median PIGC expression
or methylation. Cox regression analysis was performed to assess
whether the overexpression of PIGC was an independent risk factor
for survival in patients with HCC. P<0.05 was considered to
indicate a statistically significant difference.
Results
PIGC transcriptional levels of PIGC
are higher in liver cancer compared with normal tissue
After investigating the transcriptional levels in
different types of cancer using the Oncomine database, it was
revealed that PIGC expression levels were higher in liver and brain
cancer compared with their healthy counterparts, whilst this gene
expression was downregulated in HCC compared with normal tissues.
The present study exclusively analyzed the levels of PIGC mRNA in
HCC in four datasets from the Oncomine database. Using the Roessler
Liver Data (P<0.0001; fold change, 2.623), Roessler Liver 2 Data
(P<0.0001; fold change, 2.407), Wurmbach Liver Data
(P<0.0001; Fold change, 2.116) and Chen Liver Data (P<0.0001;
fold change, 1.882), the levels of PIGC expression were observed to
be significantly higher in liver cancer compared with that in
healthy tissue (Fig. 1), indicating
that PIGC is upregulated during the progression of HCC.
Associations between PIGC mRNA and
clinical characteristics
Using data mining from the TCGA (TCGA-LIHC) and the
Cbioportal website, the present study included a total of 370 cases
of patients with liver cancer with clinical follow-up information
and PIGC mRNA expression. By setting the median PIGC mRNA value as
the cut-off, it was revealed that the expression of PIGC was
significantly associated with sex (χ2=6.496; P=0.011),
AFP level (χ2=22.544; P<0.0001), hepatitis
(χ2=5.716; P=0.017), G stage (χ2=14.978;
P<0.0001), N stage (χ2=5.345; P=0.021) and M stage
(χ2=9.264; P=0.002) (Fig.
2). However, no significant associations between the levels of
PIGC mRNA and age, alcohol consumption or TNM stage were observed
(Table I).
 | Table I.Clinical characteristics of HCC
stratified by PIGC mRNA expression. |
Table I.
Clinical characteristics of HCC
stratified by PIGC mRNA expression.
|
| PIGC mRNA
expression |
|
|
|---|
|
|
|
|
|
|---|
| Clinical
parameter | Low | High | χ2
value | P-value |
|---|
| Age, years |
|
| 0.302 | 0.583 |
|
≤55 | 60 | 65 |
|
|
|
>55 | 125 | 120 |
|
|
| Sex |
|
| 6.496 | 0.011a |
|
Male | 136 | 113 |
|
|
|
Female | 49 | 72 |
|
|
| AFP level |
|
| 22.544 |
<0.001a |
|
Normal | 122 | 91 |
|
|
|
High | 15 | 49 |
|
|
| Hepatitis |
|
| 5.716 | 0.017a |
| No | 130 | 107 |
|
|
|
Yes | 47 | 67 |
|
|
| Drinking |
|
| 3.612 | 0.057 |
| No | 118 | 135 |
|
|
|
Yes | 67 | 50 |
|
|
| T stage |
|
| 1.793 | 0.181 |
|
T1+T2 | 145 | 134 |
|
|
|
T3+T4 | 39 | 50 |
|
|
| N stage |
|
| 5.354 | 0.021a |
| N0 | 116 | 136 |
|
|
|
N1+NX | 69 | 48 |
|
|
| M stage |
|
| 9.264 | 0.002a |
| M0 | 63 | 37 |
|
|
|
M1+MX | 122 | 148 |
|
|
| G stage |
|
| 14.978 |
<0.001a |
|
G1+G2 | 134 | 98 |
|
|
|
G3+G4 | 51 | 87 |
|
|
| TNM stage |
|
| 1.502 | 0.22 |
|
I+II | 133 | 123 |
|
|
|
III+IV | 40 | 50 |
|
|
High expression levels of PIGC mRNA
predicts worse survival in patients with HCC
Based on the Kaplan-Meier plots (log-rank test), it
was demonstrated that a high expression level of PIGC was
significantly associated with an unfavorable OS [hazard ratio
(HR)=1.877; P=0.0044]. Similarly, it was revealed that the
overexpression of PIGC in HCC was significantly associated with
decreased disease-free survival (DFS) (HR=2.018; P=0.0121 (Fig. 3A and B). Following the multivariate
analysis with Cox regression, the results showed that high
expression of PIGC was an independent prognostic factor for poor OS
[HR=2.203; 95% confidence interval (CI)=1.476–3.287; P<0.0001]
and DFS (HR=1.525; 95% CI=1.114–2.087; P=0.008) in patients with
HCC (Tables II and III), suggesting that PIGC may be a novel
marker with prognostic significance for HCC. Taken together, the
aforementioned results suggested that PIGC mRNA may be a potential
independent prognostic marker for patients with HCC.
 | Table II.Cox regression analyses of PIGC mRNA
level and OS. |
Table II.
Cox regression analyses of PIGC mRNA
level and OS.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Parameter | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years | 1.152
(0.795–1.67) | 0.455 | – | – |
|
≤55 |
|
|
|
|
|
>55 |
|
|
|
|
| Sex | 0.816
(0.573–1.163) | 0.260 | – | – |
|
Male |
|
|
|
|
|
Female |
|
|
|
|
| AFP level | 1.056
(0.646–1.727) | 0.828 | – | – |
|
Normal |
|
|
|
|
|
High |
|
|
|
|
| Hepatitis | 0.562
(0.37–0.816) | 0.007a | 0.665
(0.411–1.075) | 0.096 |
| No |
|
|
|
|
|
Yes |
|
|
|
|
| Drinking | 1.026
(0.704–1.479) | 0.892 | – | – |
| No |
|
|
|
|
|
Yes |
|
|
|
|
| T stage | 2.489
(1.75–3.54) |
<0.0001a | 1.375
(0.396–4.778) | 0.616 |
|
T1+T2 |
|
|
|
|
|
T3+T4 |
|
|
|
|
| N stage | 1.152
(1.049–2.179) | 0.027a | 1.14
(0.663–1.961) | 0.635 |
| N0 |
|
|
|
|
|
N1+NX |
|
|
|
|
| M stage | 1.563
(1.0792.264) | 0.018a | 1.686
(0.972–2.926) | 0.063 |
| M0 |
|
|
|
|
|
M1+MX |
|
|
|
|
| G stage | 1.004
(0.772–1.306) | 0.977 | – | – |
|
G1+G2 |
|
|
|
|
|
G3+G4 |
|
|
|
|
| TNM stage | 2.488
(1.689–3.548) |
<0.0001a | 1.74
(0.506–5.985) | 0.380 |
|
I+II |
|
|
|
|
|
III+IV |
|
|
|
|
| PIGC mRNA | 1.654
(1.166–2.347) | 0.005a | 2.203
(1.476–3.287) |
<0.0001a |
|
Low |
|
|
|
|
|
High |
|
|
|
|
 | Table III.Cox regression analysis of PIGC mRNA
level and DFS. |
Table III.
Cox regression analysis of PIGC mRNA
level and DFS.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Parameter | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years | 0.966
(0.707–1.321) | 0.828 | – | – |
|
≤55 |
|
|
|
|
|
>55 |
|
|
|
|
| Sex | 0.885
(0.645–1.124) | 0.45 | – | – |
|
Male |
|
|
|
|
|
Female |
|
|
|
|
| AFP level | 1.108
(0.734–1.673) | 0.625 | – | – |
|
Normal |
|
|
|
|
|
High |
|
|
|
|
| Hepatitis | 0.889
(0.643–1.228) | 0.475 | – | – |
| No |
|
|
|
|
|
Yes |
|
|
|
|
| Drinking | 1.011
(0.732–1.395) | 0.948 | – | – |
| No |
|
|
|
|
|
Yes |
|
|
|
|
| T stage | 2.270
(1.643–3.135) |
<0.001a | 1.088
(0.432–2.738) | 0.857 |
|
T1+T2 |
|
|
|
|
|
T3+T4 |
|
|
|
|
| N stage | 1.249
(0.904–1.724) | 0.178 | – | – |
| N0 |
|
|
|
|
|
N1+NX |
|
|
|
|
| M stage | 1.151
(0.826–1.602) | 0.406 | – | – |
| M0 |
|
|
|
|
|
M1+MX |
|
|
|
|
| G stage | 1.105
(0.811–1.507) | 0.526 | – | – |
|
G1+G2 |
|
|
|
|
|
G3+G4 |
|
|
|
|
| TNM stage | 2.354
(1.692–3.275) |
<0.001a | 2.156
(0.874–5.317) | 0.095 |
|
I+II |
|
|
|
|
|
III+IV |
|
|
|
|
| PIGC mRNA | 1.493
(1.107–2.012) | 0.009a | 1.525
(1.114–2.087) | 0.008a |
|
Low |
|
|
|
|
|
High |
|
|
|
|
PIGC expression is negatively
regulated by DNA methylation
By using methHC, the present study generated a
contrastive figure showing that the levels of DNA methylation were
significantly higher in liver tumor tissues compared with that in
healthy tissues (transcript:NM_002642; P<0.005; Fig. S1A). In addition, by investigating
the association between PIGC expression and DNA methylation, the
present study observed a negative correlation (Spearman's
rho=−0.453), indicating that PIGC expression gradually decreased
with an increase in PIGC promoter DNA methylation (Fig. S1B). Furthermore, data on PIGC
promoter methylation was downloaded from the Cbioportal website and
upon analysis, the results matched the follow-up information with
TCGA-LIHC data. The present study also investigated whether PIGC
methylation status was associated with survival outcomes of
patients with HCC. In the two-group analysis stratified by the
median status of PIGC DNA methylation, no associations between PIGC
methylation and OS or DFS were observed (Fig. S2).
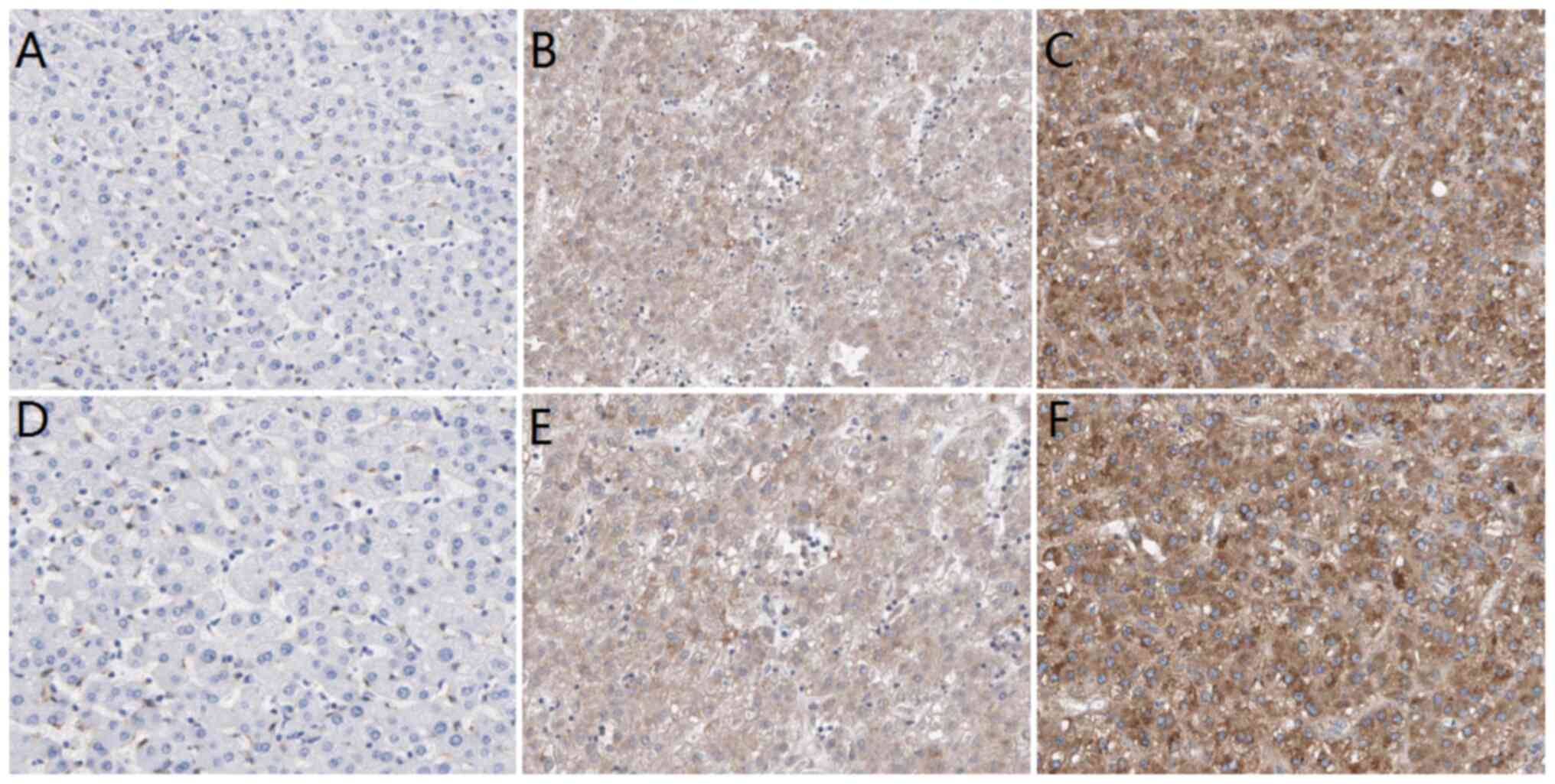
Expression of PIGC protein is
upregulated in HCC
In order to analyze the associations between PIGC
protein expression and clinicopathological parameters, the present
study set the cut-off value of H-score as 5 points according to
Kaplan-Meier curves. If the H-score was <5 points, then the
result was defined as low expression; if the H-score was >5
points, then the result was regarded as high expression. The IHC
results revealed that PIGC protein was expressed in liver cancerous
and non-cancerous tissues, as demonstrated by the brown particles
localized in the cytoplasmic membranous with no staining of nucleus
(Fig. 4). However, the staining
intensity in HCC and non-cancerous tissues was markedly different.
In 88 cases of liver cancerous tissues, 63 cases (71.59%) showed
high PIGC expression and 25 cases (28.41%) showed low PIGC
expression. However, 88 cases of matched tissues exhibited low
expression levels of PIGC protein without any case of adjacent
non-cancerous tissue showing strong staining. These results suggest
that the PIGC protein is highly expressed in HCC tissue and
expressed at low levels in corresponding non-cancerous tissues.
Thus, PIGC protein may be a useful biomarker for the diagnosis of
liver cancer. The χ2 analysis showed that PIGC was
significantly associated with N stage (χ2=5.099;
P=0.024), M stage (χ2=6.974; P=0.008) and TNM stage
(χ2=4.985; P=0.026), but there were no significant
associations observed with G stage, T stage, age or sex (Table IV).
 | Table IV.Associations between PIGC protein
expression and clinical features. |
Table IV.
Associations between PIGC protein
expression and clinical features.
|
| PIGC
expression |
|
|
|---|
|
|
|
|
|
|---|
| Factor | High (n=63) | Low (n=25) | χ2
value | P-value |
|---|
| Sex |
|
| 0.880 | 0.348 |
|
Male | 42 | 14 |
|
|
|
Female | 21 | 11 |
|
|
| Age, years |
|
| 0.503 | 0.478 |
|
<60 | 33 | 11 |
|
|
|
≥60 | 30 | 14 |
|
|
| G stage |
|
| 1.267 | 0.260 |
|
G1+G2 | 23 | 6 |
|
|
|
G3+G4 | 40 | 19 |
|
|
| T stage |
|
| 0.329 | 0.566 |
|
T1-T2 | 14 | 7 |
|
|
|
T3-T4 | 49 | 18 |
|
|
| N stage |
|
| 5.099 | 0.024a |
| N0 | 19 | 14 |
|
|
|
N1-N2 | 44 | 11 |
|
|
| M stage |
|
| 6.974 | 0.008a |
| M0 | 44 | 24 |
|
|
| M1 | 19 | 1 |
|
|
| TNM stage |
|
| 4.985 | 0.026a |
|
I–II | 17 | 13 |
|
|
|
III–IV | 46 | 12 |
|
|
Prognostic value of PIGC protein and
Cox regression analysis
Kaplan-Meier analysis was performed to investigate
the association between PIGC protein expression and OS in patients
with HCC. The Kaplan-Meier survival curve revealed that patients
with HCC with high expression levels of PIGC exhibited a worse OS
compared with those with low PIGC expression (P=0.0324; Fig. 5). The univariate Cox regression
analysis model revealed that PIGC protein expression (HR=3.311; 95%
CI=1.156–9.486; P=0.026) and M stage (HR=2.618; 95% CI=1.247–5.496;
P=0.011) were prognostic factors for patients with HCC. However,
the results showed that PIGC protein expression was not an
independent prognostic factor in patients with HCC following the
multivariate Cox regression analysis (HR=2.697; 95% CI=0.909–8.01;
P=0.074 (Table V).
 | Table V.Cox regression analysis for OS in 88
hepatocellular carcinoma cases. |
Table V.
Cox regression analysis for OS in 88
hepatocellular carcinoma cases.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | HR (95%CI) | P-value | HR (95%CI) | P-value |
|---|
| Sex | 0.746
(0.366–0.746) | 0.420 |
|
|
| Age | 1.04
(0.512–2.11) | 0.914 |
|
|
| G stage | 0.878
(0.424–1.818) | 0.726 |
|
|
| T stage | 1.569
(0.598–4.118) | 0.361 |
|
|
| N stage | 1.261
(0.593–2.682) | 0.547 |
|
|
| M stage | 2.618
(1.247–5.496) | 0.011 | 2.206
(0.942–4.356) | 0.071 |
| TNM stage | 1.204
(0.566–2.56) | 0.629 |
|
|
| PIGC
expression | 3.311
(1.156–9.486) | 0.026 | 2.697
(0.909–8.01) | 0.074 |
PPI network analysis
The STRING database was utilized to reveal the PPI
information about PIGC. As presented in Fig. S3, it was revealed that the PPI
network of PIGC consisted of 21 nodes and 104 edges (PPI
enrichment, P<1.0×10−16). Furthermore, KEGG pathway
enrichment analysis was performed in the present study. According
to five KEGG pathways (Table VI),
it was revealed that PIGC is primarily involved in ‘GPI-anchor
biosynthesis’ [false discovery rate (FDR)=1.72×10−32].
It was also observed that 3 genes (CDIPT, CDS1 and CDS2) may be
involved in the phosphatidylinositol signaling pathway
(FDR=0.00484), which is highly associated with the proliferation
and metastasis of malignant tumors. The KEGG pathway enrichment
analysis indicated that PIGC may participate in the occurrence and
progression of HCC.
 | Table VI.KEGG pathway enrichment analysis of
PIGC from STRING. |
Table VI.
KEGG pathway enrichment analysis of
PIGC from STRING.
| Pathway ID | Pathway
description | Observed gene
count | False discovery
rate | Matching proteins
in network |
|---|
| 563 |
Glycosylphosphatidylinositol-anchor
biosynthesis | 13 |
1.72×10−32 | DPM2, GPAA1, PIGA,
PIGB, PIGC, PIGF, PIGG, PIGH, PIGL, PIGO, PIGP, PIGQ, PIGY |
| 1100 | Metabolic
pathways | 19 |
4.98×10−20 | ALG1, CDIPT, CDS1,
CDS2, DPAGT1, DPM1, DPM2, DPM3, GPAA1, PIGA, PIGB, PIGC, PIGF,
PIGH, PIGL, PIGO, PIGP, PIGQ, PIGY |
| 510 | N-Glycan
biosynthesis | 5 |
1.09×10−07 | ALG1, DPAGT1, DPM1,
DPM2, DPM3 |
| 4070 |
Phosphatidylinositol signaling system | 3 |
4.84×10−03 | CDIPT, CDS1,
CDS2 |
| 564 | Glycerophospholipid
metabolism | 3 |
6.13×10−03 | CDIPT, CDS1,
CDS2 |
Discussion
To the best of our knowledge, the present study is
the first to demonstrate the association between the expression of
PIGC and prognosis significance of patients with HCC. The present
study revealed that PIGC was highly expressed in HCC, both in terms
of mRNA and protein levels, and the upregulation was significantly
associated with unfavorable prognosis in patients with HCC.
Subsequently, a negative association between DNA methylation and
PIGC expression was observed according to the Cbioportal website,
implying that PIGC expression is negatively regulated by DNA
methylation. Based on the aforementioned results, the present study
concluded that PIGC is an oncogene regulated by DNA methylation,
and may be utilized as a potential therapeutic target for the
treatment of HCC.
It has been well established that there are 10 genes
encoding enzymes in the process of GPI synthesis that have already
been discovered, and PIGC is one of these important genes (7). The transfer of N-acetylglucosamine from
UDP-GlcNAc to GPI is the first step in the biosynthesis of GPI, and
this crucial step is regulated by PIGC (17). GPI anchoring is essential for
embryogenesis, neurogenesis and immune responses. Studies have
revealed that mutations of PIGC can lead to the death of embryos
and the disruption of PIGC is lethal in yeasts (18,19).
Furthermore, GPI anchors are bound to proteins expressed on the
surface of eukaryotic cells (20).
Over 150 functionally diverse proteins are GPI-anchored, including
enzymes, proteins involved in endocytosis (21), adhesion molecules, receptors and
complement regulators (20). The
overexpression of Glypican 3 (GPC3), a member of GPI-anchored
proteoglycans, aids in suppressing hepatocyte proliferation and
liver regeneration in transgenic mice (22). Grozdanov et al (23) reported that GPC3 is upregulated in
the majority of liver cancer types, but not expressed in normal
hepatocytes and benign liver disease. They concluded that GPC3
contributes to the proliferation and metastasis of HCC by
activating Wnt signaling. Phosphatidylinositol-glycan biosynthesis
class X protein (PIGX) is also an important gene in the
biosynthetic pathway of GPI anchoring (23). A recent study reported that PIGX is
highly expressed in breast cancer (24). C4.4A, a glycolipid-anchored membrane
protein, is overexpressed in patients with esophageal cancer and
associated with a poor prognosis (25).
According to the KEGG pathway enrichment analysis,
the upregulation of PIGC was associated with the activation of
biological processes involved in tumor progression, including the
phosphatidylinositol signaling pathway. Phosphatidylinositol
signaling, a complicated network of phospholipid messengers and
enzymes, represents one of the most substantial signaling systems
involved in a variety of lesions, including cancer (26). Previous studies have revealed that
the phosphorylation of PI (3–5) P3
mediated by PI3K leads to the recruitment of Akt, causing the
activation of downstream signaling cascades (PI3K/Akt) (27,28).
PI3K/Akt signaling is an important proliferative pathway involving
various cytokines, growth factors and receptors (29). Furthermore, the PI3K/Akt signaling
pathway occupies a central position in the regulation of cell
invasion and metastasis, and its activation is a crucial feature of
the epithelial-mesenchymal transition in the progression of cancer
(30). Hence, it is plausible that
PIGC may participate in the generation and progression of HCC
mediated by phosphatidylinositol signaling (PI3K/Akt pathway),
highlighting novel potential targets for therapeutic interventions.
Further biochemical, animal and even clinical studies are required
in order to clarify the potential mechanism of PIGC involved in the
tumorigenesis of HCC.
From the TCGA-LIHC data, the present study observed
that PIGC mRNA expression was significantly associated with M and N
stages, which was in line with the results from the IHC assay.
Although the Cox regression analysis showed that PIGC mRNA
overexpression was an independent risk factor for shorter OS,
similar results in PIGC protein levels were not observed. The
different results between PIGC mRNA and protein may be explained by
the following reasons. Firstly, compared with the TCGA-LIHC data,
the number of patients with HCC included in the present study from
our hospital were relatively small. Secondly, this discrepancy may
be due to ethnic differences. Finally, there may be some unknown
regulation processes that occur during the translation process of
PIGC.
Several limitations exist in the present study.
Firstly, as only 88 cases of specimens stained by IHC were included
in the present study, the results should be interpreted with
caution. Secondly, as only the OS data were recorded and the DFS
data were neglected during follow-up, it is not possible to
determine the association between PIGC protein expression and the
prognosis of patients with HCC. Thirdly, the potential correlations
between PIGC methylation and prognostic factors in HCC were not
investigated. Finally, the genes co-expressed alongside PIGC were
not investigated. Hence, the potential mechanism of PIGC
dysregulation in the pathogenesis and development of HCC needs to
be further investigated through medical experiments.
In conclusion, PIGC is a novel oncogene regulated by
DNA methylation. An elevated expression of PIGC was found to be
significantly correlated with an unfavorable survival. PIGC may be
utilized as a new prognostic biomarker and is a potential target
for personalized medicine in the future.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by Hubei Municipal
Health and Family Planning Commission (grant no. WJ2017Q034).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XP and CL contributed to the design of the present
study, developed the methodology, collected the bioinformatics
data, performed the experiments, analyzed the results and wrote the
manuscript. AH, RL and YC contributed to the collection of patient
data. XP and WD contributed to the design of the study, critically
revised the manuscript and approved the final version to be
published. All authors agreed to be accountable for all aspects of
the study.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Renmin Hospital of Wuhan University (Wuhan,
China). Written patient consent was obtained.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Heimbach JK, Kulik LM, Finn RS, Sirlin CB,
Abecassis MM, Roberts LR, Zhu AX, Murad MH and Marrero JA: AASLD
guidelines for the treatment of hepatocellular carcinoma.
Hepatology. 67:358–380. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bertuccio P, Turati F, Carioli G,
Rodriguez T, La Vecchia C, Malvezzi M and Negri E: Global trends
and predictions in hepatocellular carcinoma mortality. J Hepatol.
67:302–309. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maucort Boulch D, De Martel C, Franceschi
S and Plummer M: Fraction and incidence of liver cancer
attributable to hepatitis B and C viruses worldwide. Int J Cancer.
142:2471–2477. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choi J, Han S, Kim N and Lim YS:
Increasing burden of liver cancer despite extensive use of
antiviral agents in a hepatitis B virus-endemic population.
Hepatology. 66:1454–1463. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264–1273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu H, Yang C, Lu W and Zeng Y: Prognostic
significance of glypican-3 expression in hepatocellular carcinoma.
Medicine (Baltimore). 97:e97022018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Watanabe R, Inoue N, Westfall B, Taron CH,
Orlean P, Takeda J and Kinoshita T: The first step of
glycosylphosphatidylinositol biosynthesis is mediated by a complex
of PIG-A, PIG-H, PIG-C and GPI1. EMBO J. 17:877–885. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Edvardson S, Murakami Y, Nguyen TT,
Shahrour M, St-Denis A, Shaag A, Damseh N, Le Deist F, Bryceson Y,
Abu-Libdeh B, et al: Mutations in the phosphatidylinositol glycan C
(PIGC) gene are associated with epilepsy and intellectual
disability. J Med Genet. 54:196–201. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jaeken J and Péanne R: What is new in CDG?
J Inherit Metab Dis. 40:569–586. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schleinitz D, Klöting N, Lindgren CM,
Breitfeld J, Dietrich A, Schön MR, Lohmann T, Dreßler M, Stumvoll
M, Mccarthy MI, et al: Fat depot-specific mRNA expression of novel
loci associated with waist-hip ratio. Int J Obes (Lond).
38:120–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang L, Gao Z, Hu L, Wu G, Yang X, Zhang
L, Zhu Y, Wong BS, Xin W, Sy MS and Li C:
Glycosylphosphatidylinositol anchor modification machinery
deficiency is responsible for the formation of pro-prion protein
(PrP) in BxPC-3 protein and increases cancer cell motility. J Biol
Chem. 291:3905–3917. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim N, Park WY, Kim JM, Park JH, Kim JS,
Jung HC and Song IS: Gene expression of AGS cells stimulated with
released proteins by Helicobacter pylori. J Gastroen
Hepatol. 23:643–651. 2008. View Article : Google Scholar
|
|
13
|
Kawakami F, Sircar K, Rodriguez-Canales J,
Fellman BM, Urbauer DL, Tamboli P, Tannir NM, Jonasch E, Wistuba
II, Wood CG, et al: Programmed cell death ligand 1 and
tumor-infiltrating lymphocyte status in patients with renal cell
carcinoma and sarcomatoid dedifferentiation. Cancer. 123:4823–4831.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cao Y, Song J, Chen J, Xiao J, Ni J and Wu
C: Overexpression of NEK3 is associated with poor prognosis in
patients with gastric cancer. Medicine (Baltimore). 97:e96302018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Camp RL, Dolled-Filhart M and Rimm DL:
X-tile: A new bio-informatics tool for biomarker assessment and
outcome-based cut-point optimization. Clin Cancer Res.
10:7252–7259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tai CJ, Su TC, Jiang MC, Chen HC, Shen SC,
Lee WR, Liao CF, Chen YC, Lin SH, Li LT, et al: Correlations
between cytoplasmic CSE1L in neoplastic colorectal glands and depth
of tumor penetration and cancer stage. J Transl Med. 11:292013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Inoue N, Watanabe R, Takeda J and
Kinoshita T: PIG-C, one of the three human genes involved in the
first step of glycosylphosphatidylinositol biosynthesis is a
homologue of Saccharomyces cerevisiae GPI2. Biochem Biophys Res
Commun. 226:193–199. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shamseldin HE, Tulbah M, Kurdi W, Nemer M,
Alsahan N, Al Mardawi E, Khalifa O, Hashem A, Kurdi A, Babay Z, et
al: Identification of embryonic lethal genes in humans by
autozygosity mapping and exome sequencing in consanguineous
families. Genome Biol. 16:1162015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leidich SD, Kostova Z, Latek RR, Costello
LC, Drapp DA, Gray W, Fassler JS and Orlean P:
Temperature-sensitive yeast GPI anchoring mutants gpi2 and gpi3 are
defective in the synthesis of N-acetylglucosaminyl
phosphatidylinositol. Cloning of the GPI2 gene. J Biol Chem.
270:13029–13035. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kinoshita T and Fujita M: Biosynthesis of
GPI-anchored proteins: Special emphasis on GPI lipid remodeling. J
Lipid Res. 57:6–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lakhan SE, Sabharanjak S and De A:
Endocytosis of glycosylphosphatidylinositol-anchored proteins. J
Biomed Sci. 16:932009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu B, Bell AW, Paranjpe S, Bowen WC,
Khillan JS, Luo JH, Mars WM and Michalopoulos GK: Suppression of
liver regeneration and hepatocyte proliferation in
hepatocyte-targeted glypican 3 transgenic mice. Hepatology.
52:1060–1067. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grozdanov PN, Yovchev MI and Dabeva MD:
The oncofetal protein glypican-3 is a novel marker of hepatic
progenitor/oval cells. Lab Invest. 86:1272–1284. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakakido M, Tamura K, Chung S, Ueda K,
Fujii R, Kiyotani K and Nakamura Y: Phosphatidylinositol glycan
anchor biosynthesis, class X containing complex promotes cancer
cell proliferation through suppression of EHD2 and ZIC1, putative
tumor suppressors. Int J Oncol. 49:868–876. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ohtsuka M, Yamamoto H, Masuzawa T,
Takahashi H, Uemura M, Haraguchi N, Nishimura J, Hata T, Yamasaki
M, Miyata H, et al: C4.4A expression is associated with a poor
prognosis of esophageal squamous cell carcinoma. Ann Surg Oncol.
20:2699–2705. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thapa N, Tan X, Choi S, Lambert PF,
Rapraeger AC and Anderson RA: The hidden conundrum of
phosphoinositide signaling in cancer. Trends Cancer. 2:378–390.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thorpe LM, Yuzugullu H and Zhao JJ: PI3K
in cancer: Divergent roles of isoforms, modes of activation and
therapeutic targeting. Nat Rev Cancer. 15:7–24. 2014. View Article : Google Scholar
|
|
28
|
Vanhaesebroeck B, Stephens L and Hawkins
P: PI3K signalling: The path to discovery and understanding. Nat
Rev Mol Cell Biol. 13:195–203. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu P, Cheng H, Roberts TM and Zhao JJ:
Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev
Drug Discov. 8:627–644. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song LB, Li J, Liao WT, Feng Y, Yu CP, Hu
LJ, Kong QL, Xu LH, Zhang X, Liu WL, et al: The polycomb group
protein Bmi-1 represses the tumor suppressor PTEN and induces
epithelial-mesenchymal transition in human nasopharyngeal
epithelial cells. J Clin Invest. 119:3626–3636. 2009. View Article : Google Scholar : PubMed/NCBI
|