Introduction
Tumor suppressors and tumor promoters regulate cell
functions in an opposite manner. For example, while tumor promoters
generally support cell growth, survival, and invasion, tumor
suppressors inhibit these cell functions. Accordingly, networks of
the two opposing groups have evolved for their coordinated
regulation, which is essential for cells to adapt to changes in the
external environment and internal physiology. An imbalance in such
networks can cause tumor initiation and progression (1), and therefore, elucidating the
functional interaction between tumor suppressors and tumor
promoters is required for an improved understanding of not only
cell function regulation, but also the process leading to tumor
initiation and progression.
Slug, a member of the Snail family of transcription
factors (2), is upregulated in
various types of cancers (3–7). Since Slug upregulation facilitates
epithelial-mesenchymal transition and the subsequent invasion and
metastasis of cancer cells (2,3), it is
considered as a tumor promoter. Slug is downregulated by Mdm2, a
ring-finger-containing E3 ligase that ubiquitinates Slug, leading
to its proteasome-dependent degradation. It has been reported that
the action of Mdm2 on Slug is facilitated by the binding of Slug to
p53, a tumor suppressor/transcription factor (8). Previous studies from our group have
shown that p53 can form a complex with its transcriptional target
p21WAF1/CIP1, and the p53/p21 complex rather than p53
alone is the functional unit that binds to Slug to promote
Mdm2-mediated degradation of Slug (9). The p53/p21 complex consistently
suppresses cancer cell invasion (9),
suggesting that the complex acts as a tumor suppressor that can
degrade a tumor promoter, such as Slug, and inhibit the spread of
cancer cells. In this regard, it is interesting to speculate that
Slug might not be unilaterally attacked by p53 and p21, but instead
might have a tool to counter p53 and p21 to maintain the balance
between itself and the p53/p21 complex. To date, however, this
question has not been addressed.
Mdm2 can also directly bind to p53 and p21, inducing
proteasomal degradation (10–14).
While Mdm2 ubiquitinates p53 for p53 degradation (10–12),
Mdm2 facilitates the binding of p21 to the proteasome without
ubiquitinating p21 (13,14). Our recent studies revealed that Mdm2
binds to p53 in the p53/p21 complex more efficiently than p53 alone
(15). Accordingly, the binding of
p21 to p53 promoted Mdm2-mediated p53 degradation. However, it
remains unclear whether p53 also influences p21 stability.
Considering that the regulation of p53 and p21 protein stability is
important for controlling their activities in cells (10–14),
Slug may influence the protein stability of p53 and p21. Here, we
investigated this possibility using HCT116 colon cancer cells,
because in contrast to numerous other cancer cells in which p53 is
mutated, HCT116 cells express p53 wild-type. Moreover, p53- and
p21-knockout variants of HCT116 cells are also available (16). Therefore, HCT116 cells and their
variants have been widely used as models to investigate the
functions of p53 and p21. Our data demonstrate that Slug
facilitates the degradation of p53 and p21 proteins by inducing
Mdm2 expression, which acts as a mediator of such actions of Slug.
While Slug requires p21 to induce p53 degradation, we found that
p53 does not influence Slug-induced p21 degradation. Our data
support not only the existence of mutual interactions between Slug
and p53/p21 to balance their tumor-promoting and -suppressing
functions, respectively, but also provide new insights into the
mechanism wherein the stability of p53 and p21 proteins is
regulated.
Materials and methods
Antibodies, small-interfering RNAs,
and chemicals
The following antibodies were used in the study:
Anti-Slug (Abnova), anti-p53 and anti-Mdm2 (Santa Cruz
Biotechnology, Inc.), anti-p21 (Cell Signaling Technology), and
anti-β-actin (Sigma-Aldrich; Merck KGaA). Small-interfering RNAs
(siRNAs) were purchased from Ambion. When two sets of siRNAs were
used, they were numbered as siRNA-1 and −2. Their catalog numbers
are as follows: Slug siRNA-1 and −2, S13128 and S224653; Mdm2
siRNA, S224037. Cycloheximide was purchased from Sigma-Aldrich;
Merck KGaA.
Cell culture and treatments
Sources and authentication of HCT116 colon cancer
cells, their p53- or p21-knockout variants, H460 and A549 lung
cancer cells have been described previously (17,18).
Cells were regularly screened for mycoplasma contamination using
MycoAlert Mycoplasma Detection Kits (Lonza, Switzerland). Cells
were cultured in McCoy's 5A (HCT116 cells and their variants) or
RPMI-1640 medium (H460 and A549 cells) supplemented with 10%
heat-inactivated FBS. For gene knockdown, siRNAs targeting the
specified genes were introduced into cells using Lipofectamine
RNAiMAX (Invitrogen; Thermo Fisher Scientific, Inc.). The treated
cells were used for experiments after 24 h of recovery.
Western blot analysis
Cell lysates, prepared via a previously described
method (19), were subjected to
SDS-PAGE. The resolved proteins were electrotransferred onto
nitrocellulose membranes (Millipore) and analyzed using the
indicated antibodies and an ECL detection system (Bio-Rad)
(19).
Quantitative real-time PCR
Total RNA was extracted using the Hybrid-R RNA
preparation kit (GeneAll Biotechnology, Korea) and reverse
transcribed into cDNA using an RT-for-PCR kit (GeneAll
Biotechnology). Quantitative real-time PCR was carried out using
the SYBR-Green Real-time PCR Premix (Enzynomics, Korea) using the
following primers: Mdm2, 5′-TAGTATTTCCCTTTCCTTTGATGA-3′ and
5′-CACTCTCCCCTGCCTGATAC-3′; Slug, 5′-CATGCCTGTCATACCACAAC-3 and
5′-GGTGTCAGATGGAGGAGGG-3′; GAPDH, 5′-CATCTCTGCCCCCTCTGCTGA-3′ and
5′-GAATGACCTTGCCCAGAGCCT-3′. The results of real-time PCR
amplifications were analyzed using an IQ-5 Real-Time System
(Bio-Rad). GAPDH was used as an internal control for
normalization.
Data presentation and statistical
analysis
The results shown in this study are representative
of at least three independent experiments. Statistical significance
(P<0.05) of the graphic data was determined using
Student's t-test or two-way ANOVA with Sidak's multiple
comparisons test (GraphPad Software).
Results
Slug decreases the cellular levels of
p53 and p21
Considering that p53 and p21 cooperate to promote
Slug protein degradation (9), cells
may have established a system for Slug to counter such actions of
p53 and p21. To investigate this possibility, we knocked down Slug
expression in HCT116 colon cancer cells using two sets of specific
siRNAs. Slug knockdown using either siRNA resulted in an increase
in the cellular levels of p53 and p21, as analyzed by western
blotting (Fig. 1), suggesting that
Slug reduces the levels of p53 and p21 proteins in the cells.
Hereafter, only si-Slug-1 was used and is referred to as Slug
siRNA.
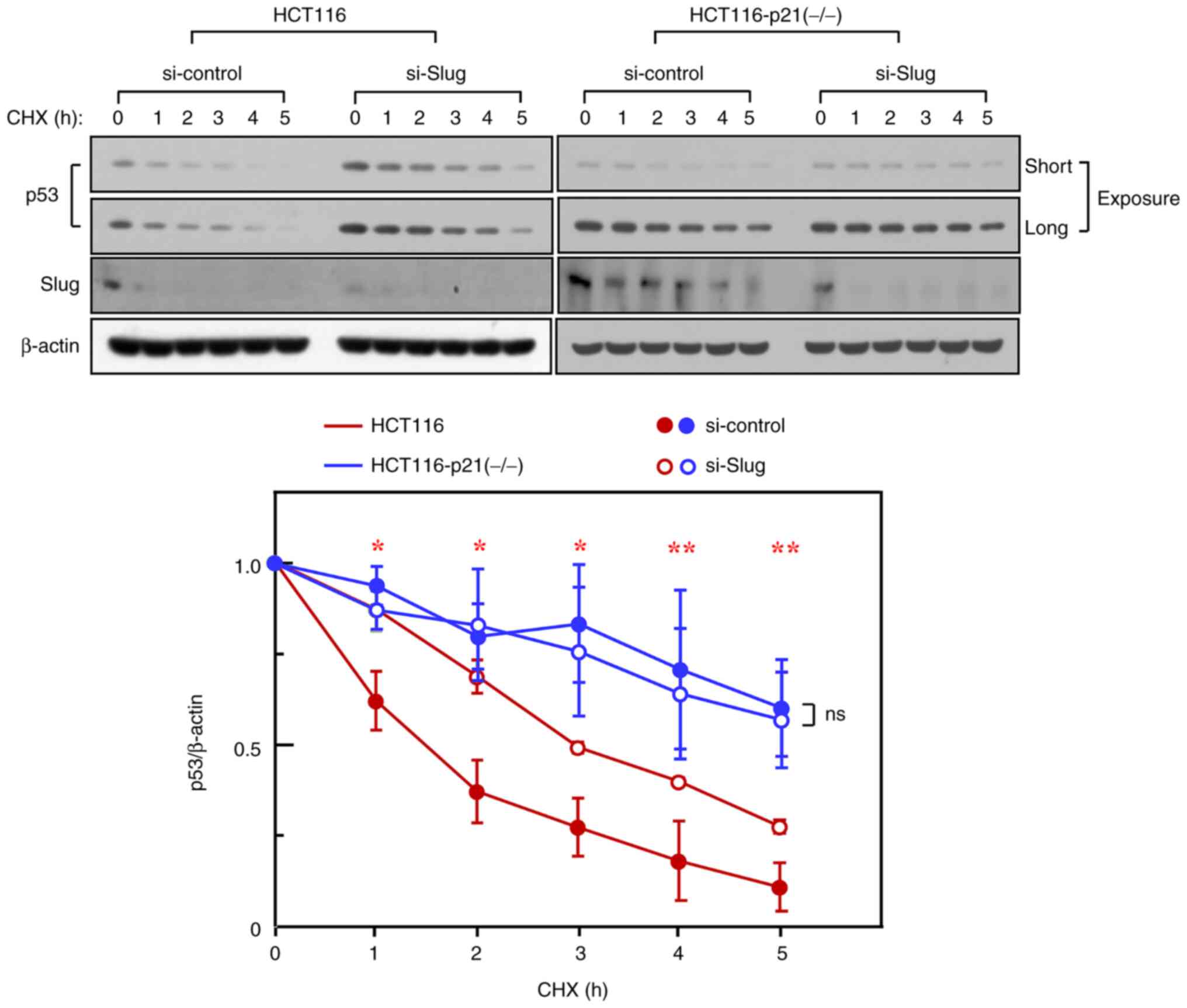
Slug reduces p53 protein stability in
a p21-dependent manner
Given that the cellular levels of p53 are controlled
mainly via the regulation of its protein stability (10–12), the
reduction in p53 levels by Slug might reflect the ability of Slug
to reduce p53 protein stability. To investigate this possibility,
HCT116 cells were incubated in the presence of cycloheximide, an
inhibitor of protein synthesis. The results showed the subsequent
reduction of p53 protein, thereby indicating its degradation. The
degradation of p53 was effectively reduced upon Slug knockdown
(Fig. 2), suggesting that Slug
promotes p53 protein degradation.
We have previously reported that p53 and p21 can
form a complex via their direct interaction (9,20). In
this regard, it was interesting to speculate whether p21 influences
Slug-induced p53 degradation. To address this question, we used
HCT116 p21-knockout variant cells. The stability of p53 protein in
these variants was much higher than that in HCT116 parental cells,
as reported recently (15). Notably,
in contrast to HCT116 parental cells, Slug knockdown in
p21-knockout cells did not further increase p53 stability (Fig. 2), indicating that Slug requires p21
to promote p53 degradation.
Slug reduces p21 protein stability in
a p53-independent manner
We next investigated the possible influence of Slug
on p21 protein stability and the probable requirement of p53 for
such an action of Slug. HCT116 parental cells and their
p53-knockout variants were used for this purpose. Slug knockdown
increased p21 protein stability in the parental cells, indicating
that Slug also promotes p21 degradation. However, in contrast to
the effect of p21 knockout on p53 stability, p53 knockout did not
significantly influence the basal stability of p21 as well as the
Slug knockdown-induced increase in p21 stability (Fig. 3). Therefore, Slug does not require
p53 to promote p21 degradation.
Slug requires Mdm2 to reduce p53 and
p21 levels
Since Mdm2 is involved in the degradation of both
p53 and p21 (10–14), we investigated whether Mdm2 plays a
role in Slug-induced degradation of p53 and p21. Towards this, we
knocked down Mdm2 in HCT116 cells. As expected, Mdm2 knockdown
enhanced the levels of p53 and p21 (Fig.
4A, lanes 1 and 3). However, the levels of both proteins were
not further increased upon the additional knockdown of Slug
(Fig. 4A, lanes 3 and 4). This
observation is in contrast with the ability of Slug knockdown to
increase p53 and p21 levels in control cells (Fig. 4A, lanes 1 and 2), suggesting that
Slug requires Mdm2 to promote p53 and p21 degradation.
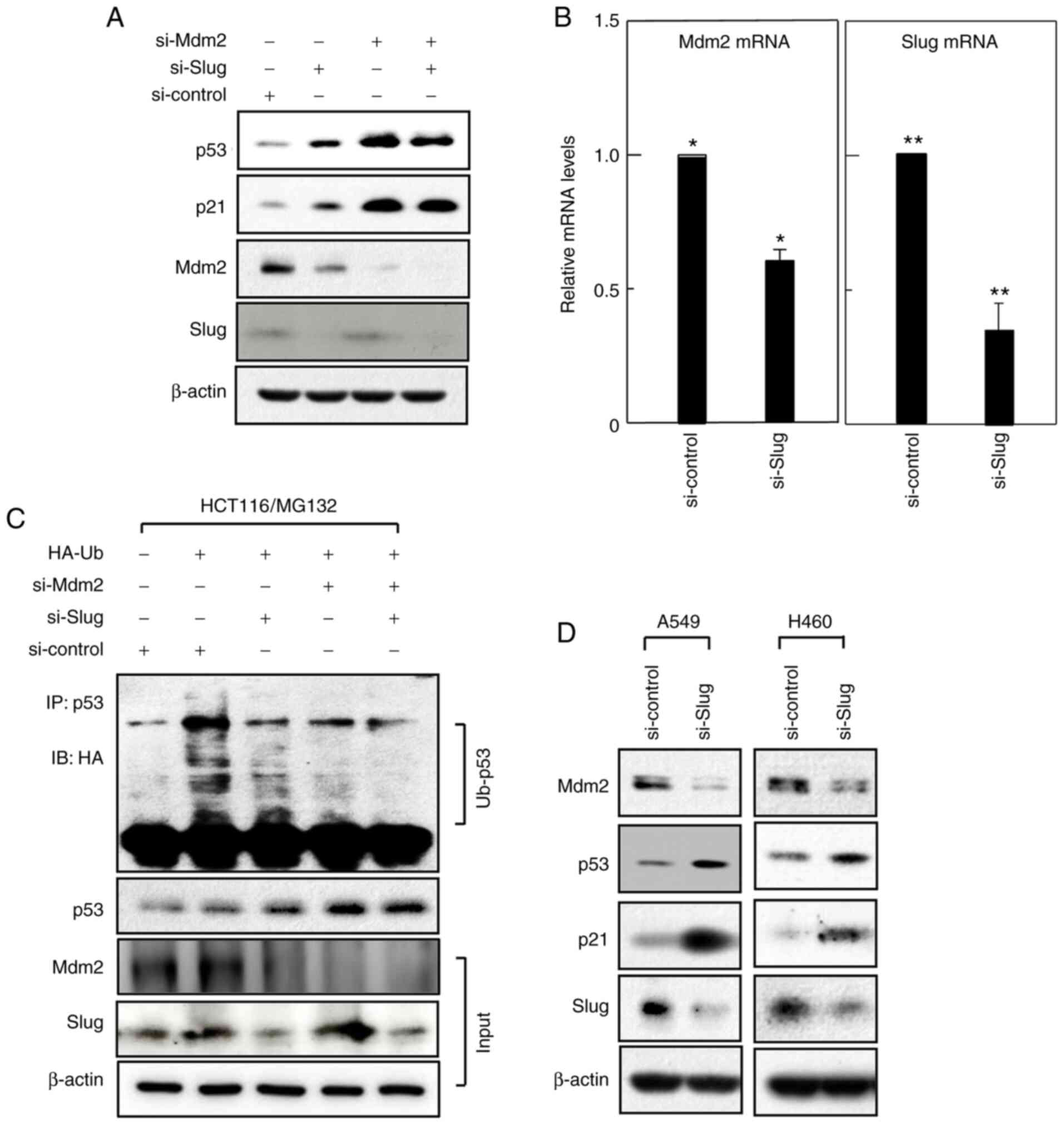 | Figure 4.Slug increases Mdm2 expression. (A)
HCT116 cells were treated with siRNAs targeting Slug or Mdm2 in the
combination indicated. After 24 h of incubation, expression levels
of the specified proteins were compared using western blotting. (B)
Mdm2 and Slug mRNA levels in HCT1116 cells, treated with control or
Slug siRNA, were compared by reverse transcription-quantitative
PCR. (C) HCT116 cells were transfected with the expression vector
for HA-tagged ubiquitin along with siRNAs targeting Slug or Mdm2 in
the indicated combinations. The transfectants were treated with
MG132 (10 µM) for 2 h. The precipitated proteins were analyzed by
western blotting using anti-HA and anti-p53 antibodies. Effects of
siRNAs were confirmed by analyzing cell lysates using anti-Mdm2 and
anti-Slug antibodies (input). (D) A549 and H460 cells were treated
with control or Slug siRNA for 24 h. Levels of the indicated
proteins were compared. *P<0.005, si-control vs. si-Slug for
Mdm2 mRNA. **P<0.05, si-control vs. si-Slug for Slug mRNA.
siRNA, small interfering RNA; Slug, zinc finger protein SNAI2;
HA-Ub, HA-ubiquitin; IP, immunoprecipitation; IB,
immuonoblotting. |
Slug induces Mdm2 expression
While analyzing Mdm2 expression, we unexpectedly
observed that Slug knockdown reduced Mdm2 protein levels (Fig. 4A). This led us to investigate whether
Slug also influences Mdm2 mRNA levels. Analysis by quantitative
real-time PCR revealed that Mdm2 mRNA levels in HCT116 cells were
significantly reduced upon Slug knockdown (Fig. 4B), suggesting that Slug induces Mdm2
expression. These findings also suggest that Slug promotes p53 and
p21 degradation by inducing Mdm2.
Slug increases p53 ubiquitination
While Mdm2 promotes p21 degradation without
ubiquitinating p21 (13,14), Mdm2-mediated ubiquitination of p53 is
required for p53 degradation (10–12).
Therefore, to further confirm the involvement of Mdm2 in the
actions of Slug, we investigated whether Slug also influences p53
ubiquitination. As expected, levels of p53 ubiquitination were
greatly reduced upon Mdm2 knockdown (Fig. 4C), consistent with the fact that Mdm2
is a major E3 ligase of p53. Notably, Slug knockdown also
effectively reduced p53 ubiquitination, indicating the ability of
Slug to promote p53 ubiquitination. These data support the notion
that Mdm2 can act downstream of Slug.
Slug influences Mdm2, p53, and p21
levels in multiple cell types
To investigate whether Slug regulates Mdm2, p53, and
p21 levels in other cell types, we knocked down Slug in A549 and
H460 lung cancer cells. As in the case of HCT116 cells, Slug
knockdown decreased Mdm2 levels and increased p53 and p21 levels
(Fig. 4D). Therefore, the ability of
Slug to induce Mdm2 expression and thereby promote p53 and p21
degradation may not be confined to a particular cell type.
Discussion
Our studies using HCT116 colon cancer cells have
shown that Slug reduces the cellular levels of p53 and p21 by
decreasing their protein stability. This phenomenon appears to be
mediated by Mdm2, since Slug increases Mdm2 expression and the
prevention of this event by Mdm2 knockdown abolishes the ability of
Slug to downregulate p53 and p21. Considering these results, we
propose that Slug induces Mdm2, which in turn, acts on p53 and p21,
promoting their degradation, at least in HCT116 cells. However, the
application of this model to other cell types may be possible
because we further verified the ability of Slug to increase and
decrease Mdm2 and p53/p21 levels, respectively, using A549 and H460
lung cancer cells.
We have recently reported that Mdm2 acts more
efficiently on p53 in the p53/p21 complex than p53 alone (15). In this regard, our finding that Slug
requires p21 for inducing p53 degradation is consistent with the
view that Mdm2 mediates Slug-induced p53 degradation. This view was
further supported by the finding that Slug promotes p53
ubiquitination. In sharp contrast, we found that p53 is dispensable
for Slug-induced p21 degradation, suggesting that Mdm2 can act on
p21 in the absence of p53. The ability of Mdm2 to induce p21
degradation in p53-null cells has also been reported by other
investigators (14). Notably, our
direct comparative analysis of p21 stability in control and
p53-knockout cells revealed that the presence of p53 does not
further facilitate the ability of Slug (i.e., Mdm2 in this case) to
induce p21 degradation. Our model for the role of Mdm2 in
Slug-induced p53 and p21 degradation is schematically depicted in
Fig. 5.
It should be noted that we verified the ability of
Slug to induce Mdm2 expression by knocking down Slug. To further
confirm this, we overexpressed Slug in HCT116 cells. However, this
treatment did not noticeably influence the levels of Mdm2 and p53,
as analyzed by western blotting (data not shown). Although the
reason for these unexpected results is not clear, it may reflect
that Slug is constitutively expressed in the cells to functionally
saturated levels. Slug has been consistently reported to be
upregulated in numerous cancer cells (3–7). The
constitutive upregulation of Slug may be the reason that in sharp
contrast to Slug knockdown, further elevation of Slug levels does
not markedly influence its downstream target molecules.
Mdm2 is generally considered as an oncogenic protein
since it degrades tumor suppressors such as p53 and p21 (21). However, the targets of Mdm2 are not
confined to cellular proteins, and Mdm2 can also bind to mRNAs. For
example, we showed that Mdm2 can stabilize Slug mRNA by binding to
it (22). This observation, along
with the ability of Slug to increase Mdm2 mRNA levels suggests the
existence of an amplification loop between Slug and Mdm2 at least
at the mRNA level to activate the process leading to p53 and p21
degradation (Fig. 5).
It is well known that Mdm2 is a transcriptional
target of p53 (23). This raises the
question as to why a tumor suppressor, such as p53, induces the
expression of an oncogenic protein, such as Mdm2. Notably, Mdm2
does not always act as an oncogenic component and often performs
contrasting functions by contributing to tumor suppression
(24). For example, as stated
earlier, Mdm2 can induce the proteolytic degradation of Slug, which
is promoted by the action of the p53/p21 complex (8,9)
(Fig. 5). Moreover, Mdm2 can
facilitate the translocation of p53 from the nucleus to the cytosol
(25,26), where p53 binds to Bcl-2 family
proteins, suppressing and promoting cancer cell invasion and
apoptosis, respectively, depending on the stress context (17,27–29). The
dual role of Mdm2 is consistent with the induction of its
expression by components with contrasting functions, such as p53
and Slug.
Given that Slug can act as a transcriptional
repressor (2), Slug may reduce
levels of p53 and p21 proteins not only by decreasing their
stability but also by downregulating their mRNA levels. Although
this latter possibility was beyond the scope of this study, future
investigation of this possibility may provide a global view of the
regulation of p53 and p21 levels by Slug.
In conclusion, we have demonstrated the ability of
Slug to promote p53 and p21 degradation. Given that p53 and p21 can
also promote Slug degradation (9),
the balance between these two opposing components (p53/p21 vs.
Slug) may be critical for cells to adapt to changes in the external
environment and internal physiology, and the failure to do so may
contribute to tumor initiation and progression.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from The National
Research Foundation of Korea (grant no. 2019R1A2C2084508) and The
Korea Institute of Radiological and Medical Sciences, funded by the
Ministry of Science and ICT, Republic of Korea (grant no.
50531-2021).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
JK, JL, UK, JKP and HDU performed the experiments
and interpretated the results. HDU conceived and designed the
current study and wrote the manuscript. All authors have read and
approved the final manuscript. JK and HDU confirm the authenticity
of all raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
siRNA
|
small-interfering RNA
|
References
|
1
|
Serrano M and Massagué J: Networks of
tumor suppressors. Workshop: Tumor suppressor networks. EMBO Rep.
1:115–119. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nieto MA: The snail superfamily of
zinc-finger transcription factors. Nat Rev Mol Cell Biol.
3:155–166. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Uygur B and Wu WS: SLUG promotes prostate
cancer cell migration and invasion via CXCR4/CXCL12 axis. Mol
Cancer. 10:1392011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Castro Alves C, Rosivatz E, Schott C,
Hollweck R, Becker I, Sarbia M, Carneiro F and Becker KF: Slug is
overexpressed in gastric carcinomas and may act synergistically
with SIP1 and snail in the down-regulation of E-cadherin. J Pathol.
211:507–515. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Uchikado Y, Natsugoe S, Okumura H,
Setoyama T, Matsumoto M, Ishigami S and Aikou T: Slug Expression in
the E-cadherin preserved tumors is related to prognosis in patients
with esophageal squamous cell carcinoma. Clin Cancer Res.
11:1174–1180. 2005.PubMed/NCBI
|
|
7
|
Alves CC, Carneiro F, Hoefler H and Becker
KF: Role of the epithelial-mesenchymal transition regulator slug in
primary human cancers. Front Biosci. 14:3035–3050. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang SP, Wang WL, Chang YL, Wu CT, Chao
YC, Kao SH, Yuan A, Lin CW, Yang SC, Chan WK, et al: p53 controls
cancer cell invasion by inducing the MDM2-mediated degradation of
Slug. Nat Cell Biol. 11:694–704. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim J, Bae S, An S, Park JK, Kim EM, Hwang
SG, Kim WJ and Um HD: Cooperative actions of p21WAF1 and p53 induce
Slug protein degradation and suppress cell invasion. EMBO Rep.
15:1062–1068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee JT and Gu W: The multiple levels of
regulation by p53 ubiquitination. Cell Death Differ. 17:86–92.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marine JC and Lozano G: Mdm2-mediated
ubiquitylation: p53 and beyond. Cell Death Differ. 17:93–102. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wade M, Wang YV and Wahl GM: The p53
orchestra: Mdm2 and Mdmx set the tone. Trends Cell Biol.
20:299–309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jin Y, Lee H, Zeng SX, Dai MS and Lu H:
MDM2 promotes p21waf1/cip1 proteasomal turnover independently of
ubiquitylation. EMBO J. 22:6365–6377. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Z, Wang H, Li M, Agrawal S, Chen X
and Zhang R: MDM2 is a negative regulator of p21WAF1/CIP1,
independent of p53. J Biol Chem. 279:16000–16006. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee J, Kim J, Kim EM, Kim U, Kang AR, Park
JK and Um HD: p21WAF1/CIP1 promotes p53 protein degradation by
facilitating p53-Wip1 and p53-Mdm2 interaction. Biochem Biophys Res
Commun. 543:23–28. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bunz F, Dutriaux A, Lengauer C, Waldman T,
Zhou S, Brown JP, Sedivy JM, Kinzler KW and Vogelstein B:
Requirement for p53 and p21 to sustain G2 arrest after DNA damage.
Science. 282:1497–1501. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim EM, Jung CH, Kim J, Hwang SG, Park JK
and Um HD: The p53/p21 complex regulates cancer cell invasion and
apoptosis by targeting Bcl-2 family proteins. Cancer Res.
77:3092–3100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jung CH, Kim EM, Song JY, Park JK and Um
HD: Mitochondrial superoxide dismutase 2 mediates
γ-irradiation-induced cancer cell invasion. Exp Mol Med. 51:1–10.
2019. View Article : Google Scholar
|
|
19
|
Kim DK, Cho ES, Seong JK and Um HD:
Adaptive concentrations of hydrogen peroxide suppress cell death by
blocking the activation of SAPK/JNK pathway. J Cell Sci.
114:4329–4334. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim EM, Kim J and Um HD: Bcl-2 protein
targeting by the p53/p21 complex-response. Cancer Res.
78:2772–2774. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao Y, Yu H and Hu W: The regulation of
MDM2 oncogene and its impact on human cancers. Acta Biochim Biophys
Sin (Shanghai). 46:180–189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jung CH, Kim J, Park JK, Heang SG, Moon
SK, Kim WJ and Um HD: Mdm2 increases cellular invasiveness by
binding to and stabilizing the Slug MRNA. Cancer Lett. 335:270–277.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mello SS and Attardi LD: Deciphering p53
signaling in tumor suppression. Curr Opin Cell Biol. 51:65–72.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Manfredi JJ: The Mdm2-p53 relationship
evolves: Mdm2 swings both ways as an oncogene and a tumor
suppressor. Genes Dev. 24:1580–1599. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boyd SD, Tsai KY and Jacks T: An intact
HDM2 RING-finger domain is required for nuclear exclusion of p53.
Nat Cell Biol. 2:563–568. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Geyer RK, Yu ZK and Maki CG: The MDM2
RING-finger domain is required to promote p53 nuclear export. Nat
Cell Biol. 2:569–573. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim EM, Park JK, Hwang SG, Kim WJ, Liu ZG,
Kang SW and Um HD: Nuclear and cytoplasmic p53 suppress cell
invasion by inhibiting respiratory complex-I activity via Bcl-2
family proteins. Oncotarget. 5:8452–8465. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mihara M, Erster S, Zaika A, Petrenko O,
Chittenden T, Pancoska P and Moll UM: p53 has a direct apoptogenic
role at the mitochondria. Mol Cell. 11:577–590. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chipuk JE, Kuwana T, Bouchier-Hayes L,
Droin NM, Newmeyer DD, Schuler M and Green DR: Direct activation of
bax by p53 mediates mitochondrial membrane permeabilization and
apoptosis. Science. 303:1010–1014. 2004. View Article : Google Scholar : PubMed/NCBI
|