Introduction
Gliomas are the most common primary central nervous
system (CNS) tumors, corresponding to ~80% of all malignant primary
brain tumors in adults (United States of America, 2013–2017)
(1). According to the 2016 World
Health Organization (WHO) classification of CNS tumors, diffuse
gliomas are classified based on histological (astrocytoma,
oligoastrocytoma, oligodendroglioma and glioblastoma) and genetic
features [isocitrate dehydrogenase mutations,
alpha-thalassemia/mental retardation, X-linked (ATRX) loss, TP53
mutation and 1p/19q-codeletion] (2).
In addition, the WHO classified CNS tumors into 4 grades (I–IV)
that have distinct prognosis and require different therapeutic
decisions (3,4). Glioblastomas (grade IV), which
correspond to ~50% of gliomas are highly malignant gliomas with a
poor prognosis (5,6). The current therapeutic options for
these tumors include surgery, radiation, immunotherapy and
chemotherapy with non-specific and highly toxic agents (7–9).
Currently, alkylating agents (temozolomide, carmustine and
lomustine), mTOR inhibitors (everolimus) and monoclonal antibodies
(bevacizumab and naxitamab-gqgk) are approved by the Food and Drug
Administration authority for the treatment of high-grade gliomas
(https://www.cancer.gov) (10). Inspite of considerable advances in
the understanding of the molecular basis of gliomas (6,11),
progress in improving the clinical outcomes of the disease is still
slow and very few new therapies have been developed.
Reversine
[2-(4-morpholinoanilino)-6-cyclohexylaminopurine] is an adenosine
triphosphate analog that acts as a potent multi-kinase inhibitor
selective to aurora kinases, MPS1 (monopolar spindle 1) and JNK
(12–14). The antineoplastic activity of
reversine has been demonstrated in hematological and solid tumors,
in which it triggered cell cycle arrest, polyploidy, apoptosis and
autophagy (15).
Aurora kinases, a family of serine/threonine
kinases, consisting of aurora kinase A (AURKA), aurora kinase B
(AURKB) and aurora kinase C (AURKC) serve an essential role in cell
cycle progression and cellular division (16). High expression of AURKA, AURKB and
AURKC has observed in several types of cancer, including gliomas
and is associated with a poor prognosis (17–19).
AURKA and AURKB are members with the most detailed
characterizations: AURKA serves a role in the formation of the
mitotic spindle during mitosis, while AURKB serves a key role in
the organization of the centromere-kinetochore region,
microtubule-kinetochore attachments and cytokinesis (16,20,21). Due
to the differential expression and biological functions related to
the malignant phenotype attributed to these proteins, aurora
kinases have been proposed as potential targets for pharmacological
intervention, particularly in cancer (22–24).
In gliomas, high AURKA and AURKB expression is
associated with a poor prognosis and chemoresistance (18,25),
which led to the study of the reversine effects in these disease
models. Currently, reversine is in the preclinical phase of study
in oncology, but the data accumulated thus far has indicated
interesting antineoplastic activity (15). Hence, the identification of the most
sensitive type of tumors to reversine, including predictors of
response for this drug, may identify a profile of cancer patients
eligible for clinical trials. The present study aimed to target
aurora kinases using reversine and to identify the cellular and
molecular mechanisms underlying its anti-glioma effects.
Materials and methods
Cell culture, reagents and
chemicals
Oligodendroglioma (HOG) and glioblastoma (T98G,
U251MG and U87MG) cell lines were kindly provided by Professor
Regina Pekelmann Markus (University of São Paulo, São Paulo,
Brazil). The authenticity of the cells was determined by Short
Tandem Repeat (STR). The STR data from U87MG cells used in the
present study is compatible with a glioblastoma of unknown origin
(https://web.expasy.org/cellosaurus/CVCL_0022). All
cell lines were cultured in the Roswell Park Memorial Institute
medium (RPMI)-1640 and supplemented with 10% fetal bovine serum
(FBS), glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin.
The cells were maintained at 37°C in a 5% CO2 humidified
incubator and regularly split to maintain their exponential growth
state (up to six passages). Reversine
[2-(4-morpholinoanilino)-6-cyclohexylaminopurine] was obtained from
Target Molecule Corp. and prepared as a 50 mM stock solution in
dimethyl sulfoxide (Me2SO4; DMSO). Z-VAD-FMK was obtained from
Cayman Chemical Company and prepared as a 20 mM stock solution in
DMSO. Venetoclax (ABT-199) was obtained from Target Molecule Corp.
and prepared as a 50 mM stock solution in DMSO. Obatoclax
(GX15-070) was obtained from Chemietek and prepared as a 10 mM
stock solution in DMSO.
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was obtained using the TRIzol®
reagent (Thermo Fisher Scientific Inc.) from cells according to the
manufacturers' protocol. cDNA was synthesized from 1 µg of RNA
using a High-Capacity cDNA Reverse Transcription kit (Thermo Fisher
Scientific Inc.) according to the manufacturers' protocol. qPCR was
performed using an ABI 7500 Sequence Detector System (Thermo Fisher
Scientific Inc.) in conjunction with a SybrGreen System (Thermo
Fisher Scientific Inc.) for the expression of aurora kinase A
(AURKA), aurora kinase B (AURKB), BCL2 apoptosis regulator (BCL2),
BCL2 like (BCL2L1 1), baculoviral IAP repeat containing 5 (BIRC5),
BCL2 interacting protein 3 (BNIP3), BCL2 interacting protein 3 like
(BNIP3L), BCL2 associated agonist of cell death (BAD), BCL2
associated X, apoptosis regulator (BAX), BCL2 binding component 3
(BBC3), phorbol-12-myristate-13-acetate-induced protein 1 (PMAIP1),
cyclin dependent kinase inhibitor 1A (CDKN1A), cyclin dependent
kinase inhibitor 1B (CDKN1B) and growth arrest and DNA damage
inducible alpha (GADD45A) genes (Table
SI) according to the manufacturers' protocol. The thermocycling
conditions included an initial denaturation at 95°C for 10 min,
followed by 40 cycles of denaturation (95°C for 15 sec), annealing
(60°C for 30 sec) and extension (60°C for 30 sec). Hypoxanthine
phosphoribosyltransferase 1 (HPRT1) and β-actin were used as
reference genes. Relative quantification values were calculated
using the 2−ΔΔCt equation (26). A negative ‘No Template Control’ was
included for each primer pair as control for contamination or
unspecific amplification. Data were visualized using the multiple
experiment viewer (MeV) v.4.9.0 software (27).
Western blotting
Total protein extraction was performed using a
buffer containing 100 mM Tris (pH 7.6), 1% Triton X-100, 150 mM
NaCl, 2 mM PMSF, 10 mM Na3VO4, 100 mM NaF, 10
mM Na4P2O7, and 4 mM EDTA from
untreated U87MG, HOG, T98G and U251MG cells, or HOG, T98G and
U251MG cells treated with vehicle or reversine (1.6, 3.2 or 6.4 µM)
for 24 h or for cells treated with 1.6 µM reversine for 24, 48 and
72 h. Extracted proteins were quantified by the Bradford method.
Equal amounts of protein (30 µg) were separated by 8–15% SDS-PAGE
(28) and transferred onto
nitrocellulose membranes (GE Healthcare). Antibodies against AURKA
(cat. no. sc-25425), AURKB (cat. no. sc-25426), phosphorylated
(p)-histone H3S10 (sc-8659-R) and p-histone H2A.XS139
(γH2AX; sc-51748) were purchased from Santa Cruz Biotechnology,
Inc. Antibodies against PARP1 (cat. no. 9542), SQSTM1/p62 (cat. no.
88588), LC3BI/II (cat. no. 2775), β-actin (cat. no. 4970) and
α-tubulin (cat. no. 2144) were obtained from Cell Signaling
Technology, Inc. All primary antibodies were used at 1:1,000
dilution and incubated for 16 h at 4°C. α-tubulin and β-actin were
used as the loading controls. Secondary antibodies anti-mouse (cat.
no. 7076) and anti-rabbit (cat. no. 7074) conjugated with
horseradish peroxidase were obtained from Cell Signaling
Technology, Inc. and used at 1:2,000 dilution with 2 h of
incubation at room temperature. The SuperSignal™ West Dura Extended
Duration Substrate system (Thermo Fisher Scientific, Inc.) and
G:BOX Chemi XX6 gel document system (Syngene Europe) were used for
blot visualization. Band intensities were determined using
UN-SCAN-IT gel 6.1 software (SilkScientific).
Cell viability assay
Cell viability was determined by a sulforhodamine B
(SRB) assay. Briefly, a total of 4×103 glioma cells
(HOG, T98G and U251MG) per well were seeded in a 96-well plate in
an RPMI-1640 medium with 10% FBS in the presence of vehicle (DMSO)
or different concentrations of reversine (0.4, 0.8, 1.6, 3.1, 6.3,
12.5, 25.0 and 50.0 µM) for 24, 48 and 72 h. Dose-response
cytotoxicity for HOG, T98G, and U251MG cells treated with vehicle
or graded concentrations of venetoclax (0.1, 0.5, 1, 5, 10 and 50
µM) or obatoclax (0.01, 0.03, 0.1, 0.3, 1 and 3 µM) for 48 h was
performed. For drug combination studies, HOG, T98G and U251MG cells
were treated with vehicle or graded concentrations of reversine
(0.8, 1.6, 3.2 and 6.4 µM) in combination or not with Z-VAD-FMK (20
µM), venetoclax (5 µM), or obatoclax (HOG, 10 nM; T98G, 30 nM and
U251MG, 100 nM) for 48 h. The cells were then fixed with 10%
trichloroacetic acid at 4°C for at least 1 h. Subsequently, the
plates were washed with distilled water three times and a 0.2%
solution of SRB diluted in 1% acetic acid was added to the plates
and incubated for 30 min at 37°C. The non-associated dye was
removed by washing with 1% acetic acid three time and the plates
were dried at room temperature. Next, the plates were incubated
with a 10 mM TRIS pH 10.5 solution under stirring for 30 min at
4°C. Cell viability was evaluated by measuring the absorbance at
570 nm. The inhibitory concentration (IC)50 values were
calculated by performing a nonlinear regression analysis in
GraphPad Prism 5 (GraphPad Software, Inc.).
Clonogenic assay
HOG, T98G and U251MG cells (1×103
cells/35 mm2 plate) were incubated with vehicle or
different concentrations of reversine (0.2, 0.4, 0.8 and 1.6 µM)
for 24 h and then the medium was replaced with a drug-free medium
(RPMI-1640 with 10% FBS). Colonies were detected after 10–15 days
of culture by adding 0.5% crystal violet (Sigma-Aldrich; Merck
KGaA) to a 10% ethanol solution for 15 min at room temperature.
Images were acquired using the G:BOX Chemi XRQ (Syngene Europe) and
analyzed using ImageJ 1.45s software (US National Institutes of
Health).
Cell cycle analysis
A total of 2×105 cells/well (HOG, T98G
and U251MG) were seeded in 6-well plates in the presence of vehicle
or reversine (0.8 and 1.6 µM), harvested after 24 h, fixed with 70%
ethanol for at least 2 h at 4°C, and stored at 4°C for up to 7
days. Fixed cells were stained with 20 µg/ml propidium iodide (PI)
containing 10 µg/ml RNase A for 30 min at room temperature in a
light-protected area. DNA content distribution was acquired using a
FACSCalibur (Becton-Dickinson) flow cytometer and analyzed using
FlowJo software v.X.0.7 (Treestar, Inc.).
Immunofluorescence analysis
HOG, T98G and U251MG cells treated with vehicle or
1.6 µM reversine for 24 h, were fixed with ice-cold 100% methanol,
permeabilized with 0.5% Triton X-100 in PBS for 30 min at room
temperature and blocked with 1% bovine serum albumin (BSA) in PBS
for 1 h at room temperature. Next, the cells were incubated with
anti-α-tubulin Alexa Fluor® 488 conjugate (cat. no.
53-4502-82; 1:200 in 1% BSA in PBS; Thermo Fisher Scientific Inc.)
for 16 h at 4°C protected from light followed by washing once with
PBS. Finally, the slides were mounted in ProLong Gold Antifade
Mountant with DAPI (Thermo Fisher Scientific Inc.) for 1 h at room
temperature. Images were captured using a fluorescent microscope
(Lionheart FX Automated microscope; BioTek Instruments Inc.;
magnification, ×400).
Trypan blue exclusion
Cell viability was measured using a trypan blue dye
(Sigma-Aldrich; Merck KGaA) exclusion test after the incubation of
HOG, T98G and U251MG cells (1×105 cells/ml) with vehicle
or 0.4, 1.6 and 6.4 µM of reversine for 72 h. The supernatants (500
µl) were collected and the cells were removed after the addition of
100 µl of PBS (phosphate buffered saline), 50 µl of trypsin and 100
µl of RPMI-1640 medium. Each step was performed twice. The cell
solution was mixed and an aliquot of 10 µl of trypan blue was added
to 90 µl of the cell solution. Cells of this solution were counted
using a light microscope (magnification, ×100) and Neubauer chamber
(OPTIK-Labor), and cells dyed with trypan blue were considered
non-viable cells.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism v5 (GraphPad Software, Inc.). For multiple comparisons, ANOVA
followed by the post hoc Bonferronis test was used. For the
comparison of 2 groups, the paired Student's t-test was used.
P<0.05 was considered to indicate a statistically significant
difference. The IC50 values were calculated by
performing a nonlinear regression analysis in GraphPad Prism v5.
Each experiment was repeated three times and data were presented as
the means ± SD.
Results
AURKA and AURKB expression in glioma
cell lines
First, AURKA and AURKB mRNA and protein levels as
well histone H3 phosphorylation were investigated in a panel of
glioma cell lines. T98G cells exhibited the highest levels of AURKA
mRNA (P<0.001, HOG vs. U87MG or U251MG; P<0.05, T98G vs.
U87MG or U251MG) and protein (P<0.01, T98G vs. other cell
lines), while HOG cells exhibited the highest levels of AURKB mRNA
(P<0.001, HOG vs. other cell lines; P<0.05, T98G vs. U87MG)
and protein (P<0.001, HOG vs. other cell lines; P<0.01, T98G
vs. U87MG cell line) (Fig. 1A-C).
U87MG and U251MG cells showed low levels of AURKA and AURKB
(Fig. 1A-C). Higher levels of
p-histone H3 were observed in T98G cells (P<0.01, T98G vs. other
cell lines) (Fig. 1C), indicating
increased AURKB activity. Based on these findings, HOG, T98G and
U251MG were selected for additional functional assays.
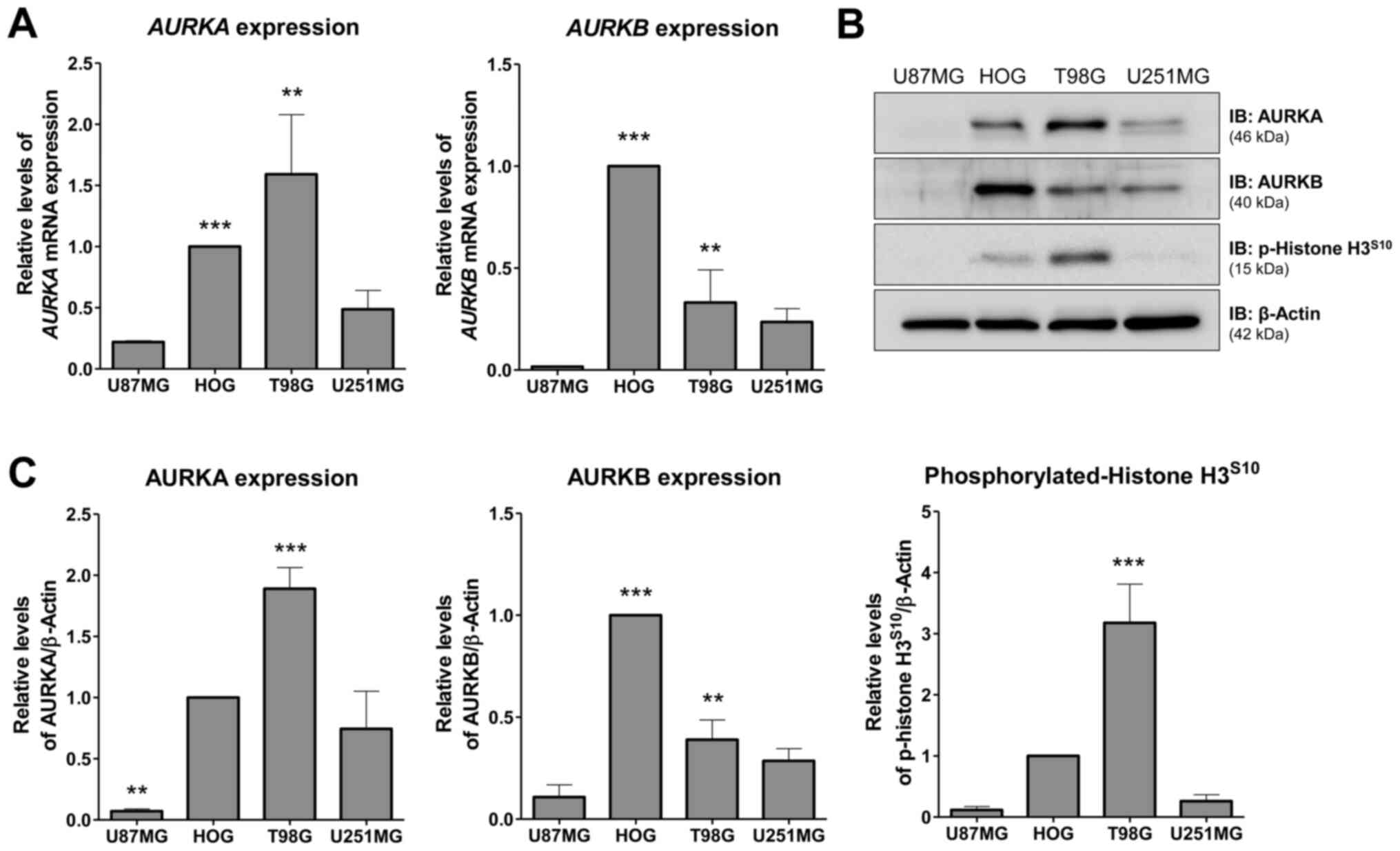 | Figure 1.Expression of AURKA and AURKB in
glioma cell lines. (A) RT-qPCR analysis of AURKA and AURKB mRNA
expression in U87MG, HOG, T98G and U251MG cells. Bar graphs
represent the mean ± SD of at least 3 independent samples.
AURKA expression analysis: ***P<0.001, HOG vs. U87MG or
U251MG; **P<0.05, T98G vs. U87MG or U251MG. AURKB
expression analysis: ***P<0.001, HOG vs. other cell lines;
**P<0.05, T98G vs. U87MG. (B) Western blot analysis for AURKA,
AURKB and p-histone H3S10 in total cell extracts from
U87MG, HOG, T98G, and U251MG; membranes were reprobed with the
antibody for the detection of β-actin. (C) Bar graphs represent the
mean ± SD of 3 independent experiments quantifying band intensities
of indicated proteins. AURKA protein levels: ***P<0.01, T98G vs.
other cell lines; **P<0.01, U87MG vs. other cell lines. AURKB
protein levels: ***P<0.001, HOG vs. other cell lines;
**P<0.01, T98G vs. U87MG cell line. Phosphorylated-histone
H3S10 levels: ***P<0.01, T98G vs. other cell lines.
ANOVA test and Bonferroni post-test. AURKA, aurora kinase A; AURKB,
aurora kinase B; p, phosphorylated; RT-q, reverse
transcription-quantitative. |
Reversine reduces cell viability and
clonogenicity in glioma cells
Next, the effects of reversine on the viability of
glioma cells were investigated. Reversine reduced the viability in
a dose- and time-dependent manner in all the glioma cell lines,
with HOG and T98G cells being more sensitive compared with U251MG
cells (Fig. 2A). The 24, 48 and 72 h
IC50 were, respectively, 12, <0.4 and <0.4 µM for
HOG cells; 11, 3.6 and 0.4 µM for T98G cells; and 13, 7.5, and 6.9
µM for U251MG cells (Fig. 2A).
Similar results were observed in the colony formation assay. In HOG
and T98G cells, reversine exposure for 24 h strongly reduced
clonogenicity capacity (>80% of reduction in colony formation at
doses ≥0.4 µM), while reversine reduced clonogenicity to a lower
degree in U251MG (reduction ranged from 33–48%) (Fig. 2B and C) compared with vehicle-treated
cells. These data suggested that glioma cells expressing higher
AURKA or AURKB levels may be more sensitive to the antineoplastic
effects of reversine.
 | Figure 2.Reduction of cell viability and
clonogenicity by reversine in glioma cells. (A) Dose- and
time-response cytotoxicity analyzed by the sulforhodamine B (SRB)
assay for HOG, T98G and U251MG cells treated with vehicle (DMSO) or
graded concentrations of reversine (0.4, 0.8, 1.6, 3.1, 6.3, 12.5,
25 and 50 µM) for 24, 48 and 72 h. Values are expressed as the
percentage of viable cells for each condition relative to
vehicle-treated cells. Results are shown as mean ± SD of at least 3
independent experiments. (B) Colony formation after reversine
exposure for 24 h and placement in drug-free media for an
additional 10–15 days. Representative images of colony formation
after vehicle or reversine (0.2, 0.4, 0.8, and 1.6 µM) treatment
are illustrated. (C) Bar graph represents mean ± SD of relative
number of colonies (% of control). *P<0.05, **P< 0.01 and
***P<0.001. IC, inhibitory concentration. |
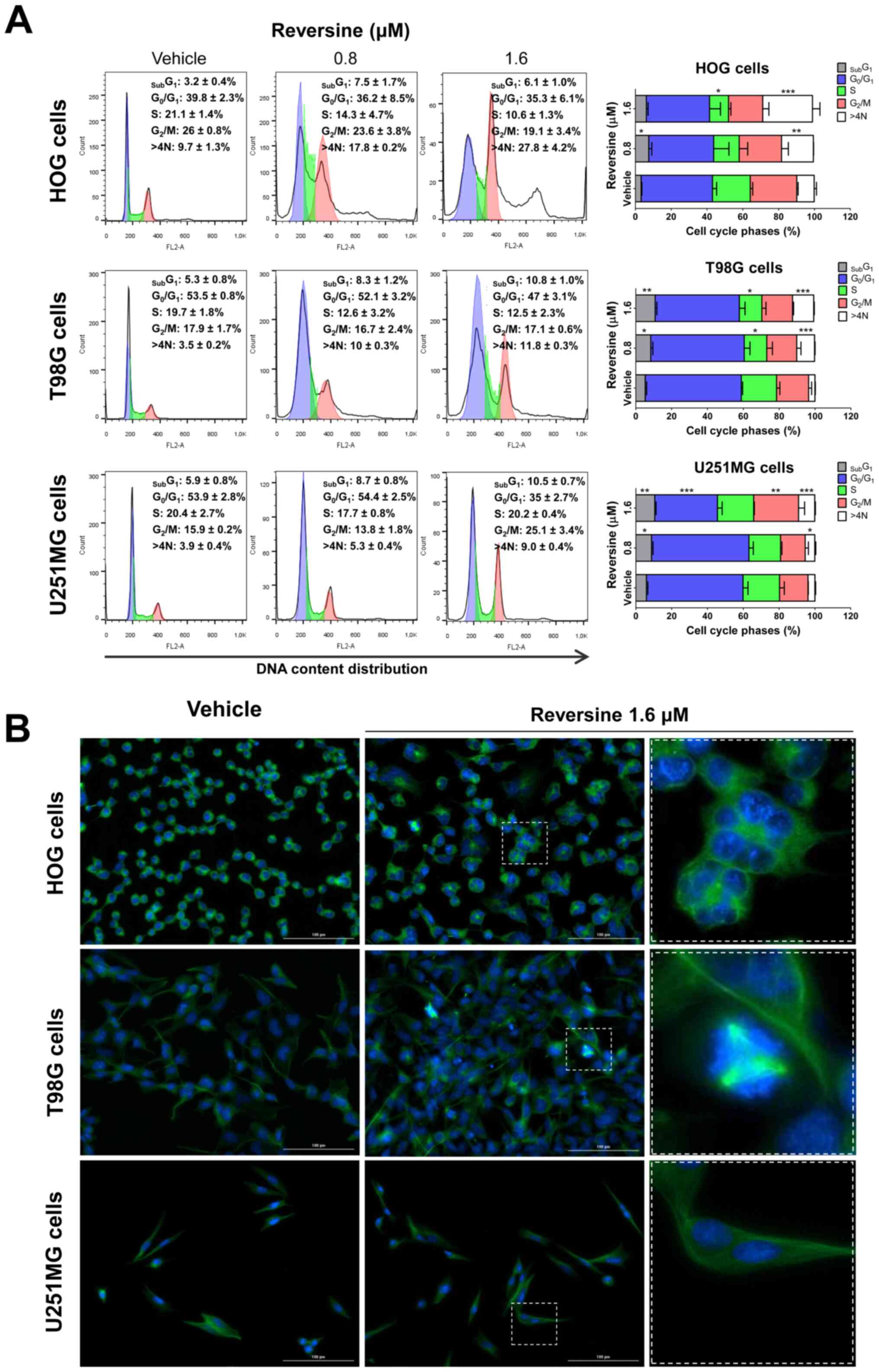
Reversine triggers mitotic aberrations
and polyploidy in glioma cells
As aurora kinases serve a key role in mitosis and
cytokinesis (16), the impact of
reversine on cell cycle progression was investigated. In HOG and
T98G cells, reversine treatment reduced S-phase cells and induced
polyploidy in a dose-dependent manner (P<0.05), which was more
evident for HOG cells (Fig. 3A). In
U251MG cells, treatment with reversine increased G2/M
arrest and polyploidy in a dose-dependent manner (P<0.05;
Fig. 3A). In T98G and U251MG cells,
reversine exposure increased the sub-G1 cell population
(P<0.05; Fig. 3A). The
morphological analysis via immunofluorescence corroborated the flow
cytometry quantitative data and revealed additional qualitative
details. An increased frequency of polyploidy cells was observed in
reversine-treated HOG cells (Fig.
3B). In T98G cells, mitotic aberrations, including cells with
multiple mitotic spindles were observed with reversine treatment
(Fig. 3B). Few changes were observed
in U251MG cells exposed to reversine, which was consistent with the
lower frequency of changes observed by flow cytometry and the lower
sensitivity of this cell line to reversine (Fig. 3B) compared with vehicle-treated
cells.
Apoptosis and DNA damage markers are
induced by reversine exposure in glioma cells
Next, the effect of reversine on cell signaling was
investigated, particularly aurora kinase activity, DNA damage,
apoptosis and autophagy using western blotting and RT-qPCR.
Reversine reduced AURKA and/or AURKB expression/activity and
increased γH2AX and cleaved-PARP1 (Fig.
4A and B). No consistent modulation in autophagy markers, LC3B,
or SQSTM1/p62 were observed in the glioma cells (Fig. 4A and B). These results suggested that
a mitotic catastrophe followed by apoptosis may be the main
mechanism involved in the reduction of cell viability induced by
reversine in glioma cells. To obtain additional insights into the
differences in sensitivity to reversine observed among the glioma
cell lines, a panel of antiapoptotic, pro-apoptotic and DNA
damage-induced cell cycle arrest-related genes was investigated in
HOG cells with 1.6 µM reversine exposure for 24 h (Fig. 4C). Based on the gene expression
profile, PMAIP1, CDKN1A and GADD45A genes were selected for further
investigation in all glioma cells by RT-qPCR. In HOG cells, PMAIP1,
CDKN1A and GADD45A genes were significantly upregulated (all
P<0.01), while only GADD45A was significantly upregulated
in T98G cells (P<0.01; Fig. 4C)
compared with vehicle-treated cells. None of the 3 selected genes
was modulated in U251MG cells (Fig.
4C). It is notable that U251MG cells present a homozygous
deletion of CDKN1A (29),
which is consistent with the absence of amplification of this gene
in the present study (Fig. 4C). The
trypan blue exclusion assay and PARP1 cleavage corroborated the
molecular findings, indicating an increase in cell death with
reversine exposure, as observed by increased proportion of cells
dyed with trypan blue and the ratio of cleaved-PARP1 (all
P<0.05; Figs. 4D and S1).
 | Figure 4.Reversine triggered apoptosis and DNA
damage markers in glioma cells. Western blot analysis for AURKA,
AURKB, p-histone H3S10, γH2AX, PARP1 (total and
cleaved), LC3BI/II and SQSTM1/p62 in total cell extracts from HOG,
T98G and U251MG cells treated with (A) vehicle (DMSO) or graded
concentrations of reversine (vehicle, 1.6, 3.2 or 6.4 µM) for 24 h
or (B) graded time of exposure (24, 48 and 72 h) to reversine at
1.6 µM. Membranes were reprobed with the antibody for the detection
of α-tubulin. (C) Heatmap illustrates the RT-qPCR analysis of BCL2,
BCL2L1, BIRC5, BNIP3, BNIP3L, BAD, BAX, BBC3, PMAIP1, CDKN1A,
CDKN1B and GADD45A gene expression in HOG cells upon treatment with
reversine (1.6 µM; mean, n=4) for 24 h. The data are represented as
the fold-change of vehicle-treated HOG cells and down and
upregulated genes are shown by blue and red, respectively. Bar
graph represents mean ± SD of the fold-change of vehicle-treated
cells (dotted line) for PMAIP1, CDKN1A and GADD45A in
HOG, T98G, and U251MG cell lines upon reversine exposure for 24 h.
**P<0.1 and ***P<0.001. (D) Trypan blue exclusion dye assay
in HOG, T98G, and U251MG cells upon vehicle or 1.6 µM reversine
treatment for 72 h. Bar graph represents relative mean ± SD of
viable (gray) and non-viable (blue) cells. *P<0.05 and
**P<0.01. p, phosphorylated; RT-q, reverse
transcription-quantitative; AURKA, aurora kinase A; AURKB, aurora
kinase B; γH2AX, phosphorylated histone 2AX; PARP1,
poly(ADP-ribose) polymerase 1; SQSTM1/p62, sequestosome 1; LC3BII,
microtubule associated protein 1 light chain 3 beta, BCL2, BCL2
apoptosis regulator; BCL2L1, BCL2 like 1; BIRC5, baculoviral IAP
repeat containing 5; BNIP3, BCL2 interacting protein 3; BNIP3L,
BCL2 interacting protein 3 like; BAD, BCL2 associated agonist of
cell death; BAX, BCL2 associated X, apoptosis regulator; BBC3, BCL2
binding component 3; PMAIP1,
phorbol-12-myristate-13-acetate-induced protein 1; CDKN1A, cyclin
dependent kinase inhibitor 1A; CDKN1B, cyclin dependent kinase
inhibitor 1B; GADD45A, growth arrest and DNA damage inducible gene
45 alpha. |
To further explore the molecular findings using
pharmacological tools, Z-VAD-FMK (pan-caspase inhibitor) (30), venetoclax (selective BCL2 inhibitor)
(31) and obatoclax (mimetic BH3)
(32) were used in combination with
reversine in glioma cells. Z-VAD-FMK treatment partially attenuated
the reduction of cell viability induced by reversine (Fig. S2). Venetoclax (IC50
ranged from 6.1 to 13.6 µM) and obatoclax (IC50 ranged
from 0.10 to 0.21 µM) treatment reduced glioma cell viability and
potentiated the antineoplastic effects of reversine in glioma cells
(Fig. S3). These data indicated
that caspase-mediated apoptosis contributes to the
reversine-induced reduction of cell viability in glioma cells, as
previously reported for other solid tumors (i.e. colorectal cancer
and renal carcinoma) (28,29) and adds new insights into BCL2-related
processes in this context.
Discussion
In the present study, the cellular and molecular
effects of reversine were investigated in glioma cells. Reversine
has emerged as a potential anticancer agent and its antineoplastic
effects have already been reported for hematological neoplasms
(28,33–35),
oral squamous cell carcinoma (36),
thyroid cancer (37,38), breast cancer (12,39),
cervical carcinoma (40), non-small
cell lung cancer (41), urothelial
carcinoma (42), renal carcinoma
(43), colon carcinoma (14,44),
osteosarcoma (45) and others
(15). The main mechanisms of cell
death attributed to the reduction in cell viability induced by
reversine are mitotic catastrophe, apoptosis and autophagy
(33,36,39). In
solid tumors, the IC50 for reversine ranges from >1
to 20 µM and hence the data obtained related to glioma in the
present study (IC50>0.4–13 µM) suggested that
reversine is potent for this type of tumor. From a molecular point
of view, reversine acts as a selective multikinase inhibitor for
AURKA, AURKB, JNK and MPS1 (14,28,33,46).
A functional role and a prognostic relevance for the
expression of aurora kinases in gliomas have been identified,
providing evidence that these proteins are potential therapeutic
targets in this disease (18,25). In
gliomas, AURKA expression is high in advanced stages of the disease
and its high expression is associated with proliferation (e.g.
Ki-67) and angiogenesis (e.g. HIF1α) markers and predicts a worse
prognosis (25). Similarly, AURKB
expression is high in glioblastomas, is related to chemoresistance
to temozolomide and negatively impacts the clinical outcome
(18). The genetic or
pharmacological inhibition of aurora kinases reduces tumor
viability and growth and increases sensitivity to chemotherapy and
radiotherapy in vitro and in vivo models of
glioblastoma (18,47,48). For
instance, the combined treatment of VX680 (an aurora kinase
inhibitor) and radiation enhances the efficacy of radiotherapy by
targeting radioresistant cells in mice xenografted with human
glioma (47).
In the present study, HOG cells, which had the
highest AURKB levels, also demonstrated the greatest sensitivity to
reversine. AURKB serves an essential role in cytokinesis (16), which corroborates with the
significant increase in polyploidy observed in HOG cells after
exposure to reversine in the present study. Similarly, T98G cells,
which had the highest AURKA levels in the present study,
demonstrated multiple mitotic aberrations including the formation
of cells with multiple mitotic spindles. AURKA serves a central
role in the organization of spindle orientation (16), which corroborates with the
morphological findings in T98G cells in the present study. The
lower sensitivity of U251MG cells, which also expressed lower AURKA
and AURKB levels in the present study, suggested that the
expression of aurora kinases may serve as a marker of response to
reversine, which could facilitate the use of this drug in the
context of personalized medicine.
Despite the recent finding that reversine induces
autophagy in several cancer models (35,36,38,41,42), in
glioma cells in the present study no consistent modulation was
observed in autophagy markers, LC3B, or STSQM1/p62 with reversine
exposure. Autophagy is a complex and conserved process that acts as
a double-edged sword, promoting cell death or serving as a
mechanism of resistance to apoptosis (49). Hence, in glioma cells in the present
study, the absence of autophagy modulation observed could explain
the greater sensitivity to reversine because mitotic catastrophe
followed by apoptosis, seems to be the main mechanism in the
reduction of cell viability.
Among the modulated genes upon reversine exposure in
HOG cells, the increase in cell cycle arrest markers (CDKN1A
and GADD45A) and the pro-apoptotic gene responsive to DNA
damage (PMAIP1) were observed in the present study. The
CDKN1A and GADD45A genes encode a cyclin-dependent kinase inhibitor
and a sensor of oncogenic stress, respectively, which are both
important for cell cycle progression and the maintenance of
centrosome stability (50–52). The PMAIP1 gene (also known as NOXA)
is a pro-apoptotic member of the BCL2 protein family, which acts as
a BH3-only protein and is involved in the intrinsic apoptosis
pathway (53). Notably, in the
present study, the combination of reversine with venetoclax
(selective BCL2 inhibitor) or obatoclax (mimetic BH3) demonstrated
potentiating effects in reducing cell viability in glioma cells.
Together, these data provided additional evidence for the
reversine-modulated molecular network and new potential drug
response markers.
Despite new insights from a cellular and molecular
perspective on the reversine action in glioma models, a limitation
of the present study is that the experiments were only carried out
in 2D cell line models. Future studies using 3D culture, primary
glioma cells or animal models are therefore required and may
provide solid evidence about the antineoplastic potential of
reversine for the treatment of gliomas.
In conclusion, based on the findings of the present
study, reversine potently induces mitotic catastrophe and apoptosis
in glioma cells. The exploratory molecular analysis suggested that
the expression of aurora kinases as well as the induction of genes
responsive to DNA damage, CDKN1A, PMAIP1 and GADD45A
may be related to an improved anticancer response to reversine in
glioma cells. The preclinical findings of the present study
highlighted that reversine may be a putative novel drug in the
antineoplastic arsenal against gliomas.
Supplementary Material
Supporting Data
Acknowledgements
The authors thank Prof. Regina Pekelmann Markus
(University of São Paulo, São Paulo, Brazil) for providing the cell
lines used in the present study.
Funding
This study was supported by grants (grant nos.
2019/23864-7, 2018/19372-9 and 2015/17177-6) from the São Paulo
Research Foundation (FAPESP) and grant no. 402587/2016-2 from the
Conselho Nacional de Desenvolvimento Científico e Tecnológico
(CNPq). This study was financed in part by the Coordenação de
Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) -
Finance Code 001.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
CH and KL designed, performed and analyzed the
experiments and prepared the manuscript. BODA, LBLDM, KGDF and LCF
participated in the experiments and analysis. LVCL provided input,
participated in the interpretation of the manuscript data and
prepared the manuscript. JMN supervised and participated in the
overall design of study and the experiments and analyses and
prepared the manuscript. LVCL and JMN confirmed the authenticity of
all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ostrom QT, Patil N, Cioffi G, Waite K,
Kruchko C and Barnholtz-Sloan JS: CBTRUS Statistical Report:
Primary Brain and Other Central Nervous System Tumors Diagnosed in
the United States in 2013–2017. Neuro Oncol. 22 (Suppl 2):iv1–iv96.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
Classification of Tumors of the Central Nervous System: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Straube C, Schmidt-Graf F, Wiestler B,
Zimmer C, Meyer B and Combs SE: The algorithms of adjuvant therapy
in gliomas and their effect on survival. J Neurosurg Sci.
63:179–186. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goldbrunner R, Ruge M, Kocher M, Lucas CW,
Galldiks N and Grau S: The Treatment of Gliomas in Adulthood. Dtsch
Arztebl Int. 115:356–364. 2018.PubMed/NCBI
|
|
5
|
Gittleman H, Boscia A, Ostrom QT, Truitt
G, Fritz Y, Kruchko C and Barnholtz-Sloan JS: Survivorship in
adults with malignant brain and other central nervous system tumor
from 2000–2014. Neuro Oncol. 20 (Suppl 7):vii6–vii16. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brennan CW, Verhaak RG, McKenna A, Campos
B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ,
Berman SH, et al TCGA Research Network, : The somatic genomic
landscape of glioblastoma. Cell. 155:462–477. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al European Organisation for Research and Treatment of Cancer
Brain Tumor and Radiotherapy Groups: National Cancer Institute of
Canada Clinical Trials Group, : Radiotherapy plus concomitant and
adjuvant temozolomide for glioblastoma. N Engl J Med. 352:987–996.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ali MY, Oliva CR, Noman ASM, Allen BG,
Goswami PC, Zakharia Y, Monga V, Spitz DR, Buatti JM and Griguer
CE: Radioresistance in Glioblastoma and the Development of
Radiosensitizers. Cancers (Basel). 12:122020. View Article : Google Scholar
|
|
9
|
Gilbert MR, Dignam JJ, Armstrong TS, Wefel
JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S,
Won M, et al: A randomized trial of bevacizumab for newly diagnosed
glioblastoma. N Engl J Med. 370:699–708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fisher JP and Adamson DC: Current
FDA-approved therapies for high-grade malignant Gliomas.
Biomedicines. 9:92021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brat DJ, Verhaak RG, Aldape KD, Yung WK,
Salama SR, Cooper LA, Rheinbay E, Miller CR, Vitucci M, Morozova O,
et al Cancer Genome Atlas Research Network, : Comprehensive,
integrative genomic analysis of diffuse lower-grade gliomas. N Engl
J Med. 372:2481–2498. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang D, Huang Y, Huang Z, Weng J, Zhang S
and Gu W: Relation of AURKB over-expression to low survival rate in
BCRA and reversine-modulated aurora B kinase in breast cancer cell
lines. Cancer Cell Int. 19:1662019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song HK, Noh EM, Kim JM, You YO, Kwon KB
and Lee YR: Reversine inhibits MMP-3, IL-6 and IL-8 expression
through suppression of ROS and JNK/AP-1 activation in
interleukin-1β-stimulated human gingival fibroblasts. Arch Oral
Biol. 108:1045302019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jemaà M, Abassi Y, Kifagi C, Fezai M,
Daams R, Lang F and Massoumi R: Reversine inhibits Colon Carcinoma
Cell Migration by Targeting JNK1. Sci Rep. 8:118212018. View Article : Google Scholar
|
|
15
|
Piccoli M, Ghiroldi A, Monasky MM, Cirillo
F, Ciconte G, Pappone C and Anastasia L: Reversine: A synthetic
purine with a dual activity as a cell dedifferentiating agent and a
selective anticancer drug. Curr Med Chem. 27:3448–3462. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Willems E, Dedobbeleer M, Digregorio M,
Lombard A, Lumapat PN and Rogister B: The functional diversity of
Aurora kinases: A comprehensive review. Cell Div. 13:72018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Diekema DS: Is taller really better?
Growth hormone therapy in short children. Perspect Biol Med.
34:109–123. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alafate W, Wang M, Zuo J, Wu W, Sun L, Liu
C, Xie W and Wang J: Targeting Aurora kinase B attenuates
chemoresistance in glioblastoma via a synergistic manner with
temozolomide. Pathol Res Pract. 215:1526172019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang A, Gao K, Chu L, Zhang R, Yang J and
Zheng J: Aurora kinases: Novel therapy targets in cancers.
Oncotarget. 8:23937–23954. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marumoto T, Zhang D and Saya H: Aurora-A -
a guardian of poles. Nat Rev Cancer. 5:42–50. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Steigemann P, Wurzenberger C, Schmitz MH,
Held M, Guizetti J, Maar S and Gerlich DW: Aurora B-mediated
abscission checkpoint protects against tetraploidization. Cell.
136:473–484. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pérez Fidalgo JA, Roda D, Roselló S,
Rodríguez-Braun E and Cervantes A: Aurora kinase inhibitors: A new
class of drugs targeting the regulatory mitotic system. Clin Transl
Oncol. 11:787–798. 2009. View Article : Google Scholar
|
|
23
|
Libertini S, Abagnale A, Passaro C, Botta
G and Portella G: Aurora A and B kinases - targets of novel
anticancer drugs. Recent Patents Anticancer Drug Discov. 5:219–241.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Borisa AC and Bhatt HG: A comprehensive
review on Aurora kinase: Small molecule inhibitors and clinical
trial studies. Eur J Med Chem. 140:1–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lehman NL, O'Donnell JP, Whiteley LJ,
Stapp RT, Lehman TD, Roszka KM, Schultz LR, Williams CJ, Mikkelsen
T, Brown SL, et al: Aurora A is differentially expressed in
gliomas, is associated with patient survival in glioblastoma and is
a potential chemotherapeutic target in gliomas. Cell Cycle.
11:489–502. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saeed AI, Sharov V, White J, Li J, Liang
W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, et
al: TM4: A free, open-source system for microarray data management
and analysis. Biotechniques. 34:374–378. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lima K, Carlos JAEG, Alves-Paiva RM,
Vicari HP, Souza Santos FP, Hamerschlak N, Costa-Lotufo LV, Traina
F and Machado-Neto JA: Reversine exhibits antineoplastic activity
in JAK2V617F-positive myeloproliferative neoplasms. Sci Rep.
9:98952019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hama S, Matsuura S, Tauchi H, Yamasaki F,
Kajiwara Y, Arita K, Yoshioka H, Heike Y, Mandai K and Kurisu K:
p16 Gene transfer increases cell killing with abnormal nucleation
after ionising radiation in glioma cells. Br J Cancer.
89:1802–1811. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Van Noorden CJ: The history of Z-VAD-FMK,
a tool for understanding the significance of caspase inhibition.
Acta Histochem. 103:241–251. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Souers AJ, Leverson JD, Boghaert ER,
Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH,
Fairbrother WJ, et al: ABT-199, a potent and selective BCL-2
inhibitor, achieves antitumor activity while sparing platelets. Nat
Med. 19:202–208. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nguyen M, Marcellus RC, Roulston A, Watson
M, Serfass L, Murthy Madiraju SR, Goulet D, Viallet J, Bélec L,
Billot X, et al: Small molecule obatoclax (GX15-070) antagonizes
MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc
Natl Acad Sci USA. 104:19512–19517. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
D'Alise AM, Amabile G, Iovino M, Di
Giorgio FP, Bartiromo M, Sessa F, Villa F, Musacchio A and Cortese
R: Reversine, a novel Aurora kinases inhibitor, inhibits colony
formation of human acute myeloid leukemia cells. Mol Cancer Ther.
7:1140–1149. 2008. View Article : Google Scholar
|
|
34
|
Rodrigues Alves AP, Machado-Neto JA,
Scheucher PS, Paiva HH, Simões BP, Rego EM and Traina F: Reversine
triggers mitotic catastrophe and apoptosis in K562 cells. Leuk Res.
48:26–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Carlos J, Lima K, Coelho-Silva JL, de Melo
Alves-Paiva R, Moreno NC, Vicari HP, de Souza Santos FP,
Hamerschlak N, Costa-Lotufo LV, Traina F, et al: Reversine exerts
cytotoxic effects through multiple cell death mechanisms in acute
lymphoblastic leukemia. Cell Oncol (Dordr). 43:1191–1201. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee YR, Wu WC, Ji WT, Chen JY, Cheng YP,
Chiang MK and Chen HR: Reversine suppresses oral squamous cell
carcinoma via cell cycle arrest and concomitantly apoptosis and
autophagy. J Biomed Sci. 19:92012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hua SC, Chang TC, Chen HR, Lu CH, Liu YW,
Chen SH, Yu HI, Chang YP and Lee YR: Reversine, a 2,6-disubstituted
purine, as an anti-cancer agent in differentiated and
undifferentiated thyroid cancer cells. Pharm Res. 29:1990–2005.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lu CH, Liu YW, Hua SC, Yu HI, Chang YP and
Lee YR: Autophagy induction of reversine on human follicular
thyroid cancer cells. Biomed Pharmacother. 66:642–647. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kuo CH, Lu YC, Tseng YS, Shi CS, Chen SH,
Chen PT, Wu FL, Chang YP and Lee YR: Reversine induces cell cycle
arrest, polyploidy, and apoptosis in human breast cancer cells.
Breast Cancer. 21:358–369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Qin HX, Yang J, Cui HK, Li SP, Zhang W,
Ding XL and Xia YH: Synergistic antitumor activity of reversine
combined with aspirin in cervical carcinoma in vitro and in vivo.
Cytotechnology. 65:643–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lu YC, Lee YR, Liao JD, Lin CY, Chen YY,
Chen PT and Tseng YS: Reversine induced multinucleated cells, cell
apoptosis and autophagy in human non-small cell lung cancer cells.
PLoS One. 11:e01585872016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fang CY, Chen JS, Chang SK and Shen CH:
Reversine induces autophagic cell death through the AMP-activated
protein kinase pathway in urothelial carcinoma cells. Anticancer
Drugs. 29:29–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cheng L, Wang H, Guo K, Wang Z, Zhang Z,
Shen C, Chen L and Lin J: Reversine, a substituted purine, exerts
an inhibitive effect on human renal carcinoma cells via induction
of cell apoptosis and polyploidy. OncoTargets Ther. 11:1025–1035.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Park YL, Ha SY, Park SY, Choi JH, Jung MW,
Myung DS, Kim HS and Joo YE: Reversine induces cell cycle arrest
and apoptosis via upregulation of the Fas and DR5 signaling
pathways in human colorectal cancer cells. Int J Oncol.
54:1875–1883. 2019.PubMed/NCBI
|
|
45
|
Kim JS, Cho IA, Kang KR, Lim H, Kim TH, Yu
SK, Kim HJ, Lee SA, Moon SM, Chun HS, et al: Reversine induces
caspase-dependent apoptosis of human osteosarcoma cells through
extrinsic and intrinsic apoptotic signaling pathways. Genes
Genomics. 41:657–665. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hiruma Y, Koch A, Dharadhar S, Joosten RP
and Perrakis A: Structural basis of reversine selectivity in
inhibiting Mps1 more potently than aurora B kinase. Proteins.
84:1761–1766. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li N, Maly DJ, Chanthery YH, Sirkis DW,
Nakamura JL, Berger MS, James CD, Shokat KM, Weiss WA and Persson
AI: Radiotherapy followed by aurora kinase inhibition targets
tumor-propagating cells in human glioblastoma. Mol Cancer Ther.
14:419–428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hong X, O'Donnell JP, Salazar CR, Van
Brocklyn JR, Barnett KD, Pearl DK, deCarvalho AC, Ecsedy JA, Brown
SL, Mikkelsen T, et al: The selective Aurora-A kinase inhibitor
MLN8237 (alisertib) potently inhibits proliferation of glioblastoma
neurosphere tumor stem-like cells and potentiates the effects of
temozolomide and ionizing radiation. Cancer Chemother Pharmacol.
73:983–990. 2014.PubMed/NCBI
|
|
49
|
Galluzzi L, Pietrocola F, Bravo-San Pedro
JM, Amaravadi RK, Baehrecke EH, Cecconi F, Codogno P, Debnath J,
Gewirtz DA, Karantza V, et al: Autophagy in malignant
transformation and cancer progression. EMBO J. 34:856–880. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xiao BD, Zhao YJ, Jia XY, Wu J, Wang YG
and Huang F: Multifaceted p21 in carcinogenesis, stemness of tumor
and tumor therapy. World J Stem Cells. 12:481–487. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Salvador JM, Brown-Clay JD and Fornace AJ
Jr: Gadd45 in stress signaling, cell cycle control, and apoptosis.
Adv Exp Med Biol. 793:1–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liebermann DA, Tront JS, Sha X, Mukherjee
K, Mohamed-Hadley A and Hoffman B: Gadd45 stress sensors in
malignancy and leukemia. Crit Rev Oncog. 16:129–140. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Morsi RZ, Hage-Sleiman R, Kobeissy H and
Dbaibo G: Noxa: Role in cancer pathogenesis and treatment. Curr
Cancer Drug Targets. 18:914–928. 2018. View Article : Google Scholar : PubMed/NCBI
|