Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common type of cancer worldwide and the cause of 8.2% of
cancer-related fatalities in 2008 (1). Hepatectomy remains the first option for
patients with HCC, while non-surgical approaches, such as
chemotherapy, radiation, radiofrequency ablation (RFA),
transarterial chemoembolization (TACE) and percutaneous ethanol
injection, have been used to inhibit tumor progression and
recurrence (2). Due to inadequate
resection, unprecedented tumor formation or intrahepatic metastases
that were not detected during resection, the majority of patients
experience recurrence within 5 years of surgery (3).
TACE is globally performed as an effective treatment
for HCC, as it inhibits residual tumor growth, suppresses
metastasis, prevents relapse and prolongs patient survival time
(4). The patients with large HCC
tumor size, Child Pugh A/B or intrahepatic metastases are
considered as candidates for receiving TACE 1–2 months after
resection (5,6). However, not all patients are suitable
candidates for TACE, as it can also result in a deterioration of
liver function and prognosis after surgery (7). Individual analysis of the molecular
mechanism, prediction of the effects of different treatments and
selection of the most appropriate treatment based on the
histological characteristics is the main focus of personalized and
precision medicine (8).
Cochlin (COCH) is a secreted protein identified in
glaucomatous but not normal trabecular meshwork, that has been
shown to be responsive to altered fluid shear dynamics (9). COCH is mainly detected in the normal
inner ear and its mutation has been found to be associated with
hearing loss, glaucoma and DFNA9 (an autosomal dominant cause of
non-syndromic adult-onset sensorineural hearing loss with
associated variable vestibular dysfunction), while it is expressed
at lower levels in the eye, spleen, cerebellum, lung, brain and
thymus (10–12). Through RNA-seq analysis of 20 HCC
tissues and adjacent non-neoplastic tissues, we observed that COCH
was highly expressed in HCC samples (preliminary research, data not
shown). However, to the best of our knowledge, whether COCH is
associated with the tumorigenesis and progression of HCC has not
been reported to date. Therefore, the aim of the present study was
to investigate the prognostic value of COCH and its association
with the effects of TACE in patients with HCC.
Materials and methods
Patients and tissue samples
A total of 135 patients with HCC were recruited from
the Shanghai Eastern Hepatobiliary Surgery Hospital (Shanghai,
China) between January 2005 and December 2007. All the patients
underwent hepatectomy with or without postoperative TACE. The tumor
tissues were embedded in paraffin and underwent tissue microarray
(TMA) analysis. The patients were selected according to the
following inclusion criteria: World Health Organization performance
status 0–1, Child-Pugh class A, absence of ascites, no chemotherapy
or radiotherapy prior to curative resection, and confirmation of
HCC diagnosis by pathological examination (13,14). The
following histological features were examined: Thin beam, thick
beam or pseudoglandular duct, the degree of differentiation, the
degree of necrosis and infiltration, cell type and microvascular
invasion. Hepatectomy was performed as previously described,
Tumor-Node-Metstasis (TNM) stage was then determined (5,15). Tumor
tissue, adjacent non-neoplastic tissues and non-neoplastic distant
tissues were collected after hepatectomy. The tissues outside the
capsule (distance ≤1 cm) were defined as adjacent non-neoplastic
tissues, while the tissues outside the capsule (distance >1 cm)
were defined as non-neoplastic distant tissues. The protocol of the
present study was approved by the Ethics Committee of Shanghai
Eastern Hepatobiliary Surgery Hospital. Patients provided written
informed consent for the publication of any associated data and
accompanying images.
Adjuvant TACE
Patients received hepatic arterial angiography and
adjuvant TACE within 1–2 months of hepatectomy. Patients without a
tumor in the residual liver received preventive TACE (10 mg
hydroxycamptothecin, 20 mg pirarubicin and 1 ml lipiodol). Patients
with tumor in the residual liver received therapeutic TACE (10 mg
hydroxycamptothecin, 20 mg pirarubicin, 100 mg oxaliplatin and 5 ml
Lipiodol). Positron emission tomography-computed tomography
(PET-CT) or magnetic resonance imaging evaluation was performed 1
month after the treatment, in order to decide whether subsequent
TACE treatment should be performed.
Follow-up
The follow-up visits took place once every 3–6
months in the first 5 years after surgery. A complete physical
examination was performed at each follow-up visit. Serum
α-fetoprotein measurements, liver function tests and an abdominal
ultrasound were performed. Furthermore, PET-CT or magnetic
resonance imaging was performed upon suspicion of recurrence or
metastasis. Patients with recurrence received repeat hepatectomy,
chemotherapy, radiotherapy or local ablative therapy, depending on
the size, location and number of recurrent tumors, as well as the
liver function. Overall survival (OS) time was defined as the time
from hepatectomy to the date of death or the date of the last
follow-up. Disease-free survival (DFS) time was defined as the time
from hepatectomy to recurrence or the date of the last
follow-up.
TMA and immunohistochemical
analysis
The clinical tissue samples were fixed with 10%
formaldehyde at room temperature for 24 h and embedded in paraffin.
The section thickness was 3–5 µm. Hematoxylin and eosin staining
(room temperature for 50 sec for both) was performed on the tumor
tissues, adjacent non-neoplastic tissues and non-neoplastic distant
tissues to determine optimal contents. Tissue samples (1 mm in
diameter) were punched from paraffin-embedded tissues and then
arranged in a TMA module with 0.2-mm intervals (Shanghai Biochip
Company, Ltd.). An immunohistochemical assay was performed as
previously reported (16). The
antibody against COCH was purchased from Abcam (cat. no. ab171410;
1:100 dilution). Secondary antibody was purchased from Agilent
Technologies, Inc. (anti-Rabbit-HRP; cat. no. K400311-2; 1:100
dilution). Stained sections were evaluated by three different
researchers who were blinded to the clinical characteristics. The
immunohistochemical staining intensity was scored as follows based
on the coloration intensity and the percentage of stained cells:
The staining intensity was score as: 0, negative; 1, weak; 2,
moderate; or 3, strong. The percent positivity was scored as
0–100%. The percentage and staining intensity scores were
multiplied to yield immunoreactive score: Scores of 0 or 1 were
defined as low expression of COCH, while scores of 2 and 3 were
defined as high expression of COCH (17). Cases in which there were
disagreements on the immunohistochemistry staining intensity score
were discussed with other researchers until a consensus was
reached.
RNA collection, cDNA synthesis and
reverse transcription-quantitative (RT-q)PCR analysis
Total RNA was extracted from cell lines,
fresh-frozen tumor specimens and healthy control tissues using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.).
cDNA synthesis was performed using random hexamers (Roche
Diagnostics) and SuperScriptII reverse transcription (Invitrogen;
Thermo Fisher Scientific, Inc.). RT-qPCR was performed using an ABI
7900 Fast Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) and SYBR Green PCR kit (Takara Bio, Inc.). The
temperature protocol of RT-PCR was: 95°C for 1 min, 35 cycles (95°C
for 30 secs, 60°C for 30 secs and 72°C for 30 secs). 18S was
qualified as reference gene (18).
The primer sequences were as follows: COCH, forward:
5′-AGAAAACACCCGAGAAGAAAACT-3′ and reverse:
5′-CCAATTCCCAACATTAGAGCCA-3′ and 18S, forward:
5′-CGGCTACGACATCCAAGGAA-3′ and reverse: 5′-GCTGGAATTAGCGCGGCT-3′.
The quantity of mRNA was determined using the 2−ΔΔCq
method (19).
Statistical analysis
All the statistical analyses were conducted using
SPSS version 20.0 (IBM Corp.). The differences between tumor
tissues, adjacent non-neoplastic tissues and non-neoplastic distant
tissues were determined by Kruskal-Wallis test, followed by Dunn's
test. The associations between COCH expression and clinical data
were determined using the χ2 test (once expected values
were ≤5, Fisher's exact test was chosen). The differences in OS and
DFS times between groups were determined by Kaplan-Meier analysis
with log-rank tests. A univariate analysis was performed to
determine the variants with statistical significance. The Cox
regression model was used to analyze the effect of independent
factors on OS and DFS time, based on the variants selected by
univariate analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient histological
characteristics
The characteristics of the patients (n=135) are
summarized in Table I. All the
patients were diagnosed through radiological and pathological
examination, and had undergone hepatectomy, with or without TACE.
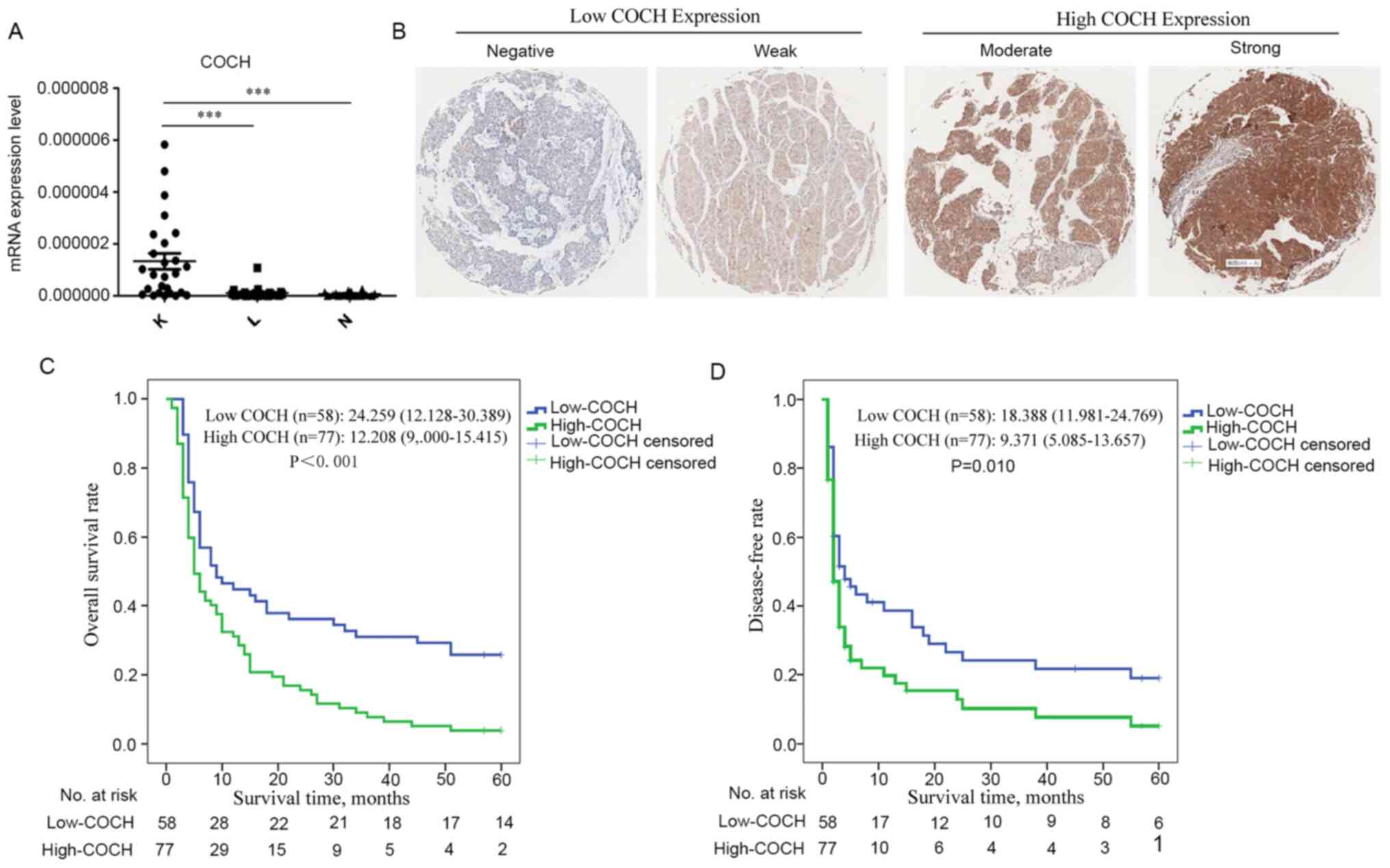
Reverse transcription-PCR analysis of 27 patients revealed that the
mRNA level of COCH was higher in the tumor tissues compared with
that in the adjacent and distant non-neoplastic tissues (Fig. 1A). The patients were divided into two
groups according to the expression of COCH, which was determined by
the immunostaining intensity of TMA slides (Fig. 1B). The immunostaining results were
analyzed and evaluated by three individual researchers
independently.
 | Table I.Patient characteristics (n=135). |
Table I.
Patient characteristics (n=135).
| Characteristic | Value |
|---|
| Age, years |
|
| Mean ±
SD | 48.8±10.294 |
| Median
(range) | 49 (26–75) |
| Sex, n |
|
|
Male | 118 (87.4) |
|
Female | 17
(12.6) |
| HBsAg, n |
|
|
Positive | 126 (93.3) |
|
Negative | 9
(6.7) |
| Largest tumor size,
cm |
|
| ≤5 | 21
(15.6) |
|
>5 | 114 (84.4) |
| Serum AFP,
ng/ml |
|
|
≤400 | 34
(25.2) |
|
>400 | 101 (74.8) |
| Tumor number,
n |
|
|
Single | 122 (90.4) |
|
Multiple | 13
(9.6) |
| Portal vein tumor
thrombus, n |
|
|
Negative | 44
(32.6) |
|
Positive | 91
(67.4) |
| Tumor capsule,
n |
|
|
Complete | 39
(28.9) |
|
Incomplete | 96
(71.1) |
| BCLC stage, n |
|
| A | 13
(9.6) |
| B | 31
(23) |
| C | 91
(67.4) |
| TNM stage, n |
|
|
I/II | 30
(22.2) |
|
III/IV | 105 (77.8) |
| Adjuvant TACE,
n |
|
|
Yes | 39
(28.9) |
| No | 96
(71.1) |
High COCH expression predicts a poor
prognosis of HCC
In all patients, COCH expression levels were found
to be significantly associated with portal vein tumor thrombosis
(PVTT; P=0.039) and BCLC stage (P=0.049) (Table II). Kaplan-Meier analysis revealed
that patients with high COCH expression exhibited a markedly
shorter OS time compared with patients with low COCH expression
(high-COCH patients: Median OS time, 12.208 months; 95% confidence
interval (CI), 9.000–15.415; and low-COCH patients: Median OS time,
24.259 months; 95% CI, 18.218–30.389; P<0.001; Fig. 1C). Furthermore, patients with high
COCH expression exhibited earlier recurrence of HCC (high-COCH
patients: Median DFS time, 9.371 months; 95% CI, 5.085–13.657; and
low-COCH patients: Median DFS time, 18.388 months; 95% CI,
11.981–24.769; P=0.010; Fig.
1D).
 | Table II.Association between COCH protein
expression and clinicopathological characteristics. |
Table II.
Association between COCH protein
expression and clinicopathological characteristics.
|
| COCH expression in
HCC |
|
|---|
|
|
|
|
|---|
| Characteristic | Total, n | Low, n (%) | High, n (%) | P-value |
|---|
| Total patients | 135 | 58 (42.96) | 77 (57.03) |
|
| Age, years |
|
|
|
|
|
≤49 | 69 | 30 (22.22) | 39 (28.89) | 0.902 |
|
>49 | 66 | 28 (20.74) | 38 (28.15) |
|
| Sex |
|
|
|
|
|
Male | 118 | 49 (36.30) | 69 (51.11) | 0.374 |
|
Female | 17 | 9
(0.07) | 8
(0.06) |
|
| HBsAg |
|
|
|
|
|
Negative | 9 | 1
(0.01) | 8
(0.06) | 0.077a |
|
Positive | 126 | 57 (42.22) | 69 (51.11) |
|
| Serum AFP,
ng/ml |
|
|
|
|
|
≤400 | 34 | 18 (13.33) | 16 (11.85) | 0.174 |
|
>400 | 101 | 40 (29.63) | 61 (45.18) |
|
| Largest tumor size,
cm |
|
|
|
|
| ≤5 | 21 | 10 (0.07) | 11 (0.08) | 0.639 |
|
>5 | 114 | 48 (35.56) | 66 (48.89) |
|
| Tumor capsule |
|
|
|
|
|
Complete | 39 | 15 (11.11) | 24 (17.78) | 0.501 |
|
Incomplete | 96 | 43 (31.85) | 53 (39.26) |
|
| Portal vein tumor
thrombus |
|
|
|
|
|
Positive | 92 | 34 (25.19) | 58 (42.96) | 0.039b |
|
Negative | 43 | 24 (17.78) | 19 (14.07) |
|
| Tumor number |
|
|
|
|
|
Single | 122 | 54 (0.40) | 68 (0.50) | 0.350 |
|
Multiple | 13 | 4
(0.03) | 9
(0.07) |
|
| BCLC stage |
|
|
|
|
| A | 13 | 8
(0.06) | 5
(0.04) | 0.049b |
| B | 31 | 16 (0.12) | 15 (11.11) |
|
| C | 91 | 34 (21.16) | 57 (42.22) |
|
| TNM stage |
|
|
|
|
|
I/II | 30 | 17 (12.59) | 13 (0.10) | 0.086 |
|
III/IV | 105 | 41 (30.37) | 64 (47.41) |
|
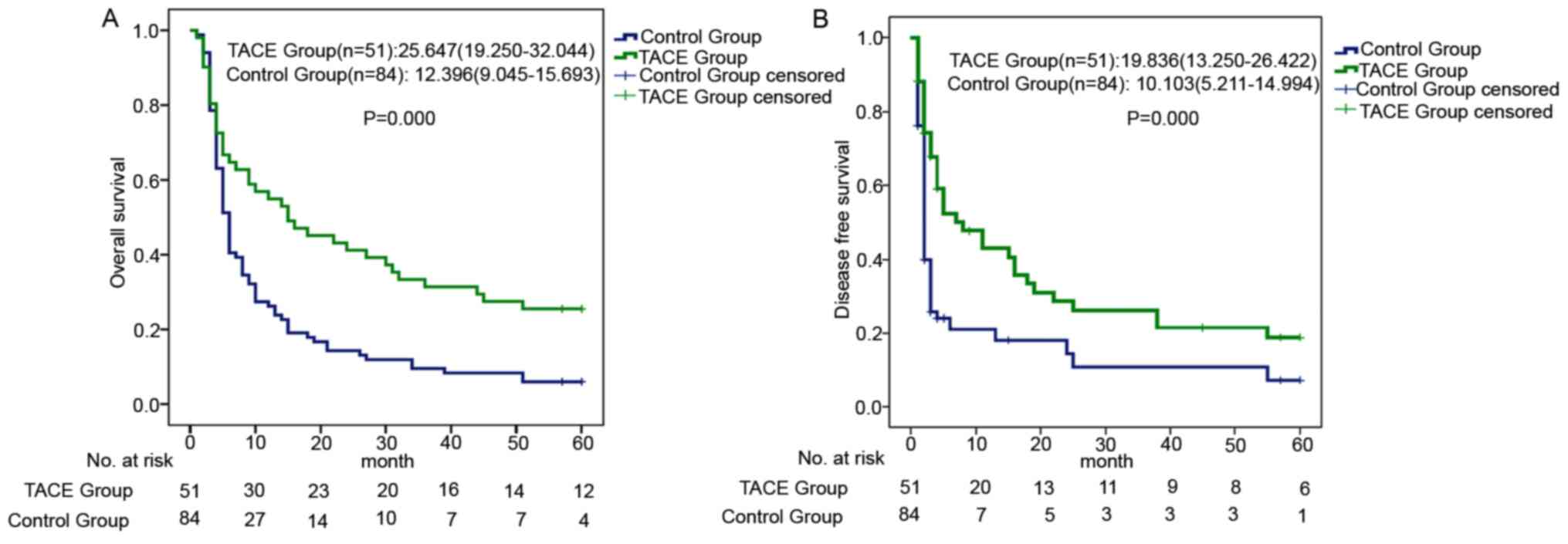
COCH expression level may predict the
effect of adjuvant TACE
The aim of adjuvant TACE is mainly to prevent HCC
recurrence. As shown in Fig. 2A and
B, adjuvant TACE prolonged the OS (adjuvant TACE group: Median
OS time, 25.647 months; 95% CI, 19.250–32.044; and control group:
Median OS time, 12.396 months; 95% CI, 9.045–15.693; P<0.001;
Fig. 2A) and 5-year DFS (adjuvant
TACE group: Median DFS time, 19.836 months; 95% CI, 13.250–26.422;
and control group: Median DFS time, 10.103 months; 95% CI,
5.211–14.994; P<0.001; Fig. 2B)
times of the patients.
The results shown in Fig.
1 indicated that COCH predicted a poor patient prognosis and
early cancer recurrence. The present study also investigated the
association between COCH expression and the effectiveness of TACE.
TACE treatment did not decrease the recurrence rate of patients
with high COCH expression compared with that of patients who did
not receive TACE (control group vs. TACE group: Median DFS time,
8.254 months vs. 12.402 months; 95% CI, 2.725–13.782 vs.
4.915–19.890; P=0.087; Fig. 3B).
However, patients with low COCH expression exhibited a
significantly lower recurrence rate after TACE (low COCH group vs.
high COCH group: Median DFS time, 27.348 months vs. 12.386 months;
95% CI, 17.310–37.385 vs. 3.895–20.878; P=0.002; Fig. 3D). As recurrence significantly
affects the prognosis of patients with HCC, the OS time of patients
in different COCH expression groups, with and without TACE, was
analyzed. TACE treatment was found to not be suitable for patients
with high COCH expression, as it did not reduce recurrence or
prolong OS time (control group vs. TACE group: Median OS time,
11.000 months vs. 14.214 months; 95% CI, 7.574–14.426 vs.
7.923–20.505; P=0.485; Fig. 3A).
However, TACE improved the OS time of the HCC patients with low
COCH expression (control group vs. TACE group: Median OS time,
39.565 months vs. 14.200 months; 95% CI, 30.422–48.708 vs.
7.947–20.453; P<0.001; Fig.
3C).
Univariate and multivariate analysis
of prognostic factors
Cox regression analysis was employed to analyze the
association between COCH level and the effects of TACE. As shown in
Table III, univariate Cox
regression analysis indicated that adjuvant TACE, the size and
number of tumors, completeness of the tumor capsule and HBV
infection (shown as HBsAg in the table) were associated with
recurrence in patients with low COCH expression. Multivariate Cox
regression analysis based on the factors identified as
statistically significant on the univariate Cox regression analysis
revealed that TACE was an independent biomarker for 5-year DFS in
HCC patients with low COCH expression (hazard ratio, 0.4727; 95%
CI, 0.3503–2.139; P=0.0324). In addition, tumor number (P=0.0033)
and tumor size (P=0.0393) were independent predictors of 5-year
DFS. However, the univariate analysis revealed that no variable was
significantly associated with tumor recurrence in patients with
high COCH expression.
 | Table III.Univariate and multivariate Cox
regression analyses of 5-year disease-free survival in patients
with different COCH expression levels. |
Table III.
Univariate and multivariate Cox
regression analyses of 5-year disease-free survival in patients
with different COCH expression levels.
|
| Low COCH
expression | High COCH
expression |
|---|
|
|
|
|
|---|
| Variables | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Univariate
analysis |
|
|
|
|
|
Adjuvant TACE (yes vs.
no) | 0.386
(0.198–0.753) | 0.005a | 0.632
(0.365–1.093) | 0.101 |
| Age
(>49 years vs. ≤49 years) | 1.429
(0.772–2.646) | 0.256 | 0.954
(0.575–1.583) | 0.857 |
| Sex
(male vs. female) | 0.669
(0.263–1.704) | 0.399 | 0.821
(0.352–1.913) | 0.647 |
|
HBsAg* (negative vs. positive) | 21.931
(0.016–29732.5) | 0.401 | 1.308
(0.562–3.043) | 0.534 |
| Serum
AFP (>400 ng/ml vs. ≤400 ng/ml) | 1.326
(0.678–2.596) | 0.41 | 1.192
(0.640–2.222) | 0.580 |
| Largest
tumor size (>5 cm vs. ≤5 cm) | 2.903
(1.124–7.497) | 0.028a | 2.044
(0.919–4.546) | 0.080 |
| Portal
vein tumor thrombus (negative vs. positive) | 1.173
(0.634–2.170) | 0.611 | 1.593
(0.869–2.919) | 0.132 |
| Tumor
capsule (complete vs. incomplete) | 0.440
(0.202–0.959) | 0.039a | 0.749
(0.430–1.303) | 0.306 |
| Tumor
number (single vs. multiple) | 4.035
(1.364–11.937) | 0.012a | 0.882
(0.401–1.942) | 0.755 |
| BCLC
stage (A vs. B vs. C) | 1.292
(0.851–1.960) | 0.229 | 1.474
(0.951–2.284) | 0.083 |
| TNM
(I+II vs. III+IV) | 1.513
(0.759–3.015) | 0.239 | 1.753
(0.856–3.590) | 0.125 |
| Multivariate
analysis |
|
|
|
|
|
Adjuvant TACE (yes vs.
no) | 0.4727
(0.3503–2.319) | 0.0324a | NA | NA |
| Largest
tumor size (>5 cm vs. ≤5 cm) | 2.7752
(0.4953–2.061) | 0.0393a | NA | NA |
| Tumor
capsule (complete vs. incomplete) | 0.4576
(0.4044–1.933) | 0.0532 | NA | NA |
| Tumor
number (single vs. multiple) | 5.3590
(0.5713–2.939) | 0.0033a | NA | NA |
Discussion
Partial hepatectomy is the recommended first-line
treatment for primary HCC. However, the local recurrence rate in
the first 5 years following resection is as high as 70% (20). Satellite lesions, cirrhosis and tumor
size are considered to be closely associated with postoperative
recurrence (21). Several treatment
options may be used to prevent the recurrence of HCC, including
repeat hepatectomy, RFA and TACE (3). RFA and TACE may be considered more
suitable for patients with Child-Pugh grade A or B, and for those
with a greater size or number of tumors (22). However, based on the heterogeneity of
HCC, not all patients will benefit from TACE. Patients with large
tumors or venous invasion are at higher risk of recurrence and are
advised to receive TACE in clinical practice (23). Molecular analysis of in situ
and recurrent tumors may improve our understanding of the
mechanisms underlying recurrence and help identify prognostic
biomarkers (24,25). Several systematic analyses based on
>10,000 patients with HCC demonstrated that patients receiving
TACE experienced a survival benefit compared with the control group
(26,27). However, other studies have reported
different results regarding recurrence and survival after receiving
TACE. A Cochrane analysis of 6 trials observed no superior
effectiveness of TACE compared with the control group (28,29).
This controversy focuses not only on patient recruitment for TACE,
but also on the need for more large-scale trials (30,31).
Therefore, patient selection is crucial when considering TACE.
The present study demonstrated that COCH was a
suitable predictor of survival and recurrence in patients with HCC.
The expression of COCH was closely associated with PVTT, HBV
infection and BCLC stage. However, when the efficacy of adjuvant
TACE was analyzed, only patients with low COCH expression appeared
to benefit from the treatment. To the best of our knowledge, the
present study is the first to report that COCH is associated with
recurrence and may be useful in evaluating prognosis. It may also
serve as a factor determining whether TACE should be administered
to prevent recurrence and prolong OS time. Measuring COCH
expression may also help evaluate the effect of TACE following
hepatectomy and to determine whether to select TACE as a first-line
adjuvant therapy. However, the univariate analysis in patients with
high COCH expression revealed that the recurrence rate was not
associated with any variables, including BCLC and TNM stage, which
was reported to be associated with recurrence (3,32). This
discrepancy may be due to the limited number of included patients.
A larger study is required to confirm the results of the present
study.
The mechanisms underlying the beneficial effect of
TACE treatment on patients with low COCH expression remain elusive.
The results of immunohistochemical analysis revealed that COCH was
expressed in both the nucleus and the cytoplasm. It has not yet
been reported whether COCH is more highly expressed in HCC and
whether its expression is associated with the survival and
recurrence of HCC, but the expression of COCH in normal liver
tissue is low (33). TACE inhibits
recurrence mainly by suppressing the early metastasis of tumor
cells (34). However, it is
difficult to detect the small intrahepatic metastases that
contribute to early tumor recurrence, before or after hepatectomy.
Theoretically, therapies focusing on undetected intrahepatic
metastases are crucial for preventing the recurrence of HCC.
However, some studies highlight the need for the careful selection
of patients for TACE, as the treatment may damage liver cells and
compromise liver function, which is important to help optimize the
benefit of the overall HCC treatment course (7,35). The
side effects of TACE may affect patient survival, which may explain
why patients with high COCH expression do not benefit from TACE
treatment.
In the present study, COCH was identified as a
potential biomarker of HCC prognosis. Patients with high COCH
expression exhibited poor OS times, early recurrence and no obvious
response to adjuvant postoperative TACE. By contrast, patients with
low COCH expression exhibited better OS and DFS times, as well as a
better response to TACE. However, the predictive value of COCH for
the clinical selection of TACE usage requires further verification
by large-scale clinical trials, and the underlying mechanism must
also be further investigated.
Acknowledgements
The authors would like to thank Dr Liwei Dong, Miss
Xiao-Fan Feng and Mr. Meng-Qi Zhuang for their technical assistance
(all National Center for Liver Cancer, Shanghai, China).
Funding
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81702339, 81802499 and
81802311), Jiangsu Province's Science and Technology Support
Program (Social Development) Project (no. BE2017658) and the
Applied Foundational Research of Medical and Health Care of Suzhou
City (nos. SYSD2018206, SYS2019079 and SYS2018104).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CW performed the experiments and performed the
statistical analysis. ZWD and CGZ collected the pathological data
of the patients and prepared the tissue microarray. SW analyzed
revised the clinical data and the manuscript. ZHL and ZMZ performed
the molecular experiments. JP and JW designed and conceived the
experiment and confirmed the authenticity of the raw data. CY
conceived the study and wrote the manuscript. All authors have read
and approved the manuscript.
Ethics approval and consent to
participate
HCC samples were obtained from the Shanghai Eastern
Hepatobiliary Surgery Hospital, and studies on human tissues were
approved by the Ethics Board of the Shanghai Eastern Hepatobiliary
Surgery Hospital. Written consent was obtained from all
patients.
Patient consent for publication
Patients provided written informed consent for the
publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bruix J and Sherman M; American
Association for the Study of Liver Diseases, : Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tabrizian P, Jibara G, Shrager B, Schwartz
M and Roayaie S: Recurrence of hepatocellular cancer after
resection: Patterns, treatments, and prognosis. Ann Surg.
261:947–955. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Forner A, Reig M and Bruix J:
Hepatocellular carcinoma. Lancet. 391:1301–1314. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Omata M, Cheng AL, Kokudo N, Kudo M, Lee
JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, et al:
Asia-pacific clinical practice guidelines on the management of
hepatocellular carcinoma: A 2017 update. Hepatol Int. 11:317–370.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chan SL, Mo FK, Johnson PJ, Liem GS, Chan
TC, Poon MC, Ma BB, Leung TW, Lai PB, Chan AT, et al: Prospective
validation of the Chinese University prognostic index and
comparison with other staging systems for hepatocellular carcinoma
in an Asian population. J Gastroenterol Hepatol. 26:340–347. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang B, Zheng B, Yang M, Zeng Z, Yang F,
Pu J, Li C and Liao Z: Liver resection versus transarterial
chemoembolization for the initial treatment of Barcelona clinic
liver cancer stage B hepatocellular carcinoma. Hepatol Int.
12:417–428. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marquardt JU, Galle PR and Teufel A:
Molecular diagnosis and therapy of hepatocellular carcinoma (HCC):
An emerging field for advanced technologies. J Hepatol. 56:267–275.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chance MR, Chang J, Liu S, Gokulrangan G,
Chen DH, Lindsay A, Geng R, Zheng QY and Alagramam K: Proteomics,
bioinformatics and targeted gene expression analysis reveals
up-regulation of cochlin and identifies other potential biomarkers
in the mouse model for deafness in Usher syndrome type 1F. Hum Mol
Genet. 19:1515–1527. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ikezono T, Matsumura T, Matsuda H, Shikaze
S, Saitoh S, Shindo S, Hasegawa S, Oh SH, Hagiwara Y, Ogawa Y, et
al: The diagnostic performance of a novel ELISA for human CTP
(Cochlin-tomoprotein) to detect perilymph leakage. PLoS One.
13:e01914982018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jung J, Kim HS, Lee MG, Yang EJ and Choi
JY: Novel COCH p.V123E mutation, causative of DFNA9 sensorineural
hearing loss and vestibular disorder, shows impaired cochlin
post-translational cleavage and secretion. Hum Mutat. 36:1168–1175.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nyström A, Bornert O, Kühl T, Gretzmeier
C, Thriene K, Dengjel J, Pfister-Wartha A, Kiritsi D and
Bruckner-Tuderman L: Impaired lymphoid extracellular matrix impedes
antibacterial immunity in epidermolysis bullosa. Proc Natl Acad Sci
USA. 115:E705–E714. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Suman A, Barnes DS, Zein NN, Levinthal GN,
Connor JT and Carey WD: Predicting outcome after cardiac surgery in
patients with cirrhosis: A comparison of Child-Pugh and MELD
scores. Clin Gastroenterol Hepatol. 2:719–723. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang K, Liu J, Yan ZL, Li J, Shi LH, Cong
WM, Xia Y, Zou QF, Xi T, Shen F, et al: Overexpression of
aspartyl-(asparaginyl)- beta-hydroxylase in hepatocellular
carcinoma is associated with worse surgical outcome. Hepatology.
52:164–173. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dong LW, Hou YJ, Tan YX, Tang L, Pan YF,
Wang M and Wang HY: Prognostic significance of Beclin 1 in
intrahepatic cholangiocellular carcinoma. Autophagy. 7:1222–1229.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Q, Tan YX, Ren YB, Dong LW, Xie ZF,
Tang L, Cao D, Zhang WP, Hu HP and Wang HY: Zinc finger protein
ZBTB20 expression is increased in hepatocellular carcinoma and
associated with poor prognosis. BMC Cancer. 11:2712011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bas A, Forsberg G, Hammarström S and
Hammarström ML: Utility of the housekeeping genes 18S rRNA,
beta-actin and glyceraldehyde-3-phosphate-dehydrogenase for
normalization in real-time quantitative reverse
transcriptase-polymerase chain reaction analysis of gene expression
in human T lymphocytes. Scand J Immunol. 59:566–573. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marrero JA, Kulik LM, Sirlin CB, Zhu AX,
Finn RS, Abecassis MM, Roberts LR and Heimbach JK: Diagnosis,
staging, and management of hepatocellular carcinoma: 2018 Practice
guidance by the american association for the study of liver
diseases. Hepatology. 68:723–750. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim JM, Lee SH, Shin WY, Lee KY, Kim JM
and Ahn SI: Intrahepatic recurrence of single nodular
hepatocellular carcinoma after surgical resection: An analysis by
segmental distribution. ANZ J Surg. 88:E840–E844. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang K, Liu G, Li J, Yan Z, Xia Y, Wan X,
Ji Y, Lau WY, Wu M and Shen F: Early intrahepatic recurrence of
hepatocellular carcinoma after hepatectomy treated with
re-hepatectomy, ablation or chemoembolization: A prospective cohort
study. Eur J Surg Oncol. 41:236–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang L, Li J, Yan J, Cao J, Liu C, Zhang
X, Wu M and Yan Y: Early recurrence after curative resection in
oligonodular hepatocellular carcinoma. Hepatogastroenterology.
60:28–31. 2013.PubMed/NCBI
|
|
24
|
Feng AL, Zhu JK, Yang Y, Wang YD, Liu FY,
Zhu M and Liu CZ: Repeated postoperative adjuvant TACE after
curative hepatectomy improves outcomes of patients with HCC. Minim
Invasive Ther Allied Technol. 1–6. Dec 27–2019.(Epub ahead of
print). doi: 10.1080/13645706.2019.1707689. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen J, Huang J, Chen M, Yang K, Chen J,
Wang J, Xu L, Zhou Z and Zhang Y: Transcatheter arterial
chemoembolization (TACE) versus hepatectomy in hepatocellular
carcinoma with macrovascular invasion: A meta-analysis of 1683
patients. J Cancer. 8:2984–2991. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Llovet JM and Bruix J: Systematic review
of randomized trials for unresectable hepatocellular carcinoma:
Chemoembolization improves survival. Hepatology. 37:429–442. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lencioni R, de Baere T, Soulen MC, Rilling
WS and Geschwind JF: Lipiodol transarterial chemoembolization for
hepatocellular carcinoma: A systematic review of efficacy and
safety data. Hepatology. 64:106–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oliveri RS, Wetterslev J and Gluud C:
Transarterial (chemo)embolisation for unresectable hepatocellular
carcinoma. Cochrane Database Syst Rev. CD0047872011.PubMed/NCBI
|
|
29
|
Forner A, Llovet JM and Bruix J:
Chemoembolization for intermediate HCC: Is there proof of survival
benefit? J Hepatol. 56:984–986. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Farinati F, Giacomin A, Vanin V, Giannini
E and Trevisani F: TACE treatment in hepatocellular carcinoma: What
should we do now? J Hepatol. 57:221–222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li KW, Li X, Wen TF and Lu WS: The effect
of postoperative TACE on prognosis of HCC: An update.
Hepatogastroenterology. 60:248–251. 2013.PubMed/NCBI
|
|
32
|
Zhang Y, Chen SW, Liu LL, Yang X, Cai SH
and Yun JP: A model combining TNM stage and tumor size shows
utility in predicting recurrence among patients with hepatocellular
carcinoma after resection. Cancer Manag Res. 10:3707–3715. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li L, Ikezono T, Watanabe A, Shindo S,
Pawankar R and Yagi T: Expression of full-length Cochlin p63s is
inner ear specific. Auris Nasus Larynx. 32:219–223. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Galle PR, Tovoli F, Foerster F, Wörns MA,
Cucchetti A and Bolondi L: The treatment of intermediate stage
tumours beyond TACE: From surgery to systemic therapy. J Hepatol.
67:173–183. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Miksad RA, Ogasawara S, Xia F, Fellous M
and Piscaglia F: Liver function changes after transarterial
chemoembolization in US hepatocellular carcinoma patients: The
LiverT study. BMC Cancer. 19:7952019. View Article : Google Scholar : PubMed/NCBI
|