Introduction
Nasopharyngeal carcinoma (NPC), that originates from
the epithelium of the nasopharynx, is a malignant head and neck
tumor characterized by local invasion and early distant metastasis
(1). Recently, more and more
researchers have realized that non-coding RNAs (ncRNAs), including
microRNAs (miRNAs/miRs) and long non-coding RNAs (lncRNAs), are new
sets of clinical biomarkers and potential tumor treatment targets
(2). A lncRNA is a transcript of
more than 200 nucleotides in length, lacking the ability of protein
coding. lncRNAs seem to participate in a variety of biological
processes, such as cell proliferation, invasion, apoptosis and
cancer progression (3). There is
evidence that lncRNA disorders are involved in cell transformation
and development of a variety of cancers, including NPC (4,5). A
recent study demonstrated that silencing of lncRNA SRRM2-AS
inhibited NPC cell proliferation, colony formation and
angiogenesis, blocked cell cycle progression and enhanced apoptosis
(6). Xue and Cao found that CASC15
enhanced NPC cell proliferation and metastasis via sponging
miR-101-3p in vitro and in vivo (7). The inhibitory influence of XIST on
miR-491-5p was found to suppress the growth of NPC tumors in
vivo (8). miRNAs are a type of
conserved endogenous ncRNAs, which can negatively regulate gene
expression at the post transcriptional level (9). As confirmed, miRNAs can function as
proto-oncogenes or tumor-suppressor genes to participate in various
cell biological processes, such as cell proliferation, migration
and autophagy (10). miR-30b is one
member of the miR-30 family, which was found to play essential
roles in proliferation, invasion, and autophagy in osteosarcoma
cells (11). However, there is no
direct evidence to support the involvement of miR-30b in NPC
progression and processes.
X-inactive specific transcript (XIST) is an lncRNA
derived from the XIST gene (12).
XIST is highly expressed in a variety of tumors including ovarian
cancer, non-small cell lung cancer, glioblastoma, breast cancer and
liver cancer (13–17). Silencing of XIST was found to inhibit
cell growth, metastasis and promote cell apoptosis, and knockdown
of XIST can also inhibit tumor growth and promote high survival
rate in nude mice, which indicates that XIST plays a pivotal role
in the occurrence, development and progress of malignant tumors
(17). However, the role of XIST in
NPC and its potential biological mechanism remains to be explored.
Overexpression of miR-30b was confirmed to inhibit cell migration
in NSCLC (18). Interestingly,
although miR-30b has been reported to play an inhibitory role in
certain types of cancer, it has also been shown to play a role as
an oncogene in melanoma (19,20). The
matrix metalloproteinases (MMPs) are a family of zinc and
calcium-dependent proteolytic enzymes and play an important role in
osteogenic differentiation (20).
Relevant experiments show that targeting cysteine rich protein with
kazal motifs (RECK) may be an effective way to prevent the
progression of oral cancer (21).
The molecular mechanism of lncRNA XIST involved in the regulation
of invasion and migration of NPC cells by miR-30b remains
unclear.
Materials and methods
Tissue specimen
Thirty-five pairs of matched tumor tissues and
adjacent non-tumor tissues were collected from NPC patients (15
women and 20 men; age range, 44–70 years; median age, 61 years),
who were surgically operated on at Liaocheng People's Hospital from
February 2010 to October 2016. This study was approved by the
Ethics Committee of Liaocheng People's Hospital. Prior to the
study, all participants provided informed consent.
Cell culture
Human nasopharyngeal epithelial cell line NP69 and
human NPC cell lines (SUNE1 and HK1) were purchased from the
American Type Culture Collection (ATCC) and maintained in RPMI-1640
medium (Invitrogen; Thermo Fisher Scientific, Inc.) supplemented
with 10% fetal bovine serum (FBS; Invitrogen; Thermo Fisher
Scientific, Inc.) and 1% penicillin/streptomycin in 5%
CO2 in a 37°C incubator. The primary normal human nasal
epithelial cell line was cultured in epithelial cell growth medium
(Promocell) at 37°C in 5% CO2.
Cell transfection
siRNA of XIST (si-XIST), siRNA control (siNC),
miR-30b mimic (miR-30b), miRNA control (MIR con) and miR-30b
inhibitor were purchased from Genepharma Co., Ltd. NPC cells were
cultured into a 6-well plate and cultured in a complete growth
medium. Antibiotics were not used for at least 24 h before
transfection. Then, the cells were transiently transfected with
siRNA or co-transfected with si-XIST and miR-30b inhibitor or mimic
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.). The transfected cells were collected and analyzed. The
sequence of siXIST, siNC, miR-30b mimic and inhibitor were as
follows: si-XIST, 5′-GACCUUGUCAUGUGGAUAUTT−3′ (forward) and
5′-AUAUCCACAUGACAAGGUCTT−3′ (reverse); si-NC,
5′-UUCUCCGAACGUGUCACGUTT−3′ (forward) and
5′-ACGUGACACGUUCGGAGAATT−3′ (reverse); miR-30b mimic,
5′-UGUAAACAUCCUACACUCAGCU-3′ (forward), and
3′-ACAUUUGUAGGAUGUGAGUCGA-5′ (reverse); miR-30b inhibitor,
5′-AGCUGAGUGUAGGAUGUUUACA-3′; miR-30b mimic control,
5′-UCACAACCUCCUAGAAAGAGUAGA−3′; miR-30b inhibitor control,
5′-UCACAACCUCCUAGAAAGAGUAGA−3′.
Quantitive real-time PCR (qPCR)
assay
The total RNA from NPC cells was separated by TRIzol
reagent. The synthesis of cDNA required the use of the M-MLV
Reverse Transcriptase Kit (Toyobo). RT-qPCR was performed with the
SYBR Green Real-Time PCR analysis Kit (Takara). U6 and GAPDH were
respectively used for internal controls for RT-qPCR and western
blot analysis. The qPCR cycling conditions were as follows: 95°C
for 10 min, followed by 40 cycles at 95°C for 15 sec and 60°C for
45 sec, and a final extension step of 95°C for 15 sec, 60°C for 1
min, 95°C for 15 sec and 60°C for 15 sec. Calculation of target
gene expression and protein level was performed using the
2−ΔΔCq method (22).
Primer sequences were as follows: XIST forward (F),
5′-AATGGAACGGGCTGAGTTTTAG-3′ and reverse (R),
5′-TCATCCGCTTGCGTTCATAG-3′; miR-30b F, 5′-UGUAAACAUCCUACACUCAGCU-3′
and R, 5′-ACAUUUGUAGGAUGUAGUCGA−3′; RECK F,
5′-TGTTGACCTGTTTAGCGGATGT-3′ and R, 5′-GAAAAGTTCTGTTGGCCTGTTGT-3′;
GAPDH F, 5′-AGGCTGTTGGGAAAGTTCTTC−3′ and R,
5′-ACTGTTGGAACTCGGAATGC-3′; U6 F, 5′-TGCGGGTGCTCGCTTCGGCAGC-3′ and
R, 5′-CCAGTGCAGGGTCCGAGGT-3′.
CCK-8 assay
Cell proliferation was analyzed and determine using
the CCK-8 assay. In short, 1×103 cells/well were laid in
triplicate on a 96-well culture plate (Costar, Corning Inc.),
cultured for 24 h, and then transfected with si-XIST, si-NC, or
si-XIST + miR-30b inhibitor. At 24, 48 and 72 h after transfection,
10 µl CCK-8 solution was added to each well. The cells were
incubated for an additional 1 h, and then the proliferative
activity of the cells was measured at 450 nm by VersaMax (Molecular
Devices, LLC).
Detection of double luciferase
reporter assay
The putative binding site for miR-4301 in HOTTIP was
predicted using a bioinformatics tool starBase (http://starbase.sysu.edu.cn/index.php).
The wild-type (WT) XIST 3′-UTR (untranslated region) and mutated
(MUT) XIST 3-UTR without miR-30b were designed and constructed. The
mature miR-30b and its negative control (NC) sequences were
cotransfected with XIST 3-UTR-WT and XIST into cells. The
fluorescent enzyme activity reagent (Promega Corp.) of the sample
was detected by double fluorescent enzyme reporter gene assay.
After 48 h, the cells were harvested and detected for luciferase
activity. The double fluorescein reporter gene assay system
(Promega Corp.) was used. Luciferase co-transfection was used as a
standard control.
Western blot analysis
The total protein was extracted from NPC cells and
tissues RIPA lysis buffer (Beyotime Biotechnology). Measurement of
total protein concentration was performed using the BCA kit
(Pierce; Thermo Fisher Scientific, Inc.). Equal amount of protein
(12 µg per lane) from the cell lysates was separated by 10%
SDS-PAGE gels and transferred to polyvinylidene fluoride (PVDF)
membranes (Millipore Corp.). At room temperature, the PVDF membrane
was blocked with 5% fat-free milk for 1 h. Subsequently, the
membranes were rinsed with TBST twice and incubated with primary
antibodies, including RECK (cat. no. ab238162, 1:1,000; Abcam);
E-cadherin (cat. no. 3195, 1:2,000; Cell Signaling Technology,
Inc.), N-cadherin (cat. no. 13116, 1:2,000; Cell Signaling
Technology, Inc.), Vimentin (cat. no. 5741, 1:2,000; Cell Signaling
Technology, Inc.) and glyceraldehyde-3-phosphate dehydrogenase
(GAPDH, cat. no. ab9485, 1:10,000; Abcam, the loading control) at
room temperature for 3 h. The membranes were then incubated with
the horseradish peroxidase-conjugated secondary antibody (cat. no.
ab6721; 1:10,000; Abcam) at room temperature for 1 h. Then,
enhanced chemiluminescence was used to develop, immobilize and
analyze the results. The relative expression of the target protein
was calculated according to the internal reference protein of
GAPDH.
Transwell assays
Transwell chambers (8-µm pore size; Costar, Inc.)
were used to assess the migration and invasion of NPC cells. For
the migration assays, 5×104 cells were added into the
upper chamber. For the invasion assays, 1×105 cells were
added into the upper chamber precoated with Matrigel (BD
Bioscience). Matrigel was dissolved overnight at 4°C, diluted with
serum-free medium at a ratio of 1:3, and added at 50 µl/hole to the
top chamber of a Transwell chamber. Then the plate was air dried in
an incubator for 4–5 h. An amount 500 µl medium with 15% FBS was
then placed into the basolateral chamber. The chamber was
maintained at 37°C in a 5% CO2 incubator for 48 h. The
cells on the lower surface of the membrane were then fixed with 4%
paraformaldehyde (25°C for 10 min), stained with 0.5% crystal
violet (25°C for 30 min). The images were captured in four randomly
selected fields under a inverted microscope (CKX41; Olympus, Japan)
and images were captured at ×200 magnification.
Statistical analysis
Results are displayed as mean ± SD from experiments
conducted in triplicate. Student's t-test and and one-way analysis
of variance (ANOVA) with Tukey's post hoc test were used to analyze
differences between two groups and multiple groups, respectively.
All statistical analyses were performed using SPSS 20.0 software
(IBM Corp.) and GraphPad Prism 5.02 Software (GraphPad Software,
Inc.). P-value <0.05 was considered to indicate a statistically
significant result.
Results
Expression of XIST in NPC and adjacent
tissues and cell lines
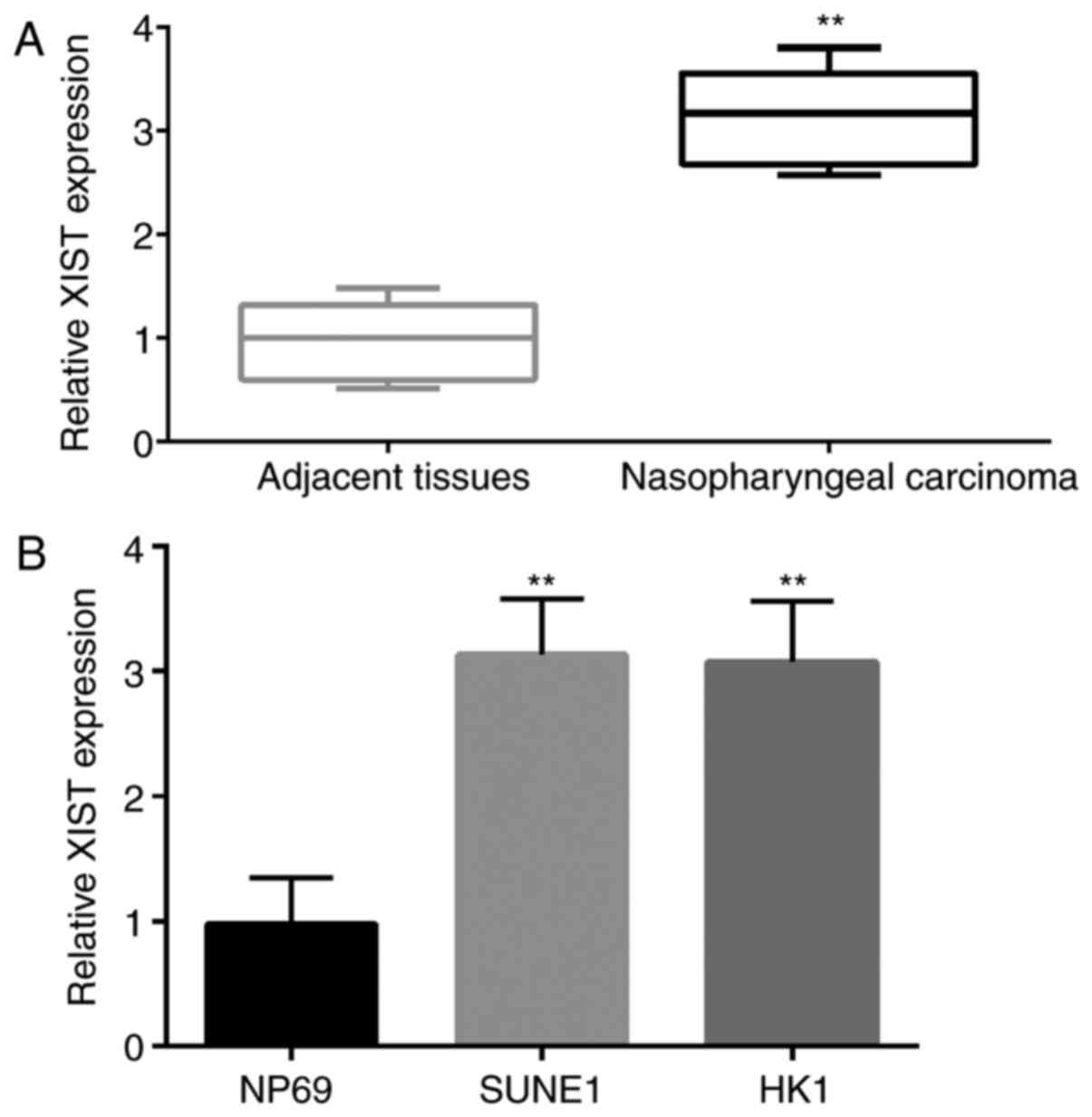
First, the expression of XIST in 35 NPC and adjacent
tissues and 2 types of NPC cell lines (SUNE1 and HK1) was detected
by qPCR. The results showed that the expression level of XIST in
tumor tissue was significantly higher than that in the adjacent
normal tissue (Fig. 1A). Similar to
this result, the expression level of XIST in NPC cell lines (SUNE1
and HK1) was significantly higher than that in the NP69 cells
(Fig. 1B).
Knockdown of lncRNA XIST inhibits the
proliferation and metastasis of NPC cells
In our study, SUNE1 HK1 cell lines were selected to
knockdown XIST in vitro. qPCR was used to detect the
expression of XIST following knockdown of XIST (Fig. 2A) and confirm the knockdown
efficiency. CCK-8 assay demonstrated that the growth ability of
SUNE1 and HK1 cells were significantly inhibited by the knockdown
of XIST (Fig. 2B). Transwell assay
showed that the number of invasive and migratory cells in the SUNE1
(Fig. 2C and E) and HK1 (Fig. 2D and F) cell lines was significantly
decreased after knockdown of XIST.
Expression level of miR-30b in NPC
tissues and cell lines
First, the expression of miR-30b in 35 NPC tissues
and adjacent tissues and NPC cell lines (SUNE1 and HK1) was
detected by qPCR. The results stated clearly that the expression
level of miR-30b in tumor tissues was significantly lower than that
in the adjacent normal tissues (Fig.
3A). Similar to this result, the expression level of miR-30b in
the NPC cell lines was significantly lower than that in the NP69
cells (Fig. 3B).
lncRNA XIST is targeted by miR-30b and
negatively regulates its expression
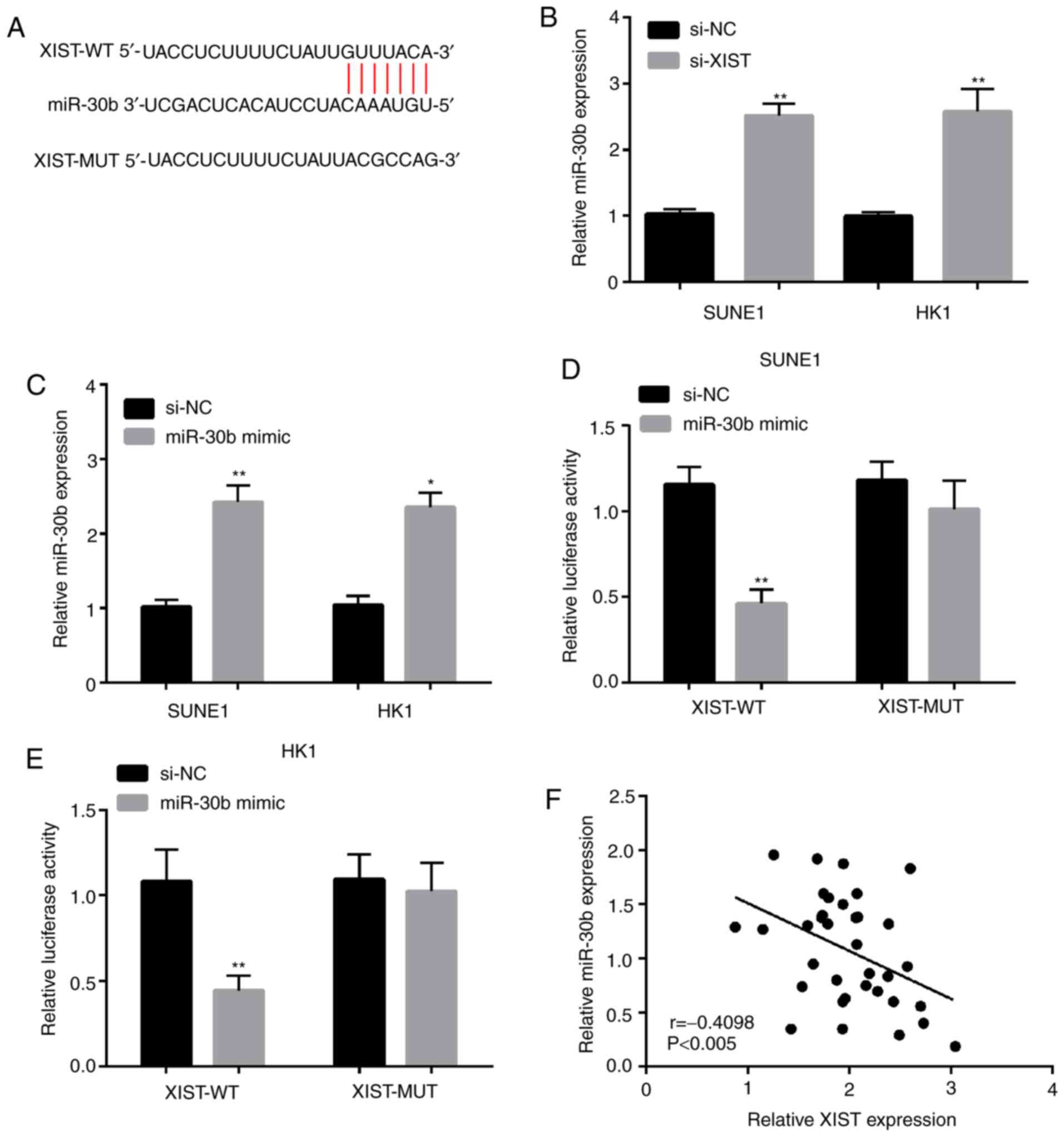
To explore the regulatory mechanisms of lncRNA XIST,
the prediction of target sites between lncRNA XIST and miR-30b was
performed by StarBase v3.0 (Fig.
4A). miR-30b was selected from these miRNAs that interacted
with lncRNA XIST. In addition, the miR-30b expression was explored
by qPCR in SUNE1 and HK1 cell lines following lncRNA XIST
knockdown. The results demonstrated that miR-30b expression was
significantly increased in SUNE1 and HK1 cell lines transfected
with si-XIST (Fig. 4B). As shown in
Fig. 4C, miR-30b expression was
higher when cells were transfected with miR-30b mimic in the SUNE1
and HK1 cell lines. The luciferase reporter gene assay revealed
that co-transfection of XIST-WT and miR-30b mimic significantly
decreased luciferase activity. However, no significant differences
were observed in luciferase activity after co-transfection of
XIST-MUT and miR-30b mimic (Fig. 4D and
E). In addition, the outcome of the linear correlation analysis
demonstrated that miR-30b expression was negatively correlated with
lncRNA XIST expression in the NPC tissues (Fig. 4F). In a word, these results imply
that lncRNA XIST may serve as a competitive endogenous (ce)RNA to
directly bind to miR-30b and negatively regulate its
expression.
Knockdown of lncRNA XIST or miR-30b suppresses cell
proliferation, migration and invasion in SUNE1 and HK1 cells. As
shown in Fig. 5A, we therefore
transfected the si-NC, si-XIST and miR-30b inhibitor into the SUNE1
and HK1 cell lines. The expression of miR-30b was upregulated by
si-XIST, and this tendence was reversed by the miR-30b inhibitor.
Meanwhile, we tested the expression of epithelial to mesenchymal
transition (EMT) markers at the protein level in the SUNE1 and HK1
cell lines following transfection with si-XIST and/or the miR-30b
inhibitor. The outcome indicated that the E-cadherin (E) protein
level was apparently increased in the SUNE1 and HK1 cells
transfected with si-XIST, which was obviously counteracted when the
miR-30b inhibitor was co-transfected. In addition, the proein
levels of N-cadherin (N) and vimentin (V) were downregulated by
XIST knockdown, and the tendence was reversed by the miR-30b
inhibitor (Fig. 5B). As expected,
the results showed that lncRNA XIST knockdown significantly
suppressed cell invasion (Fig. 5C and
D) and migration (Fig. 5E and F)
in the SUNE1 and HK1 cells, respectively, and co-transfection of
si-XIST and the miR-30b inhibitor reversed the si-XIST-mediated
suppressive effects.
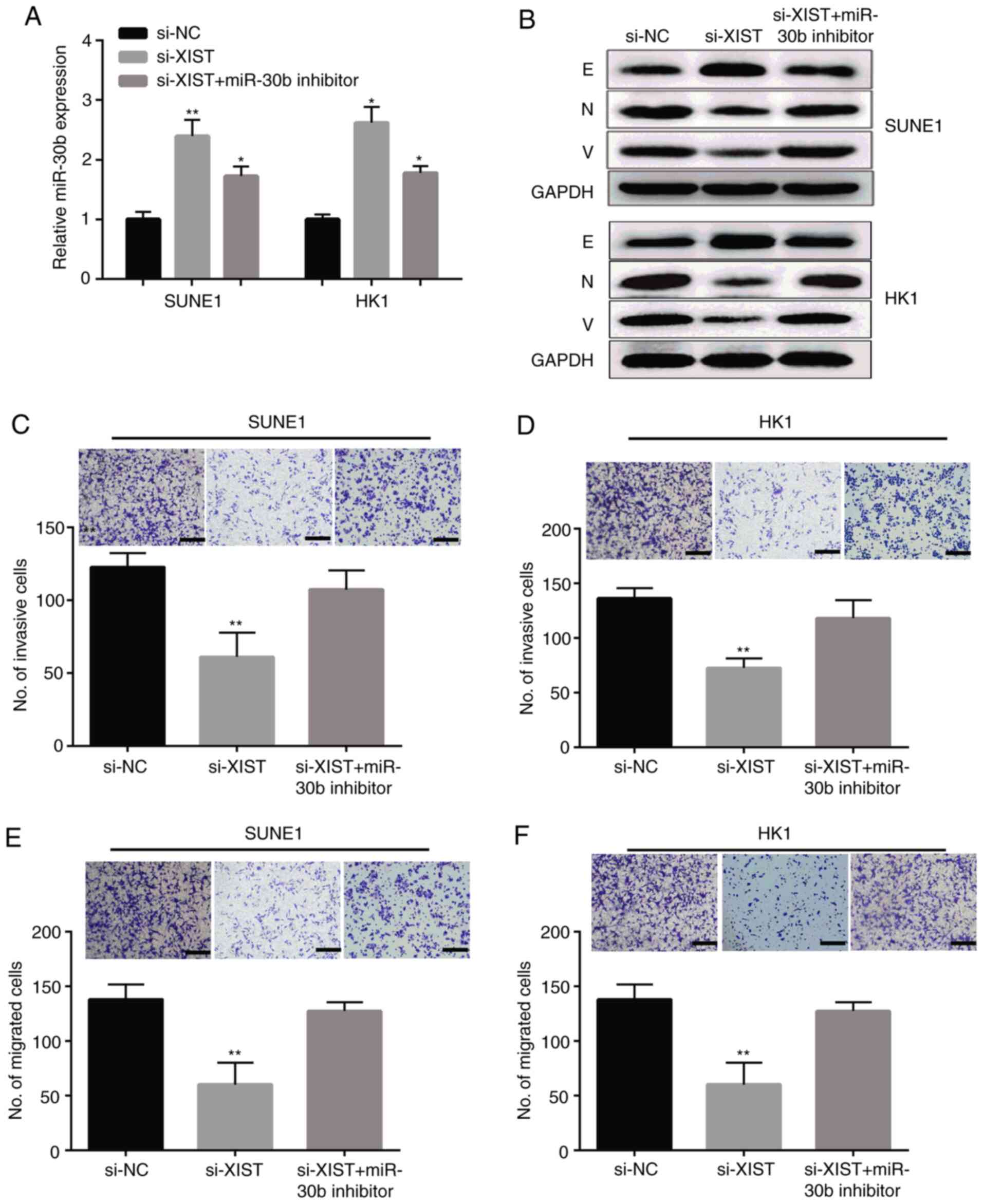 | Figure 5.lncRNA XIST knockdown suppresses NPC
cell invasion and migration by targeting miR-30b. (A) Expression of
miR-30b in NPC SUNE1 and HK1 cells transfected with si-XIST, si-NC,
or si-XIST and miR-30b inhibitor. (B) The protein levels of
EMT-related marker protein in SUNE1 and HK1 cells transfected with
si-XIST, si-NC, or si-XIST and miR-30b inhibitor. E, E-cadherin; N,
N-cadherin; V, vimentin. (C-F) SUNE1 and HK1 cell migration and
invasion after transfection with si-XIST, si-NC, si-XIST and
miR-30b inhibitor (×100 magnification). **P<0.01, *P<0.05,
compared to the si-NC group. XIST, X-inactive specific transcript;
NPC, nasopharyngeal carcinoma; NC, negative control; EMT,
epithelial-to-mesenchymal transition. |
RECK is a direct miR-30b target in NPC
cells
In order to study the mechanism of the effect of
miR-30b on NPC cells, we analyzed the target genes of miR-30b and
predicted that RECK is a direct target of miR-30b by using
TargetScan (http://www.targetscan.org/vert_72/) (Fig. 6A). The analysis of luciferase
reporter gene displayed that the co-expression of miR-30b with
RECK-3′-UTR reporter gene plasmid significantly inhibited
luciferase activity in the SUNE1 and HK1 cells, but no significant
change was observed in the mutant plasmid (Fig. 6B and C). The expression level of RECK
was significantly decreased by the miR-30b mimic in SUNE1 and HK1
cells as shown in Fig. 6D. Protein
level results showed that the expression of RECK was reduced by
si-XIST, which were obviously counteracted when the miR-30b
inhibitor was co-transfected into the SUNE1 and HK1 cells (Fig. 6E).
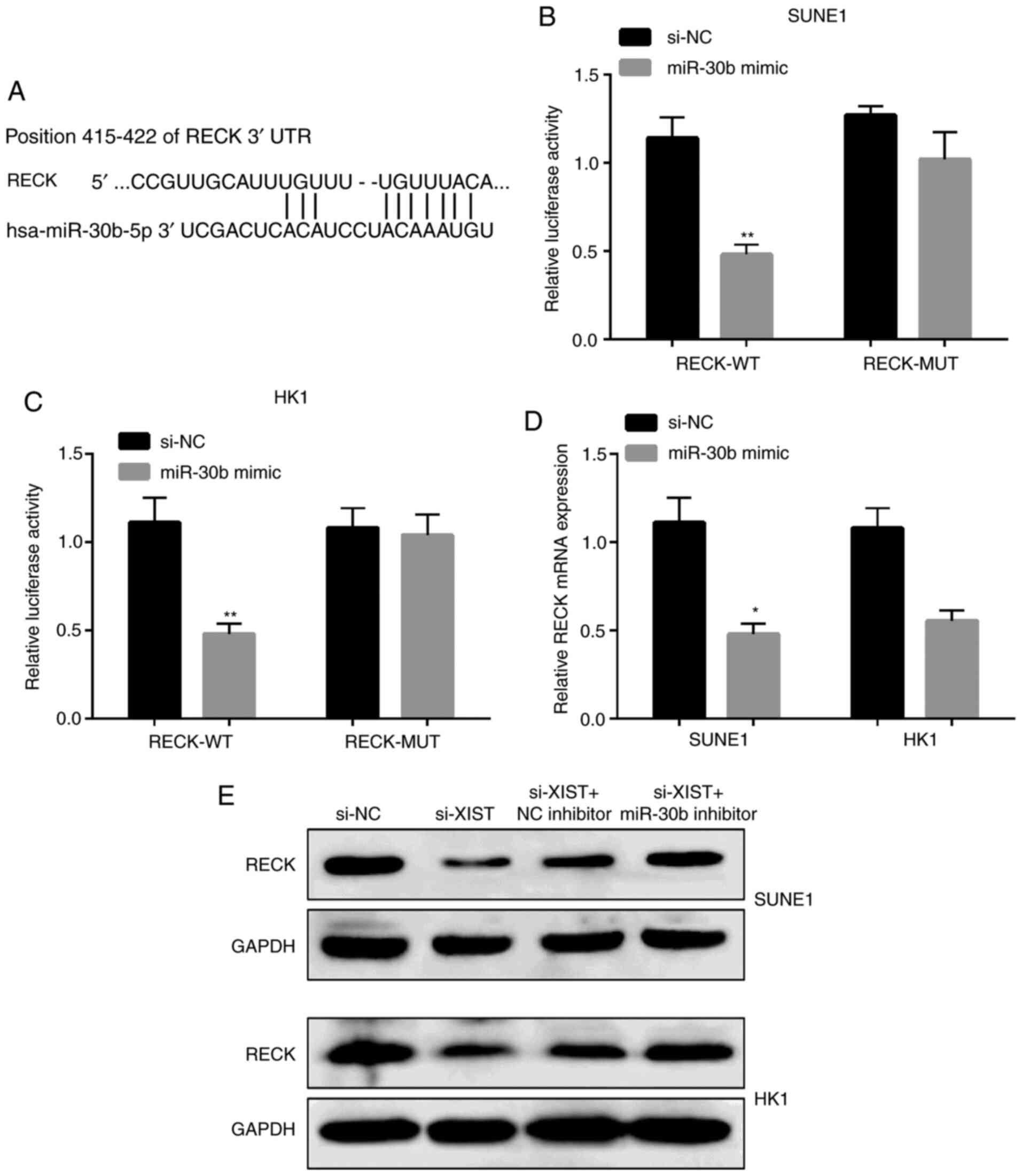 | Figure 6.RECK is a direct miR-30b target in
NPC cells. (A) Display of the RECK 3′UTR-WT or -MUT sequences with
miR-30b sequences. (B and C) Luciferase activity of RECK 3′UTR-WT
or -MUT in SUNE1 and HK1 cells after decreasing miR-30b. (D) RECK
mRNA expression in NPC cells after transfection with the miR-30b
mimic. (E) Protein levels of RECK in NPC cells after transfection
with si-XIST, si-NC, si-XIST and miR-30b inhibitor. **P<0.01,
*P<0.05, compared with the NC. RECK, reversion inducing cysteine
rich protein with kazal motifs; XIST, X-inactive specific
transcript; NPC, nasopharyngeal carcinoma; WT, wild-type; MUT,
mutated; NC, negative control. |
Discussion
Nasopharyngeal carcinoma (NPC), that originates from
the epithelium of the nasopharynx, is a malignant head and neck
tumor characterized by local invasion and early distant metastasis
(1). Despite the possibility of
initial radical treatment, approximately 30% of NPC patients
present with metastasis or disease relapse (23). When the tumor undergoes metastasis
after treatment, the prognosis of NPC patients is poor (24). Statistical data show that despite
significant progress in the 5-year survival rate of
molecular-targeted NPC therapy, patients with NPC still do not meet
the estimated improvement expectations (25). Therefore, there is an urgent need to
study the molecular mechanism underlying the occurrence and
development of NPC and new effective treatment strategies. Long
non-coding RNAs (lncRNAs) can mediate gene expression and affect
tumor development, progression and treatment (26). One study found that lnRNA SRRM2-AS
silencing prevented the angiogenesis of NPC cells by upregulating
MYLK and activating NPR (6). It was
also found that the high expression of lncRNA MALAT1 was related to
the poor prognosis of pancreatic cancer patients (27). Knockdown of lncRNA TUG1 was found to
significantly inhibit tumor proliferation and angiogenesis in
vivo, reduce the activity of hepatoblastoma cells in
vitro, and inhibit various phenomena of tumor cell metastasis
(28). Wang et al
demonstrated that the high expression of lncRNA XIST may play a
significant role in the process of cancer cell lesions by promoting
the proliferation of GBM cells (29). The abnormal expression of lncRNA XIST
has been found in various cancers. Upregulation of the expression
of XIST affecting the behavior of glioma cells (15). Similar to these results, our study
showed that according to qPCR results, when compared with normal
nasopharyngeal epithelial cells, lncRNA XIST was upregulated in NPC
cells. The results of CCK-8 and Transwell assays demonstrated that
inhibition of lncRNA XIST suppressed the proliferation, migration
and invasion of NPC cells, which suggests that lncRNA XIST may
affect the process of NPC metastasis. Our results suggest that
lncRNA XIST is upregulated and enhances the development and
progression of NPC. However, the specific mechanism of lncRNA XIST
in NPC needs further study.
Zhuang et al found that lncRNA XIST can
significantly inhibit the interaction of its target gene miR-92b.
XIST inhibited the proliferation and metastasis of HCC cells by
targeting miR-92b (17). Ma et
al demonstrated that lncRNA XIST also controls the downstream
target MACC1 through competitive endogenous (ce)RNA, so as to boost
the proliferation and invasion of GC cells (30). miR-30b, which belongs to the miR-30
family, is located in the genomic region of chromosome 8q24. miRNA
expression profiles have highlighted miR-30b downregulation as a
common event in human malignancies. For example, overexpression of
miR-30b inhibits cell migration and invasion in non-small cell lung
cancer (31), and high levels of
miR-30b inhibit the growth of gastric cancer cells and promote
apoptosis (32). In our research, we
detected the expression of lncRNA XIST and miR-30b, and found that
they were negatively correlated in NPC cells. Furthermore, on the
basis of the analysis of bioinformatics results, we discovered that
lncRNA XIST includes a target combined site with miR-30b.
Luciferase assay showed that lncRNA XIST interacted directly with
miR-30b and regulated it. These results indicate that the
competitive binding of lncRNA XIST to miR-30b and its expression
are negatively regulated.
It has been confirmed that miRNAs can play a
significant role in many types of cancer. Overexpression of
miR-491-5p was found to significantly inhibit NPC cell
proliferation, migration and invasion in vitro and tumor
growth in vivo by targeting Notch3 (33). For example, miR-96 and miR-21 are
highly expressed in non-small cell lung cancer (NSCLC) tumor
tissues, and the regulation of target genes can significantly
affect the migration and invasion of NSCLC cells (34,35). A
previous studies has shown that miR-21 can alleviate osteoporosis
by targeting the RECK gene, which suggests that RECK may be a new
target for the treatment of osteoporosis (36). RECK has been proven to be a target of
miR-30b by bioinformatics analysis and fluorescein reporter gene
analysis. More importantly, miR-30b mimic suppressed RECK mRNA
expression. Knockdown of lncRNA XIST significantly reduced RECK
expression, which was reversed by the miR-30b inhibitor. We suggest
that lncRNA XIST regulates RECK expression by negatively regulating
miR-30b. In addition, our results showed that si-XIST or miR-30b
inhibitor inhibited cell migration and invasion, suggesting that
the lncRNA XIST/miR-30b/RECK1 axis participates in the progression
of NPC (Fig. S1). The lack of using
external data portal (GEO, ICGC and Arrayexpress) and online
database (PROGgeneV2, GEPIA, UCSC xena, SurvExpress, UALCAN,
Linkedomics, cBioportal, OncomiR and Oncomine) may be a limitation
of the present study. Results using these databases could help to
confirm the findings of our study.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LS and HQ conceived and designed the study, and
drafted the manuscript. LS, MZ and HQ collected, analyzed and
interpreted the experimental data. LS revised the manuscript for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Liaocheng People's Hospital (no. 201002008, Liaocheng, Shandong,
China). Signed written informed consents were obtained from the
patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen YP, Chan ATC, Le QT, Blanchard P, Sun
Y and Ma J: Nasopharyngeal carcinoma. Lancet. 394:64–80. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khoury S and Tran N: Circulating
microRNAs: Potential biomarkers for common malignancies. Biomark
Med. 9:131–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang KC, Yang YW, Liu B, Sanyal A,
Corces-Zimmerman R, Chen Y, Lajoie BR, Protacio A, Flynn RA, Gupta
RA, et al: A long noncoding RNA maintains active chromatin to
coordinate homeotic gene expression. Nature. 472:120–124. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prensner JR and Chinnaiyan AM: The
emergence of lncRNAs in cancer biology. Cancer Discov. 1:391–407.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang QQ and Deng YF: Genome-wide analysis
of long non-coding RNA in primary nasopharyngeal carcinoma by
microarray. Histopathology. 66:1022–1030. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen S, Lv L, Zhan Z, Wang X, You Z, Luo X
and You H: Silencing of long noncoding RNA SRRM2-AS exerts
suppressive effects on angiogenesis in nasopharyngeal carcinoma via
activating MYLK-mediated cGMP-PKG signaling pathway. J Cell
Physiol. 235:7757–7768. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xue MY and Cao HX: Long non-coding RNA
CASC15 promotes nasopharyngeal carcinoma cell proliferation and
metastasis by downregulating miR-101-3p. Eur Rev Med Pharmacol Sci.
23:8897–8904. 2019.PubMed/NCBI
|
|
8
|
Cheng Q, Xu X, Jiang H, Xu L and Li Q:
Knockdown of long non-coding RNA XIST suppresses nasopharyngeal
carcinoma progression by activating miR-491-5p. J Cell Biochem.
119:3936–3944. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han Q, Li L, Liang H, Li Y, Xie J and Wang
Z: Downregulation of lncRNA X inactive specific transcript (XIST)
suppresses cell proliferation and enhances radiosensitivity by
upregulating mir-29c in nasopharyngeal carcinoma cells. Med Sci
Monit. 23:4798–4807. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gu Z, Hou Z, Zheng L, Wang X, Wu L and
Zhang C: LncRNA DICER1-AS1 promotes the proliferation, invasion and
autophagy of osteosarcoma cells via miR-30b/ATG5. Biomed
Pharmacother. 104:110–118. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brown CJ, Ballabio A, Rupert JL,
Lafreniere RG, Grompe M, Tonlorenzi R and Willard HF: A gene from
the region of the human X inactivation centre is expressed
exclusively from the inactive X chromosome. Nature. 349:38–44.
1991. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ren C, Li X, Wang T, Wang G, Zhao C, Liang
T, Zhu Y, Li M, Yang C, Zhao Y and Zhang GM: Functions and
mechanisms of long noncoding RNAs in ovarian cancer. Int J Gynecol
Cancer. 25:566–569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tantai J, Hu D, Yang Y and Geng J:
Combined identification of long non-coding RNA XIST and HIF1A-AS1
in serum as an effective screening for non-small cell lung cancer.
Int J Clin Exp Pathol. 8:7887–7895. 2015.PubMed/NCBI
|
|
15
|
Yao Y, Ma J, Xue Y, Wang P, Li Z, Liu J,
Chen L, Xi Z, Teng H, Wang Z, et al: Knockdown of long non-coding
RNA XIST exerts tumor-suppressive functions in human glioblastoma
stem cells by up-regulating miR-152. Cancer Lett. 359:75–86. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang YS, Chang CC, Lee SS, Jou YS and
Shih HM: Xist reduction in breast cancer upregulates AKT
phosphorylation via HDAC3-mediated repression of PHLPP1 expression.
Oncotarget. 7:43256–43266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhuang LK, Yang YT, Ma X, Han B, Wang ZS,
Zhao QY, Wu LQ and Qu ZQ: MicroRNA-92b promotes hepatocellular
carcinoma progression by targeting Smad7 and is mediated by long
non-coding RNA XIST. Cell Death Dis. 7:e22032016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He S, Lai R, Chen D, Yan W, Zhang Z, Liu
Z, Ding X and Chen Y: Downregulation of miR-221 inhibits cell
migration and invasion through targeting Methyl-CpG binding domain
protein 2 in human oral squamous cell carcinoma cells. Biomed Res
Int. 2015:7516722015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gaziel-Sovran A, Segura MF, Di Micco R,
Collins MK, Hanniford D, Vega-Saenz de Miera E, Rakus JF, Dankert
JF, Shang S, Kerbel RS, et al: miR-30b/30d regulation of GalNAc
transferases enhances invasion and immunosuppression during
metastasis. Cancer Cell. 20:104–118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fan X, Wang E, Wang X, Cong X and Chen X:
MicroRNA-21 is a unique signature associated with coronary plaque
instability in humans by regulating matrix metalloproteinase-9 via
reversion-inducing cysteine-rich protein with Kazal motifs. Exp Mol
Pathol. 96:242–249. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mannello F, Tonti GA, Bagnara GP and Papa
S: Role and function of matrix metalloproteinases in the
differentiation and biological characterization of mesenchymal stem
cells. Stem Cells. 24:475–481. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee V, Kwong D, Leung TW, Lam KO, Tong CC
and Lee A: Palliative systemic therapy for recurrent or metastatic
nasopharyngeal carcinoma-How far have we achieved? Crit Rev Oncol
Hematol. 114:13–23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ran Y, Wu S and You Y: Demethylation of
E-cadherin gene in nasopharyngeal carcinoma could serve as a
potential therapeutic strategy. J Biochem. 149:49–54. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu A, Wu K, Li J, Mo Y, Lin Y, Wang Y,
Shen X, Li S, Li L and Yang Z: Let-7a inhibits migration, invasion
and epithelial-mesenchymal transition by targeting HMGA2 in
nasopharyngeal carcinoma. J Transl Med. 13:1052015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xiong XD, Ren X, Cai MY, Yang JW, Liu X
and Yang JM: Long non-coding RNAs: An emerging powerhouse in the
battle between life and death of tumor cells. Drug Resist Updat.
26:28–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li L, Chen H, Gao Y, Wang YW, Zhang GQ,
Pan SH, Ji L, Kong R, Wang G, Jia YH, et al: Long noncoding RNA
MALAT1 promotes aggressive pancreatic cancer proliferation and
metastasis via the stimulation of autophagy. Mol Cancer Ther.
15:2232–2243. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dong R, Liu GB, Liu BH, Chen G, Li K,
Zheng S and Dong KR: Targeting long non-coding RNA-TUG1 inhibits
tumor growth and angiogenesis in hepatoblastoma. Cell Death Dis.
7:e22782016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Z, Yuan J, Li L, Yang Y, Xu X and
Wang Y: Long non-coding RNA XIST exerts oncogenic functions in
human glioma by targeting miR-137. Am J Transl Res. 9:1845–1855.
2017.PubMed/NCBI
|
|
30
|
Ma L, Zhou Y, Luo X, Gao H, Deng X and
Jiang Y: Long non-coding RNA XIST promotes cell growth and invasion
through regulating miR-497/MACC1 axis in gastric cancer.
Oncotarget. 8:4125–4135. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen S, Li P, Yang R, Cheng R, Zhang F,
Wang Y, Chen X, Sun Q, Zang W, Du Y, et al: MicroRNA-30b inhibits
cell invasion and migration through targeting collagen triple helix
repeat containing 1 in non-small cell lung cancer. Cancer Cell Int.
15:852015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu ED, Li N, Li BS, Li W, Zhang WJ, Mao
XH, Guo G, Zou QM and Xiao B: miR-30b, down-regulated in gastric
cancer, promotes apoptosis and suppresses tumor growth by targeting
plasminogen activator inhibitor-1. PLoS One. 9:e1060492014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Q, Li Q, Xu T, Jiang H and Xu LG:
miR-491-5p suppresses cell growth and invasion by targeting Notch3
in nasopharyngeal carcinoma. Oncol Rep. 35:3541–3547. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu ZL, Wang H, Liu J and Wang ZX:
MicroRNA-21 (miR-21) expression promotes growth, metastasis, and
chemo- or radioresistance in non-small cell lung cancer cells by
targeting PTEN. Mol Cell Biochem. 372:35–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li J, Li P, Chen T, Gao G, Chen X, Du Y,
Zhang R, Yang R, Zhao W, Dun S, et al: Expression of microRNA-96
and its potential functions by targeting FOXO3 in non-small cell
lung cancer. Tumour Biol. 36:685–692. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao W, Dong Y, Wu C, Ma Y, Jin Y and Ji
Y: MiR-21 overexpression improves osteoporosis by targeting RECK.
Mol Cell Biochem. 405:125–133. 2015. View Article : Google Scholar : PubMed/NCBI
|