Introduction
Endometrial cancer (EC) is the most common
gynaecological cancer in developed countries, and the incidence of
EC is increasing rapidly worldwide (1,2). Over
189,000 cases of endometrial cancer are diagnosed worldwide per
year and ~45,000 mortality cases (3,4). EC is a
multi-factorial disease of which pathogenesis has not been fully
elucidated. Most cases of EC are diagnosed at an early-stage and
have a good prognosis after surgery alone; however, the 5-year
survival rate is only 17% for patients with distant metastatic
disease (5,6). It is therefore crucial to understand
the underlying mechanisms of EC metastasis in order to develop
effective strategies for EC diagnosis and therapy.
MicroRNAs (miRs) are a class of short non-coding
RNAs that regulate gene expression at the post-transcriptional
level by binding to the 3′ untranslated region (3′UTR) of target
mRNA (7). Abnormal expression of
miRNAs has been found in various types of tumor and is frequently
associated with numerous aspects of tumor progression, including
proliferation, differentiation, invasion, migration, apoptosis and
senescence (8). Emerging evidence
has revealed that several miRNAs are dysregulated in EC (9–11) and
can act as either potent oncogenes or tumor suppressor genes
(12–14). For example, miR-23a and miR-135a have
been demonstrated to inhibit EC development (12,14).
Conversely, miR-29b can inhibit the proliferation and decrease the
migratory and invasive abilities of EC cells (13). miR-20a-5p is a member of the
miR-17-92 cluster, which plays a complex role in tumorigenesis
(15). Dysregulation of miR-20a-5p
has been observed in various cancers, including colorectal cancer,
cervical cancer, ovarian cancer and hepatocellular carcinoma
(16). Ramón et al (17) reported that miR-20a-5p is
significantly downregulated in cancerous endometrium compared with
control endometrium. Furthermore, a negative correlation between
vascular endothelial growth factor A protein expression and miR-20a
expression is observed in EC specimens (17). However, the function and underlying
mechanism of miR-20a-5p in EC remain poorly understood.
Janus kinase 1 (Jak1) is a member of a class of
protein-tyrosine kinases which are involved in autoimmune diseases
and malignancies (18,19). Jak1 phosphorylates the proteins named
signal transducers and activators of transcription in response to
interferon (20) and serves a
critical role in cancer progression.
The present study aimed to examine the expression of
miR-20a-5p in human EC tissues and to determine the association
between miR-20a-5p expression and the clinicopathological
characteristics of patients with EC. The effects of miR-20a-5p on
cell proliferation, invasive ability and adhesion were
investigated. Whether miR-20a-5p could inhibit EC progression by
targeting janus kinase 1 (Jak1) was also evaluated. The findings
from this study might provide a better understanding of EC
pathogenesis.
Materials and methods
Tissue collection
The present study was approved by the Ethics
Committee of The First Affiliated Hospital of Hebei North
University (Zhangjiakou, Hebei, China). All patients provided
written informed consent in compliance with the code of ethics of
the World Medical Association (Declaration of Helsinki). Human EC
tissues and paracancerous tissues were collected from 47 patients
with EC who underwent surgical resection. None of the patients had
received chemotherapy or radiotherapy prior to surgery. The tissue
samples were immediately frozen in liquid nitrogen.
Cell culture and transfection
The normal human endometrial stromal cell line hESC
(cat. no. BNCC340262) and the EC cell lines Ishikawa, KLE, HHUA and
RL95-2 were purchased from BeNa Culture Collection. All cells were
cultured in Dulbecco's modified Eagle's medium/F12 (Invitrogen;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Invitrogen; Thermo Fisher Scientific, Inc.). HEK293 cells were
purchased from the American Type Culture Collection and cultured in
RPMI Medium 1640 (Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS. Cells were placed at 37°C in a
humidified incubator containing 5% CO2. Ishikawa cells
were transfected with 2 µM miR-negative control (NC), 2 µM
miR-20a-5p-mimic, 2 µM miR-20a-5p-inhibitor, 4 µg pcDNA3.1 or 4 µg
Jak1-pcDNA3.1 (Shanghai GenePharma Co., Ltd.) using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturers' instructions. After 48 h, cells were observed under
a fluorescence microscope and the transfection efficiency was
>80% (Fig. S1).
Reverse transcription-quantitative
(RT-q) PCR
miRNAs were isolated from tissues or cultured cells
using mirVanaTM miR isolation kit (Ambion; Thermo Fisher
Scientific, Inc.). Total RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). cDNA was synthesized
from 5 ng of total RNA using the First Strand cDNA Synthesis kit
(Fermentas; Thermo Fisher Scientific, Inc.) according to the
manufacturers' instructions. The sequences of primers were as
follows: Jak1, forward 5′-AGCGATGTCCTTACCACACC-3′, reverse
5′-CCTCAACACACTCAGGAGCA-3′; GAPDH, forward
5′-TCAACGACCACTTTGTCAAGCTCA-3′, reverse
5′-GCTGGTGGTCCAGGGGTCTTACT-3′; miR-20a-5p, forward
5′-TAAAGTGCTTATAGTGCAGGTAG-3′, reverse 5′-TGGTGTCGTGGAGTCG-3′; and
U6, forward 5′-CTCGCTTCGGCAGCACA-3′ and reverse
5′-AACGCTTCACGAATTTGCGT-3′. Amplification and detection were
performed with a SYBR-Green PCR kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.) on the ABI PRISM 7700 Sequence Detection
System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
thermocycling conditions were as follows: 95°C for 4 min, followed
by 40 cycles of 95°C for 30 sec, 59°C for 30 sec and 72°C for 1 min
with a final extension at 72°C for 5 min. The relative expression
levels were normalized to endogenous controls U6 and GAPDH and were
expressed as 2−ΔΔCq (21).
Western blotting
Tissues and cells were collected and lysed using
RIPA buffer (Beyotime Institute of Biotechnology) at 4°C. Protein
concentration was determined using the BCA Protein Assay kit
(Beyotime Institute of Biotechnology). Proteins (40 µg) were
separated by 10% SDS-PAGE and transferred onto PVDF membranes (EMD
Millipore). Subsequently, membranes were blocked with 5% skimmed
milk at 4°C overnight. After washing in Tris-buffered
saline-Tween-20 (0.05%) solution, membranes were incubated with
mouse monoclonal primary antibodies against Jak1 (1:400; cat. no.
sc376996; Santa Cruz Biotechnology, Inc.) and GAPDH (1:1,000; cat.
no. sc365062; Santa Cruz Biotechnology, Inc.) at 4°C overnight.
Membranes were then incubated with IgG-horseradish
peroxidase-conjugated secondary antibody (1:5,000; cat. no. sc2005;
Santa Cruz Biotechnology, Inc.) at 37°C for 1 h. Pierce SuperSignal
West Pico Chemiluminescent Substrate (Pierce Biotechnology, Inc.)
was used to detect the signal on the membrane. The data were
analyzed via densitometry using ImageJ software (version 1.8.0;
National Institutes of Health) and normalized to expression of the
internal control GAPDH.
Dual luciferase reporter assay
Plasmid constructs carrying wild type or mutant Jak1
3′UTR in the psiCHECK vector were cotransfected with miR-20a-5p
mimic or miR NC into HEK293 cells using Lipofectamine 2000.
miR-20a-5p mimic and miR NC were synthesized by Shanghai GenePharma
Co., Ltd. and the sequences were as follows: miR-20a-5p mimic,
sense, 5′-UAAAGUGCUUAUAGUGCAGGUAG-3′; miR-20a-5p mimic, antisense,
5′-ACCUGCACUAUAAGCACUUUAUU-3′. miR NC, 5′-UUGUACUACACAAAAGUACUG-3′.
After 48 h, the luciferase activity was measured using the
Dual-Luciferase Reporter 1000 System (Promega Corporation).
Renilla luciferase activity was normalized to firefly
luciferase activity to control transfection efficiency.
MTT assay
Cell proliferation was determined using a MTT Assay
Kit from Abcam according to the manufacturers' instructions.
Briefly, cells at the density of 1×104/well were
cultured in 96-well plates for 24, 48, 72 and 96 h and were
incubated with 10 µl of MTT reagent for 3 h at 37°C. Subsequently,
MTT solvent was added to dissolve purple formazan crystals and
cells were further incubated at 37°C for 15 min. Absorbance was
read at 570 nm using a microplate reader (Bio-Rad Laboratories,
Inc.).
Cell invasion assay
Transwell inserts (Corning) were precoated with
Matrigel (1:20; Corning) 37°C for 30 min before seeding the cells.
At 48 h post-transfection, cells were collected with serum-free
media and seeded into the upper chamber at a final concentration of
5×104 cells/ml whereas medium containing 10% FBS was
added into the lower chamber. After incubation at 37°C for 8 h, the
non-invasive cells in the upper chamber were removed gently using
cotton swabs. The cells that have invaded the bottom of the
membranes were fixed with ethanol, stained with hematoxylin. Cells
were observed under an inverted light microscope (CKX31, Olympus
Corporation; magnification, ×400). The number of invaded cells was
quantified by counting five randomly chosen microscopic fields.
Fibronectin adhesion assay
At 48 h post-transfection, cells were seeded at the
density of 5×104 cells per well into 96-well plates that
were precoated with 1% human plasma fibronectin-purified protein
(EMD Millipore). After incubation at 37°C for 2 h, cells were
washed with PBS three times and fixed with 3.7% formaldehyde at
room temperature for 40 min. Cells were stained with 0.1% crystal
violet solution at room temperature for 30 min and the crystal
violet was then solubilized in 10% acetic acid solution. The
absorbance was determined at 570 nm using a microplate reader
(Bio-Rad).
Statistical analysis
Statistical analysis was performed using SPSS 19.0
(IBM Corp.). The data were presented as the means ± standard
deviation and analyzed by Student's t-test or ANOVA followed
by Bonferroni's post hoc test. P<0.05 was considered to indicate
a statistically significant difference.
Results
miR-20a-5p is downregulated in EC
tissues and associated with the clinicopathological characteristics
of patients with EC
Expression of miR-20a-5p in EC and paracancerous
tissues was examined by RT-qPCR. As presented in Fig. 1, miR-20a-5p was significantly
downregulated in EC tissues compared with paracancerous tissues
(P<0.01). Furthermore, miR-20a-5p expression was found to be
significantly associated with the depth of myometrial invasion,
FIGO stage, histologic grade and lymph node metastasis in patients
with EC (Table I).
 | Table I.The association between miR-20a-5p
expression and clinicopathologic features of EC patients. |
Table I.
The association between miR-20a-5p
expression and clinicopathologic features of EC patients.
| Clinicopathologic
features | Cases, n=47 | miR-20a-5p
expressionb | P-value |
|---|
| Age, years |
|
|
|
|
≤55 | 26 | 0.448±0.08 |
|
|
>55 | 21 | 0.413±0.11 | 0.227 |
| Depth of myometrial
invasion |
|
|
|
|
<1/2 | 35 | 0.413±0.097 |
|
|
≥1/2 | 12 | 0.490±0.067 | 0.015 |
| FIGO
stagea |
|
|
|
|
I+II | 34 | 0.414±0.097 |
|
|
III+IV | 13 | 0.481±0.076 | 0.03 |
| Histologic
grade |
|
|
|
| G1 | 30 | 0.404±0.098 |
|
| G2 | 7 | 0.430±0.043 |
|
| G3 | 10 | 0.518±0.065 | 0.003 |
| Lymph node
metastasis |
|
|
|
|
Yes | 8 | 0.484±0.063 |
|
| No | 39 | 0.422±0.100 | 0.036a |
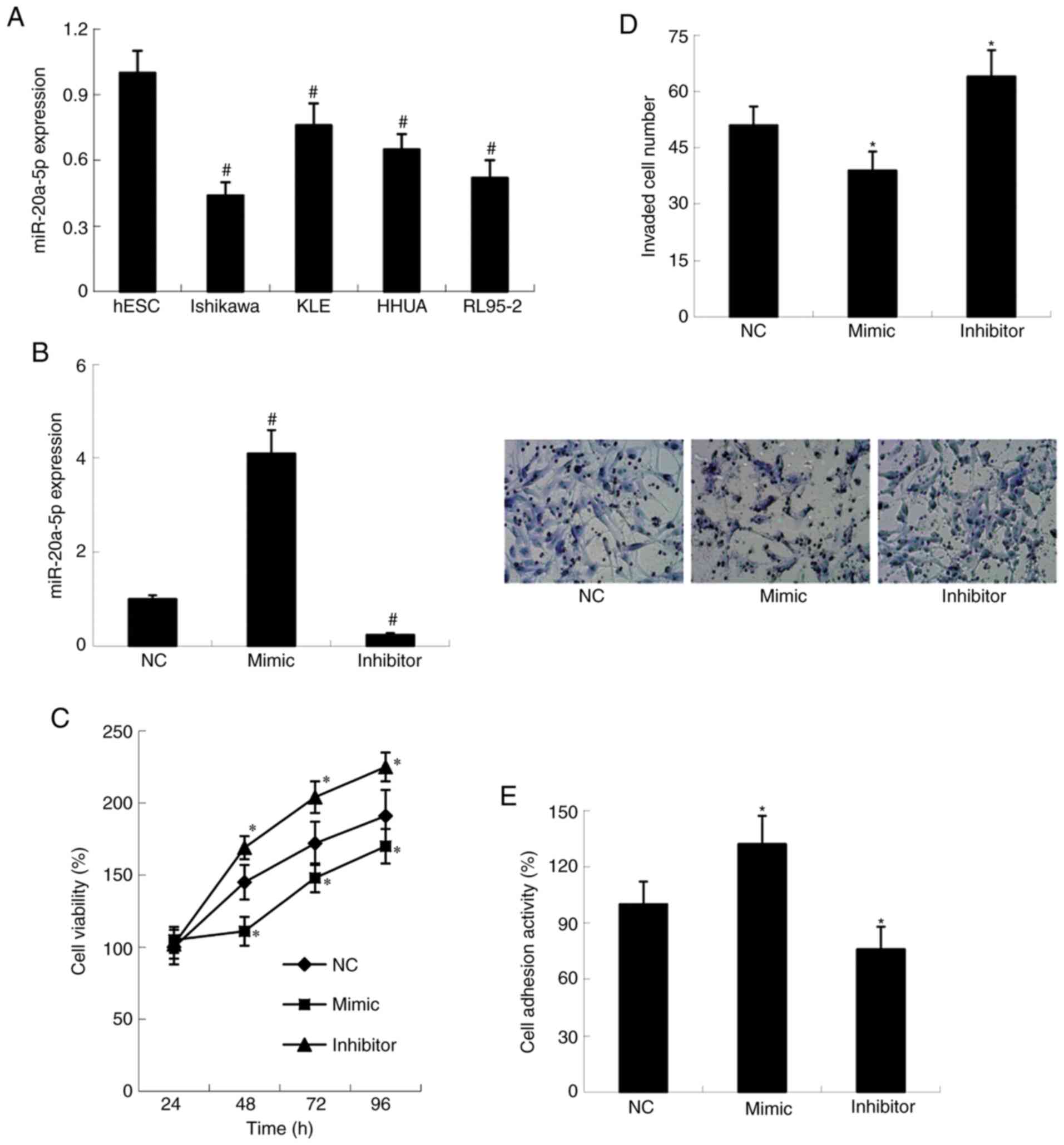
miR-20a-5p inhibits cell viability and
invasive ability, and stimulates cell adhesion
Expression of miR-20a-5p in endometrial stromal cell
line hESC and EC derived cell lines Ishikawa, KLE, RL95-2 and HHUA
was examined by RT-qPCR. As presented in Fig. 2A, miR-20a-5p expression was
significantly decreased in EC derived cells compared with hESC
cells (P<0.01). To investigate the effect of miR-20a-5p on cell
proliferation, invasive ability and adhesion, Ishikawa cell line,
which exhibited the lowest miR-20a-5p level, was chosen for
subsequent gain-of-function and loss-of-function experiments. miR
NC, miR-20a-5p mimic and miR-20a-5p inhibitor were transfected into
Ishikawa cells. As demonstrated in Fig.
2B, miR-20a-5p expression was significantly increased in mimic
group and decreased in inhibitor group compared with NC group
(P<0.01). Furthermore, Ishikawa cells transfected with
miR-20a-5p-mimic showed decreased proliferation (P<0.05) and
invasion ability (P<0.05), and increased adhesion ability
compared with NC group (P<0.05). Ishikawa cells transfected with
miR-20a-5p-inhibitor showed the opposite results (Fig. 2C-E).
Jak1 is upregulated and associated
with miR-20a-5p expression in EC tissues
Jak1 mRNA and protein expression in EC and
paracancerous tissues was examined by RT-qPCR and western blotting,
respectively. As presented in Fig.
3A, the mRNA and protein expression of Jak1 was significantly
increased in EC tissues compared with paracancerous tissues
(P<0.05). Furthermore, Jak1 mRNA expression was negatively
correlated with miR-20a-5p expression in EC tissues (P<0.01;
Fig. 3B).
miR-20a-5p directly targets Jak1
To verify whether miR-20a-5p could directly target
Jak1, Jak1 3′UTR reporter assay was performed in HEK293 cells. As
seen in Fig. 4A, the luciferase
activity of wild type Jak1 3′UTR was significantly decreased in
cells transfected with miR-20a-5p-mimic compared with cells
transfected with miR-NC (P<0.01); however, no change was
observed in the mutant Jak1 3′UTR. Furthermore, results from
RT-qPCR and western blotting demonstrated that the mRNA and protein
expression of Jak1 was significantly decreased in Ishikawa cells
transfected with miR-20a-5p-mimic but increased in cells
transfected with miR-20a-5p-inhibitor (P<0.01 for mRNA
expression; P<0.05 for protein expression; Fig. 4B).
Jak1 overexpression reverses the
effects of miR-20a-5p on cell proliferation, invasive ability and
adhesion
Jak1-pcDNA3.1 and the empty vector pcDNA3.1 were
transfected into Ishikawa cells. The results demonstrated that Jak1
mRNA and protein expression was significantly increased in the
Jak1-pcDNA3.1 group compared with pcDNA3.1 group (P<0.01 for
mRNA, P<0.05 for protein; Fig.
S2). To further investigate whether Jak1 was involved in
mediating the effects of miR-20a-5p on cell proliferation, invasive
ability and adhesion, Jak1-pcDNA3.1 was transfected into Ishikawa
cells in the presence of miR-20a-5p mimic. The results demonstrated
that Jak1 protein expression was significantly increased in cells
transfected with the miR-20a-5p mimic and Jak1-pcDNA3.1 compared
with cells transfected with miR-20a-5p-mimic only (P<0.01);
however, the increased expression of miR-20a-5p in
miR-20a-5p-mimic-transfected cells was not affected by
Jak1-pcDNA3.1 transfection (Fig. 5A and
B). Furthermore, the inhibitory effect of miR-20a-5p-mimic on
cell proliferation and invasive ability, and the promotive effect
of miR-20a-5p-mimic on cell adhesion were reversed by transfection
with Jak1-pcDNA3.1 (P<0.05; Fig.
5C-E).
Discussion
Alteration in miR-20a-5p expression patterns is
associated with endometrial growth, differentiation and
carcinogenesis of the endometrium (22–24).
Previous studies reported that miR-20a-5p is significantly
decreased in the plasma and serum of patients with endometriosis,
and that it serves important roles in the pathogenesis of
endometriosis (22,23). Conversely, miR-20a-5p expression was
found to be upregulated in endometriotic stromal cells (24). In addition, in vitro
application of hypoxia results in downregulation of miR-20a-5p in
Ishikawa cells (25). In the present
study, downregulation of miR-20a-5p was observed in EC tissues and
cell lines compared with paracancerous tissues and endometrial
stromal cells, respectively. Furthermore, miR-20a-5p expression was
significantly associated with the depth of myometrial invasion,
FIGO stage, histologic grade and lymph node metastasis in patients
with EC. Patients with lower miR-20a-5p expression exhibited lower
FIGO stage and histologic grade, as well as less myometrial
invasion and lymph node metastasis.
miR-20a-5p serves various roles according to the
type of cancer. Previous studies have demonstrated that miR-20a-5p
can induce radioresistance in hepatocellular carcinoma and
nasopharyngeal cancer cells (26,27).
Zhao et al (28) reported
that miR-20a-5p can inhibit cell proliferation, mobility and
invasiveness, and facilitate apoptosis in breast cancer. However,
contrasting report indicated that miR-20a-5p promotes invasion and
metastasis, and induces epithelial-mesenchymal transition of
colorectal cancer cells (29). In
the present study, miR-20a-5p was demonstrated to inhibit cell
proliferation and cell invasive ability, as well as to induce cell
adhesion ability. These findings were in agreement with a previous
study reporting the suppressor role of miR-20a-5p in EC cell lines
(30).
Numerous target genes of miR-20a-5p have been
identified in previous studies, including cyclin D1, E2F
transcription factor 3, interleukin-8 and dual specificity
phosphatase 2 (16,31). In the present study, miR-20a-5p
expression and Jak1 expression levels were negatively correlated in
EC tissues. Furthermore, Jak1 was demonstrated to be a direct
target of miR-20a-5p following dual luciferase reporter assay. A
previous study has reported that activated Jak1 might contribute to
carcinogenesis in leukemias, lymphomas and myeloproliferative
neoplasms (32). Sexl et al
(33) reported that Jak1-deficient
cells are more tumorigenic than wild-type cells. Jak1 can also act
as either an oncogene or a tumor suppressor under certain
conditions (34). The present study
demonstrated that miR-20a-5p could decrease Jak1 expression in EC
cells, and that Jak1 overexpression could reverse the effects of
miR-20a-5p-mimic on EC cell proliferation, invasive ability and
adhesion. These results supported the notion that Jak1 could
contribute to EC progression (35,36) and
suggested that miR-20a-5p may play a tumor suppressive role in EC
partly by decreasing Jak1 expression.
In the present study, miR-20a-5p expression was
detected in four EC derived cell lines, and the results
demonstrated that all these EC cell lines had significantly
decreased miR-20a-5p expression compared with normal human
endometrial stromal cells. Since Ishikawa cell line showed the
lowest miR-20a-5p expression, it was selected for subsequent
experiments. The present study was however limited because the
gain- and loss-of-function experiments were only performed in the
Ishikawa cell line. Functional experiments performed in additional
cell lines is therefore required.
In conclusion, to the best of our knowledge, the
present study was the first to demonstrate that miR-20a-5p
expression was significantly correlated with the depth of
myometrial invasion, FIGO stage, histologic grade and lymph node
metastasis in patients with EC. In addition, miR-20a-5p expression
and Jak1 mRNA expression were negatively correlated in EC tissues.
Jak1 was confirmed as a novel target of miR-20a-5p, and miR-20a-5p
acted as a tumor suppressor in EC at least partly through
decreasing Jak1 expression. This study provided new insights into
the underlying mechanisms of EC progression, and the findings
suggested that miR-20a-5p and Jak1 may serve as potential
therapeutic targets in EC. Further investigation is required to
confirm the effect of miR-20a-5p in animal models of EC and to
determine additional target genes of miR-20a-5p.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was funded by Key Project of Science and
Technology Plan of Hebei Health Committee (grant no. 20212598).
Availability of data and materials
The data sets generated and analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
QX and YH conceived and designed the experiments,
analyzed the data and prepared the manuscript. HM and JW conducted
the experiments. YK contributed to data collection and analysis.
All authors read and approved the final version.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The First Affiliated Hospital of Hebei North University
(Zhangjiakou, Hebei, China).
Patient consent for publication
All patients provided written informed consent in
compliance with the code of ethics of the World Medical Association
(Declaration of Helsinki).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Braun MM, Overbeek-Wager EA and Grumbo RJ:
Diagnosis and management of endometrial cancer. Am Fam Physician.
93:468–474. 2016.PubMed/NCBI
|
|
2
|
McAlpine JN, Temkin SM and Mackay HJ:
Endometrial cancer: Not your grandmother's cancer. Cancer.
122:2787–2798. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsikouras P, Bouchlariotou S, Vrachnis N,
Dafopoulos A, Galazios G, Csorba R and von Tempelhoff GF:
Endometrial cancer: Molecular and therapeutic aspects. Eur J Obstet
Gynecol Reprod Biol. 169:1–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Colombo N, Creutzberg C, Amant F, Bosse T,
González-Martín A, Ledermann J, Marth C, Nout R, Querleu D, Mirza
MR, et al: ESMO-ESGO-ESTRO consensus conference on endometrial
cancer: Diagnosis, treatment and follow-up. Ann Oncol. 27:16–41.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu TX and Rothenberg ME: MicroRNA. J
Allergy Clin Immunol. 141:1202–1207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mohr AM and Mott JL: Overview of microRNA
biology. Semin Liver Dis. 35:3–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hutt S, Tailor A, Ellis P, Michael A,
Butler-Manuel S and Chatterjee J: The role of biomarkers in
endometrial cancer and hyperplasia: A literature review. Acta
Oncol. 58:342–352. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stope MB, Koensgen D, Weimer J, Paditz M,
Burchardt M, Bauerschlag D and Mustea A: The future therapy of
endometrial cancer: microRNA's functionality, capability, and
putative clinical application. Arch Gynecol Obstet. 294:889–895.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rižner TL: Discovery of biomarkers for
endometrial cancer: Current status and prospects. Expert Rev Mol
Diagn. 16:1315–1336. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li HL, Sun JJ, Ma H, Liu SJ, Li N, Guo SJ,
Shi Y, Xu YY, Qi ZY, Wang YQ, et al: MicroRNA-23a inhibits
endometrial cancer cell development by targeting SIX1. Oncol Lett.
18:3792–3802. 2019.PubMed/NCBI
|
|
13
|
Kong J, He X, Wang Y and Li J: Effect of
microRNA-29b on proliferation, migration, and invasion of
endometrial cancer cells. J Int Med Res. 47:3803–3817. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang J, Zhang L, Jiang W, Zhang R, Zhang
B, Silayiding A and Duan X: MicroRNA-135a promotes proliferation,
migration, invasion and induces chemoresistance of endometrial
cancer cells. Eur J Obstet Gynecol Reprod Biol X. 5:1001032020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fuziwara CS and Kimura ET: Insights into
Regulation of the miR-17-92 Cluster of miRNAs in Cancer. Front Med
(Lausanne). 2:642015.PubMed/NCBI
|
|
16
|
Agrawal S, Tapmeier T, Rahmioglu N,
Kirtley S, Zondervan K and Becker C: The miRNA Mirage: How close
are we to finding a non-invasive diagnostic biomarker in
endometriosis? A systematic review. Int J Mol Sci. 19:5992018.
View Article : Google Scholar
|
|
17
|
Ramón LA, Braza-Boïls A, Gilabert J,
Chirivella M, España F, Estellés A and Gilabert-Estellés J:
MicroRNAs related to angiogenesis are dysregulated in endometrioid
endometrial cancer. Hum Reprod. 27:3036–3045. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schwartz DM, Bonelli M, Gadina M and
O'Shea JJ: Type I/II cytokines, JAKs, and new strategies for
treating autoimmune diseases. Nat Rev Rheumatol. 12:25–36. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kleppe M, Kwak M, Koppikar P, Riester M,
Keller M, Bastian L, Hricik T, Bhagwat N, McKenney AS, Papalexi E,
et al: JAK-STAT pathway activation in malignant and nonmalignant
cells contributes to MPN pathogenesis and therapeutic response.
Cancer Discov. 5:316–331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mitchell TJ and John S: Signal transducer
and activator of transcription (STAT) signalling and T-cell
lymphomas. Immunology. 114:301–312. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang L, Huang W, Ren C, Zhao M, Jiang X,
Fang X and Xia X: Analysis of serum microRNA profile by solexa
sequencing in women with endometriosis. Reprod Sci. 23:1359–1370.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jia SZ, Yang Y, Lang J, Sun P and Leng J:
Plasma miR-17-5p, miR-20a and miR-22 are down-regulated in women
with endometriosis. Hum Reprod. 28:322–330. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin SC, Wang CC, Wu MH, Yang SH, Li YH and
Tsai SJ: Hypoxia-induced microRNA-20a expression increases ERK
phosphorylation and angiogenic gene expression in endometriotic
stromal cells. J Clin Endocrinol Metab. 97:E1515–E1523. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eismann J, Hirschfeld M, Erbes T, Rücker
G, Jäger M, Ritter A, Weiss D, Gitsch G and Mayer S: Hypoxia- and
acidosis-driven aberrations of secreted microRNAs in endometrial
cancer in vitro. Oncol Rep. 38:993–1004. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Zheng L, Ding Y, Li Q, Wang R,
Liu T, Sun Q, Yang H, Peng S, Wang W and Chen L: miR-20a induces
cell radioresistance by activating the PTEN/PI3K/Akt signaling
pathway in hepatocellular carcinoma. Int J Radiat Oncol Biol Phys.
92:1132–1140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang D, Bian G, Pan Y, Han X, Sun Y, Wang
Y, Shen G, Cheng M, Fang X and Hu S: miR-20a-5p promotes
radio-resistance by targeting Rab27B in nasopharyngeal cancer
cells. Cancer Cell Int. 17:322017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao W, Geng D, Li S, Chen Z and Sun M:
LncRNA HOTAIR influences cell growth, migration, invasion, and
apoptosis via the miR-20a-5p/HMGA2 axis in breast cancer. Cancer
Med. 7:842–855. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng D, Zhao S, Tang H, Zhang D, Sun H,
Yu F, Jiang W, Yue B, Wang J, Zhang M, et al: MicroRNA-20a-5p
promotes colorectal cancer invasion and metastasis by
downregulating Smad4. Oncotarget. 7:45199–45213. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang Y and Yang N: MicroRNA-20a-5p
inhibits epithelial to mesenchymal transition and invasion of
endometrial cancer cells by targeting STAT3. Int J Clin Exp Pathol.
11:5715–5724. 2018.PubMed/NCBI
|
|
31
|
Chen LT and Jiang CY: MicroRNA expression
profiles identify biomarker for differentiating the embolic stroke
from thrombotic stroke. Biomed Res Int. 2018:45141782018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Roskoski R Jr: Janus kinase (JAK)
inhibitors in the treatment of inflammatory and neoplastic
diseases. Pharmacol Res. 111:784–803. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sexl V, Kovacic B, Piekorz R, Moriggl R,
Stoiber D, Hoffmeyer A, Liebminger R, Kudlacek O, Weisz E,
Rothammer K and Ihle JN: Jak1 deficiency leads to enhanced
Abelson-induced B-cell tumor formation. Blood. 101:4937–4943. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yeh YT, Ou-Yang F, Chen IF, Yang SF, Su
JH, Hou MF and Yuan SS: Altered p-JAK1 expression is associated
with estrogen receptor status in breast infiltrating ductal
carcinoma. Oncol Rep. 17:35–39. 2007.PubMed/NCBI
|
|
35
|
van der Zee M, Sacchetti A, Cansoy M,
Joosten R, Teeuwssen M, Heijmans-Antonissen C, Ewing-Graham PC,
Burger CW, Blok LJ and Fodde R: IL6/JAK1/STAT3 signaling blockade
in endometrial cancer affects the ALDHhi/CD126+
stem-like component and reduces tumor burden. Cancer Res.
75:3608–3622. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ren Y, Zhang Y, Liu RZ, Fenstermacher DA,
Wright KL, Teer JK and Wu J: JAK1 truncating mutations in
gynecologic cancer define new role of cancer-associated protein
tyrosine kinase aberrations. Sci Rep. 3:30422013. View Article : Google Scholar : PubMed/NCBI
|