Introduction
Cervical cancer (CC) is the most common female
genital tract malignancy, and has an impact on the
psychophysiological health of female patients (1). CC ranks fourth in terms of incidence
and mortality rates among all malignancies in women globally; it
was estimated that there were 569.8 thousand newly diagnosed cases
of CC and 311.4 thousand deaths related to CC in 2018 in China
(2). Among the cases of mortality,
85% were found in developing countries (2,3). The
incidence of CC in China is ~10.30/100,000 individuals, and the
mortality is 2.62/100,000 individuals. The incidence and mortality
rates of CC rank the seventh and eighth among all female
malignancies, respectively in China (4). CC remains the primary disease
threatening the health and life of Chinese women.
Clinical staging of CC by the International
Federation of Gynecology and Obstetrics is still highly subjective
and has limitations (5). By using
this staging system, the high-risk factors influencing prognosis,
such as infiltration of the parametrium and lymphatic vessels,
vaginal involvement and lymph node metastases, are not easy to
distinguish and the heterogeneity is large (6,7). To
address the aforementioned limitations of FIGO staging system, MRI,
CT and PET/CT scans have been used as auxiliary diagnostic tools
for staging, efficacy observation and evaluation of failure mode
(an effective tool for risk assessment and improvement), prognosis
and efficacy of CC treatment (8–10).
18F-fluorodeoxyglucose (18F-FDG) positron
emission tomography/computed tomography (PET/CT) is a
state-of-the-art molecular imaging technique, which not only
provides precise anatomical information, but also detects the
metabolic changes of the tissues and organs at the molecular level.
This technique has been increasingly applied to the diagnosis and
treatment of CC (9,11,12). In
a previous systematic review and meta-analysis of 72 studies that
included 5,042 patients (13), the
diagnostic performance of PET/CT scan for lymph node metastases for
early stage CC was better than that of MRI and CT scans. The
diagnostic sensitivity and specificity of PET/CT scan were 74.7%
(95% CI, 63.3–84.0) and 97.6% (95% CI, 95.4–98.9), respectively.
The value of MRI and CT were 55.2% (95% CI, 49.2–61.7), 57.5% (95%
CI, 53.5–61.4) and 93.2% (95% CI, 91.4–94.0), 92.3% (95% CI,
91.1–93.5), respectively.
Serum tumor markers have been gradually introduced
in the early diagnosis of CC. Some genes are uniquely expressed in
different cancer types, and squamous cell carcinoma antigen
(SCC-Ag) is the most common marker for CC (14,15). A
number of studies have shown that serum SCC-Ag levels are
associated with staging, tumor size, degree of cervical invasion
and lymph node metastases in squamous cell carcinoma of the cervix
(16–19).
In the present study, a prospective follow-up
investigation was performed on patients who received treatment for
early metaphase CC. The follow-up lasted for 7 to 42 months. The
purpose of the study was to discuss the clinical value of PET/CT
combined with serum SCC-Ag in diagnosing postoperative recurrence
and metastases in patients with CC. The research findings shed new
light on an earlier, more accurate and more comprehensive detection
method for recurrent/metastatic CC lesions, and also on the
development of subsequent individualized treatment to improve the
survival rate and quality of life of patients with CC.
Patients and methods
Patients
The present study conformed to the Declaration of
Helsinki and was approved by the Ethics Committee of Southwest
Medical University (Luzhou, China). Early and middle stage patients
with CC who received treatment at The Affiliated Hospital of
Southwest Medical University between January 1st 2015 and December
31st 2017 and who had complete data were followed up. The inclusion
criteria were as follows: i) confirmed as CC by surgical pathology,
including squamous cell carcinoma, adenosquamous carcinoma and
adenocarcinoma of the cervix; ii) classified as stage IA-III B
according to the 2009 FIGO staging system (5); iii) IA-IIA patients were mainly treated
with surgery (extensive panhysterectomy with pelvic lymph node
dissection); iv) IIB-IIIB patients were mainly treated with
platinum-based chemotherapy or radiotherapy; v) no
contraindications for imaging, with consent for PET/CT; vi) no
other malignancies, and no diseases influencing serum SCC-Ag
levels, such as dermatological and respiratory diseases. The
exclusion criteria were as follows: i) distant metastases confirmed
before treatment; ii) patients who were pregnant or lactating. A
total of 273 patients were eligible and provided written informed
consent.
Methods and follow-up
All included patients were followed up once every 2
months in the first 6 months and then once every 3 months. The
follow-up was ≥6 months, with the last follow-up conducted in June
2019. During the follow-up, 19 patients left the study, including 9
patients who did not receive PET/CT scan, and 8 patients were lost
to follow-up. Thus, 246 patients were included in the final
results. A total of 90.11% of patients completed the follow-up, and
the median follow-up duration was 22 months (range, 7–42
months).
During each follow-up, the serum SCC-Ag level was
detected. For the first two follow-up visits, PET/CT was
immediately performed for patients suspected of tumor recurrence
and metastases based on clinical manifestations, gynecological
examination, transvaginal ultrasonography, routine imaging
examination or serum tumor marker determination. For those patients
who did not receive a PET/CT scan during the first two follow-up
visits, a PET/CT scan was performed in the third follow-up visit.
The flow diagram of the follow-up and study is shown in Fig. 1.
Serum SCC-Ag determination
The serum SCC-Ag level was detected at 3 days before
the PET/CT scan (regardless of whether such a test had been
performed previously). From each patient, 3 ml of venous blood was
collected, and the plasma was separated to harvest the serum
(placed in water bath for 30 min at 7°C and centrifuged at 4,000 ×
g for 10 min at 4°C). The serum SCC-Ag level was detected by using
a fully automated microplate reader (Abbott i-2000 automatic immune
analyzer) and quantitative assay kit for SCC-Ag (cat. no. CF051163;
Tellgen Corp.). The normal SCC-Ag range was 0–1.5 ng/ml. The
presence of recurrence or metastases was considered if SCC-Ag
>1.5 ng/ml.
PET/CT scan
The PET/CT scan was performed with the GEMINI TF16
PET/CT scanner (Philips Healthcare). 18F-FDG was
provided by HTA Co., Ltd., with radiochemical purity >95%.
Before the scan, the patients fasted for at least 6 h and required
to maintain a stationary status. Plasma glucose levels were
determined in the peripheral venous blood to ensure that the blood
glucose level was kept at 3.9–11.1 mmol/l. The contrast agent
(18F-FDG) was injected intravenously at a dose of
3.7–5.5 MBq/kg of body weight, and then the patients lay down to
rest in a quiet room for 45 to 60 min. During the rest, the
patients were told to drink purified water (~1,200 ml) and then
empty their bladder prior to the PET/CT scan. The patients took a
supine position on the examination couch. During the body scan, the
patients were told to fold the two forearms over the forehead, and
for the head scan (both upper limbs were flat on both sides of the
body during head scan), the patients were to breathe smoothly and
keep an immobile body position, so that the CT and PET images could
be fused perfectly. A low-dose plain CT scan was first performed
(voltage, 120 kV; current, 10 mA; rotation time of the bulb tube,
0.3 sec per round; slice thickness, 5 mm). The scan scope was from
the top of the skull to the middle and upper segments of the thigh.
Next, PET images (3D model) were collected and reconstructed.
Usually, each scan was performed at 6–8 bed positions (depending on
the height of patients); the collection time per bed position was
2–3 min for the body surface and 3–5 min for the brain.
The images were reviewed independently by two
nuclear medicine physicians with >15 years of experience. A
consensus was reached by discussion in cases where opinion
diverged. If the former 2 doctors had differing opinions, another
doctor (chief physician with >20 years of experience) made the
final decision. The quantitative analysis was performed with a
semi-quantitative indicator, the maximum standardized uptake value
(SUVmax), which is now widely used in the clinic.
Lesions with high 18F-FDG uptake were located compared
to that in comparable normal contralateral structures and/or
surrounding soft tissues. The region of interest was delineated
along the periphery of the lesions on the most clearly visualized
sections in terms of radioactivity uptake. The computer workstation
then automatically calculated the mean SUV (SUVmean) and
SUVmax. The presence of metastases and tumor recurrence
was considered if the SUVmax was ≥2.5. For patients with
multiple lesions, representative lesions were chosen to determine
the SUVmax. For the qualitative analysis, the existence
of residual or recurrent lesions was considered if soft-tissue
density shadows appeared in the primary site, with abnormally high
radioactivity uptake. In addition, if new lesions were revealed by
scans of the whole body and other positions, with abnormally high
radioactivity uptake, the presence of metastases was considered.
Typical PET/CT images of postoperative metastases and recurrence in
patients with CC are shown in Fig.
2; the images of those without postoperative metastases and
recurrence are shown in Fig. 3.
Combined diagnostic method
When PET/CT was combined with serum SCC-Ag
determination, any positive indicator was deemed positive for the
combined diagnostic method. If both tests were negative, no tumor
recurrence or metastasis was considered.
Gold standards
Criteria for metastases or recurrence of CC were as
follows: i) the patients received a secondary surgery or puncture,
and the diagnosis was confirmed by pathology; and ii) the patients
did not receive a secondary surgery or puncture, but a judgment was
made with reference to the follow-up results (medical history,
gynecological examination, cytological smear of vaginal stumps,
tumor markers and imaging). The patients with metastases or
recurrence received follow-up examinations for >6 months, and
the lesions increased in number or volume progressively. The
results of the PET/CT scan and serum SCC-Ag determination were
compared against the final clinical diagnosis made by surgical
pathology and follow-up. Those with positive results from the
PET/CT scan and serum SCC-Ag determination were considered
true-positive cases if tumor recurrence was confirmed within 3
months by surgical pathology; otherwise, they were regarded as
false-positive cases. If no lesions were found during the 3-month
follow-up for the negative patients, they were considered
true-negative cases, while the presence of lesions meant that they
were considered false-negative cases.
Statistical analysis
SPSS 20.0 software (IBM Corp.) was used to perform
the statistical analysis. For the baseline information of the
patients, the counts were expressed as means (percentages) and the
quantitative data were expressed as mean ± standard deviation. The
results of pathology and clinical follow-up were taken as the gold
standard for diagnosis. The consistency between the results of
different diagnostic methods and the gold standard was measured by
κ test. Κ value ≥0.7 indicated high consistency, 0.4–0.7 indicated
moderate consistency and <0.4 indicated low consistency. The
diagnostic sensitivity, specificity, positive predictive value and
negative predictive value were calculated for PET/CT, serum SCC-Ag
determination and the combined method. The accuracy [accuracy=(true
positive + true negative)/total sample size ×100%) and misdiagnosis
rate (misdiagnosis rate=false positive/true negative ×100%] of the
diagnostic results were calculated. The diagnostic results of the
three methods were compared by the χ2 test (McNemar
χ2 test was used for paired data and Pearson
χ2 test was used for independent sample data). To
account for multiple comparisons, Bonferroni correction was applied
to correct all the P-values. The diagnostic efficacy was compared
by the ROC curve analysis. CIs for area under the ROC curve values
were estimated on the basis of a 95% confidence level. The areas
under the ROC curve were compared by the Z test. The scatter plots
to establish the association between SUVmax and serum
SCC-Ag levels in the patients with CC were also drawn, and the
correlation between the two was analyzed by Pearson's correlation
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Baseline information of patients with
CC
The 246 patients with CC receiving treatment were
aged 39 to 73 years old, with a mean age of 51.4±10.2 years.
According to the FIGO staging system, there were 22 patients with
stage IA (8.9%), 45 patients with stage IB (18.3%), 39 patients
with stage IIA (15.9%), 55 patients with stage IIB (22.4%), 27
patients with stage IIIA (11.0%) and 58 patients with stage IIIB
(23.6%) disease. Pathology of the primary lesions confirmed
squamous cell carcinoma in 210 patients (85.4%), adenocarcinoma in
26 patients (10.6%) and adenosquamous carcinoma in 10 patients
(4.1%) (Table I).
 | Table I.Baseline information of cervical
cancer patients (n=246). |
Table I.
Baseline information of cervical
cancer patients (n=246).
| Characteristic | Value |
|---|
| Mean age (range),
years | 51.4±10.2
(29–73) |
| FIGO clinical
staging, n (%) |
|
| IA | 22 (8.9) |
| IB | 45
(18.3) |
|
IIA | 39
(15.9) |
|
IIB | 55
(22.4) |
|
IIIA | 27
(11.0) |
|
IIIB | 58
(23.6) |
| Pathological
classification, n (%) |
|
|
Squamous cell carcinoma | 210 (85.4) |
|
Adenocarcinoma | 26
(10.6) |
|
Adenosquamous carcinoma | 10 (4.1) |
| Tumor size, n
(%) |
|
| ≥4.6
cm | 130 (52.8) |
| <4.6
cm | 116 (47.2) |
| Metastases or
recurrence, n (%) |
|
|
Yes | 137 (55.7) |
| No | 109 (44.3) |
| PET/CT scan, n
(%) |
|
|
Positive | 126 (51.2) |
|
Negative | 120 (48.8) |
| Serum SCC-Ag
determination, n (%) |
|
|
Positive | 151 (61.4) |
|
Negative | 95
(38.6) |
There were 130 patients with primary lesions ≥4.6 cm
(52.8%) and 116 patients with primary lesions <4.6 cm
(47.2%).
During the follow-up, tumor recurrence or metastases
were confirmed in a total of 137 patients (55.7%), including 18
deaths. The average SUVmax was 7.17±6.88. PET/CT scan
revealed 126 positive results (51.2%). The average serum SCC-Ag
level was 5.68±4.68 ng/ml, and 151 patients (61.4%) were positive
according to serum SCC-Ag determination (Table I).
Comparison of the results of the three
diagnostic methods
The results of pathological examination and
follow-up were taken as the gold standard, and the diagnostic
results of the three methods (PET/CT scan, serum SCC-Ag
determination and the combination of both) were compared against
the gold standard. As shown in Table
II, with the PET/CT scan, 10 patients had a false-positive
diagnosis, with a misdiagnosis rate of 5.74%; 21 patients were
affected by a false-negative diagnosis, with a missed diagnosis
rate of 8.14%; and the accuracy of PET/CT scan method was 87.39%.
With the serum SCC-Ag detection method, 28 patients had a
false-positive diagnosis, with a misdiagnosed rate of 16.09%. A
false-negative diagnosis affected 14 patients, with the missed
diagnosis rate of 4.42%. The accuracy of this method was 82.92%.
For the combined method, 8 patients had a false-positive diagnosis,
with a misdiagnosis rate of 4.59%, while a false-negative diagnosis
affected 9 patients, with a missed diagnosis rate of 3.49%. The
accuracy of this combined method was 93.09%. The results of the
three diagnostic methods were compared against those of
pathological examination. According to the consistency test, the κ
coefficient was 0.747 for PET/CT scan, and 0.860 for the combined
method. The serum SCC-Ag method had general consistency (κ
coefficient <0.7). A statistically significant difference was
observed for both PET/CT and combined methods, indicating high
consistency of these two methods with the gold standard, indicating
that both methods have a high diagnostic value.
 | Table II.Comparison of postoperative
metastases and recurrence diagnosed by the PET/CT, serum SCC-Ag or
combined methods. |
Table II.
Comparison of postoperative
metastases and recurrence diagnosed by the PET/CT, serum SCC-Ag or
combined methods.
|
|
| Pathology and
follow-up |
|
|
|---|
|
|
|
|
|
|
|---|
| Examination
method | Examination
results | Positive, n | Negative, n | κ coefficient | P-value |
|---|
| PET/CT | Positive | 116 | 10 | 0.747 | <0.001 |
|
| Negative | 21 | 99 |
|
|
| Serum SCC-Ag | Positive | 123 | 28 | 0.649 | <0.001 |
|
| Negative | 14 | 81 |
|
|
| Combined
method | Positive | 128 | 8 | 0.860 | <0.001 |
|
| Negative | 9 | 101 |
|
|
Comparison of diagnostic efficacy of
the combined diagnostic method
Table III provides
the diagnostic efficacy of the three methods and the corresponding
95% CIs. It can be seen from the table that the diagnostic
sensitivity of the combined method for postoperative metastases and
recurrence was 93.43% (95% CI, 0.875–0.967), the specificity was
92.67% (95% CI, 0.856–0.965), the positive predictive value was
94.12% (95% CI, 0.884–0.972) and the negative predictive value was
91.81% (95% CI, 0.846–0.959). The values of these indicators were
all higher than those for either PET/CT scan or serum SCC-Ag
determination methods alone. The specificity and positive
predictive values were subjected to the χ2 test, and
P-values were both below 0.05, indicating a significant difference.
Two methods were compared at a time (PET-CT with combined method,
SCC-Ag with combined method, respectively) by McNemar's
χ2 test or Pearson's chi-squared test. The statistical
significance of these results is presented in Table III.
 | Table III.Comparison of diagnostic efficacy of
the combined diagnostic method. |
Table III.
Comparison of diagnostic efficacy of
the combined diagnostic method.
| Examination
method | Sensitivity, % (95%
CI) | Specificity, % (95%
CI) | Positive predictive
value, % (95% CI) | Negative predictive
value, % (95% CI) |
|---|
| PET/CT | 84.67%
(0.722–0.900) | 90.83%
(0.834–0.953) | 92.06%
(0.855–0.959) | 82.50%
(0.742–0.886) |
| Serum SCC-Ag | 89.78%
(0.831–0.941) | 74.31%
(0.649–0.819) | 81.45%
(0.741–0.871) | 85.26%
(0.762–0.914) |
| Combined
method | 93.43%
(0.875–0.967) | 92.67%
(0.856–0.965) | 94.12%
(0.884–0.972) | 91.81%
(0.846–0.959) |
| P1a | 0.043b | 0.815b | 0.511c | 0.036c |
| P2a | 0.405b |
<0.001b |
<0.001c | 0.138c |
Comparison of ROC curve of the
combined diagnostic method
The ROC curve was plotted using the results of the
gold standard pathology and follow-ups, and the area under the ROC
curve was calculated. The area under the ROC curve for the combined
method was 0.930±0.019 (95% CI, 0.893–0.968; P<0.001), which was
larger than that for the PET/CT scan (0.878±0.023; 95% CI,
0.832–0.924; z-test value=2.07; P<0.05) and the SCC-Ag method
(0.819±0.03; 95% CI, 0.761–0.877; z-test value=3.51; P<0.05).
The results showed that the combined method had a higher diagnostic
value for postoperative metastases and tumor recurrence in patients
with CC (area under the ROC curve >0.9) (Fig. 4 and Table
IV).
 | Table IV.Comparison of receiver operating
characteristic curve of the combined method for postoperative
cervical cancer tumor recurrence and metastases. |
Table IV.
Comparison of receiver operating
characteristic curve of the combined method for postoperative
cervical cancer tumor recurrence and metastases.
|
|
|
|
| Asymptotic 95%
CI |
|---|
|
|
|
|
|
|
|---|
| Test variable | Area | Sth.
Errora | Asymptotic
Sig.b | Lower bound | Upper bound |
|---|
| PET/CT
diagnosis | 0.878c | 0.023 | 0.000 | 0.832 | 0.924 |
| SCC-Ag
diagnosis | 0.819c | 0.030 | 0.000 | 0.761 | 0.877 |
| Combined
methodd | 0.930 | 0.019 | 0.000 | 0.893 | 0.968 |
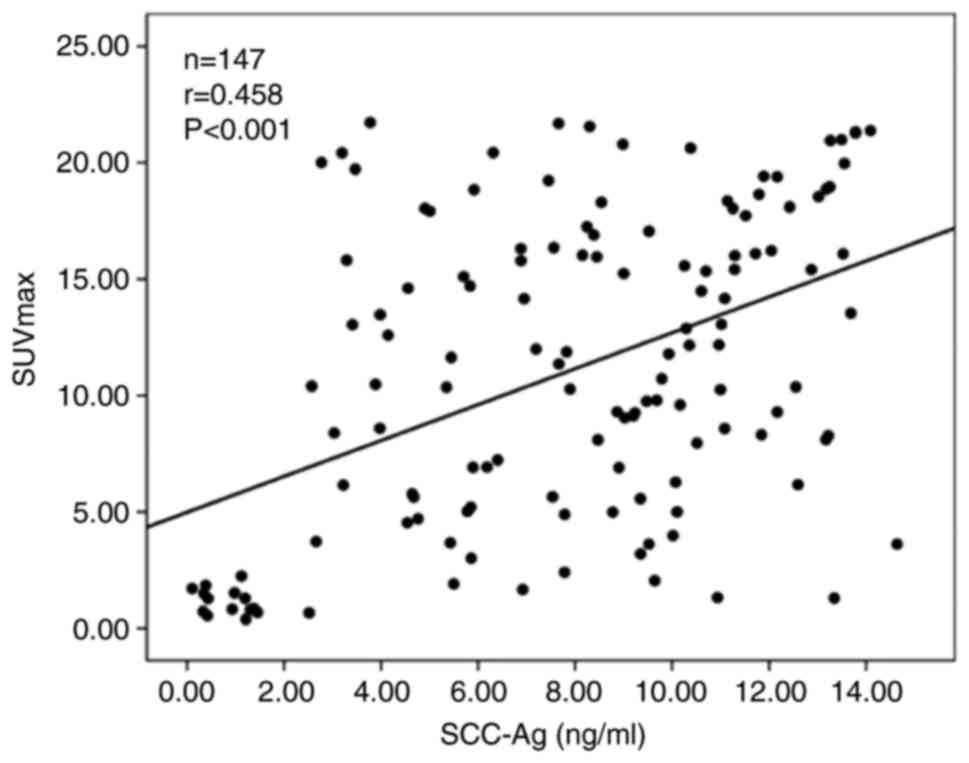
Correlation between SUVmax
and serum SCC-Ag levels in patients with CC with postoperative
metastases and tumor recurrence
The average SUVmax was 11.08±6.59 in 137
patients with CC with postoperative metastases and tumor
recurrence, and the average serum SCC-Ag level was 6.84±5.43 ng/ml.
The scatter plot of the association between the average serum
SCC-Ag level and SUVmax was drawn for the 137 patients
with CC with postoperative metastases and tumor recurrence
(Fig. 5). The results showed that
the serum SCC-Ag level was positively correlated with
SUVmax (r=0.458; P<0.001), indicating that the higher
the levels of serum SCC-Ag patients with metastasis and tumor
recurrence are, the higher the SUVmax is.
Discussion
At present, the primary treatment for early stage CC
is surgery, which may be combined with radiotherapy, chemotherapy,
hyperthermia and gene therapy (20,21). The
optimal individualized therapy should be developed based on the
actual clinical situation of the patients. Approximately 80% of the
recurrence cases occur within 2–3 years after the treatment for CC.
Metastases largely occur in the pelvic and abdominal cavities
(22,23). Tumor recurrence or metastases in
gynecological malignancies are usually asymptomatic, making early
discovery difficult, and there is still a lack of effective
monitoring tools (24). Therefore,
the diagnosis of CC recurrence and metastases by follow-up, use of
tumor markers and imaging techniques is of high clinical
significance. The clinical value of PET/CT scan with serum SCC-Ag
in diagnosing postoperative metastases and recurrence in patients
with CC was discussed in the current study.
18F-FDG PET/CT is a fusing imaging
technique that acquires morphological and functional images
simultaneously. This technique can also show the position and scope
of the lesions, and determine the presence of pelvic lymph node
metastases and distant metastases (25). Due to these benefits,
18F-FDG PET/CT has been increasingly used for the
diagnosis of CC recurrence and metastases. The present study
further confirmed the clinical value of PET/CT in diagnosing CC
recurrence and metastases. The area under the ROC curve was
0.878±0.023 (95% CI, 0.832–0.924; P<0.001), and the sensitivity
and specificity of the PET/CT scan method were 84.67% (95% CI,
0.722–0.900) and 90.83% (95% CI, 0.834–0.953), respectively, which
were consistent with other relevant studies (26–28).
Previous studies have shown that PET/CT has certain
limitations in diagnosing CC recurrence and metastases (29,30): i)
For lymph nodes <0.5 cm, PET/CT scan has limited diagnostic
value; ii) 18F-FDG is not specific for tumors and may
produce false-positive results when diagnosing inflammatory lesions
and lymph node micrometastases; iii) PET/CT images fail to recover
the true activity in involved tissues once the volume of the
disease is <2.5 times the spatial resolution of the scanner
(31); and iv) given individual
factors and variation of technical skills across the operators,
SUVmax measurement is highly heterogenous. In the
present study, missed diagnosis by PET/CT scan affected 21 patients
with metastases and recurrence, with a missed diagnosis rate of
8.14%. This indicated a relatively low diagnostic sensitivity and
failure to achieve an accurate result.
Serum SCC-Ag is the most widely used marker for
residual CC recurrence and metastases after treatment (14,15). The
serum SCC-Ag level is closely associated with the infiltration and
metastases of squamous cell carcinoma of the cervix, and it is an
independent risk factor of CC recurrence (30). The present study indicated that the
area under the ROC curve for the serum SCC-Ag level method was
0.819±0.03 (95% CI, 0.761–0.877; P<0.001), and the sensitivity
was also high 89.78% (0.831–0.941). Admittedly, the serum SCC-Ag
level had a low specificity for diagnosing CC recurrence and
metastases, which was only 74.31% (95% CI, 0.649–0.819). Moreover,
the serum SCC-Ag determination alone is not able to indicate the
location of tumor recurrence and metastases.
Previous studies highly recommend PET/CT as a
routine imaging examination for patients with CC with a progressive
increase in serum SCC-Ag levels for unknown reasons, and also for
patients with negative serum SCC-Ag levels (32,33). The
present study showed that the serum SCC-Ag level was positively
correlated with SUVmax (r=0.458; P<0.001). Given the
aforementioned benefits from both methods, PET/CT scan combined
with serum SCC-Ag determination could be have a greater clinical
value for diagnosing recurrence and metastases during the
postoperative follow-up (34). The
combined approach not only ensures a high sensitivity, but also a
high specificity, reducing missed diagnosis and misdiagnosis, and
providing more valuable information for the choice of appropriate
salvage treatment for patients with recurrence. The diagnostic
sensitivity of the combined method for postoperative
metastases/recurrence in patients with early CC was 93.43% (95% CI,
0.875–0.967) and the specificity was 92.67% (95% CI, 0.856–0.965);
the positive predictive value was 94.12% (95% CI, 0.884–0.972), the
negative predictive value was 91.81% (95% CI, 0.846–0.959) and the
area under the ROC curve was 0.930±0.019 (95% CI, 0.893–0.968;
P<0.001). All of the aforementioned indicators for the combined
method were significantly different from those using either PET/CT
scan or serum SCC-Ag determination alone.
In view of the popularity and high cost of PET/CT,
it is recommended that whole body PET-CT imaging should be
performed as early as possible for the patients whose serum SCC Ag
gradually increases during clinical follow-up, while other
conventional imaging tests are negative.
There are a number of limitations to the present
study. As a retrospective study, there may be a patient selection
bias. The study was not stratified regarding the tumor stage and
lymph node size, which may be confounding factors or represent
bias. In the present study, a SUVmax of 2.5 as the
standard threshold of tumor metastasis and recurrence, which is a
truncated value. could have been affected by the differences in
individual factors, such as the equipment utilized and the
operator's technical level. Therefore, the diagnosis of this
threshold is affected by a great heterogeneity and would require
further quantification.
Taken together, for patients with early metaphase CC
receiving treatment, PET/CT scan combined with serum SCC-Ag
determination during the follow-up is capable of an earlier, more
comprehensive and more accurate detection of recurrence/metastatic
lesions. This combined diagnostic method is of high clinical
application value to provide objective and valuable information for
the development of subsequent treatment schemes.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC conceived and designed the current study and
contributed to writing the manuscript. CQ and SH performed the
experiments. CQ and YC confirm the authenticity of all the raw
data. LC, LZ and HD analyzed and interpreted the data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved and monitored by the Ethics
Committee of The Affiliated Hospital, Southwest Medical University
(Luzhou, China). Written informed consent was provided by all the
patients who participated in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cohen PA, Jhingran A, Oaknin A and Denny
L: Cervical cancer. Lancet. 393:169–182. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Ervik M, Lam F, Colombet M, Mery
L, Piñeros M, Znaor A, Soerjomataram I and Bray F: Global cancer
observatory: Cancer today. International Agency for Research on
Cancer; Lyon: 2018
|
|
3
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pecorelli S: Revised FIGO staging for
carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol
Obstet. 105:103–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martins EB, Chojniak R, Kowalski LP,
Nicolau UR, Lima EN and Bitencourt AG: Diffusion-weighted MRI in
the assessment of early treatment response in patients with
squamous-cell carcinoma of the head and neck: Comparison with
morphological and PET/CT Findings. PLoS One. 10:e01400092015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harashavardhan NS, Naveen Kumar S, Uday
Kumar NS, Ezhilarasi R and Bhavani B: A prospective analysis of
magentic resonance imaging and computed tomography in staging with
uterine cervix carcinoma: A single centre experience. J Chalmeda
Anand Rao Institute Med Sci. 16:158–162. 2018.
|
|
8
|
Lee SI, Catalano OA and Dehdashti F:
Evaluation of gynecologic cancer with MR imaging, 18F-FDG PET/CT,
and PET/MR imaging. J Nucl Med. 56:436–443. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gee MS, Atri M, Bandos AI, Mannel RS, Gold
MA and Lee SI: Identification of distant metastatic disease in
uterine cervical and endometrial cancers with FDG PET/CT: Analysis
from the ACRIN 6671/GOG 0233 multicenter trial. Radiology.
287:176–184. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dolgun ZN, Altintas AS, Ivan C and
Balkanli P: Comparison of preoperative magnetic resonance imaging
results with postoperative pathologic results in early stage
uterine cervical cancer. Eur J Gynaecol Oncol. 39:935–938.
2018.
|
|
11
|
Herrera FG and Prior JO: The role of
PET/CT in cervical cancer. Front Oncol. 3:342013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ramlov A, Kroon PS, Jurgenliemk-Schulz IM,
Leeuw AA, Gormsen LC, Fokdal LU, Tanderup K and Lindegaard JC:
Impact of radiation dose and standardized uptake value of (18)FDG
PET on nodal control in locally advanced cervical cancer. Acta
Oncol. 54:1567–1573. 2015. View Article : Google Scholar : PubMed/NCBIPubMed/NCBI
|
|
13
|
Selman TJ, Mann C, Zamora J, Appleyard TL
and Khan K: Diagnostic accuracy of tests for lymph node status in
primary cervical cancer: A systematic review and meta-analysis.
CMAJ. 178:855–862. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Salvatici M, Achilarre MT, Sandri MT,
Boveri S, Vanna Z and Landoni F: Squamous cell carcinoma antigen
(SCC-Ag) during follow-up of cervical cancer patients: Role in the
early diagnosis of recurrence. Gynecol Oncol. 142:115–119. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shimura K, Mabuchi S, Yokoi T, Sasano T,
Sawada K, Hamasaki T and Kimura T: Utility of serum squamous cell
carcinoma antigen levels at the time of recurrent cervical cancer
diagnosis in determining the optimal treatment choice. J Gynecol
Oncol. 24:321–329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fu J, Wang W, Wang Y, Liu C and Wang P:
The role of squamous cell carcinoma antigen (SCC Ag) in outcome
prediction after concurrent chemoradiotherapy and treatment
decisions for patients with cervical cancer. Radiat Oncol.
14:1462019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choi KH, Lee SW, Yu M, Jeong S, Lee JW and
Lee JH: Significance of elevated SCC-Ag level on tumor recurrence
and patient survival in patients with squamous-cell carcinoma of
uterine cervix following definitive chemoradiotherapy: A
multi-institutional analysis. J Gynecol Oncol. 30:e12019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang EY, Huang YJ, Chanchien CC, Lin H,
Wang CJ, Sun LM, Tseng CW, Tsai CC, Ou YC, Fu HC, et al:
Pretreatment carcinoembryonic antigen level is a risk factor for
para-aortic lymph node recurrence in addition to squamous cell
carcinoma antigen following definitive concurrent chemoradiotherapy
for squamous cell carcinoma of the uterine cervix. Radiat Oncol.
7:132012.
|
|
19
|
Kang S, Nam BH, Park JY, Seo SS, Ryu SY,
Kim JW, Kim SC, Park SY and Nam JH: Risk assessment tool for
distant recurrence after platinum-based concurrent chemoradiation
in patients with locally advanced cervical cancer: A Korean
gynecologic oncology group study. J Clin Oncol. 30:2369–2374. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pieterse QD, Kenter GG, Maas CP, de Kroon
CD, Creutzberg CL, Trimbos JB and Kuile MM: Self-reported sexual,
bowel and bladder function in cervical cancer patients following
different treatment modalities: Longitudinal prospective cohort
study. Int J Gynecol Cancer. 23:1717–1725. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiao XB, Hu J and Zhu LR: The safety of
ovarian preservation in early-stage adenocarcinoma compared with
squamous cell carcinoma of uterine cervix: A systematic review and
meta-analysis of observational studies. Int J Gynecol Cancer.
26:1510–1514. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim TH, Kim MH, Kim BJ, Park SI, Ryu SY
and Cho CK: Prognostic importance of the site of recurrence in
patients with metastatic recurrent cervical cancer. Int J Radiat
Oncol Biol Phys. 98:1124–1131. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bhatla N, Aoki D, Sharma DN and
Sankaranarayanan R: Cancer of the cervix uteri. Int J Gynaecol
Obstet. 143 (Suppl 2):S22–S36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Salani R, Khanna N, Frimer M, Bristow RE
and Chen LM: An update on post-treatment surveillance and diagnosis
of recurrence in women with gynecologic malignancies: Society of
gynecologic oncology (SGO) recommendations. Gynecol Oncol.
146:3–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yilmaz B, Dağ S, Ergul N and Çermik TF:
The efficacy of pretreatment and after treatment 18F-FDG PET/CT
metabolic parameters in patients with locally advanced squamous
cell cervical cancer. Nucl Med Commun. 40:219–227. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu J, Yuan S, Wang L, Sun X, Hu X, Meng X
and Yu J: Diagnostic and predictive value of using RGD PET/CT in
patients with cancer: A systematic review and meta-analysis. Biomed
Res Int. 2019:85347612019.PubMed/NCBI
|
|
27
|
Kitajima K, Suzuki K, Nakamoto Y, Onishi
Y, Sakamoto S, Senda M, Kita M and Sugimura K: Low-dose
non-enhanced CT versus full-dose contrast-enhanced CT in integrated
PET/CT studies for the diagnosis of uterine cancer recurrence. Eur
J Nucl Med Mol Imaging. 37:1490–1498. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sironi S, Picchio M, Landoni C, Galimberti
S, Signorelli M, Bettinardi V, Perego P, Mangioni C, Messa C and
Fazio F: Post-therapy surveillance of patients with uterine
cancers: Value of integrated FDG PET/CT in the detection of
recurrence. Eur J Nucl Med Mol Imaging. 34:472–479. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bentivegna E, Uzan C, Gouy S, Leboulleux
S, Duvillard P, Lumbroso J, Haie-Meder C, Schlumberger M and Morice
P: Correlation between [18f]fluorodeoxyglucose positron-emission
tomography scan and histology of pelvic nodes in early-stage
cervical cancer. Anticancer Res. 30:1029–1032. 2010.PubMed/NCBI
|
|
30
|
Chou HH, Chang TC, Yen TC, Ng KW, Hsueh S,
Ma SY, Chang CJ, Huang HJ, Chao A, Wu TI, et al: Low value of
[18F]-fluoro-2-deoxy-D-glucose positron emission tomography in
primary staging of early-stage cervical cancer before radical
hysterectomy. J Clin Oncol. 24:123–128. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Driscoll DO, Halpenny D, Johnston C,
Sheehy N and Keogan M: 18F-FDG-PET/CT is of limited value in
primary staging of early stage cervical cancer. Abdom Imaging.
40:127–133. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maffione AM, Piva M, Tsamita CS, Nanni C,
Castellucci P, Ambrosini V, Lopci E, Musto A, Rampin L, Grassetto
G, et al: Positron-emission tomography in gynaecologic
malignancies. Arch Gynecol Obstet. 280:521–528. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chong A, Ha JM, Jeong SY, Song HC, Min JJ,
Bom HS and Choi HS: Clinical usefulness of (18)F-FDG PET/CT in the
detection of early recurrence in treated cervical cancer patients
with unexplained elevation of serum tumor markers. Chonnam Med J.
49:20–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pan L, Cheng J, Zhou M, Yao Z and Zhang Y:
The SUVmax (maximum standardized uptake value for F-18
fluorodeoxyglucose) and serum squamous cell carcinoma antigen
(SCC-ag) function as prognostic biomarkers in patients with primary
cervical cancer. J Cancer Res Clin Oncol. 138:239–246. 2012.
View Article : Google Scholar : PubMed/NCBI
|