Introduction
Acute myeloid leukemia (AML) is a genetically
heterogeneous and highly aggressive hematological malignancy with
an average onset age of 67 years (1). Patients with AML harbor distinct
genetic and molecular abnormalities caused by various chromosomal
aberrations in about 50–60% of de novo AML cases and 80–95%
of secondary cases (2). Classically,
mutated genes in AML are classified according to their
characteristics, and the gene group that affects cell proliferation
includes KIT, FLT3, and RAS (3,4). MAPK,
induced Ras signaling, is a major oncogenic pathway in AML.
Alterations in RAS are involved in the
progression of various cancers. Missense RAS mutations
causing a gain-of-function phenotype occur in 25% of human cancers
(5) and 20–25% of AMLs. KRAS
and NRAS mutations are present in 5 and 11% of AMLs,
respectively (6). Patients with
RAS-mutated AML have shorter overall survival and AML-free
survival, higher median age, and lower complete remission rates
(7). In addition, abnormalities in
Ras proteins that regulate various functions, including growth,
migration, adhesion, survival, and differentiation in normal cells,
affect leukemia primarily and result in secondary effects such as
chemotherapy resistance and cancer recurrence (8–11).
Therefore, the RAS status of a patient has prognostic value
and is a potent cancer biomarker (12–15).
However, it has been challenging to target Ras directly. While
drugs targeting the downstream signaling pathway, including RAF,
mitogen-activated protein kinase/ERK (extracellular
signal-regulated kinase) kinase (MEK), are being developed mainly
for solid tumors (12,16–19),
none of them are proven successful (20–22).
Developed as a pan-RAF inhibitor of RAF, downstream of Ras,
LY3009120 inhibits the activity of all RAF isoforms (A-RAF, B-RAF,
and c-RAF) and RAF dimers, and it is currently in the first phase
of clinical trials for solid tumors (23).
Therefore, in this study, using RAS mutant
AML cells, we investigated the synergistic effects of MAPK
inhibitors in combination with low-dose cytarabine or azacytidine,
currently used as first-line treatments for AML in elderly
patients.
Materials and methods
Cell culture and treatment
The three AML cell lines (HL-60, NB4, and KG-1) used
in the study were obtained from the American Type Culture
Collection (ATCC) and the German Collection of Microorganisms and
Cell Cultures GmbH (DSMZ). KG-1 and HL-60 cell lines were
authenticated using the AmpFISTR®
Identifiler® PCR Amplification Kit and the NB4 cell line
using the PowerPlex®21 System Kit. Cells were cultured
in RPMI-1640 medium supplemented with 10% heat-inactivated fetal
bovine serum (Gibco) and 1% penicillin/streptomycin (Gibco) and
incubated overnight at 37°C in 5% CO2. Cells were seeded
for 24 h and treated with cytarabine, azacytidine, LY3009120,
LXH254, dabrafenib, and trametinib. For single treatment, each
inhibitor was treated at indicated concentrations ranging from
0.001 to 10 µM for 72 h. After that, the combined treatment was
treated with cytarabine 50 nM or/and LY3009120 6/50 nM
concentration for 72 h. Each experiment was repeated five
times.
Patient samples
Bone marrow mononuclear cells (MNCs) from patients
with AML were isolated using the Ficoll gradient method and
cryopreserved in Cell banker 2, a serum-free medium. The MNCs of
the patients were thawed at 37°C in Iscove's modified Dulbecco's
media containing 10% fetal bovine serum and incubated for 24 h.
Agents and antibodies
Cytarabine (Cytosar-U®, Ara-C,
Arabinosylcytosine); the pan-RAF inhibitors, LY3009120 and LXH254;
the B-RAF inhibitor, dabrafenib; and the MEK inhibitor, trametinib
were purchased from Selleck Chemicals, and azacitidine was procured
from Sigma-Aldrich. The following antibodies were purchased from
Cell Signaling Technology: phospho-c-RAF (Ser338; cat. no. 9427),
c-RAF (cat. no. 9422), B-RAF (cat. no. 9434), phospho-MEK1/2
(Ser217/221; cat. no. 9121), MEK1/2 (cat. no. 9122), phospho-p44/42
mitogen-activated protein kinase (MAPK; Erk1/2; Thr202/Tyr204; cat.
no. 9101), p44/42 MAPK (Erk1/2; cat. no. 4696), caspase-3 (cat. no.
9662), caspase-9 (cat. no. 9502), and poly(ADP-ribose) polymerase
(PARP; cat. no. 9542). GAPDH (SC-25743) antibodies were purchased
from Santa Cruz Biotechnology. The secondary antibodies, goat
anti-rabbit IgG (H+L) and goat anti-mouse IgG (H+L), were purchased
from Jackson ImmunoResearch Laboratories.
Proliferation assay
Cells were cultured in 96-well plates for 1 day and
treated with each drug six times for 72 h. Cell proliferation was
assessed using the Quanti-Max WST-8 Cell Viability Assay kit
(Biomax). DMSO was used as a control for all experiments. The
optical density of each well was measured at 450 nm using a
microplate reader (Molecular Devices) (24). Each experiment was repeated five
times.
Western blotting
Protein concentrations were measured using the Micro
BCA™ Protein Assay Kit (Thermo Fisher Scientific). Equal amounts of
protein (15 µg) were loaded on 10 and 12% polyacrylamide gels
containing SDS, transferred to PVDF membranes, and blocked for 1 h
in 5% skim milk. The membranes were incubated overnight at 4°C with
the appropriate primary antibody and then with the HRP-conjugated
secondary antibody for 1 h at 37°C. GAPDH was used as a control
marker in all the blots.
Cell cycle analysis
To investigate the cell cycle profiles of the
treated and untreated cells after the treatment, the cells were
collected, washed with DPBS, and fixed with 70% ethanol. The fixed
cells were treated with 0.4 mg/ml RNase (Promega) and 5 µl of 50
µg/ml propidium iodide (Invitrogen) (25). The stained cells were analyzed using
FACSCanto II (BD Biosciences) and the apoptotic cells were detected
using flow cytometry. For cell cycle analysis, flow cytometry data
was analyzed using ‘ModFit’ software. Each experiment was repeated
three times.
Apoptosis assay
Apoptosis induction after single or combined
treatment with cytarabine and LY3009120 was measured using the Dead
Cell Apoptosis Kit with Annexin V/FITC and PI (Thermo Fisher
Scientific), and DMSO was used as a control. Cells were collected
24 or 72 h after their respective treatment and stained with
Annexin V/PI dye (26). Each
experiment was repeated three times.
Statistical analysis
Statistical significance among the groups was
determined using GraphPad Prism version 5.0 (GraphPad Software
Inc.). Statistical analyses for the efficacy of the drugs were
performed using the two-tailed unpaired Student's t-test. One-way
ANOVA followed by Dunnett's post hoc test were used for multiple
comparisons. Combination index (CI) values were calculated using
the Chou-Talalay equation (27). CI
values were used to validate combinatorial effects between the two
drugs; CI>1.00 indicates antagonism, CI=1.00 indicates
additivity, and CI<1.00 indicates synergism (28). Western blot bands were quantified
using ImageJ software (1.53a), and comparisons between the two
groups were analyzed using Student's t-test using a GraphPad.
*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. P<0.05
was considered to indicate a statistically significant
difference.
Results
Pan-Raf inhibitor specifically
inhibits proliferation of RAS-mutant AML cells
We evaluated the effects of cytarabine, azacytidine,
and the MAPK inhibitors, LY3009120, LXH254, dabrafenib, and
trametinib on the proliferation of three AML cell lines. To
determine whether the inhibitors had differential effects on the
AML cells with RAS mutations and those with wild-type (WT)
RAS, we treated the HL-60, NB4, and KG-1 cells with varying
concentrations of the inhibitors. We found a slight increase in
proliferation after treatment with low concentrations of all the
inhibitors except trametinib, but a dose-dependent decrease in all
cell lines (Fig. 1A-F)
(****P<0.0001). In addition, after treatment with 0.1 µM of the
pan-RAF inhibitors, LY3009120 and LXH254, the proliferation rate in
the RAS-mutated cells was significantly lower than that in
the WT RAS KG-1 cells (Fig. 1C
and D). In contrast, dabrafenib and trametinib treatments did
not show a difference in the proliferation rate of the
RAS-mutated and WT RAS-containing cells (Fig. 1E and F). The IC50 value for each
inhibitor can be found in Table
SI.
Treatment with a combination of
low-dose cytarabine and LY3009120 decreases cell proliferation in
RAS-mutated AML cells
After testing the individual effects of cytarabine,
azacitidine, and the MAPK inhibitors, we tested whether the
combination of either low-dose cytarabine or azacitidine with
pan-RAF inhibitors has a synergistic effect in RAS-mutated
AML cells. The dose of cytarabine was chosen based on that used in
clinical practice and previous studies (29–32). We
tested the combinations of low-dose cytarabine (0–70 nM) or
azacitidine (0–200 nM) with LY3009120 or LXH254 (0–100 nM) in
HL-60, NB4, and KG-1 cells (Figs. S1A
and B and S2A and B).
Analysis of the CI value for the combined treatment
of cytarabine and LY3009120 showed synergism in HL-60 and NB4 cells
(Table I). In the HL-60 cells, a
stronger synergistic effect was seen after combination treatment
with low-dose cytarabine and LY3009120 compared to that seen after
individual treatments. In the NB4 cells treated with the
combination, the proliferation rate was slightly reduced with mild
synergism. Furthermore, the inhibitory effect of the combination of
azacytidine with a pan-RAF inhibitor was not as prominent as that
of the cytarabine combination (Fig. S1A
and B). In comparison with each single treatment group,
antagonism was exhibited in the IC values under the combined
treatment conditions with no change in cell proliferation.
 | Table I.Evaluation of the synergistic effect
of low-dose cytarabine and LY3009120. |
Table I.
Evaluation of the synergistic effect
of low-dose cytarabine and LY3009120.
| A, HL-60
(NRAS Q61L) cells |
|---|
|
|---|
| Cytarabine, nM | LY3009120, nM | Fa | CI |
|---|
| 50 | 6.25 | 0.47 | 0.49 |
| 50 | 12.50 | 0.44 | 0.53 |
| 50 | 25.00 | 0.36 | 0.71 |
| 50 | 50.00 | 0.35 | 0.96 |
| 50 | 100.00 | 0.39 | 1.01 |
|
| B, NB4
(KRAS A18D) cells |
|
| Cytarabine,
nM | LY3009120,
nM | Fa | CI |
|
| 50 | 6.25 | N/A | N/A |
| 50 | 12.50 | N/A | N/A |
| 50 | 25.00 | 0.50 | 1.00 |
| 50 | 50.00 | 0.70 | 0.75 |
| 50 | 100.00 | 0.71 | 1.18 |
These results reveal that the two pan-RAF
inhibitors, LY3009120 and LXH254, specifically affect
RAS-mutated AML cells. However, they do not exhibit the same
synergism with cytarabine, and only LY3009120 shows a synergistic
effect with cytarabine in the RAS-mutated AML cell
lines.
Low-dose cytarabine in combination
with LY3002190 inhibits oncogenic MAPK signaling in RAS-mutated AML
cell lines
Next, we investigated whether the observed synergism
affected the Ras/RAF/MEK/ERK signaling pathways. Here, synergistic
doses were selected based on proliferation inhibition data
(Table I) and treated the HL-60 and
NB4 cells with cytarabine and/or LY3002190 for 24 h before
assessing the signaling pathway components by western blotting. The
combination treatments led to a significant decrease in the levels
of phosphorylated c-RAF in HL-60 cells (Fig. 2A), whereas the levels of p-c-RAF
increased in NB4 cells after single treatments by limited
paradoxical activation. Finally, the level of phosphorylated MEK
and ERK decreased with combined treatment in both the
RAS-mutated cells (Fig. 2A).
While the differences in the NB4 cells were not as statistically
significant as that in the HL-60 cells, a significantly reduced
value was confirmed (Fig.
2B)(*P<0.05).
 | Figure 2.Inhibition of the MAPK pathway
protein in RAS mutant cells following combined treatment
with LY3009120 and low-dose cytarabine. (A) NRAS-mutated
HL-60 and KRAS-mutated NB4 cells were treated with
cytarabine and/or LY3009120 for 24 h, and the levels of the MAPK
pathway molecules, including p-c-RAF, c-RAF, B-RAF, p-MEK1/2,
MEK1/2, p-ERK1/2 and ERK1/2, were analyzed via western blotting.
The HL-60 cell line was treated with 50 nM cytarabine, 6 nM
LY3009120 or 50 nM cytarabine + 6 nM LY3009120. The NB4 cell line
was treated with 50 nM cytarabine, 50 nM LY3009120 or 50 nM
cytarabine + 50 nM LY3009120. (B) Semi-quantification of protein
phosphorylation in HL-60 and NB4 cells. *P<0.05. p-,
phosphorylated. |
These results indicate that compared to the
individual drug treatments, the combined treatment with cytarabine
and LY3009120 showed a marked effect on the phosphorylation of
proteins required for active MAPK signaling.
Combined treatment with low-dose
cytarabine and LY3009120 results in cell cycle inhibition
To investigate whether the combination treatment
alters cell cycle progression in RAS-mutated AML cells, we
performed cell cycle analysis under the same conditions. Cells were
treated cytarabine, LY3009120, or a combination of both (as
described earlier) for 24 and 72 h, stained with PI, and analyzed
using flow cytometry.
Notably, in the HL-60 cells treated for 24 h, the
percentage of cells in the G0G1 phase increased while that of cells
in the S phases decreased after combined treatment. NB4 cells
showed a slight increase in the percentage G0G1 phase cells and
decrease in the S phase cells after combined treatment for 24 h
(Fig. 3A) (*P<0.05;
***P<0.001, ****P<0.0001).
In the HL-60 cells treated for 72 h, the percentage
of cells in the G0G1 phase increased remarkably and that of the
cells in the S phases decreased after the combined treatment
(Fig. 4A). In the NB4 cells treated
for 72 h, there was an increase in the G0G1 cells in the
combination treatment group as opposed to the individual treatment
groups, and there was a slight increase in the percentage of S
phase cells in the combination treatment group (Fig. 4A). Figs.
3B and 4B show that the cell
cycle arrest was statistically significant) (*P<0.05,
**P<0.01, ***P<0.001, ****P<0.0001).
In HL-60 cells, cell cycle inhibition was observed
after treatment for 24 h. Compared to the HL-60 cells, the NB4
cells showed weaker cell cycle arrest at 24/72 h. These results are
consistent with the results of the combined treatment observed in
the proliferation assays.
Combined treatment with low-dose
cytarabine and LY3009120 causes cell death in RAS-mutated AML
cells
We used Annexin V and PI staining and analyzed the
apoptotic HL-60 and NB4 cells after individual and combined
treatments with cytarabine and LY3002190 for 24 and 72 h. Of the
HL-60 cells analyzed after 24 h treatment, there was a slight
increase in the early apoptotic cells in the combined treatment
group and not in the control and individual treatment groups
(Fig. 5A). At 72 h after treatment
of the HL-60 cells, there was an increase in the early/late
apoptotic cells in the combined treatment group (Fig. 5B). In contrast, NB4 cells showed only
slight changes in the apoptotic cells at both 24 and 72 h (Fig. 5) (****P<0.0001).
Next, to identify the precise mechanism underlying
cell death, we examined the expression of molecules related to cell
death signaling. Specifically, we analyzed the cleavage of PARP,
caspase-3, and caspase-9. After co-treatment of cytarabine and
LY3009120 in HL-60 cells, the levels of caspase-3 and caspase-9
decreased and the level of cleaved PARP increased. In NB4 cells, no
change in caspase was seen, but PARP levels decreased and the
cleaved form increased in the combination treatment group (Fig. 6). We additionally confirmed changes
in autophagy-related molecules, but did not confirm the significant
difference of the combination treatment (Fig. S3).
These results and the immunoblotting data confirm
the underlying mechanisms of synergism in HL-60 cells. Furthermore,
immunoblotting analysis for NB4 cells showed a slight molecular
change consistent with the slight increase in apoptotic cells.
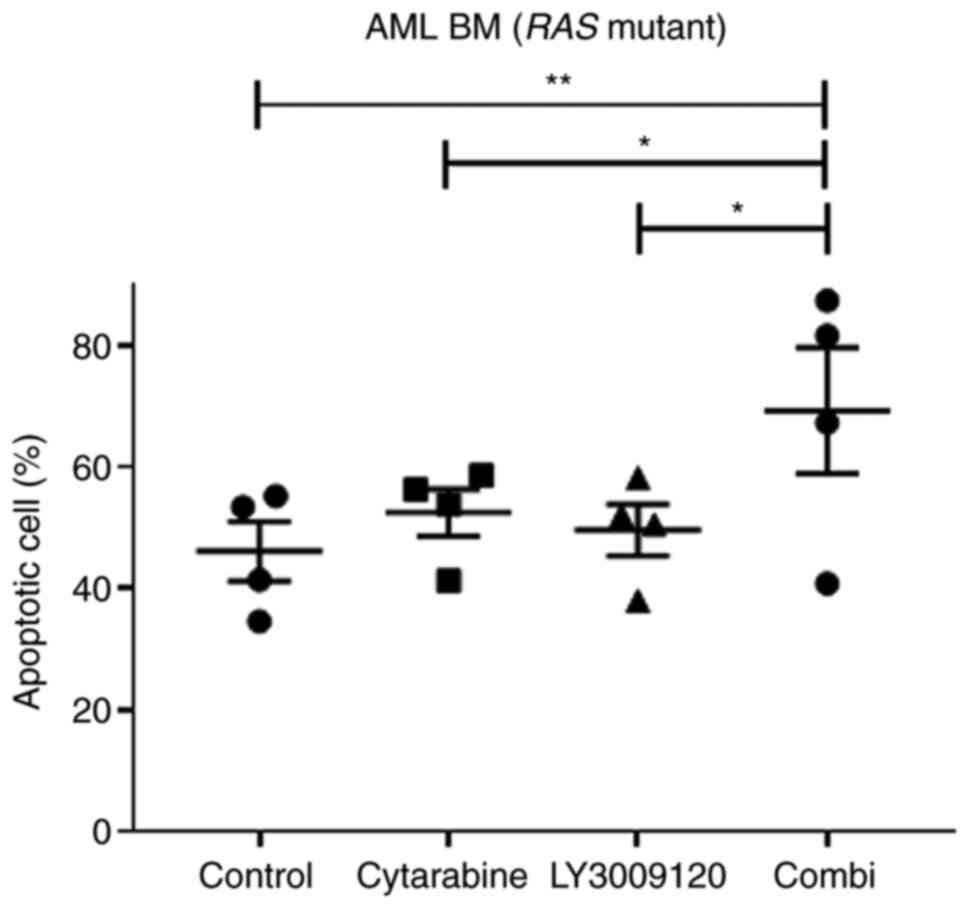
The combination treatment of low-dose
cytarabine and LY3009120 induced apoptosis in primary AML cells
bearing the RAS mutation
We tried to confirm the apoptosis effect of the
combination treatment of low-dose cytarabine and LY3009120 in
primary AML cells and HL-60 and NB4 cell lines. We analyzed
apoptosis in the primary cells of three patients with AML with
NRAS mutants and one patient with KRAS mutants
(Table SII). Even if it was
analyzed using a small number of samples, in the primary cells of
patients with AML with NRAS or KRAS mutations, the
ratio of apoptotic cells increased after the combination treatment
with the two drugs (Figs. 7 and
S4 and S5) (*P<0.05, **P<0.01).
These results confirm that the combination treatment
of low-dose cytarabine and LY3009120 could be a potential new
treatment for patients with AML with RAS mutations by
confirming its effect on primary cells as well as AML cell
lines.
Discussion
AML predominantly affects elderly people, and the
RAS mutation often results in a poor prognosis of AML. Since
cytarabine and azacitidine are commonly used to treat AML in the
elderly, we investigated the combined effect of these two drugs
with the pan-RAF inhibitor LY3009120 in AML cells carrying the
RAS mutations. The AML cells we used had several genetic
modifications, including RAS mutations, and the WT
RAS KG-1 cells are less sensitive to anticancer drugs. Thus,
in this study, we intended to confirm the combination effect of the
anticancer agents and the Raf inhibitor used for AML treatment and
confirmed the reduction of down-signaling by treatment with the Raf
inhibitor in all three cell lines. However, this drug combination
synergistically affects only the RAS-mutated AML cells, and
the synergism with cell cycle inhibition and increased apoptosis
was confirmed in the HL-60 cells with NRAS mutation. In the
NB4 cells with KRAS mutation, a slight cell cycle inhibition
was observed in terms of increased G0G1 phase cells, decreased S
phase cells, and increased apoptotic cells. Minimal effect was
observed in NB4 cells as opposed to the HL-40 cells; however, cell
cycle inhibition was observed after the 24 h treatment, which is a
relatively short time, and apoptosis induction was induced after
the 72 h treatment in NB4 cells. This indicates that the combined
treatment of drugs causes cell death after cell cycle arrest.
The pan-RAF inhibitor, LY3009120, inhibits all RAF
isoforms and binds to both protomers of the RAF dimer to prevent
phosphorylation of the downstream signaling molecules MEK and ERK,
with limited paradoxical activation in RAS-mutated cells
(23). Consistent with this, we
found a reduction in the levels of phosphorylated MEK and ERK in
cells treated with LY3009120, indicating that the RAF kinase
activity was impaired by the inhibitor, despite increased levels of
phosphorylated c-RAF at specific inhibitor concentrations in NB4
cells, a phenomenon that has been observed in other cells (33). High concentrations of inhibitors
induce nonspecific effects; however, compared with other MAPK
inhibitors, the two pan-RAF inhibitors showed a specific inhibitory
effect on RAS-mutated AML cells.
Moreover, CI analysis and analysis of levels of
various proteins in the signaling pathway revealed the synergistic
effect of cytarabine and LY3009120 in RAS-mutated AML cells.
The latter analysis showed that levels of phosphorylated MEK and
ERK were reduced in both RAS mutant cells, with changes in
ERK being the most prominent. In addition to the differences
between cell lines, we also found differences in the effects of the
pan-RAF inhibitors, LY3009120 and LXH254. However, both inhibitors
belong to the same family of type II RAF inhibitors. Only LY3009120
displayed a synergistic effect with low-dose cytarabine. We suggest
that this response is due to differences in the mechanism and/or
specificity of inhibition of the two agents. Although both the
pan-RAF inhibitors act on both protomers of the RAF dimer, LXH254
acts on both B- and c-RAF (from NCI) and LY3009120 inhibits the
kinase activity of all RAF isoforms (23). It is known that the cytotoxic
response of LY3009120 is specific for the RAS mutation, and
this response is less toxic to healthy bone marrow cells.
Furthermore, this drug effect persists without producing resistant
cells after drug treatment (34).
Therefore, the differences in the effects of these inhibitors
observed in combination with low-dose cytarabine could be
attributed to the mechanism of action of these inhibitors.
Although synergistic effects were observed in both
the AML cell lines studied, the effects were prominent in the
NRAS-mutated HL-60 cells, compared to KRAS-mutated
NB4 cells. Therefore, we further investigated the response of the
two cell lines to the RAF inhibitor LY3009120. In HL-60 cells, the
expression of phosphorylated ERK decreased in the LY3009120-treated
cells compared to the control cells. Conversely, in NB4 cells, the
expression of phosphorylated ERK and phosphorylated c-RAF increased
after LY3009120 treatment. Such paradoxical activity of ERK owing
to a RAF inhibitor is well known (35–37).
Therefore, we suggest that these paradoxical activities of
LY3009120 are reflected in the differences observed in the response
of HL-60 and NB4 cells to the combination therapy.
Despite this complex physiological phenomenon, our
immunoblotting data show that the combination of cytarabine and
LY3009120 has shown synergy in RAS mutant AML, and thus can
be considered a novel therapeutic strategy. In order to clearly
confirm the therapeutic effect, humanization experiments can show
clear results for going to a clinical trial, but further studies on
animal and patient-based studies are needed. Induction of specific
mutations and obtaining patient samples with RAS mutations
are not easy, so our in vitro study cannot determine the role of
the tumor microenvironment in actual patients, differences between
drugs, and the underlying mechanisms for cell-to-cell
reactivity.
Therefore, further studies are required on this
issue, and it would be interesting to test the synergy between
pan-RAF inhibitors and venetoclax, either with or without low-dose
cytarabine (38) in
RAS-mutated AML.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the National Research
Foundation of Korea (NRF; grant no. NRF-2014M3A6A4074727), Korea
Health Industry Development Institute (KHIDI; grant no. HI18C1876)
and Yuhan Corporation (grant no. 800-20180097).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JP acquired, analyzed and interpreted the data, and
drafted the manuscript. YK, HP, JMB, JH, DYS and SSY devised the
study concept and design, and provided technical guidance for all
aspects of the project. All authors discussed the results and
contributed to the final manuscript. SSY and YK confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was reviewed and approved by the
Institutional Review Board of Seoul National University Hospital
(approval no. IRB-1201-099-396; Seoul). Primary samples from
patients with acute myeloid leukemia were obtained with written
informed consent from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Almeida AM and Ramos F: Acute myeloid
leukemia in the older adults. Leuk Res Rep. 6:1–7. 2016.PubMed/NCBI
|
|
2
|
Papaemmanuil E, Gerstung M, Bullinger L,
Gaidzik VI, Paschka P, Roberts ND, Potter NE, Heuser M, Thol F,
Bolli N, et al: Genomic classification and prognosis in acute
myeloid leukemia. N Engl J Med. 374:2209–2221. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lagunas-Rangel FA, Chavez-Valencia V,
Gomez-Guijosa MA and Cortes-Penagos C: Acute myeloid
leukemia-genetic alterations and their clinical prognosis. Int J
Hematol Oncol Stem Cell Res. 11:328–339. 2017.PubMed/NCBI
|
|
4
|
Short NJ, Rytting ME and Cortes JE: Acute
myeloid leukaemia. Lancet. 392:593–606. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hobbs GA, Der CJ and Rossman KL: RAS
isoforms and mutations in cancer at a glance. J Cell Sci.
129:1287–1292. 2016.PubMed/NCBI
|
|
6
|
Ward AF, Braun BS and Shannon KM:
Targeting oncogenic Ras signaling in hematologic malignancies.
Blood. 120:3397–3406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kadia TM, Kantarjian H, Kornblau S,
Borthakur G, Faderl S, Freireich EJ, Luthra R, Garcia-Manero G,
Pierce S, Cortes J and Ravandi F: Clinical and proteomic
characterization of acute myeloid leukemia with mutated RAS.
Cancer. 118:5550–5559. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Steelman LS, Franklin RA, Abrams SL,
Chappell W, Kempf CR, Bäsecke J, Stivala F, Donia M, Fagone P,
Nicoletti F, et al: Roles of the Ras/Raf/MEK/ERK pathway in
leukemia therapy. Leukemia. 25:1080–1094. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Downward J: Targeting RAS signalling
pathways in cancer therapy. Nat Rev Cancer. 3:11–22. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kornblau SM, Womble M, Qiu YH, Jackson CE,
Chen W, Konopleva M, Estey EH and Andreeff M: Simultaneous
activation of multiple signal transduction pathways confers poor
prognosis in acute myelogenous leukemia. Blood. 108:2358–2365.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Steelman LS, Abrams SL, Whelan J, Bertrand
FE, Ludwig DE, Bäsecke J, Libra M, Stivala F, Milella M, Tafuri A,
et al: Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and
Jak/STAT pathways to leukemia. Leukemia. 22:686–707. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cox AD, Fesik SW, Kimmelman AC, Luo J and
Der CJ: Drugging the undruggable RAS: Mission possible? Nat Rev
Drug Discov. 13:828–851. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Coombs CC, Tallman MS and Levine RL:
Molecular therapy for acute myeloid leukaemia. Nat Rev Clin Oncol.
13:305–318. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kavanagh S, Murphy T, Law A, Yehudai D, Ho
JM, Chan S and Schimmer AD: Emerging therapies for acute myeloid
leukemia: Translating biology into the clinic. JCI Insight.
2:e956792017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu S, Jang H, Gu S, Zhang J and Nussinov
R: Drugging Ras GTPase: A comprehensive mechanistic and signaling
structural view. Chem Soc Rev. 45:4929–4952. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schmieder R, Puehler F, Neuhaus R, Kissel
M, Adjei AA, Miner JN, Mumberg D, Ziegelbauer K and Scholz A:
Allosteric MEK1/2 inhibitor refametinib (BAY 86-9766) in
combination with sorafenib exhibits antitumor activity in
preclinical murine and rat models of hepatocellular carcinoma.
Neoplasia. 15:1161–1171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dickson MA, Gordon MS, Edelman G, Bendell
JC, Kudchadkar RR, LoRusso PM, Johnston SH, Clary DO and Schwartz
GK: Phase I study of XL281 (BMS-908662), a potent oral RAF kinase
inhibitor, in patients with advanced solid tumors. Invest New
Drugs. 33:349–356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mendzelevski B, Ferber G, Janku F, Li BT,
Sullivan RJ, Welsch D, Chi W, Jackson J, Weng O and Sager PT:
Effect of ulixertinib, a novel ERK1/2 inhibitor, on the QT/QTc
interval in patients with advanced solid tumor malignancies. Cancer
Chemother Pharmacol. 81:1129–1141. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ryan MB and Corcoran RB: Therapeutic
strategies to target RAS-mutant cancers. Nat Rev Clin Oncol.
15:709–720. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Borthakur G, Popplewell L, Boyiadzis M,
Foran J, Platzbecker U, Vey N, Walter RB, Olin R, Raza A,
Giagounidis A, et al: Activity of the oral mitogen-activated
protein kinase kinase inhibitor trametinib in RAS-mutant relapsed
or refractory myeloid malignancies. Cancer. 122:1871–1879. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jain N, Curran E, Iyengar NM, Diaz-Flores
E, Kunnavakkam R, Popplewell L, Kirschbaum MH, Karrison T, Erba HP,
Green M, et al: Phase II study of the oral MEK inhibitor
selumetinib in advanced acute myelogenous leukemia: A University of
Chicago phase II consortium trial. Clin Cancer Res. 20:490–498.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maiti A, Naqvi K, Kadia TM, Borthakur G,
Takahashi K, Bose P, Daver NG, Patel A, Alvarado Y, Ohanian M, et
al: Phase II trial of MEK inhibitor binimetinib (MEK162) in
RAS-mutant acute myeloid leukemia. Clin Lymphoma Myeloma Leuk.
19:142–148. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Peng SB, Henry JR, Kaufman MD, Lu WP,
Smith BD, Vogeti S, Rutkoski TJ, Wise S, Chun L, Zhang Y, et al:
Inhibition of RAF isoforms and active dimers by LY3009120 leads to
anti-tumor activities in RAS or BRAF mutant cancers. Cancer Cell.
28:384–398. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Keum H, Kim TW, Kim Y, Seo C, Son Y, Kim
J, Kim D, Jung W, Whang CH and Jon S: Bilirubin nanomedicine
alleviates psoriatic skin inflammation by reducing oxidative stress
and suppressing pathogenic signaling. J Control Release.
325:359–369. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee SH, Kim DK, Seo YR, Woo KM, Kim CS and
Cho MH: Nickel (II)-induced apoptosis and G2/M enrichment. Exp Mol
Med. 30:171–176. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang HM, Chen CY and Wu PF:
Isophilippinolide A arrests cell cycle progression and induces
apoptosis for anticancer inhibitory agents in human melanoma cells.
J Agric Food Chem. 62:1057–1065. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chou TC: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao L, Au JL and Wientjes MG: Comparison
of methods for evaluating drug-drug interaction. Front Biosci
(Elite Ed). 2:241–249. 2010.PubMed/NCBI
|
|
29
|
Kufe DW, Griffin JD and Spriggs DR:
Cellular and clinical pharmacology of low-dose ara-C. Semin Oncol.
12 (Suppl 3):S200–S207. 1985.
|
|
30
|
Spriggs D, Griffin J, Wisch J and Kufe D:
Clinical pharmacology of low-dose cytosine arabinoside. Blood.
65:1087–1089. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen L, Guo P, Zhang Y, Li X, Jia P, Tong
J and Li J: Autophagy is an important event for low-dose cytarabine
treatment in acute myeloid leukemia cells. Leuk Res. 60:44–52.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Z, Chen J, Wang H, Ding K, Li Y, de
Silva A, Sehgal V, Burbano JL, Sundararaj R, Gamage J, et al:
Chidamide shows synergistic cytotoxicity with cytarabine via
inducing G0/G1 arrest and apoptosis in myelodysplastic syndromes.
Am J Transl Res. 9:5631–5642. 2017.PubMed/NCBI
|
|
33
|
Vakana E, Pratt S, Blosser W, Dowless M,
Simpson N, Yuan XJ, Jaken S, Manro J, Stephens J, Zhang Y, et al:
LY3009120, a panRAF inhibitor, has significant anti-tumor activity
in BRAF and KRAS mutant preclinical models of colorectal cancer.
Oncotarget. 8:9251–9266. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tambe M, Karjalainen E, Vähä-Koskela M,
Bulanova D, Gjertsen BT, Kontro M, Porkka K, Heckman CA and
Wennerberg K: Pan-RAF inhibition induces apoptosis in acute myeloid
leukemia cells and synergizes with BCL2 inhibition. Leukemia.
34:3186–3196. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hall-Jackson CA, Eyers PA, Cohen P,
Goedert M, Boyle FT, Hewitt N, Plant H and Hedge P: Paradoxical
activation of Raf by a novel Raf inhibitor. Chem Biol. 6:559–568.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hatzivassiliou G, Song K, Yen I,
Brandhuber BJ, Anderson DJ, Alvarado R, Ludlam MJ, Stokoe D, Gloor
SL, Vigers G, et al: RAF inhibitors prime wild-type RAF to activate
the MAPK pathway and enhance growth. Nature. 464:431–435. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
King AJ, Patrick DR, Batorsky RS, Ho ML,
Do HT, Zhang SY, Kumar R, Rusnak DW, Takle AK, Wilson DM, et al:
Demonstration of a genetic therapeutic index for tumors expressing
oncogenic BRAF by the kinase inhibitor SB-590885. Cancer Res.
66:11100–11105. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wei AH, Strickland SA Jr, Hou JZ, Fiedler
W, Lin TL, Walter RB, Enjeti A, Tiong IS, Savona M, Lee S, et al:
Venetoclax combined with low-dose cytarabine for previously
untreated patients with acute myeloid leukemia: Results from a
phase Ib/II study. J Clin Oncol. 37:1277–1284. 2019. View Article : Google Scholar : PubMed/NCBI
|