Introduction
Papillary thyroid carcinoma (PTC) is one of the most
common endocrine malignancies and has a 10-year survival rate of
90–98% globally (1–4). Risk factors including age (>45
years), male sex, larger tumor size and distant metastasis
contribute to poor survival based on the Surveillance, Epidemiology
and End Results (SEER) database (5).
Despite the fact that the number of metastatic lymph nodes (mLNs)
has been recognized as a negative prognostic factor, other features
of mLNs have yet to be fully understood. Recently, the
characteristics of mLNs, in particular, size, extra-nodal extension
(ENE) and micro-metastasis (<0.2 cm as the largest dimension of
the metastatic lesion) were initially proposed by the 2015 updated
version of the American Thyroid Association (ATA) guidelines
(6–8).
PTC often metastasizes to lymph nodes (LNs),
especially in the central neck region, and the size of the
metastatic lesion within the LN tends to reflect the degree of
disease progression (9,10). However, the status of the metastatic
lesion within a mLN varies to a large extent. This may include
varying metastatic deposit sizes in different LNs of varying size
(8,11). Therefore, it is reasonable to be
concerned with not only the metastatic lesion size, but also the
location within the LN. To better reflect the disease progression
of patients with PTC, the present study proposed a novel parameter:
The area proportion of the metastatic lesion within the central mLN
(APmCLN).
The post-operative risk of recurrence during
follow-up should be estimated dynamically according to the response
to therapy re-staging system (2015 version of the ATA guidelines)
(7). Previous studies have suggested
that the therapeutic response system is closely correlated with
prognosis (12–16). The objective of the current study was
to evaluate the impact of APmCLN on the response to therapy in
patients with PTC.
Materials and methods
Patients
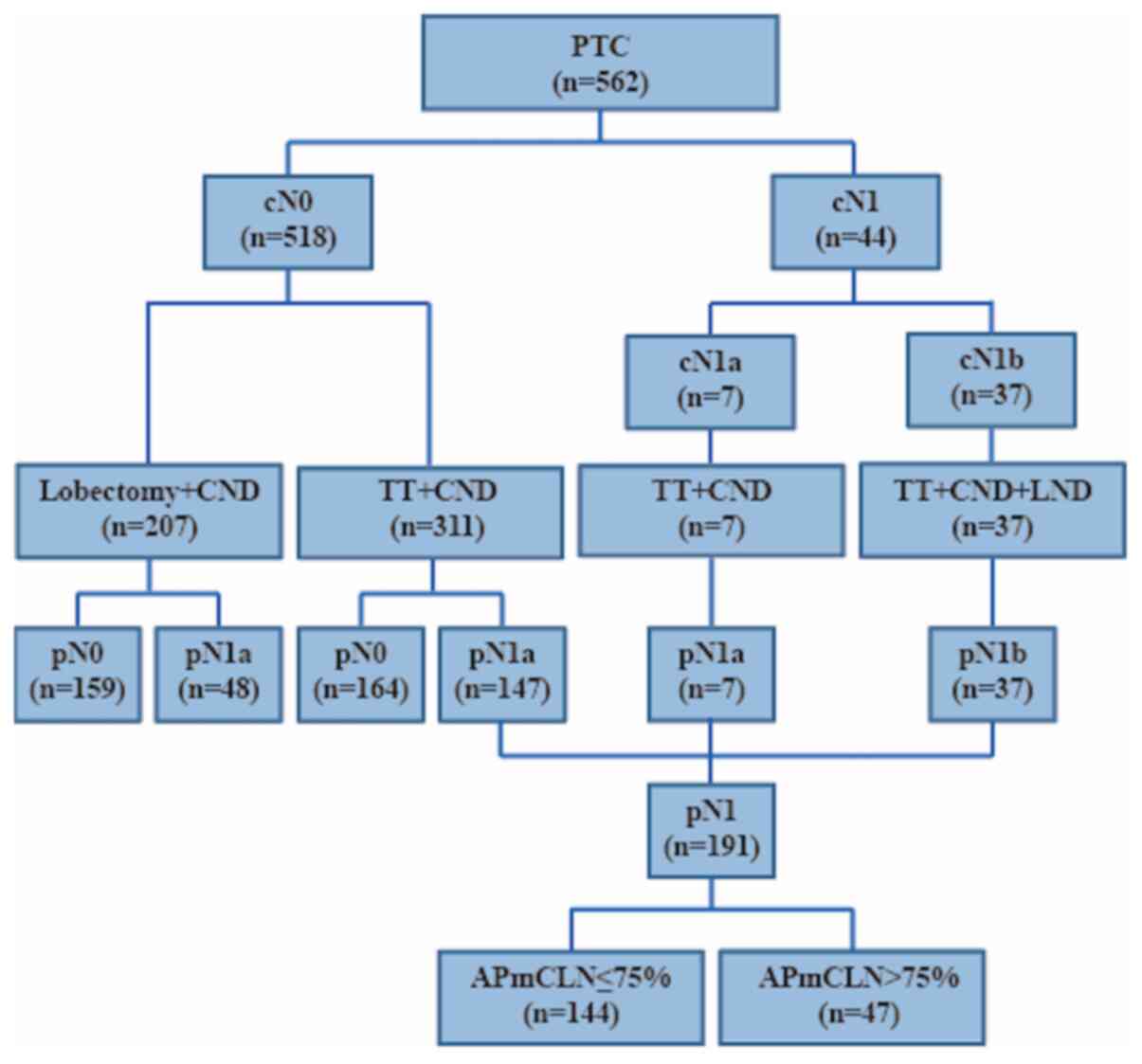
Between January 2013 and December 2015, 562 patients
with PTC were enrolled onto the study at the Affiliated Sir Run Run
Shaw Hospital (Zhejiang, China), and 355 of those underwent a total
thyroidectomy (TT) with ipsilateral or bilateral central neck
dissection (CND) (Fig. 1). Inclusion
criteria were patients with pathologically diagnosed PTC. Exclusion
criteria included no PTC diagnosis, poorly differentiated PTC
(diagnostic criteria were based on the consensus Turin proposal)
(6), coexisting other malignancies,
previous thyroidectomy and no radioactive iodine (RAI) ablation.
After surgery, a histological diagnosis of PTC and measurements of
mLN were confirmed by two experienced pathologists from the
Department of Pathology, who were independent from the present
study. Of the 355 patients that met the final selection criteria,
191 and 164 were designated as pN1 and pN0, respectively (Fig. 1) (17). In the conventional
Tumor-Node-Metastasis (TNM) staging system established by the Union
for International Cancer Control and the American Joint Commission
on Cancer (AJCC; 2010, 7th edition), pathologically confirmed lymph
node metastasis was defined as pN1, no lymph node metastasis was
defined as pN0. The study was approved by The Ethics Committee of
the Affiliated Sir Run Run Shaw Hospital, Zhejiang University
School of Medicine, and all enrolled patients provided written
informed consent.
Treatment protocol
In accordance with the 2009 ATA guidelines (6), TT was performed if the patient met one
of the following criteria: Bilateral nodularity, extra-thyroidal
extension (ETE), tumor diameter >1 cm, mulifocal lesions in the
affected lobe, regional or distant metastases, a personal history
of radiation therapy to the head and neck or a first-degree family
history of PTC. Ipsilateral CND was performed routinely for the
affected side, regardless of whether the central neck LNs were
clinically metastatic. Bilateral CND was performed for patients
whose tumor(s) was located in the isthmus or both lobes, or for
those with clinical metastasis in the neck LNs. Modified lateral
neck dissection (LND), including levels II–IV or together with V,
was performed only in patients with clinically evident nodal
disease in the lateral neck on preoperative ultrasonography or when
the ultrasound-guided fine needle aspiration of a lateral node
exhibited positive results. RAI remnant ablation was performed
postoperatively in all patients according to the 2009 ATA
guidelines (6). Only the LNs from
the central area were examined, which were derived from patients
who underwent CND with or without LND.
Histopathological examination
Tissues with thyroid, tumor(s) and LN(s) were
collected from each patient during surgery and were sent
immediately to the Department of Pathology. Tissues were fixed in
10% neutral-buffered formalin at 4°C overnight, dehydrated using
graded ethanol (100, 95, 75 and 50%) and embedded in paraffin. If
the thickness of the LNs was <6 mm, they were embedded entirely
in paraffin, cut in half, and then sliced into three 3–4-µm pieces.
If a LN was >6 mm in thickness, it was selected to be embedded
and cut in half. All of the slices were stained with hematoxylin
and eosin (H&E) prior to pathological diagnosis (18). In the event of suspicious
micro-metastasis, thyroglobulin (Tg) immunohistochemical (IHC)
staining was performed (19,20). For IHC, slides were incubated with a
primary monoclonal rabbit anti-Thyroglobulin-antibody (1:300
dilution; Abcam; ab156008) at room temperature for 60 min. Then,
the slides were incubated with a secondary anti-rabbit IgG antibody
(ImmPress Reagent Kit; MP-7405; peroxidase-conjugated) followed by
target detection using DAB plus chromogen for 10 min (Gene Tex;
GTX73338). All mLNs in the central neck were observed using light
microscopy (magnification, ×200-400) and measured by two
experienced pathologists using an ocular micrometer, and the mean
value of the measurements was recorded. In total, 2,768 central LNs
were examined, of which 670 were positive, and the one with the
largest size, lesion, or metastatic area was selected as the
representative parameter for each patient.
Definitions
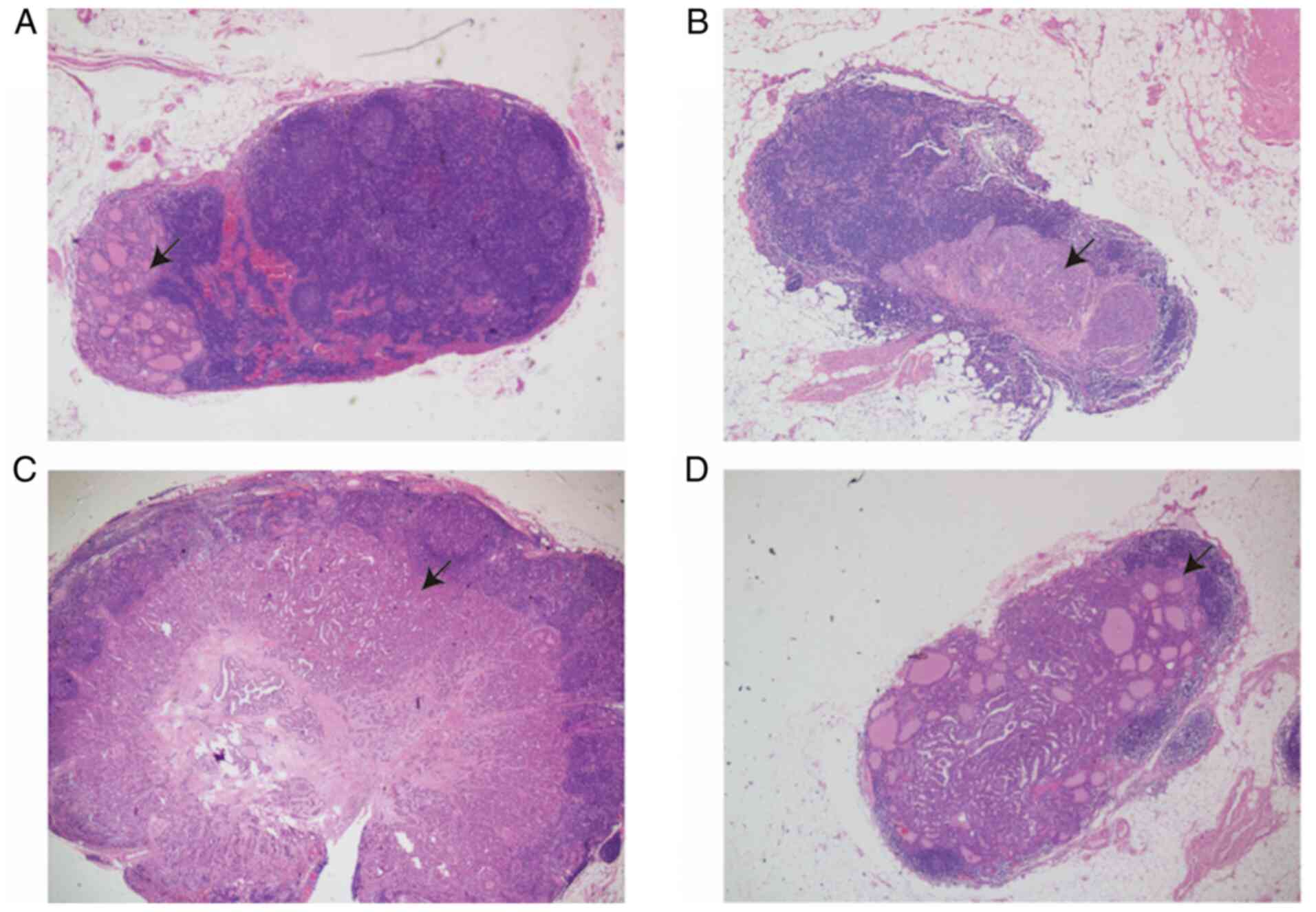
A new parameter (APmCLN) was identified and defined
as the ratio of the metastatic deposit area to the whole mLN area
in a cross-section, which was microscopically measured by a cross
and divided into four quadrants: i) ≤25% (1 Quadrant occupied by
the metastatic lesion), ii) 25–50% (2 quadrants occupied by the
metastatic lesion), iii) 50–75% (3 quadrants occupied by the
metastatic lesion) and iv) >75% (4 quadrants almost occupied by
the metastatic lesion) (Fig. 2).
When evaluating independent risk factors for treatment response,
there was no statistical significance between group i) and
ii)+iii)+iv), groups i)+ii) and iii)+iv). However, differences
between groups i) +ii) +iii) and iv) were statistically
significant. Therefore, groups i)-iii) were merged into group A and
group iv) was merged into group B.
Micro-metastasis was defined as the presence of
metastatic deposits within a LN <2 mm in diameter, which is a
parameter commonly used in breast cancer and other solid tumors
(11). Micro-metastasis has been a
proposed modification expressed in the 2015 ATA guidelines as a
low-risk parameter (7). Patients
presenting with histopathological criteria including diffuse
infiltration of the thyroid gland with lymphocytes and other
inflammation-related cells were diagnosed with chronic lymphocyte
thyroiditis (CLT) (21).
The current definition of ‘clinically apparent’ LN
metastasis (clinical N1 disease; cN1) includes any metastatic LN
identified by palpation or imaging either before initial surgery or
intraoperatively (8). When
suspicious LN appeared at level VI or II–IV, it was defined as cN1a
or cN1b, respectively.
Assessment of treatment response
The response to the therapy re-staging system has
been designed for the follow-up of patients with PTC in the 2015
ATA guidelines. According to biochemical, imaging and
cyto-pathological findings, there are four response-to-therapy
categories for patients treated with TT and RAI remnant ablation
(7). These include: i) Excellent
response, ii) biochemical incomplete response, iii) structural
incomplete response and iv) indeterminate response. The current
study rearranged these into two categories consisting of excellent
response and non-excellent response. The latter included
biochemical incomplete, structural incomplete and indeterminate
responses. According to the ATA guidelines, the recurrence rate for
the excellent response group is 1–4%, which is much lower compared
with other groups (biochemical incomplete response, 20% develop
structural disease; structural incomplete response, 50–85% continue
to have persistent disease despite additional therapy and
indeterminate response, 15–20% will have structural disease)
(7).
All patients with PTC were followed up after
completion of RAI remnant ablation. Routine neck ultrasound
examination, and measurement of serum Tg and anti-thyroglobulin
antibody (TgAb) were performed in a state of TSH (Thyroid
Stimulating Hormone) suppression every 3 months in the first year
and every 6–12 months thereafter. When biochemical or structural
incomplete response occurred, more frequent follow-up was
recommended with additional examinations (including CT scan and
Fine Needle Aspiration) were proposed. The follow-up time for all
PTCs ranged from 40 to 75 months (median, 57 months), and the
assessment of response to therapy was based on the latest
examination results. Serum Tg (normal range, 1.15–35.00 ng/ml) and
TgAb (normal range, 0–4.11 IU/ml) were measured by
electrochemiluminescence immunoassay in an Abbott
Aeroset® Automated Instrument Analyzer (Canon Medical
Systems Corp.) (22–24).
Statistical analysis
Continuous variables and categorical variables were
determined by Mann-Whitney U test and χ2 test (including
Fisher's exact tests, if needed), respectively. Bonferroni's
correction was also applied where appropriate. The results are
presented as medians with range and numbers with percentages.
Univariate logistic regression analyses were performed for sex,
age, tumor size, tumor multifocality, ETE, CLT, N stage, number and
size of central metastatic LN, the size of central metastatic
lesion and APmCLN. The variables exhibiting P<0.05 in the
univariate analysis were then selected and analyzed using
multivariate logistic regression analysis. The results were
represented as odds ratios (ORs) with 95% confidence intervals
(CIs). For all analyses, two-sided tests were employed and
P<0.05 was considered to indicate a statistically significant
difference. Statistical analyses were performed using SPSS software
version 23.0 (IBM Corp.).
Results
Characteristics of patients with PTC
with TT
The profiles of 562 patients are shown in Fig. 1. Among them, 207 underwent lobectomy,
355 underwent total thyroidectomy (TT), and these 355 patients
included 311 cases of cN0 and 44 cN1. The clinicopathological
characteristics of the 355 PTC patients are in Table I. The median age of the cohort was 42
years (range, 13–72 years). Most of the patients were female
(77.2%) and were <55 years-old (83.9%). The majority of patients
(234/355, 65.9%) were diagnosed with papillary thyroid
micro-carcinoma (PTMC, primary tumor size ≤1 cm). Multifocality,
CLT, and ETE were found in 56.1 (199/355), 26.5 (94/355) and 32.4%
(115/355) of patients, respectively. The clinical N stage included
311 cN0 (87.6%), 7 cN1a (2.0%) and 37 cN1b (10.4%) patients,
whereas the pathological N stage included 164 pN0 (46.2%), 154 pN1a
(43.4%) and and 37 pN1b (10.4%) patients. An excellent response to
primary therapy was observed in 267 patients (75.2%). For ATA risk
stratification, there were 157 (44.2%), 100 (28.2%) and 98 (27.6%)
patients in low-, intermediate- and high-risk categories,
respectively.
 | Table I.Characteristics of 355 patients with
papillary thyroid carcinoma who underwent total thyroidectomy. |
Table I.
Characteristics of 355 patients with
papillary thyroid carcinoma who underwent total thyroidectomy.
|
Characteristics | Total |
|---|
| Sexa |
|
|
Male | 81 (22.8) |
|
Female | 274 (77.2) |
| Age of diagnosis,
years |
|
| Median
(range), year | 42 (13–72) |
|
<55a | 298 (83.9) |
|
≥55a | 57 (16.1) |
| Primary tumor size,
cma |
|
| ≤1 | 234 (65.9) |
|
>1 | 121 (34.1) |
|
Multifocalitya |
|
|
Absent | 156 (43.9) |
|
Present | 199 (56.1) |
| CLTa |
|
|
Absent | 261 (73.5) |
|
Present | 94 (26.5) |
| ETEa |
|
|
Absent | 240 (67.6) |
|
Present | 115 (32.4) |
| Clinical Node
stagea |
|
|
cN0 | 311 (87.6) |
|
cN1a | 7 (2.0) |
|
cN1b | 37 (10.4) |
| Pathological Node
stagea |
|
|
pN0 | 164 (46.2) |
|
pN1a | 154 (43.4) |
|
pN1b | 37 (10.4) |
| Distant
metastasisa |
|
|
Absent | 352 (99.2) |
|
Present | 3 (0.8) |
| ATA
response-to-therapy categorya |
|
|
Excellent response | 267 (72.5) |
|
Biochemical incomplete
response | 1 (0.3) |
|
Structural incomplete
response | 15 (4.2) |
|
Indeterminate response | 72 (20.3) |
| ATA risk
stratificationa |
|
|
Low | 157 (44.2) |
|
Intermediate | 100 (28.2) |
|
High | 98 (27.6) |
Clinicopathological features
associated with the APmCLN of patients with pN1-PTC
Comparison of the postoperative pathological results
revealed several factors that were significantly different between
group A (APmCLN ≤75%) and group B (APmCLN >75%). A larger APmCLN
(>75%) was associated with aggressive characteristics, including
ETE (P=0.019), clinical node stage (P<0.001) and pathological
node stage (P<0.001). To describe the features of the mLNs, a
statistical analysis was performed for the number and size of the
central mLN and the size and ENE of the metastatic foci. It was
demonstrated that these factors were significantly different
between the two groups (P=0.001 and P<0.001, respectively).
According to the 2015 ATA guidelines, the mLN ≤5 and
micro-metastasis factors belong to the low-risk category. It was
reported that these two factors were significantly higher in group
A compared with group B (88.2 vs. 63.8% and 71.5 vs. 10.6%,
respectively; both P<0.001). Therefore, in group A, the
proportion of low- and intermediate-risk categories was 70.1%,
whereas in group B, the proportion of intermediate- and high-risk
categories was 89.4%. An APmCLN was associated with the risk
stratification of disease recurrence (Table II).
 | Table II.Characteristics of 191 patients with
pN1-papillary thyroid carcinoma according to the APmCLN. |
Table II.
Characteristics of 191 patients with
pN1-papillary thyroid carcinoma according to the APmCLN.
|
| APmCLN |
|
|---|
|
|
|
|
|---|
|
Characteristics | Group A ≤75%,
n=144 | Group B >75%,
n=47 | P-value |
|---|
| Sexa |
|
|
|
|
Male | 37 (25.7) | 19 (40.4) | 0.054 |
|
Female | 107 (74.3) | 28 (59.6) |
|
| Age of diagnosis,
years |
|
|
|
| Median
(range) | 39.5
(20.0–69.0) | 37.0
(25.0–66.0) | 0.786 |
|
<55a | 126 (87.5) | 44 (93.6) | 0.244 |
|
≥55a | 18 (12.5) | 3 (6.4) |
|
| Primary tumor size,
cma |
|
|
|
| ≤1 | 84 (58.3) | 22 (46.8) | 0.167 |
|
>1 | 60 (41.7) | 25 (53.2) |
|
|
Multifocalitya |
|
|
|
|
Absent | 62 (43.1) | 21 (44.7) | 0.845 |
|
Present | 82 (56.9) | 26 (55.3) |
|
| ETEa |
|
|
|
|
Absent | 95 (66.0) | 22 (46.8) | 0.019 |
|
Present | 49 (34.0) | 25 (53.2) |
|
| CLTa |
|
|
|
|
Absent | 108 (75.0) | 39 (83.0) | 0.259 |
|
Present | 36 (25.0) | 8 (17.0) |
|
| Clinical Node
stagea |
|
|
|
|
cN0 | 123 (85.4) | 24 (51.1) | <0.001 |
|
cN1 | 21 (14.6) | 23 (48.9) |
|
| Pathological Node
stagea |
|
|
|
|
pN1a | 128 (88.9) | 26 (55.3) | <0.001 |
|
pN1b | 16 (11.1) | 21 (44.7) |
|
| Number of central
mLN |
|
|
|
| Median
(range) | 2.0 (1.0–19.0) | 5.0 (1.0–18.0) | <0.001 |
|
≤5a | 127 (88.2) | 30 (63.8) | <0.001 |
|
>5a | 17(11.8) | 17 (36.2) |
|
| Size of central
mLN, mm |
|
|
|
| Median
(range) | 4.33
(0.67–14.90) | 6.40
(1.07–18.27) | 0.001 |
| Size of central LN
metastatic foci, mm |
|
|
|
| Median
(range) | 1.34
(0.07–6.80) | 5.33
(1.07–18.27) | <0.001 |
|
<2a | 103 (71.5) | 5 (10.6) | <0.001 |
|
≥2a | 41 (28.5) | 42 (89.4) |
|
| ENEa |
|
|
|
|
Absent | 135 (93.8) | 30 (63.8) | <0.001 |
|
Present | 9 (6.2) | 17 (36.2) |
|
| ATA
response-to-therapy categoriesa |
|
|
|
|
Excellent response | 108 (75.0) | 27 (57.4) | 0.022 |
|
Non-excellent response | 36 (25.0) | 20 (42.6) |
|
| ATA risk
stratificationa |
|
|
|
|
Low | 52 (36.1) | 5 (10.6) | 0.003 |
|
Intermediate | 49 (34.0) | 19 (40.4) |
|
|
High | 43 (29.9) | 23 (49.0) |
|
Risk factors for non-excellent
response to therapy of patients with pN1-PTC
Response to initial therapy was analyzed in all
patients. The proportion of excellent responders in groups A and B
were 75 and 57.4%, respectively. There was a significant difference
between the two groups (P=0.022; Table
II). The risk factors for non-excellent response to therapy in
patients with pN1 PTC were further analyzed (Table III). Univariate analysis indicated
that CLT (P<0.001), a higher quantity of central mLN (P=0.004),
larger sizes of central mLN (P<0.001) and their metastatic foci
(P=0.025) and higher APmCLN (P=0.023) significantly increased the
risk of classification into the non-excellent response to therapy
category. Furthermore, CLT, size of central mLN and APmCLN were
independent variables for response to therapy in multivariate
analysis (P<0.00, P=0.014 and P=0.020, respectively). Compared
with cases without CLT, those with CLT were 5.405 times (95% CI,
2.339–12.492; P<0.001) more likely to exhibit a non-excellent
response to therapy. For cases with an incremental increase of 1 mm
in central mLN size, the non-excellent risk increased by 1.283
times (95% CI, 2.339–12.492; P=0.014). For PTCs with APmCLN
>75%, the rate was 3.917 times higher compared with that of
APmCLN ≤75% (95% CI, 1.245–12.327; P=0.020). Therefore, it was
demonstrated that APmCLN (≤75 vs. >75%) represents a new
independent risk factor for predicting clinical outcome.
 | Table III.Relationships between
clinicopathological variables and non-excellent response-to-therapy
in patients with pN1-papillary thyroid carcinoma. |
Table III.
Relationships between
clinicopathological variables and non-excellent response-to-therapy
in patients with pN1-papillary thyroid carcinoma.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
|
Characteristics | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Sex, male vs.
female | 1.546
(0.754–3.171) | 0.235 |
|
|
| Age of diagnosis,
<55 vs. ≥55 years | 0.534
(0.171–1.664) | 0.279 |
|
|
| Primary tumor size,
≤1 vs. >1 cm | 1.116
(0.597–2.087) | 0.730 |
|
|
| Multifocality,
absent vs. present | 1.755
(0.918–3.356) | 0.089 |
|
|
| ETE, absent vs.
present | 0.833
(0.437–1.590) | 0.580 |
|
|
| CLT, absent vs.
present | 6.462
(3.114–13.413) | <0.001 | 5.405
(2.339–12.492) | <0.001 |
| Pathological N
stage, pN1a vs. pN1b | 1.623
(0.764–3.447) | 0.207 |
|
|
| Clinical N stage,
cN0 vs. cN1 | 1.529
(0.749–3.120) | 0.244 |
|
|
| Number of central
mLN, ≤5 vs. >5 | 3.026
(1.410–6.493) | 0.004 | 2.082
(0.821–5.280) | 0.123 |
| Size of central
mLN, mm | 1.216
(1.103–1.341) | <0.001 | 1.283
(1.051–1.566) | 0.014 |
| Size of central LN
metastatic foci, mm | 1.110
(1.013–1.215) | 0.025 | 0.823
(0.652–1.039) | 0.102 |
| APmCLN, ≤25 vs.
>25% | 1.148
(0.610–2.158) | 0.669 |
|
|
| APmCLN, ≤50 vs.
>50% | 1.826
(0.973–3.428) | 0.061 |
|
|
| APmCLN, ≤75 vs.
>75% | 2.222
(1.114–4.432) | 0.023 | 3.917
(1.245–12.327) | 0.020 |
| ENE, absent vs.
present | 1.083
(0.441–2.659) | 0.861 |
|
|
Effect of the pathological metastasis
of central LN on the Response to therapy category
It was observed that APmCLN affects the response to
initial therapy in patients with pN1-PTC. The response to therapy
in patients classified as pN0 was further analyzed. A statistically
significant difference in excellent response to therapy was
observed between patients in the APmCLN ≤75% and APmCLN >75%
(P=0.022) categories, after using Bonferroni's correction, it still
passed the statistical significance (P=0.022 <0.050/2). The
excellent response to therapy rates were 75 and 57.4%,
respectively. Notably, when comparing the pN0 and pN1-APmCLN ≤75%
groups, there was no statistical difference in the rate of
achieving an excellent response to treatment (P=0.247), and the
incidence rates for these two groups were 80.5 and 75.0%,
respectively (Table IV). Therefore,
it is reasonable to believe that APmCLN >75% will make Response
to therapy worse than APmCLN ≤75%.
 | Table IV.Response to therapy categories on the
pathological metastasis of central lymph node of patients with
papillary thyroid carcinoma treated with total thyroidectomy. |
Table IV.
Response to therapy categories on the
pathological metastasis of central lymph node of patients with
papillary thyroid carcinoma treated with total thyroidectomy.
|
| ATA
response-to-therapy categories, n (%) |
|
|---|
|
|
|
|
|---|
|
Characteristics | Excellent | Non-Excellent | P-value |
|---|
| pN0 | 132 (80.5) | 32 (19.5) | 0.247a |
| pN1-APmCLN
≤75% | 108 (75.0) | 36 (25.0) | 0.022b |
| pN1-APmCLN
>75% | 27 (57.4) | 20 (42.6) |
|
Recurrence-free survival (RFS)
according to the APmCLN
In total, five cases of disease recurrence were
identified during the median follow-up period of 57 months (range,
40–75 months) across all patients with PTC who underwent TT. The
mean time of recurrence was 25.8 months. According to the APmCLN,
the number of recurrence cases was higher in the pN1-APmCLN >75%
group compared with the pN1-APmCLN ≤75% group (5/47 vs. 0/144,
respectively), and the RFS was also significantly different between
the two groups (89.4 vs. 100%; log-rank P<0.001). In addition,
RFS of the pN1-APmCLN ≤75% and pN0 patients were both 100% and no
patients relapsed (Fig. 3).
Therefore, pN1-APmCLN >75% indicates a higher recurrence rate in
PTC patients.
Discussion
PTC is generally an indolent disease that has an
excellent prognosis. The SEER registry study concluded that the
overall survival rate at 14 years is 82% in cases of PTC without LN
metastases and 79% with nodal metastases in America (25). Therefore, an understanding of which
patients require long-term follow-up for assessing disease status
is needed. Methods to reduce the risk of recurrence and
disease-specific death is also important. Previous studies have
shown that the evaluation of clinical outcome in patients with PTC
should be dynamically adjusted throughout the observation period
(26–29). A key step in risk re-evaluation is to
assess the response to primary therapy and analyze the clinical
data obtained from imaging, biochemical and cytopathological
examination during dynamic monitoring, especially within the first
2 years of follow-up (7,15). According to the ATA guidelines, the
response to therapy re-classification describes differences in
clinical status and outcomes at various points during the observed
progress period (7). For example,
one study demonstrated a recurrence rate of 1–4% in the excellent
response group, while for the remaining three groups, the
likelihood of disease recurrence, persistence or progression is
significantly increased (7).
The weight of LN metastasis has been decreased in
the assessment of the new TNM system in patients with PTC. The
nodal status does not affect TNM staging in patients <55 years
old. In patients >55 years old with T1 or T2, N1 can promote the
stage from I to II (17). However,
LN metastasis in the central neck occurs frequently in PTC
(10) and correlates with
locoregional recurrence (8,30). Therefore, for the assessment of
recurrent risk according to the 2015 ATA guidelines, the extent of
LN involvement, including the number and size of mLN and LN
metastasis, was added and emphasized (7). In previous years, some researchers have
been concerned with the impact of other mLN parameters on PTC
biological behavior and prognosis, such as ENE (31), cancerous nodules of LNs (32) and the ratio of metastatic LNs to
total LNs removed (33,34). In the present study, APmCLN was
initially introduced as a new parameter and its effect on PTC
prognosis was analyzed.
Although five mLNs in cN0, micro-metastasis and the
central mLN (mCLN) size were added to the 2015 ATA guidelines as
new factors of recurrence risk assessment, the two parameters were
not found to affect the response of initial treatment in the
current study. The risk factors of non-excellent response to
therapy were also evaluated using univariate and multivariate
analyses. The presence of CLT, larger size mCLN and APmCLN >75%
were independent risk factors for non-excellent response to therapy
in patients with PTC with pN1.
There is no definitive conclusion as to whether CLT
is linked with a particular set of clinicopathological
characteristics or prognosis in PTC. Some studies have shown that
multifocality and LN metastasis occurs more frequently in patients
with CLT (35–37). In contrast, other studies found
associations between the presence of CLT and improved features,
such as smaller tumor size, less advanced TNM stage, lower
frequency of LN metastasis and improved prognosis (38–40). In
the present study, CLT was recognized as a risk factor for
non-excellent response to therapy in PTC. One of the
characteristics of CLT is abnormally elevated TgAb that does not
return to normal within 2 years in some patients after TT (41–43). The
increased TgAb may interfere with the measurement of Tg, which
would affect evaluation of the initial treatment response (44–46).
Therefore, it was hypothesized that CLT may not be suitable for
predicting treatment response.
The size of the mCLN is an important prognostic
factor for patients with PTC, and larger mCLNs tend to be more
aggressive. Ito et al (47)
showed that the presence of mLN >1.5 cm is associated with a
significantly lower disease-free survival rate compared with
patients with either N0 disease or patients with pN1 disease
<1.5 cm. Similarly, Sugitani et al (48) reported that in patients with pN1
disease with the largest mLN (>3 cm, 27%), the risk of
recurrence within 10 years after TT and neck dissection without RAI
ablation was significantly higher compared with pN1 patients (<3
cm, 11%). In the present study, larger-sized central mLN was
significantly associated with an increased risk of non-excellent
response to therapy. However, because of the long-term stimulation
of inflammation, the central LNs of CLT are relatively larger.
Therefore, it may be unreasonable to consider the larger mCLN size
as an independent risk factor for poor treatment response if the
inference factor of CLT is not ruled out.
To better reflect the metastatic extent of LNs, the
present study put forward the novel parameter of APmCLN, which
includes two factors. These are the size of the metastatic LN and
its metastatic foci, and whether the influence of inflammation due
to CLT may be ruled out. This was conducted so that the definition
of APmCLN is more scientific and rational. Previous studies have
reported that the sizes of mLN and LN metastasis are indicators of
tumor aggressiveness and risk factors for prognosis in PTC
(47,49–51).
However, coexisting CLT, the frequently changing size of mCLN
changes and the size of mLN foci were not independent risk factors
for non-excellent response to therapy in the current study. It is
inaccurate to predict treatment response based only on CLT and the
size of mLNs, therefore the present study included APmCLN and
systematically analyzed its clinicopathological and prognostic
value in patients with pN1-PTC after TT and CND. APmCLN >75%
exhibited worse clinicopathological features, a poor prognosis and
may be regarded as a new independent risk factor for non-excellent
response to therapy. Therefore, there was sufficient data to
conclude that APmCLN may be a reliable and practical new indicator
for predicting response to therapy and prognosis in patients with
pN1-PTC.
Several limitations were evident in the current
study. First, there were only 355 patients enrolled and only five
relapsed cases were reported in the relatively short follow-up
period. Therefore, additional studies with longer follow-up times
and multicenter data are needed. Second, APmCLN is currently a
categorical variable. The 75% critical value is obtained through
step classification and comparison between groups. If it is a
continuous variable, the cut-off value can be calculated using an
ROC curve (52–54), which is more scientific. Given the
differences in the color and cell morphology of H&E-stained
sections between tumor and normal tissue, software, such as ImageJ
and ITK-SNAP (55–58), can be used to identify the boundary,
obtain the location and range of metastases in the LN, and
calculate the area proportion of the metastatic lesion. This more
extensive analysis would allow APmCLN to be analyzed as a
continuous variable. Third, according to the ATA guidelines (2015
version) (7), some patients with
PTMC are recommend Active Surveillance rather than surgery. Active
Surveillance is applying life-long diagnostic modalities to
evaluate changes in disease status without treatment, until
progression of the disease becomes clinically apparent (59–61).
Regular follow-up should be provided for the patient to ensure that
disease progression is tolerable without any additional therapeutic
options, such as surgery. However, due to the retrospective nature
of the present study, most of the patients enrolled had undergone
surgical treatment, which was inconsistent with the current ATA
guidelines (7). The present patients
were recruited between 2013 and 2015, and treated according to the
2009 ATA guidelines (6) and the
Chinese Thyroid Association (62) in
which thyroid surgery is recommended if the lesion is considered to
be malignant.
In conclusion, APmCLN >75% was an indicator of
tumor aggressiveness and a significant independent risk factor for
non-excellent response to therapy in patients with pN1-PTC. These
results may enable physicians to further stratify patients into
various risk groups and develop effective individual follow-up and
treatment plans.
Acknowledgements
Not applicable.
Funding
This research was supported by The Innovative Talent
Support Program of Medicine and Health Technology project from the
Zhejiang Health Commission (grant no. 2021RC078) and The General
Research Project of Zhejiang Provincial Department of Education
(grant no. Y202043478).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LHS and LeX designed the study and confirm the
authenticity and legitimacy of all raw data. LHS wrote the
manuscript. LZ, JW and LJ performed the data collection and
analysis. LHS interpreted the results. YL performed the
histological examination, and confirmed the diagnosis. LiX
performed the follow-up plan and collected the data from patients.
LeX supervised the project. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by The Ethics Committee of
the Affiliated Sir Run Run Shaw Hospital, Zhejiang University
School of Medicine (Hangzhou, China). All patients provided written
informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bilimoria KY, Bentrem DJ, Ko CY, Stewart
AK, Winchester DP, Talamonti MS and Sturgeon C: Extent of surgery
affects survival for papillary thyroid cancer. Ann Surg.
246:375–384. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Adam MA, Pura J, Gu L, Dinan MA, Tyler DS,
Reed SD, Scheri R, Roman SA and Sosa JA: Extent of surgery for
papillary thyroid cancer is not associated with survival: An
analysis of 61,775 patients. Ann Surg. 260:601–607. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barney BM, Hitchcock YJ, Sharma P, Shrieve
DC and Tward JD: Overall and cause-specific survival for patients
undergoing lobectomy, near-total, or total thyroidectomy for
differentiated thyroid cancer. Head Neck. 33:645–649. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gilliland FD, Hunt WC, Morris DM and Key
CR: Prognostic factors for thyroid carcinoma. A population-based
study of 15,698 cases from the Surveillance, Epidemiology and End
Results (SEER) program 1973–1991. Cancer. 79:564–573. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Doll KM, Rademaker A and Sosa JA:
Practical guide to surgical data sets: Surveillance, epidemiology,
and end results (SEER) database. JAMA Surg. 153:588–589. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
American Thyroid Association (ATA)
Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid
Cancer, ; Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL,
Mandel SJ, Mazzaferri EL, McIver B, Pacini F, et al: Revised
American Thyroid Association management guidelines for patients
with thyroid nodules and differentiated thyroid cancer. Thyroid.
19:1167–1214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Haugen BR, Alexander EK, Bible KC, Doherty
GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM,
Schlumberger M, et al: 2015 American Thyroid Association Management
Guidelines for Adult Patients with Thyroid Nodules and
Differentiated Thyroid Cancer: The American Thyroid Association
Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid
Cancer. Thyroid. 26:1–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Randolph GW, Duh QY, Heller KS, LiVolsi
VA, Mandel SJ, Steward DL, Tufano RP and Tuttle RM; American
Thyroid Association Surgical Affairs Committee's Taskforce on
Thyroid Cancer Nodal Surgery, : The prognostic significance of
nodal metastases from papillary thyroid carcinoma can be stratified
based on the size and number of metastatic lymph nodes, as well as
the presence of extranodal extension. Thyroid. 22:1144–1152. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roh JL, Park JY and Park CI: Total
thyroidectomy plus neck dissection in differentiated papillary
thyroid carcinoma patients: Pattern of nodal metastasis, morbidity,
recurrence, and postoperative levels of serum parathyroid hormone.
Ann Surg. 245:604–610. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sivanandan R and Soo KC: Pattern of
cervical lymph node metastases from papillary carcinoma of the
thyroid. Br J Surg. 88:1241–1244. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cranshaw IM and Carnaille B:
Micrometastases in thyroid cancer. An important finding? Surg
Oncol. 17:253–258. 2008.PubMed/NCBI
|
|
12
|
Raue F and Frank-Raue K: Thyroid cancer:
Risk-stratified management and individualized therapy. Clin Cancer
Res. 22:5012–5021. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tuttle RM, Tala H, Shah J, Leboeuf R,
Ghossein R, Gonen M, Brokhin M, Omry G, Fagin JA and Shaha A:
Estimating risk of recurrence in differentiated thyroid cancer
after total thyroidectomy and radioactive iodine remnant ablation:
Using response to therapy variables to modify the initial risk
estimates predicted by the new American Thyroid Association staging
system. Thyroid. 20:1341–1349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Castagna MG, Maino F, Cipri C, Belardini
V, Theodoropoulou A, Cevenini G and Pacini F: Delayed risk
stratification, to include the response to initial treatment
(surgery and radioiodine ablation), has better outcome predictivity
in differentiated thyroid cancer patients. Eur J Endocrinol.
165:441–446. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ruben R, Pavithran PV, Menon VU, Nair V
and Kumar H: Performance of ATA risk stratification systems,
response to therapy, and outcome in an indian cohort of
differentiated thyroid carcinoma patients: A retrospective study.
Eur Thyroid J. 8:312–318. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tuttle RM and Alzahrani AS: Risk
stratification in differentiated thyroid cancer: From detection to
final follow-up. J Clin Endocrinol Metab. 104:4087–4100. 2019.
View Article : Google Scholar
|
|
17
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bassotti G, Villanacci V, Salerni B,
Maurer CA and Cathomas G: Beyond hematoxylin and eosin: The
importance of immunohistochemical techniques for evaluating
surgically resected constipated patients. Tech Coloproctol.
15:371–375. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kaczka K, Wojcik I, Matejkowska M, Kuzdak
K and Pomorski L: Lymph node metastases of papillary thyroid cancer
in immunohistochemical and molecular examination-preliminary
report. Endokrynol Pol. 56:160–167. 2005.(In Polish). PubMed/NCBI
|
|
20
|
Stavropoulos A, Varras M, Vasilakaki T,
Varra VK, Tsavari A, Varra FN, Nonni A, Kavantzas N and Lazaris AC:
Expression of p53 and PTEN in human primary endometrial carcinomas:
Clinicopathological and immunohistochemical analysis and study of
their concomitant expression. Oncol Lett. 17:4575–4589.
2019.PubMed/NCBI
|
|
21
|
Rho MH, Kim DW, Hong HP, Park YM, Kwon MJ,
Jung SJ, Kim YW and Kang T: Diagnostic value of antithyroid
peroxidase antibody for incidental autoimmune thyroiditis based on
histopathologic results. Endocrine. 42:647–652. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Spencer CA: Clinical review: Clinical
utility of thyroglobulin antibody (TgAb) measurements for patients
with differentiated thyroid cancers (DTC). J Clin Endocrinol Metab.
96:3615–3627. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Segurado OG, Volmer W and Dowell B: PSA
standardization: A review of NCCLS, Stanford and Abbott efforts.
Anticancer Res. 17:2919–2920. 1997.PubMed/NCBI
|
|
24
|
Feldt-Rasmussen U and Rasmussen AK: Serum
thyroglobulin (Tg) in presence of thyroglobulin autoantibodies
(TgAb). Clinical and methodological relevance of the interaction
between Tg and TgAb in vitro and in vivo. J Endocrinol Invest.
8:571–576. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Podnos YD, Smith D, Wagman LD and
Ellenhorn JD: The implication of lymph node metastasis on survival
in patients with well-differentiated thyroid cancer. Am Surg.
71:731–734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brito JP, Al Nofal A, Montori VM, Hay ID
and Morris JC: The impact of subclinical disease and mechanism of
detection on the rise in thyroid cancer incidence: A
population-based study in olmsted county, minnesota during 1935
through 2012. Thyroid. 25:999–1007. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aschebrook-Kilfoy B, Schechter RB, Shih
YC, Kaplan EL, Chiu BC, Angelos P and Grogan RH: The clinical and
economic burden of a sustained increase in thyroid cancer
incidence. Cancer Epidemiol Biomarkers Prev. 22:1252–1259. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Harrison MB, Graham ID, van den Hoek J,
Dogherty EJ, Carley ME and Angus V: Guideline adaptation and
implementation planning: A prospective observational study.
Implement Sci. 8:492013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Silberstein EB, Alavi A, Balon HR, Clarke
SE, Divgi C, Gelfand MJ, Goldsmith SJ, Jadvar H, Marcus CS, Martin
WH, et al: The SNMMI practice guideline for therapy of thyroid
disease with 131I 3.0. J Nucl Med. 53:1633–1651. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Baek SK, Jung KY, Kang SM, Kwon SY, Woo
JS, Cho SH and Chung EJ: Clinical risk factors associated with
cervical lymph node recurrence in papillary thyroid carcinoma.
Thyroid. 20:147–152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ricarte-Filho J, Ganly I, Rivera M, Katabi
N, Fu W, Shaha A, Tuttle RM, Fagin JA and Ghossein R: Papillary
thyroid carcinomas with cervical lymph node metastases can be
stratified into clinically relevant prognostic categories using
oncogenic BRAF, the number of nodal metastases, and extra-nodal
extension. Thyroid. 22:575–584. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen L, Zhu Y, Zheng K, Zhang H, Guo H,
Zhang L, Wu K, Kong L, Ruan W, Hu J, et al: The presence of
cancerous nodules in lymph nodes is a novel indicator of distant
metastasis and poor survival in patients with papillary thyroid
carcinoma. J Cancer Res Clin Oncol. 143:1035–1042. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee J, Lee SG, Kim K, Yim SH, Ryu H, Lee
CR, Kang SW, Jeong JJ, Nam KH, Chung WY and Jo YS: Clinical value
of lymph node ratio integration with the 8th edition of the UICC
TNM classification and 2015 ATA risk stratification systems for
recurrence prediction in papillary thyroid cancer. Sci Rep.
9:133612019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ryu IS, Song CI, Choi SH, Roh JL, Nam SY
and Kim SY: Lymph node ratio of the central compartment is a
significant predictor for locoregional recurrence after
prophylactic central neck dissection in patients with thyroid
papillary carcinoma. Ann Surg Oncol. 21:277–283. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Boi F, Pani F, Calo PG, Lai ML and
Mariotti S: High prevalence of papillary thyroid carcinoma in
nodular Hashimoto's thyroiditis at the first diagnosis and during
the follow-up. J Endocrinol Invest. 41:395–402. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Resende de Paiva C, Grønhøj C,
Feldt-Rasmussen U and von Buchwald C: Association between
Hashimoto's thyroiditis and thyroid cancer in 64,628 patients.
Front Oncol. 7:532017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dong S, Xie XJ, Xia Q and Wu YJ:
Indicators of multifocality in papillary thyroid carcinoma
concurrent with Hashimoto's thyroiditis. Am J Cancer Res.
9:1786–1795. 2019.PubMed/NCBI
|
|
38
|
Borowczyk M, Janicki A, Dworacki G,
Szczepanek-Parulska E, Danieluk M, Barnett J, Antonik M, Kałużna M,
Bromińska B, Czepczyński R, et al: Decreased staging of
differentiated thyroid cancer in patients with chronic lymphocytic
thyroiditis. J Endocrinol Invest. 42:45–52. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ieni A, Vita R, Magliolo E, Santarpia M,
Di Bari F, Benvenga S and Tuccari G: One-third of an archivial
series of papillary thyroid cancer (Years 2007–2015) Has coexistent
chronic lymphocytic thyroiditis, which is associated with a more
favorable tumor-node-metastasis staging. Front Endocrinol
(Lausanne). 8:3372017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lun Y, Wu X, Xia Q, Han Y, Zhang X, Liu Z,
Wang F, Duan Z, Xin S and Zhang J: Hashimoto's thyroiditis as a
risk factor of papillary thyroid cancer may improve cancer
prognosis. Otolaryngol Head Neck Surg. 148:396–402. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim WG, Yoon JH, Kim WB, Kim TY, Kim EY,
Kim JM, Ryu JS, Gong G, Hong SJ and Shong YK: Change of serum
antithyroglobulin antibody levels is useful for prediction of
clinical recurrence in thyroglobulin-negative patients with
differentiated thyroid carcinoma. J Clin Endocrinol Metab.
93:4683–4689. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Latrofa F, Ricci D, Montanelli L, Rocchi
R, Piaggi P, Sisti E, Grasso L, Basolo F, Ugolini C, Pinchera A and
Vitti P: Lymphocytic thyroiditis on histology correlates with serum
thyroglobulin autoantibodies in patients with papillary thyroid
carcinoma: Impact on detection of serum thyroglobulin. J Clin
Endocrinol Metab. 97:2380–2387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Spencer C and Fatemi S: Thyroglobulin
antibody (TgAb) methods-Strengths, pitfalls and clinical utility
for monitoring TgAb-positive patients with differentiated thyroid
cancer. Best Pract Res Clin Endocrinol Metab. 27:701–712. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jankovic B, Le KT and Hershman JM:
Clinical Review: Hashimoto's thyroiditis and papillary thyroid
carcinoma: Is there a correlation? J Clin Endocrinol Metab.
98:474–482. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fugazzola L, Colombo C, Perrino M and
Muzza M: Papillary thyroid carcinoma and inflammation. Front
Endocrinol (Lausanne). 2:882011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shi L, Zhou L, Wang J, Li F and Xie L:
Cytokine production of papillary thyroid carcinoma coexisting with
Hashimoto's thyroiditis. Int J Clin Exp Pathol. 10:9567–9574.
2017.PubMed/NCBI
|
|
47
|
Ito Y, Higashiyama T, Takamura Y, Miya A,
Kobayashi K, Matsuzuka F, Kuma K and Miyauchi A: Risk factors for
recurrence to the lymph node in papillary thyroid carcinoma
patients without preoperatively detectable lateral node metastasis:
Validity of prophylactic modified radical neck dissection. World J
Surg. 31:2085–2091. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sugitani I, Kasai N, Fujimoto Y and
Yanagisawa A: A novel classification system for patients with PTC:
Addition of the new variables of large (3 cm or greater) nodal
metastases and reclassification during the follow-up period.
Surgery. 135:139–148. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Perrier ND, Brierley JD and Tuttle RM:
Differentiated and anaplastic thyroid carcinoma: Major changes in
the American Joint Committee on Cancer eighth edition cancer
staging manual. CA Cancer J Clin. 68:55–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jeon MJ, Yoon JH, Han JM, Yim JH, Hong SJ,
Song DE, Ryu JS, Kim TY, Shong YK and Kim WB: The prognostic value
of the metastatic lymph node ratio and maximal metastatic tumor
size in pathological N1a papillary thyroid carcinoma. Eur J
Endocrinol. 168:219–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lee SR, Kim HO, Son BH, Shin JH and Yoo
CH: Prognostic significance of the metastatic lymph node ratio in
patients with gastric cancer. World J Surg. 36:1096–1101. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yu J, Yang L, Vexler A and Hutson AD: Easy
and accurate variance estimation of the nonparametric estimator of
the partial area under the ROC curve and its application. Stat Med.
35:2251–2282. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sorribas A, March J and Trujillano J: A
new parametric method based on S-distributions for computing
receiver operating characteristic curves for continuous diagnostic
tests. Stat Med. 21:1213–1235. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Habibzadeh F, Habibzadeh P and Yadollahie
M: On determining the most appropriate test cut-off value: The case
of tests with continuous results. Biochem Med (Zagreb). 26:297–307.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang LW, Qu AP, Yuan JP, Chen C, Sun SR,
Hu MB, Liu J and Li Y: Computer-based image studies on tumor nests
mathematical features of breast cancer and their clinical
prognostic value. PLoS One. 8:e823142013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Dello SA, van Dam RM, Slangen JJ, van de
Poll MC, Bemelmans MH, Greve JW, Beets-Tan RG, Wigmore SJ and
Dejong CH: Liver volumetry plug and play: Do it yourself with
ImageJ. World J Surg. 31:2215–2221. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhou J, Zhang Y, Chang KT, Lee KE, Wang O,
Li J, Lin Y, Pan Z, Chang P, Chow D, et al: Diagnosis of benign and
malignant breast lesions on DCE-MRI by using radiomics and deep
learning with consideration of peritumor tissue. J Magn Reson
Imaging. 51:798–809. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Nie P, Yang G, Wang Z, Yan L, Miao W, Hao
D, Wu J, Zhao Y, Gong A, Cui J, et al: A CT-based radiomics
nomogram for differentiation of renal angiomyolipoma without
visible fat from homogeneous clear cell renal cell carcinoma. Eur
Radiol. 30:1274–1284. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jeon MJ, Kim WG, Chung KW, Baek JH, Kim WB
and Shong YK: Active surveillance of papillary thyroid
microcarcinoma: Where do we stand? Eur Thyroid J. 8:298–306. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Donovan JL: Presenting treatment options
to men with clinically localized prostate cancer: The acceptability
of active surveillance/monitoring. J Natl Cancer Inst Monogr.
2012:191–196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kim TY and Shong YK: Active surveillance
of papillary thyroid microcarcinoma: A Mini-review from Korea.
Endocrinol Metab (Seoul). 32:399–406. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ya M: Interpretation of the management
guidelines for patients with thyroid nodules and differentiated
thyroid cancer (2012 Chinese edition). Lin Chung Er Bi Yan Hou Tou
Jing Wai Ke Za Zhi. 27:917–920. 2013.(In Chinese). PubMed/NCBI
|