Introduction
Differentiated thyroid cancer (DTC) is the most
frequent endocrine tumor with in most cases a good prognosis.
Standard of care consists of hemi-thyroidectomy or total
thyroidectomy often followed by 131I radioactive iodide
(RAI) ablation (1,2). Despite the increasing incidence of DTC,
less than 10% of patients with clinical disease will develop
distant metastases. From this group, 30–40% of patients with
metastatic DTC are unresponsive to 131I radioactive
iodide (RAI) treatment, have a 10-year survival rate less than 10%
and a mean life expectancy of 3–5 years. RAI refractoriness could
be the result of a tumor dedifferentiation process. (3,4). There
are currently no curative treatment options for these
RAI-refractory tumors emphasizing the need for novel therapeutic
options and molecular biomarkers (5–8).
Several studies aimed to discover novel markers that
predict RAI sensitivity. In this context, telomerase reverse
transcriptase (TERT) promoter mutations have been proposed
to assist identification of patients with poorly differentiated TC
with high risk of RAI refractoriness (9). The same holds true for the assessment
of sodium-iodide symporter expression in circulating tumor cells
(10). Also, prostate-specific
membrane antigen (PSMA) expression in tumor tissue has been
suggested to contribute in the prediction of tumor aggressiveness
and patient outcome (11). The most
intensively studied marker is Thyroglobulin (Tg). Quantitative
changes in Tg could reflect the response to RAI therapy or serve as
diagnostic or prognostic tool (12–14).
However, current methods, such as serum Tg or PSMA
expression measurements serve mostly as a diagnostic, predictive or
prognostic tool. Therefore, the identification of DTC patients at
risk for RAI refractory disease is still an unmet medical need and
additional markers need to be explored.
In the present study we aimed to identify molecular
markers in primary tumors that are associated with RAI refractory
disease. A retrospective cohort of 63 DTC patients, including all
histological subtypes, was collected for this study and consisted
of 35 RAI-sensitive DTC patients and 28 RAI-refractory (poorly)
differentiated TC patients. Extensive intratumoral mutation
profiling, gene fusions analysis, TERT promoter mutation
analysis and formalin-fixed paraffin embedded-compatible RNA
sequencing was performed in all patients. To validate potential
circulating markers, an independent cohort of 8 DTC patients was
available.
Materials and methods
Patient cohorts
Detailed pathology reports were collected of all DTC
patients diagnosed in Nijmegen and surrounding hospitals between
2000 and 2016 (1,544 patients). We selected 35 RAI-sensitive DTC
patients, ages ranged from 15 to 77 years (M=41.9±18.9), and 28
RAI-refractory DTC patients, ages ranged from 45 to 84 years
(M=61.9±10.2), that underwent total or near-total thyroidectomy and
showed residual disease after primary surgery. The eighth edition
of the American Joint Committee on Cancer (AJCC) staging system was
used to determine the TNM stage of each individual patient
(15). Patients with confirmed nodal
metastases prior to primary surgery also underwent a modified
radical lymph node dissection. RAI ablation of residual thyroid
tissue was performed 4–6 weeks after surgery. All patients included
in this study had residual disease after primary surgery as
demonstrated by diagnostic RAI scintigraphy. If indicated, patients
were repeatedly treated with RAI to reach remission. RAI
sensitivity was defined as a complete response to RAI therapy of
histologically differentiated tumor lesions resulting in remission
after the primary treatment by surgery and RAI ablation or (if
indicated) after subsequent treatments with RAI for metastases with
documented 131I uptake. Remission was defined as
undetectable thyroid-stimulating hormone stimulated Tg in the
absence of anti-Tg antibodies and no evidence of loco-regional
disease or distant metastasis on the whole-body iodide scans (WBS)
and/or neck ultrasonographic examinations at 6–9 months after the
last RAI treatment. The remission status was confirmed at the last
follow-up visit. According to the RECIST criteria, RAI
refractoriness was defined as either new evidence of recurrent
loco-regional disease or distant metastasis after successful
primary therapy or progressive disease at least 6 months after
primary treatment by surgery and RAI treatment preferably supported
by presence of metastases that do not accumulate 131I on
the last post-therapy scan. Persistent disease was defined as
detectable Tg and/or evidence of loco-regional disease or distant
metastases. Of all selected DTC patients, archived formalin-fixed
paraffin-embedded (FFPE) tissue specimens were collected for
genetic, transcriptomic and protein analyses. Collection, storage
and use of archival tissue and patient data were in compliance with
the ‘Code of Proper Secondary Use of Human Tissue in the
Netherlands’ (http://www.fmwv.nl and www.federa.org). This study was approved by the
Research Ethics Committee [Commissie Mensgebonden Onderzoek (CMO)]
of the Radboud University Medical Center under application
2015–1762 and followed the ethical guidelines of the CMO. The CMO
waived the need for consent for the use of archive samples since
the samples were analyzed anonymously.
An independent cohort including eight consecutive
newly diagnosed patients with DTC (with and without metastases),
ages ranged from 27 to 78 years (M=54.6±16.5), that were therapy
naïve and were planned to receive conventional primary treatment by
surgery followed by RAI were included in which plasma insulin-like
growth factor 2 (IGF2) concentrations were measured before surgery
and 30 days after 131I radioactive iodide therapy. This
study was also approved by the Research Ethics Committee [Commissie
Mensgebonden Onderzoek (CMO)] of the Radboud University Medical
Center under application: 2017–3628; NL62671.091.17; ClinicalTrials.gov Identifier: NCT03397238. Informed
consent was obtained from all participants and/or their legal
guardians. Their results were compared to those of six gender and
aged matched healthy volunteers.
DNA and RNA isolation from FFPE
tissues
For DNA isolation, 60 µm FFPE tissue slices were
digested overnight at 56°C in the presence of TET-lysis buffer (10
mM Tris/HCl pH 8.5, 1 mM EDTA pH 8.0, 0.01% Tween-20), 5%
Chelex-100 (Bio-Rad Laboratories, Inc.), 15 µg/ml GlycoBlue (Life
Technologies) and 400 µg proteinase K (Qiagen,), followed by
inactivation at 95°C for 10 min. For DNA isolation, the supernatant
was transferred after centrifugation (20817 rcf), cooled on ice and
precipitated in the presence of 100% EtOH and 1/33 volume 3M NaAc
(pH 5.2). Pellets were washed with cold 70% EtOH, dissolved in 80
µl Tris-EDTA and DNA concentrations were determined using the Qubit
Broad Range kit (Thermo Fisher Scientific, Inc.). For RNA
isolation, 60 µm FFPE tissue slices were digested overnight at 56°C
in the presence of 240 µl lysis buffer (Qiagen) and 400 µg
proteinase K. Next, supernatants were transferred after
centrifugation (16,000 × g) and mixed with RNA-Bee. Subsequently,
RNA was isolated by phase separation with chloroform and
precipitated by isopropanol, according to the manufacturer's
instructions (Thermo Fisher Scientific, Inc.).
Intratumoral mutation profiling
Somatic mutations in human DTC tumor tissue were
detected by our in-house Cancer Hotspot Panel based on
single-molecule molecular inversion probes, as described previously
(16). Isolated DNA from FFPE
tissues with a tumor cell percentage of >60% was subjected to
library preparation and clinically relevant regions were sequenced
of the following genes: AKT1, BIRC3, BRAF, CHEK2, CTNNB1, CXCR4,
EGFR, ERBB2, EZH2, GNA11, GNAQ, GNAS, H3F3A, H3F3B, HRAS, IDH1,
IDH2, JAK2, KIT, KRAS, MPL, MSH2, MYD88, NRAS, PDGFRA, PIK3CA,
SF3B1, SLC7A8 and ZNF2.
Gene fusion analysis
DNA/RNA was isolated from punched tumor tissue with
a tumor cell percentage of >50%. Gene fusion analysis was
performed by Next Generation Sequencing (Archer FusionPlex CTL
Panel) and data were analyzed by Archer Analysis software (version
5). Relevant fusions of the following target genes were sequenced:
ALK (5′; exons 2, 4, 6, 10, 16–23, intron 19), AXL (3′; exons
18–20), BRAF (5′; exons 7–11, 3′; exons 7, 8, 10), CCND1 (5′; exons
1–4, 3′; exons 1, 2, 4), FGFR1 (5′; exons 2, 8–10, 17, 3′; exon
17), FGFR2 (5′; exons 2, 5, 7–10, 3′; exon 17), FGFR3 (5′; exons 3,
5, 8–10, 3′; exon 17, intron 17), MET (5′; exons 2, 4–6, 13, 14,
16, 17, 21, 3′; exon 2), NRG1 (5′; exons 1, 2, 3, 6), NTRK1 (5′;
exons 2, 4, 6, 8, 10–13), NTRK2 (5′; exons 5, 7, 9, 11–17), NTRK3
(5′; exons 4, 7, 10, 13–16), PPARG (5′; exons 1, 2, 3, 5), RAF1
(5′; exons 4–7, 9–12), RET (5′; exons 2, 4, 6, 8, 9–14), ROS1 (5′;
exons 2, 4, 7, 31–37), THADA (3′; exons 24–30, 36, 37). In
addition, the FusionPlex-CTL hotspot panel also detects mutations
in BRAF (exon 11, 15), HRAS, NRAS (exon 2 and 3, codon 12, 13, 61),
KRAS (exon 2, 3 and 4, codon 12, 13, 61 and 146) and the EGFRvIII
variant.
TERT promoter mutation analysis
TERT promoter mutations C228A, C228T (at
position-124 from translation start site) and C250T (−146 from
translation start site) were detected by conventional PCR followed
by Sanger sequencing. The TERT promoter region was amplified
by the following M13-sequence extended primers: Forward 5′-TGT-
AAA- ACG- ACG- GCC- AGT- CCC- TTC- ACC- TTC- CAG- CTC-3′ and
reverse 5′-CAG- GAA- ACA- GCT- ATG- ACC- AGC- GCT-
GCC-TGA-AAC-TCG-3′. DNA was amplified by AmpliTaq PCR 360 Gold
Master Mix (Thermo Fisher, Waltham, MA, USA) and the following PCR
program: 95°C for 10 min followed by 95°C for 30 sec, 58°C for 30
sec and 72°C for 1 min (38 cycles) and a final step of 72°C for 7
min. Subsequently, Sanger sequencing was performed with M13 primers
and TERT promoter mutations were called by Sanger chromatogram
software (Sequencher 4.8, Gene Codes Corp.).
FFPE compatible RNA sequencing
Isolated RNA obtained from FFPE tissues with a tumor
cell percentage of >80% was processed for RNA sequencing by
DNAse treatment, RNA demodification, RNA fragmentation (if
necessary), first and second-strand cDNA synthesis and library
preparation according to the Ovation SoLo RNA-Seq System (NuGEN).
Subsequently, RNA sequencing was performed by Illumina NextSeq500
and analysis of the data was performed by the Centre for Molecular
and Biomolecular Informatics (CMBI). STAR, a standard aligner that
makes use of a reference genome (GRCh38), was selected for the
alignment of the sequences. To quantify the alignments made in STAR
the tool HTSeq was utilized. This produced ‘counts.txt’ files that
are easy to import into DESeq2, a package to carry out differential
gene expression analysis. Raw RNA sequencing data are deposited in
the GEO database under accession number GSE112202.
Plasma measurements
Plasma IGF2 and IGF binding protein 2 (IGFBP2)
concentrations were measured by ELISA according to manufacturer's
instructions (R&D Systems, Inc.).
Statistical analysis
Statistical significance was tested with Student's
t-test, Fisher's exact test, Mann Whitney U test, Wilcoxon
matched-pairs signed rank test, when appropriate. P-values below
0.05 were considered statistically significant. For RNA sequencing
data a false discovery rate of 0.05 was incorporated. All
statistical tests were performed using GraphPad Prism 5.0.
Results
Primary tumors from RAI-refractory DTC
patients harbor a significant higher IGF2 RNA expression compared
to RAI-sensitive DTC patients
Patient and tumor characteristics of 35
RAI-sensitive and 28 RAI-refractory DTC patients from the cohort
are listed in Table I.
RAI-refractory DTC patients were diagnosed at an older age and
displayed a less favorable TNM staging at diagnosis than
RAI-sensitive patients. This showed to be significant (P<0.001)
(Table I). To assess the mutational
status in these DTC patients, intratumoral mutational profiling,
gene fusion analysis and TERT promoter mutational analysis were
performed. There was a significantly higher proportion of tumors
bearing TERT promoter mutations in RAI-refractory DTC
patients compared to RAI-sensitive patients (50% vs. 8.6%)
(Table II). In contrast, more
RAI-sensitive patients showed gene fusions compared to
RAI-refractory patients. To gain insight into differentially
expressed genes between tumors obtained from RAI-sensitive and
RAI-refractory DTC patients, whole transcriptomics analysis was
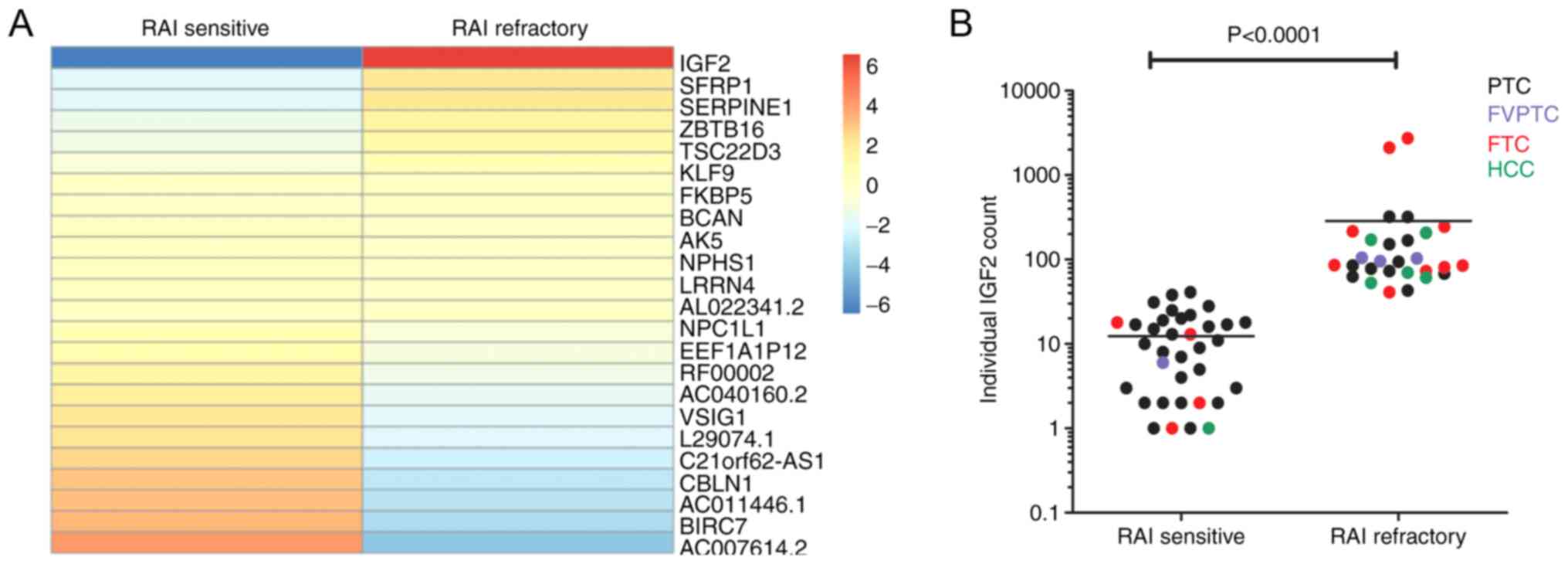
performed using RNA sequencing. Subsequently, a heatmap was
constructed to visualize expression values (Fig. 1A) and differential expression
analysis was determined within the overall comparison of
RAI-sensitive and RAI-refractory DTC patients. IGF2 showed a
significantly higher expression in RAI refractory DTC patients
compared to RAI sensitive DTC patients. Fig. 1B displays the datapoints of each
individual patient also separated for the different histological
subgroups indicating that the higher RNA expression of IGF2
in RAI refractory patients is independent of the tumor
histology.
 | Table I.Clinical characteristics of
RAI-sensitive (n=35) and RAI-refractory (n=28) patients with
DTC. |
Table I.
Clinical characteristics of
RAI-sensitive (n=35) and RAI-refractory (n=28) patients with
DTC.
| Parameter | RAI-sensitive
DTC | RAI-refractory
DTC | P-value |
|---|
| Mean age at
diagnosis ± SD, years | 41.9±18.9 | 61.9±10.2 | <0.001 |
| Sex, male/female,
n | 13/22 | 16/12 | 0.134 |
| Histology, n |
|
| 0.0045 |
|
PTC | 29 | 11 |
|
|
FTC | 4 | 10 |
|
|
HCC | 1 | 5 |
|
|
FVPTC | 1 | 2 |
|
|
T-stagea,
n |
|
| 0.0011 |
| T1 | 12 | 3 |
|
| T2 | 12 | 4 |
|
| T3 | 8 | 7 |
|
| T4 | 3 | 14 |
|
|
N-stagea,
n |
|
| NA |
| N0 | 0 | 0 |
|
| N1 | 35 | 28 |
|
|
M-stagea,
n |
|
| <0.0001 |
| M0 | 31 | 4 |
|
| M1 | 4 | 24 |
|
| Location of
metastases, n |
|
| <0.0001 |
| No
metastases | 31 | 4 |
|
|
Lung | 3 | 16 |
|
|
Bone | 0 | 2 |
|
| Lung
and bone | 1 | 5 |
|
| Lung,
liver and muscle | 0 | 1 |
|
| Number of RAI
treatments, n |
|
| 0.0032 |
|
0-1 | 22 | 6 |
|
| 2 | 9 | 12 |
|
|
>2 | 4 | 10 |
|
| Mean cumulative RAI
dose ± SD, MBq | 9,806±7,127 | 17,240±8,955 | <0.001 |
 | Table II.Genetic characteristics of
RAI-sensitive (n=35) and RAI-refractory (n=28) patients with
DTC. |
Table II.
Genetic characteristics of
RAI-sensitive (n=35) and RAI-refractory (n=28) patients with
DTC.
| Parameter | RAI-sensitive DTC,
n | RAI-refractory DTC,
n | P-value |
|---|
| Oncogenic mutation
status |
|
| 0.442 |
| BRAF
V600E | 15 | 9 |
|
|
H/K/NRAS, G12D/Q61R | 4 | 5 |
|
| Other | CCDC6:RET fusion
(n=4), CCDC6:RET fusion and PIK3CA H1047R (n=1), SQSTM1:NTRK3
fusion (n=1), ETV6:NTRK3 fusion (n=1), NCOA4:RET fusion (n=1),
PAX8:PPARG fusion (n=2) | PTEN E242X and TP53
R158G (n=1), PTEN E242X and TP53 P212fs (n=1), NRAS Q61R and AKT1
E17L (n=1), EML4:NTRK3 fusion (n=1) |
|
| Unknown | 6 | 10 |
|
| TERT promoter
mutation status |
|
| 0.0004 |
| TERT
wild-type | 32 | 14 |
|
| TERT
C228T | 3 | 14 |
|
DTC patients show significantly lower
circulating levels of IGF2 concentration after primary
treatment
To further support the role of IGF2 in the
pathogenesis of DTC we investigated the IGF2 plasma concentration
before and after treatment in an independent cohort, including
eight newly diagnosed therapy-naïve patients with DTC (4 males and
4 females, averaged 54.6±16.5 years) scheduled for conventional
treatment by surgery followed by RAI. Patients and tumor
characteristics of this cohort are listed in Table III. The IGF2 plasma concentrations
in these patients were compared to those of six age- and
gender-matched healthy volunteers (3 males and 3 females, averaged
51.7±12.4 years). The average total IGF2 concentration before
surgery (baseline) in DTC patients showed higher
(1.17*106 +/- 2.6*105 pg/ml) compared to
healthy volunteers (9.5*105 +/- 1.7*105
pg/ml) although this difference did not reach statistical
significance (Fig. 2A). Thirty days
after primary surgery the average IGF2 plasma concentration was
significantly decreased (8.3*105 +/- 1.7*105
pg/ml) compared to the level before surgery (Fig. 2B). To exclude interference between
IGFBP2 and its ligand IGF2 effecting the assay, we assessed the
plasma IGFBP2 concentrations in the same samples. The IGFBP2
concentrations after therapy were not significantly different from
those measured before surgery, which suggests an even stronger
effect of treatment on the free IGF2 circulating concentrations
(Fig. 2C).
 | Table III.Clinical characteristics of patients
with differentiated thyroid cancer from the independent cohort. |
Table III.
Clinical characteristics of patients
with differentiated thyroid cancer from the independent cohort.
| Patient no. | Age at inclusion,
years | Sex | Histology | TNM stage at
diagnosis | RAI dose, mCi |
|---|
| 1 | 42 | F | PTC | T1bN1M0 | 100 |
| 2 | 55 | M | HCC | T2mN0M1 | 200 |
| 3 | 49 | M | PTC | T1bmN1aM0 | 100 |
| 4 | 65 | M | PTC | T2N1bM0 | 200 |
| 5 | 78 | M | HCC | T4aN1bM0 | 200 |
| 6 | 50 | F | PTC | T2mN1bMx | 100 |
| 7 | 27 | F | PTC | T3N1bM0 | 200 |
| 8 | 71 | F | PTC | T1mN0M0 | 30 |
Discussion
Obliterating tumor remnants after thyroidectomy by
RAI therapy is of significant clinical importance in DTC.
Unresponsiveness to RAI is a major concern since RAI-refractory
tumors usually respond poorly to alternative therapies. Apart from
a lack of therapeutic options, markers predicting RAI
refractoriness have so far not been identified. Therefore, we
searched for differences between the molecular signatures obtained
from primary RAI sensitive and RAI refractory DTC samples. Genetic
analyses revealed an increased mutational load in RAI-refractory
DTC, including mutations in AKT1, PTEN, TP53 and TERT
promoter. Mutations or deletions of the tumor suppressor gene
PTEN are genetic alterations that can activate the PI3K-Akt
pathway (1). Other genetic
alterations activating the PI3K-Akt pathway in DTC tumors,
particularly in those having a poor prognosis, include mutations
encoding for PI3KCA, AKT1 and RAS genes. As genetic
alterations accumulate, additional mutations occur in genes such as
TP53. Additional mutations, together with the initial
genetic alterations in the MAPK or PI3K-Akt pathway contributes to
tumor progression and could ultimately lead to the development of
PDTC or anaplastic thyroid cancer (ATC) (1,17).
Previous studies have also shown that somatic TERT promoter
mutations are more prevalent in aggressive types of TC such as PDTC
and ATC (18,19). Our observations, demonstrating a
higher proportion of tumors bearing TERT promoter mutations
in RAI-refractory DTC patients, are in concordance with these
studies. Interestingly, transcriptome data from RAI refractory DTC
patients and RAI sensitive DTC patients revealed a significantly
higher RNA expression of IGF2 in primary tumors of RAI
refractory DTC patients. Moreover, we show that the IGF2 plasma
concentration in patients with DTC significantly decreased after
primary treatment by surgery and RAI. Our data suggest no clear
relationship between the increased mutational load and the
overexpression of IGF2 in RAI-refractory DTC patients.
However, due to the relatively small sample size our study probably
lacked the statistical power to robustly assess this association.
Therefore, the relationship between the increased mutational load
and overexpression of IGF2 should be investigated in future
studies including larger cohorts of patients.
The IGF2 gene is located on chromosome
11p15.5 and encodes for the IGF2 growth factor that has been shown
to play an important role in the fetal embryonic development,
growth and energy metabolism of mammals (20–24).
Apart from this, IGF2 has also been reported to be involved in
carcinogenesis. In several cancer types, increased expression of
IGF2 has been linked to poor prognosis (25). In lung cancer patients, increased
IGF2 protein expression in pleural effusion supernatants was
associated with resistance to osimertinib treatment (26). Elevated levels of IGF2 mRNA in
osteosarcoma cells were shown after chemotherapy resulting in
preservation of these cells under chemotherapeutic stress (27). In a study investigating 445
gastrointestinal tumors, high protein expression of IGF2 in the
tumor tissue was associated with a significant worse outcome
(28). In colorectal cancer,
overexpression of stromal-derived IGF2 has been shown to play a
role in development and progression, whereas increased serum
concentrations and tissue overexpression of IGF2 has been
associated with metastasis (29–31).
Moreover, in breast cancer tissues, IGF2 was found to be more
potent than in normal breast tissue for activating insulin receptor
(IR) autophosphorylation causing stimulation of cell growth
(32). Finally, a study performed by
Tominaga et al described a positive feedback loop,
IGF2-IGF1R-PI3K-ID1-IGF2, present in cancer stem-like cells causing
cells to maintain in the stem cell state (33).
A few other studies suggest a role for IGF2 in the
pathogenesis of DTC. Differences in IGF2 mRNA expression
between normal thyroid epithelial cells, thyroid adenoma and
thyroid carcinoma were demonstrated using three pairwise
comparisons with the GEO2R online tool (34). One research group published several
studies in which they demonstrate the overexpression of IGF2
mRNA in undifferentiated thyroid cancer cell lines, poorly
differentiated malignant thyrocytes, cancer thyrospheres and
thyroid cancer specimens. This overexpression of IGF2 coincided
with elevated expression of insulin receptor isoform A (IR-A) and
insulin-like growth factor 1 receptor (IGF1R). They showed that
this so-called IGF2/IR-A autocrine loop is associated with
dedifferentiation and stem-like phenotypes, resembling RAI
refractoriness (35–38). In line with these findings, we
identified higher IGF2 expression in primary tumors from RAI
refractory DTC patients via RNA sequencing. For the first time, our
study also demonstrates a significant decrease of total IGF2 plasma
concentration 30 days after primary therapy as compared to before
surgery. Collectively these data suggest that IGF2 expression could
have prognostic and therapeutic implications for several cancer
types including DTC, which strengthens the importance of our
findings and a potential role for IGF2 in acquiring RAI
refractoriness.
The mechanism behind the increase in IGF2
expression in RAI refractory tumors demonstrated in this study is
unknown. Previous studies have proposed several mechanisms to
explain this increased expression causing therapy resistance. Wang
et al showed that IGF2 produced by cancer-associated
fibroblasts (CAFs) induced autophagy in cancer cells post-radiation
thereby promoting cancer cell recovery (39). Also, drug resistance in non-small
cell lung cancer cells was observed, inflicted by
IGF2-AKT-Sox2-ABCB1 signaling in cancer cells co-cultured with CAFs
(40). The survival of osteosarcoma
cells, supported by IGF2, was dependent on enhanced autophagic flux
and glutamine availability (27). In
malignant rhabdoid tumors IGF2 has been shown to activate IGF1R and
IR followed by activation of the PI3K-AKT and RAS-ERK pathways to
promote proliferation and cell survival (41).
The present findings have potential clinical and
therapeutic consequences. Several studies have been performed where
IGF2 has been proposed as a predictive or prognostic molecular
marker (42–44). A limitation to the use of IGF2 as a
molecular marker for RAI refractoriness or for a more aggressive
tumor behavior could be the presence of an autocrine IGF signaling
loop as described by Vella and Malaguarnera (35–38).
Measurement of serum IGF2 does not account for local IGF2
production in the tumors depending on an autocrine IGF2 signaling
loop. Alternatively, measuring tumor IGF2 expression in the
tissue available after thyroidectomy as we have shown in the study
could be an potential option to be further explored (21).
Apart from serving as a molecular marker for RAI
refractoriness, IGF2 could perhaps also be targeted therapeutically
to overcome RAI refractoriness. Detecting increased circulating
levels of IGF2 could be followed by targeting IGF2 or its receptors
by using IGF monoclonal antibodies, IGF1R monoclonal antibodies or
IGF1R/IR tyrosine kinase inhibitors. Multiple phase I/II trials in
all types of cancers have been initiated using these therapeutic
options, also in combination with chemotherapeutics and other drugs
(21,45). Studies have shown that targeting the
IGF1R combined with the IR pathway may increase therapy efficacy
and prevents resistance to selective IGF1R antibodies or inhibitors
(46,47). OSI-906, a dual inhibitor of the IGF1R
and IR, has already shown antitumor activity in a phase I study and
phase II studies in combination with other drugs are ongoing
(48,49). In the case of DTC using such
inhibitors could perhaps be beneficial in combination with RAI to
overcome RAI refractoriness.
A few limitations of this study should be discussed.
The retrospective design of our study and the criteria used for
patient selection and RAI therapy indication possibly biased our
results. Moreover, as mentioned earlier, the prevalence of distant
metastasized DTC is <10%. Therefore, future studies will require
larger sample sizes in addition to confirm our findings from both
the retrospective cohort as well as the independent cohort.
Furthermore, RNA-seq data from poorly differentiated TC cell lines
such as FTC133, TPC-1 and BC-PAP showed in our hands a very low
IGF2 expression in these cells. Since these cell lines are
already RAI-refractory, overexpression or knockdown of IGF2 in
these cell lines would not answer the questions regarding the
potential role of IGF2 in acquiring RAI refractoriness.
In conclusion, important clinical, genetic and
transcriptomic differences were identified between patients with
RAI-sensitive DTC and RAI-refractory DTC. Interestingly, the
tumor-promoting growth factor IGF2 showed a significantly
higher expression in RAI refractory tumors. Plasma levels of IGF2
decreased following primary treatment in patients with DTC. These
findings suggest that tumor-promoting growth factor IGF2 could be a
potential factor in acquiring RAI refractoriness. Further studies
in independent cohorts are needed to validate our findings and
elaborate on the mechanism behind the elevated IGF2 expression and
RAI refractory in DTC patients and to explore IGF2 as a potential
therapeutic target.
Acknowledgements
Not applicable.
Funding
TP was supported by a Veni grant of the Netherlands
Organization for Scientific Research (NWO; grant no. 016.136.065)
and by the Alpe d'HuZes fund of the Dutch Cancer Society (grant no.
KUN2014-6728).
Availability of data and materials
The raw RNA datasets generated and/or analyzed
during the current study are available in the Gene Expression
Omnibus database under accession no. GSE112202 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE112202).
Authors' contributions
TC, MHT, MJ and TSP performed the experiments and
the data analysis. WEC and HM performed the gene-fusion experiments
and analysis. KR recruited the patients, kindly provided the
samples and clinical data for the independent cohort, and analysed
the data of the independent cohort. ACHvEvG performed the
pathological examinations of the tumour samples. TC, JWAS, RTNM and
TPS designed the study. TC, JWAS, RTNM and TSP wrote the
manuscript. TC and TSP confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Collection, storage and use of archived tissue and
patient data were in compliance with the ‘Code of Proper Secondary
Use of Human Tissue in the Netherlands’ (http://www.fmwv.nl and www.federa.org). The present study was approved by the
Research Ethics Committee (CMO) of the Radboud University Medical
Center under application no. 2015–1762 and followed the ethical
guidelines of the CMO. The CMO waived the need for consent for the
use of archived samples since the samples were analyzed
anonymously. The independent cohort was approved by the CMO of the
Radboud University Medical Center under application no. 2017-3628
(approval no. NL62671.091.17; ClinicalTrials.gov Identifier: NCT03397238). Written
informed consent was obtained from all participants and/or their
legal guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ATC
|
anaplastic thyroid cancer
|
|
DTC
|
differentiated thyroid cancer
|
|
FFPE
|
formalin-fixed paraffin-embedded
|
|
IGF2
|
insulin-like growth factor 2
|
|
IGFBP2
|
insulin-like growth factor binding
protein 2
|
|
PSMA
|
prostate-specific membrane antigen
|
|
RAI
|
radioactive iodide
|
|
TERT
|
telomerase reverse transcriptase
|
|
TG
|
thyroglobulin
|
References
|
1
|
Xing M: Molecular pathogenesis and
mechanisms of thyroid cancer. Nat Rev Cancer. 13:184–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fagin JA and Wells SA Jr: Biologic and
clinical perspectives on thyroid cancer. N Engl J Med.
375:1054–1067. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Durante C, Haddy N, Baudin E, Leboulleux
S, Hartl D, Travagli JP, Caillou B, Ricard M, Lumbroso JD, De
Vathaire F and Schlumberger M: Long-term outcome of 444 patients
with distant metastases from papillary and follicular thyroid
carcinoma: Benefits and limits of radioiodine therapy. J Clin
Endocrinol Metab. 91:2892–2899. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Song HJ, Qiu ZL, Shen CT, Wei WJ and Luo
QY: Pulmonary metastases in differentiated thyroid cancer: Efficacy
of radioiodine therapy and prognostic factors. Eur J Endocrinol.
173:399–408. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brose MS, Nutting CM, Jarzab B, Elisei R,
Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R,
Shong YK, et al: Sorafenib in radioactive iodine-refractory,
locally advanced or metastatic differentiated thyroid cancer: A
randomised, double-blind, phase 3 trial. Lancet. 384:319–328. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pacini F, Castagna MG, Brilli L and
Pentheroudakis G; ESMO Guidelines Working Group, : Thyroid cancer:
ESMO clinical practice guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 23 (Suppl 7):vii110–vii119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Romesser PB, Sherman EJ, Shaha AR, Lian M,
Wong RJ, Sabra M, Rao SS, Fagin JA, Tuttle RM and Lee NY: External
beam radiotherapy with or without concurrent chemotherapy in
advanced or recurrent non-anaplastic non-medullary thyroid cancer.
J Surg Oncol. 110:375–382. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schlumberger M, Tahara M, Wirth LJ,
Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff
AO, et al: Lenvatinib versus placebo in radioiodine-refractory
thyroid cancer. N Engl J Med. 372:621–630. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de la Fouchardière C, Decaussin-Petrucci
M, Berthiller J, Descotes F, Lopez J, Lifante JC, Peix JL, Giraudet
AL, Delahaye A, Masson S, et al: Predictive factors of outcome in
poorly differentiated thyroid carcinomas. Eur J Cancer. 92:40–47.
2018. View Article : Google Scholar
|
|
10
|
Zheng L, Wang G, Guo W, Pan D, Xie L, He
S, Luo C, Li H, Ran Y, Wu S, et al: NIS and epithelial-mesenchymal
transition marker expression of circulating tumor cells for
predicting and monitoring the radioactive iodine-131 therapy effect
in differentiated thyroid cancers. Mol Biol Rep. 46:4201–4212.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sollini M, di Tommaso L, Kirienko M,
Piombo C, Erreni M, Lania AG, Erba PA, Antunovic L and Chiti A:
PSMA expression level predicts differentiated thyroid cancer
aggressiveness and patient outcome. EJNMMI Res. 9:932019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song E, Kim M, Kim EY, Kim BH, Shin DY,
Kang HC, Ahn BC, Kim WB, Shong YK, Jeon MJ and Lim DJ: Lenvatinib
for radioactive iodine-refractory differentiated thyroid carcinoma
and candidate biomarkers associated with survival: A multicenter
study in Korea. Thyroid. 30:732–738. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang C, Zhang X, Li H, Li X and Lin Y:
Quantitative thyroglobulin response to radioactive iodine treatment
in predicting radioactive iodine-refractory thyroid cancer with
pulmonary metastasis. PLoS One. 12:e01796642017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giovanella L, Castellana M and Trimboli P:
Unstimulated high-sensitive thyroglobulin is a powerful prognostic
predictor in patients with thyroid cancer. Clin Chem Lab Med.
58:130–137. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tuttle RM, Haugen B and Perrier ND:
Updated American joint committee on cancer/tumor-node-metastasis
staging system for differentiated and anaplastic thyroid cancer
(eighth edition): What changed and why? Thyroid. 27:751–756. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eijkelenboom A, Kamping EJ, Kastner-van
Raaij AW, Hendriks-Cornelissen SJ, Neveling K, Kuiper RP, Hoischen
A, Nelen MR, Ligtenberg MJ and Tops BB: Reliable next-generation
sequencing of formalin-fixed, paraffin-embedded tissue using single
molecule tags. J Mol Diagn. 18:851–863. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cancer Genome Atlas Research Network, .
Integrated genomic characterization of papillary thyroid carcinoma.
Cell. 159:676–690. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jin L, Chen E, Dong S, Cai Y, Zhang X,
Zhou Y, Zeng R, Yang F, Pan C, Liu Y, et al: BRAF and TERT promoter
mutations in the aggressiveness of papillary thyroid carcinoma: A
study of 653 patients. Oncotarget. 7:18346–18355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xing M, Liu R, Liu X, Murugan AK, Zhu G,
Zeiger MA, Pai S and Bishop J: BRAF V600E and TERT promoter
mutations cooperatively identify the most aggressive papillary
thyroid cancer with highest recurrence. J Clin Oncol. 32:2718–2726.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baral K and Rotwein P: The insulin-like
growth factor 2 gene in mammals: Organizational complexity within a
conserved locus. PLoS One. 14:e02191552019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bowers LW, Rossi EL, O'Flanagan CH,
deGraffenried LA and Hursting SD: The role of the insulin/IGF
system in cancer: Lessons learned from clinical trials and the
energy balance-cancer link. Front Endocrinol (Lausanne). 6:772015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brouwer-Visser J and Huang GS: IGF2
signaling and regulation in cancer. Cytokine Growth Factor Rev.
26:371–377. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Djiogue S, Nwabo Kamdje AH, Vecchio L,
Kipanyula MJ, Farahna M, Aldebasi Y and Seke Etet PF: Insulin
resistance and cancer: The role of insulin and IGFs. Endocr Relat
Cancer. 20:R1–R17. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Frasca F, Pandini G, Scalia P, Sciacca L,
Mineo R, Costantino A, Goldfine ID, Belfiore A and Vigneri R:
Insulin receptor isoform A, a newly recognized, high-affinity
insulin-like growth factor II receptor in fetal and cancer cells.
Mol Cell Biol. 19:3278–3288. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livingstone C: IGF2 and cancer. Endocr
Relat Cancer. 20:R321–R339. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Manabe T, Yasuda H, Terai H, Kagiwada H,
Hamamoto J, Ebisudani T, Kobayashi K, Masuzawa K, Ikemura S, Kawada
I, et al: IGF2 autocrine-mediated IGF1R activation is a clinically
relevant mechanism of osimertinib resistance in lung cancer. Mol
Cancer Res. 18:549–559. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shimizu T, Sugihara E, Yamaguchi-Iwai S,
Tamaki S, Koyama Y, Kamel W, Ueki A, Ishikawa T, Chiyoda T, Osuka
S, et al: IGF2 preserves osteosarcoma cell survival by creating an
autophagic state of dormancy that protects cells against
chemotherapeutic stress. Cancer Res. 74:6531–6541. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Steigen SE, Schaeffer DF, West RB and
Nielsen TO: Expression of insulin-like growth factor 2 in
mesenchymal neoplasms. Mod Pathol. 22:914–921. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kasprzak A and Adamek A: Insulin-like
growth factor 2 (IGF2) signaling in colorectal cancer-from basic
research to potential clinical applications. Int J Mol Sci.
20:49152019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sanderson MP, Hofmann MH, Garin-Chesa P,
Schweifer N, Wernitznig A, Fischer S, Jeschko A, Meyer R, Moll J,
Pecina T, et al: The IGF1R/INSR inhibitor BI 885578 selectively
inhibits growth of IGF2-overexpressing colorectal cancer tumors and
potentiates the efficacy of anti-VEGF therapy. Mol Cancer Ther.
16:2223–2233. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Unger C, Kramer N, Unterleuthner D,
Scherzer M, Burian A, Rudisch A, Stadler M, Schlederer M, Lenhardt
D, Riedl A, et al: Stromal-derived IGF2 promotes colon cancer
progression via paracrine and autocrine mechanisms. Oncogene.
36:5341–5355. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sciacca L, Costantino A, Pandini G, Mineo
R, Frasca F, Scalia P, Sbraccia P, Goldfine ID, Vigneri R and
Belfiore A: Insulin receptor activation by IGF-II in breast
cancers: Evidence for a new autocrine/paracrine mechanism.
Oncogene. 18:2471–2479. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tominaga K, Shimamura T, Kimura N,
Murayama T, Matsubara D, Kanauchi H, Niida A, Shimizu S, Nishioka
K, Tsuji EI, et al: Addiction to the IGF2-ID1-IGF2 circuit for
maintenance of the breast cancer stem-like cells. Oncogene.
36:1276–1286. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Q, Shen Y, Ye B, Hu H, Fan C, Wang T,
Zheng Y, Lv J, Ma Y and Xiang M: Gene expression differences
between thyroid carcinoma, thyroid adenoma and normal thyroid
tissue. Oncol Rep. 40:3359–3369. 2018.PubMed/NCBI
|
|
35
|
Malaguarnera R, Frasca F, Garozzo A, Giani
F, Pandini G, Vella V, Vigneri R and Belfiore A: Insulin receptor
isoforms and insulin-like growth factor receptor in human
follicular cell precursors from papillary thyroid cancer and normal
thyroid. J Clin Endocrinol Metab. 96:766–774. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vella V and Malaguarnera R: The emerging
role of insulin receptor isoforms in thyroid cancer: Clinical
implications and new perspectives. Int J Mol Sci. 19:38142018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vella V, Nicolosi ML, Cantafio P,
Massimino M, Lappano R, Vigneri P, Ciuni R, Gangemi P, Morrione A,
Malaguarnera R and Belfiore A: DDR1 regulates thyroid cancer cell
differentiation via IGF-2/IR-A autocrine signaling loop. Endocr
Relat Cancer. 26:197–214. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vella V, Pandini G, Sciacca L, Mineo R,
Vigneri R, Pezzino V and Belfiore A: A novel autocrine loop
involving IGF-II and the insulin receptor isoform-A stimulates
growth of thyroid cancer. J Clin Endocrinol Metab. 87:245–254.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang Y, Gan G, Wang B, Wu J, Cao Y, Zhu D,
Xu Y, Wang X, Han H, Li X, et al: Cancer-associated fibroblasts
promote irradiated cancer cell recovery through autophagy.
EBioMedicine. 17:45–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang Q, Yang J, Bai J and Ren J: Reverse
of non-small cell lung cancer drug resistance induced by
cancer-associated fibroblasts via a paracrine pathway. Cancer Sci.
109:944–955. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li T, Wang J, Liu P, Chi J, Yan H, Lei L,
Li Z, Yang B and Wang X: Insulin-like growth factor 2 axis supports
the serum-independent growth of malignant rhabdoid tumor and is
activated by microenvironment stress. Oncotarget. 8:47269–47283.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
El Tayebi HM, Salah W, El Sayed IH, Salam
EM, Zekri AR, Zayed N, Salem ES, Esmat G and Abdelaziz AI:
Expression of insulin-like growth factor-II, matrix
metalloproteinases, and their tissue inhibitors as predictive
markers in the peripheral blood of HCC patients. Biomarkers.
16:346–354. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Peters G, Gongoll S, Langner C, Mengel M,
Piso P, Klempnauer J, Rüschoff J, Kreipe H and von Wasielewski R:
IGF-1R, IGF-1 and IGF-2 expression as potential prognostic and
predictive markers in colorectal-cancer. Virchows Arch.
443:139–145. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Van Arsdale AR, Arend RC, Cossio MJ,
Erickson BK, Wang Y, Doo DW, Leath CA, Goldberg GL and Huang GS:
Insulin-like growth factor 2: A poor prognostic biomarker linked to
racial disparity in women with uterine carcinosarcoma. Cancer Med.
7:616–625. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ewing GP and Goff LW: The insulin-like
growth factor signaling pathway as a target for treatment of
colorectal carcinoma. Clin Colorectal Cancer. 9:219–223. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Buck E, Gokhale PC, Koujak S, Brown E,
Eyzaguirre A, Tao N, Rosenfeld-Franklin M, Lerner L, Chiu MI, Wild
R, et al: Compensatory insulin receptor (IR) activation on
inhibition of insulin-like growth factor-1 receptor (IGF-1R):
Rationale for cotargeting IGF-1R and IR in cancer. Mol Cancer Ther.
9:2652–2664. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Malaguarnera R and Belfiore A: The insulin
receptor: A new target for cancer therapy. Front Endocrinol
(Lausanne). 2:932011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mulvihill MJ, Cooke A, Rosenfeld-Franklin
M, Buck E, Foreman K, Landfair D, O'Connor M, Pirritt C, Sun Y, Yao
Y, et al: Discovery of OSI-906: A selective and orally efficacious
dual inhibitor of the IGF-1 receptor and insulin receptor. Future
Med Chem. 1:1153–1171. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Puzanov I, Lindsay CR, Goff L, Sosman J,
Gilbert J, Berlin J, Poondru S, Simantov R, Gedrich R, Stephens A,
et al: A phase I study of continuous oral dosing of OSI-906, a dual
inhibitor of insulin-like growth factor-1 and insulin receptors, in
patients with advanced solid tumors. Clin Cancer Res. 21:701–711.
2015. View Article : Google Scholar : PubMed/NCBI
|