Nasopharyngeal carcinoma (NPC), an important type of
head and neck cancer, is highly prevalent in East Africa and Asia,
particularly in southern China (1,2). Due to
the concealed location of NPC, surgical treatment is relatively
difficult. According to the National Comprehensive Cancer Network
guidelines, radiotherapy (RT) is the primary treatment of choice
for NPC (3). RT uses an appropriate
intensity of ionizing radiation (IR) to eliminate tumor cells
(4,5). IR directly causes DNA damage and
indirectly stimulates the production of reactive oxygen species
(ROS) in tumor cells (6,7). However, according to statistics, 10–20%
of patients with NPC suffer from recurrence after primary RT due to
radiation resistance (8). Therefore,
there is an urgent requirement to discover novel methods of
radiosensitization for patients with resistance to RT.
Ferroptosis, a newly identified type of regulated
cell death (RCD), was proposed by Dixon et al (9) in 2012. Unlike other types of RCD,
ferroptosis is characterized by loss of lipid peroxidation repair
ability and the accumulation of redox-active iron (10). Morphologically, the mitochondrial
cristae decrease in number or disappear, the outer mitochondrial
membrane ruptures and the mitochondrial membrane becomes condensed.
Although the mechanism of ferroptosis has yet to be fully
elucidated, ferroptosis has a key role in a number human diseases,
such as ischemia/reperfusion injury (11), neurodegeneration (12) and various types of cancer, including
NPC (13,14). From the perspective of
radiosensitization and side effects of RT, the pharmacological
modulation of ferroptosis (stimulation or inhibition) may be of
significant clinical value.
The aim of the present review article was to discuss
the potential molecular mechanisms of ferroptosis and the microRNAs
(miRNAs/miRs) regulating ferroptosis in NPC, alongside the
potential future directions and clinical value of ferroptosis
research in RT for NPC.
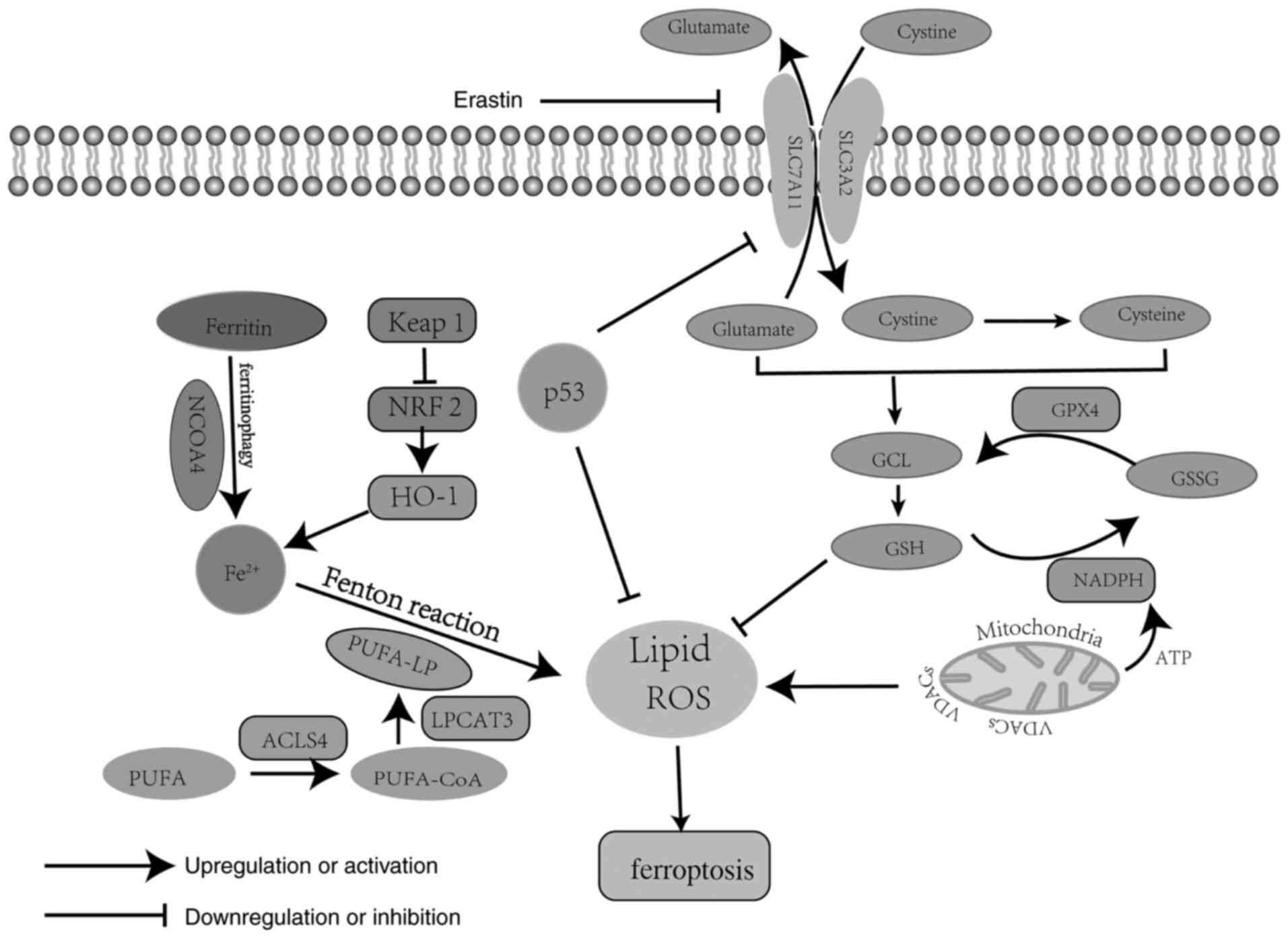
Research has revealed three critical pathways
involved in ferroptosis, which are the iron metabolism pathway, the
polyunsaturated fatty acid (PUFA) metabolism pathway and the
phospholipid hydroperoxidase glutathione peroxidase (GPX)4
metabolism pathway (Fig. 1)
(15). Iron metabolism is a redox
reaction of iron in the cytoplasm. During this process, ferric iron
(Fe3+) is absorbed into the cytoplasm via transferrin
and is then rapidly transformed into ferrous iron (Fe2+)
and stored as ferritin or in the labile iron pool; however,
Fe2+ is released due to the destruction of ferritin via
ferritinophagy, a process mediated by nuclear receptor coactivator
4 (14,16). Finally, excessive Fe2+ is
oxidized through the Fenton reaction by PUFA-containing
phospholipids (PUFA-PL), generating a large amount of ROS and
subsequently resulting in ferroptosis (17). PUFA-PL is the stress form of PUFA
acetylated by acyl-CoA synthetase and activated by acyl-CoA
synthetase long chain family member 4 (ACSL4) and
lysophosphatidylcholine acyltransferase 3, and it represents the
main lipid source of ROS (18,19). ROS
are a group of molecules with partially reduced oxygen, including
free radicals (HO• and RO•), peroxides (H2O2
and ROOH) and superoxide (O2•−), which cause cell death
by damaging DNA, RNA and lipid molecules (20). Therefore, PUFA metabolism has an
oxidative role in ferroptosis. GPX4 metabolism is key for
antagonizing lipid ROS due to the fact that GPX4 is able to
catalyze the decomposition of H2O2 and
complex lipid peroxides (21). Yang
et al (22) reported that
GPX4 is able to convert reduced glutathione (GSH) to oxidized
glutathione (GSSG), leading to weakened Ras-selective lethal small
molecule 3 (RSL3)- and erastin-induced ferroptosis.
Mechanistically, several molecules are involved in GPX4 metabolism
in ferroptosis. Among those, system Xc− and GPX4 are
considered as the main regulators of GPX4 metabolism, negatively
regulating ferroptosis (23). System
Xc− is a membrane Na+-dependent
cysteine-glutamate transporter, which is a disulphide-linked
heterodimer composed of a light-chain subunit [solute carrier
(SLC)7A11] and a heavy-chain subunit (SLC3A2) (24). Cysteine and glutamate are important
elements in GSH synthesis, and GSH generation and maintenance are
key to preventing oxidative damage due to lack of GPX4 (25). In addition, the mitochondrion is the
most important organelle involved in ferroptosis, as the energy and
electron transfer provided by the electron transfer chain are
necessary in the process of ferroptosis (26). Mitochondrial voltage-dependent anion
channels (VDACs), the transmembrane channels for transporting ion
and metabolites, are widely distributed on the outer mitochondrial
membrane (27). It was previously
demonstrated that erastin is able to target VDAC2 on the outer
mitochondrial membrane, resulting in lipid ROS release and slowing
down the oxidation of NADH (28).
NADH is mainly involved in material and energy metabolism in cells,
which supplies the energy required for ATP synthesis through the
oxidative phosphorylation process and for the conversion of GSSH to
GSH (14,29). Therefore, VDACs and NADPH oxidase
(NOX) are crucial positive regulators that promote ferroptosis, and
altering outer mitochondrial membrane permeability by antitumor
drugs may be a novel approach to tumor treatment. In summary,
ferroptosis is a non-apoptotic type of cell death that is involved
in several complex regulatory and three intersecting metabolic
pathways.
A number of pharmacological studies have attempted
to promote ferroptosis through various methods to improve the
efficacy of RT (30,31). However, to date, the association
between ferroptosis and RT has not been studied in depth. IR
randomly causes oxidative damage in all intercellular spaces,
including lipid membranes, and ferroptosis is caused by the
accumulation of toxic lipid peroxidation products (31). Therefore, there may be an interesting
connection between ferroptosis and IR. According to various
studies, RT affects the four key regulators of ferroptosis, namely
ROS, SLC7A11, ACSL4 and GPX4 (Fig.
2). Among those, ROS is considered the most important factor
implicated in ferroptosis caused by RT. Ye et al (32) reported that IR acting synergistically
with ferroptosis inducers increased ROS levels, leading to lipid
oxidation in an in vitro study using the HT-1080 human
fibrosarcoma cell line. However, regarding the expression of
SLC7A11, the conclusions have been controversial among studies. An
in vitro study involving the irradiation of ovarian cancer,
melanoma and human fibrosarcoma cells demonstrated that the
ataxia-telangiectasia mutated gene (ATM) activated by RT and IFN
derived from activated CD8+ T cells synergistically
inhibited the expression of SLC7A11 (30). However, Lei et al (33) indicated that IR markedly induced the
expression of SLC7A11, GPX4 and ACSL4. Subsequently, the expression
of SLC7A11 was considered as an adaptive response (34) and it likely involves activating
transcription factor 4 and/or the transcription factor
NF-E2-related factor 2 (NRF2), both of which are known to regulate
SLC7A11 transcription and are largely activated by IR (35–37).
Therefore, it appears that RT is able to either repress or activate
SLC7A11 expression, depending on the conditions. Mechanistically,
there are three pathways involved in RT-induced ferroptosis
(30–33). First, RT is able to induce oxidative
stress and then produce a large amount of ROS, leading to lipid
peroxidation. In addition, RT may promote PUFA-PL biosynthesis by
upregulating ACSL4 expression. Furthermore, RT also promotes
GPX4-mediated ferroptosis through DNA damage and inhibition of GSH
production. Taken together, these results indicated that the
specific mechanisms of RT-induced ferroptosis require to be further
explored and inducing ferroptosis to eliminate the radiation
resistance of tumor cells may be a direction worthy of further
investigation. These findings indicate the potential therapeutic
value of targeting ferroptosis to enhance the radiosensitivity of
NPC.
As described in the previous section, the major
cellular effect triggered by RT is to damage DNA and induce ROS
generation in cells. With regard to signaling, there appear to be
interactions between ferroptosis and other types of
radiation-induced cell death. RT damages DNA in the nucleus and,
thus, activates ATM (38). ATM is
the major regulator of the first step in DNA damage response
sensing (39). ATM activation is
able to sensitize AMP-activated protein kinase, which promotes
Beclin 1 (BECN1)-mediated autophagy, while at the same time
ferroptosis is promoted through regulating the ATM/GPX4 axis
(31,40). A previous study indicated that RT is
able to directly induce tumor cell necroptosis, which displays a
certain overlap with apoptosis (41). The intrinsic apoptotic pathway is
initiated by identifying double-strand breaks (DSBs) if DNA repair
is not successful (42). Of note,
ROS accumulation is able to prevent DNA repair and promote
ferroptosis (7). In brief, multiple
lines of evidence suggest that there is a close association between
ferroptosis and other types of RT-induced cell death, particularly
apoptosis, necroptosis and autophagic cell death.
RT currently remains the first choice of treatment
for NPC. However, radiation resistance has come to represent a
serious problem, as tumor cells are not sensitive to other forms of
death, including apoptosis. Therefore, inducing ferroptosis of
tumor cells may represent a good target for the radiosensitization
of NPC. RT causes DNA DSBs in tumor cells. Furthermore, a large
number of ROS are produced during the process of ferroptosis, which
makes it difficult to repair the DNA double strand, thus further
accelerating cell death (43).
Acquired radioresistance is currently a long-standing challenge in
RT for NPC. In view of this fact, the landing points for
investigating the therapeutic relevance of ferroptosis in RT
include the following: i) Whether the regulators of ferroptosis
modulate radiosensitivity in NPC; and ii) how to target the
regulators in NPC. These points are discussed below.
As the term suggests, the occurrence of ferroptosis
requires high levels of intracellular iron (9). In the process of ferroptosis, numerous
ROS-forming or -decomposing enzymes (cytochrome P450, xanthine
oxidase, lipoxygenase, NOX, mitochondrial complex I and III,
catalase and peroxidases) are iron-dependent (44,45).
Imbalances in iron metabolism in cells lead to iron overload and
ROS accumulation, resulting in Fenton oxidation reaction on the
lipid membrane and, eventually, ferroptosis (46). Therefore, iron is able to amplify the
production of ROS in ferroptosis (47,48). The
conversion process between Fe3+ and Fe2+ is
accompanied by the generation of energy, which benefits cellular
energy metabolism (49). Similarly,
it was demonstrated that reducing the level of intracellular iron
via the tumor suppressor gene 3-hydroxybutyrate dehydrogenase type
2 inhibited the proliferation and metastasis of NPC cells (50). Furthermore, Xu et al (51) indicated that itraconazole was able to
reduce the activity of NPC stem cells by increasing the
concentration of intracellular iron in lysosomes and lipid
peroxides. Therefore, the disruption of intracellular iron balance
may affect NPC cell survival and proliferation (52). Of note, an in vivo study
suggested that long-term treatment with iron-containing water
improved the efficiency of RT for glioma in rats via ferroptosis
(53). This method may also be
applied to RT for NPC. When ferritin is degraded via
ferritinophagy, it releases iron and promotes ferroptosis (54,55).
Previous studies suggested that transferrin and its receptor
promote ferroptotic cell death, whereas iron chelators inhibit this
type of cell death (56–58). Serum ferritin is the best single
marker reflecting iron stores in vivo (59). Compared with that of healthy
individuals, the serum ferritin level of patients with
undifferentiated NPC was reported to be higher (60). It was previously indicated that serum
ferritin levels may be valuable for predicting distant metastasis
in patients with NPC following standard intensity-modulated RT and
chemotherapy (61). Lactotransferrin
(LTF), a member of the transferrin family, may negatively regulate
the development and metastasis of NPC in vivo (62). It has been reported that LTF is
highly expressed in NPC cells and overexpression of LTF inhibited
the proliferation of NPC cells by modulating the MAPK/AKT pathway,
which is an essential pathway for tumor radiosensitization
(63–66). Therefore, this may be a viable
strategy for promoting radiosensitization of NPC through disrupting
iron metabolism.
NRF2 is considered as a main regulator of the
antioxidant response in ferroptosis, as a number of its downstream
target genes are responsible for preventing redox imbalance in
cancer cells (67). Qiang et
al (68) indicated that NRF2
serves a protective role in ferroptosis-mediated
ischemia/reperfusion-induced acute lung injury by regulating
SLC7A11 and activating STAT3. P62 may promote this process by
preventing NRF2 degradation and then increasing NRF2 nuclear
accumulation through inhibiting kelch-like ECH-associated protein 1
(KEAP1), which is able to regulate the expression of NRF2 via the
ubiquitin-proteasome route (69,70).
NRF2 was observed to be markedly upregulated in NPC tissues and may
serve as an unfavorable prognostic biomarker in patients with NPC
(71). An in vitro study
suggested that NRF2 gene knockout enhanced the radiosensitivity of
NPC cells, whereas silencing KEAP1 inhibited the radiosensitivity
of NPC cells (72). In addition,
Zhang et al (73) reported
that lowering NRF2 levels and promoting ROS production sensitized
NPC cells to RT. Huang et al (71) indicated that NRF2 expression was
upregulated through the Raf kinase inhibitor
protein/miR-450b-5p/NRF2/NAD(P)H:quinone oxidoreductase 1 axis,
which improved the radioresistance of NPC. Another study also
demonstrated that NRF2 promoted the proliferation of Epstein-Barr
virus (EBV)-transformed B cells through the EBV-related proteins
LMP1 and 2A and AKT signaling, which indicated that NRF2 may
represent a potential molecular target for EBV-related diseases,
including NPC (74).
FANCD2, a negative regulator of ferroptosis, is able
to repair DNA damage as a nuclear protein in bone marrow stromal
cells (80). Knockout of FANCD2 may
influence iron and GPX4 metabolism. In addition, an in vitro
and in vivo study revealed that FANCD2 silencing enhanced
the sensitivity of NPC cells to ionizing radiation (81). Recent results have demonstrated that
FANCD2 expression is associated with the prognosis of NPC (82). Therefore, FANCD2 may be an effective
target for radiosensitization, as well as a prognostic and
diagnostic marker of NPC.
HO-1 may be regulated by NRF2 and endoplasmic
reticulum-associated protein degradation and has a dual role in
ferroptosis (10). On the one hand,
increased expression of HO-1 may increase intracellular iron
levels; on the other hand, HO-1 was able to attenuate
erastin-induced ferroptosis in renal epithelial cells (83,84). A
study on the association between NPC and HO-1 suggested that
patients with low expression levels of HO-1 were more sensitive to
RT compared with those with high expression levels of HO-1
(85). The results suggested that
HO-1 may be a useful indicator for identifying patients with
RT-sensitive NPC. Therefore, HO-1, as a regulator of ferroptosis,
may also be an important target for radiosensitization.
p53, a key tumor suppressor gene, is activated under
different stress stimuli, including IR. p53 is able to
transcriptionally inhibit SLC7A11 expression to impair cysteine
import, ultimately promoting ferroptosis (86). p533KR, an acetylation-defective p53
mutant, is highly effective in repressing the expression of
SLC711A, but not that of other already known p53 target genes (cell
cycle-, apoptosis- or senescence-related genes) (87). However, it has also been reported
that p53 may inhibit ferroptosis by the transcriptional activation
of cyclin-dependent kinase (CDK) inhibitor 1A/p21 or inhibition of
dipeptidyl-peptidase 4 activity (88). Therefore, p53 appears to have a dual
role in ferroptosis. Previous studies have indicated that p53 also
has a key role in regulating the occurrence and development of NPC,
particularly in terms of its radiosensitivity. Wang et al
(89) reported that activating the
p53 signaling pathway via overexpressing miR-372 enhanced the
radiosensitivity of NPC. Furthermore, a clinical study suggested
that recombinant human adenovirus p53 promoted radiosensitivity in
patients with recurrent NPC (90).
The major function of miRNAs is to bind to the
3′-untranslated region of target mRNAs and subsequently inhibit
their expression (95). Previous
studies have revealed that miRNAs have an important role in the
regulation of ferroptosis. miR-182-5p and miR-324-3p were
demonstrated to promote ferroptosis via targeting GPX4 in
ischemia/reperfusion-induced renal injury and lung adenocarcinoma
(96,97). miR-17-92 and miR-424-5p abrogated
erastin- and RSL3-induced ferroptosis through targeting ACSL4 in
human umbilical vein endothelial cells and ovarian cancer cells
(98,99). In radioresistant cells, miR-7-5p
restrained ferroptosis through downregulating mitoferrin and
subsequently reducing iron levels (100). Furthermore, miR-9 and miR-137
promoted ferroptosis via reducing intracellular GSH levels; miR-9
inhibited the synthesis of GSH and miR-137 suppressed the
expression of SLC1A5, which is a component of system Xc−
(101,102). To date, several studies have
demonstrated that miRNAs regulating ferroptosis are associated with
the proliferation, invasion, migration and apoptosis of NPC cells,
particularly in terms of radiosensitivity regulation (Table I). For instance, miR-214 and
miR-182-5p were indicated to contribute to radioresistance in NPC
by regulating LTF and BNIP3 expression (103,104).
However, miR-124 and miR-9 may promote radiosensitivity of NPC via
targeting programmed cell death protein 6 (PDCD6) and suppressing
the expression of junctional adhesion molecule A (JAMA) (105–107).
Hu et al (108) indicated
that miR-214 enhanced radiosensitivity of colorectal cancer via
inhibition of autophagy-related 12-mediated autophagy. Based on the
results reported to date, miR-214 is considered to act as an
oncogene in NPC, which is able to promote the proliferation of NPC
cells and inhibit apoptosis by targeting BAX, LTF, Bcl-2-like
protein 11, WW domain-containing oxidoreductase and phosphatase and
tensin homolog (109–112). The expression levels of miR-214
were indicated to be upregulated in NPC, particularly in
metastasis-prone NPC tissues compared with those in normal
nasopharyngeal epithelial tissues (112). Therefore, miR-214 may serve as a
potential novel diagnostic and RT sensitization biomarker for NPC.
miR-124 has been detected in copious amounts in the brain and it
may participate in the pathogenesis of several disorders. Deng
et al (113) suggested that
miR-124 was able to radiosensitize glioblastoma multiforme cells by
targeting CDK4. In addition, miR-124 has been reported to be
downregulated in NPC (114).
miR-124 may enhance cell radiosensitivity by targeting JAMA and
PDCD6 (105). Current research
suggested that miR-124 is able to suppress stem-like properties and
enhance radiosensitivity in NPC cells by directly targeting JAMA
(106). These results may provide
novel insight into the molecular mechanisms underlying RT failure
in NPC and enable the design of novel therapeutic approaches
(115,116).
To date, a number of small-molecule compounds have
been confirmed to induce ferroptosis, which are expected to be
developed into novel antitumor small-molecule drugs. According to
the different targets of small-molecule compounds, ferroptosis
inducers may be divided into four categories as follows: i)
Inhibition of system Xc−; ii) inhibition of GPX4
activity; iii) degradation of GPX4 and coenzyme Q10; and iv)
induction of lipid peroxide production (18). In NPC, ferroptosis may be induced by
disulfiram/copper, itraconazole attenuates, cephalosporin
antibiotics and cucurbitacin B (13,51,117,118).
Among those, itraconazole is able to sequester iron in lysosomes,
thereby causing ferroptosis and reversing the radiation resistance
of NPC spheres. Therefore, whether ferroptosis inducers exert
radiosensitizing effects and their potential value in RT for NPC
warrants further investigation. An in vitro study indicated
that the ferroptosis inducers imidazole ketone erastin and RSL3 act
synergistically with radiation to promote ferroptotic cell death in
a variety of tumor cell lines (32).
In addition, another study suggested that erastin enhances the
radiosensitivity of HeLa and NCI-H1975 adenocarcinoma cells via GSH
depletion (119). Therefore,
ferroptosis inducers may reduce the GSH concentration to enhance
the radiosensitivity of radioresistant tumors, including NPC. A
recent study indicated that the ferroptosis inducer erastin is able
to trigger autophagy by increasing intracellular iron levels
(120). Of note, a large number of
studies have indicated that the activation of cytotoxic autophagy
is able to enhance the sensitivity of tumor cells to RT (121). Therefore, the application of
ferroptosis inducers to induce cytotoxic autophagy in NPC cells may
be a promising method for radiosensitization.
The role of ferroptosis, a relatively newly
identified type of cell death, has not been extensively
investigated in NPC to date. The aim of the present review was to
discuss the molecular mechanisms of ferroptosis in cancer. The
metabolism of iron, PUFA and GPX4 have key roles in ferroptosis and
there is a potential utility for the modulation of ferroptosis in
the radiosensitization of NPC (51,122).
Of note, the core regulators of ferroptosis, including miRNAs,
serve important functions in RT for NPC. Radiation-resistant cells
have been suggested to be more susceptible to ferroptosis due to
their metabolic characteristics and cellular signaling pathways
(123). Therefore, ferroptosis
inducers may be of value in the radiosensitization of NPC.
Itraconazole is a promising ferroptosis inducer for
radiosensitization of NPC, which may reverse the radioresistance of
NPC spheroids (51). In brief,
targeting ferroptosis may provide a novel strategy to improve RT
sensitivity of NPC.
However, several issues remain to be addressed,
including elucidating the exact mechanism of action of ferroptosis,
determining the possible association between autophagy and
ferroptosis in the radiosensitization of NPC, determining how to
use nanotechnology materials to target ferroptosis regulators in
NPC to enhance RT sensitivity and discovering additional
ferroptosis inducers and regulatory genes. These questions must be
addressed and successfully resolved before ferroptosis may be
applied in the clinical setting.
Not applicable.
No funding was received.
Data sharing is not applicable.
HLL mainly took charge of researching the literature
and writing the manuscript; XSH had a guiding role in the review
and was involved in revising the manuscript critically for
important intellectual content; NHD and JXX provided ideas in the
revision process. All authors read and approved the final
manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Chen YP, Chan ATC, Le QT, Blanchard P, Sun
Y and Ma J: Nasopharyngeal carcinoma. Lancet. 394:64–80. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Long M, Fu Z, Li P and Nie Z: Cigarette
smoking and the risk of nasopharyngeal carcinoma: A meta-analysis
of epidemiological studies. BMJ Open. 7:e0165822017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu YP, Lv X, Zou X, Hua YJ, You R, Yang
Q, Xia L, Guo SY, Hu W, Zhang MX, et al: Minimally invasive surgery
alone compared with intensity-modulated radiotherapy for primary
stage I nasopharyngeal carcinoma. Cancer Commun (Lond). 39:752019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jaffray DA: Image-guided radiotherapy:
From current concept to future perspectives. Nat Rev Clin Oncol.
9:688–699. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu G, Zeng X, Wu B, Zhao J and Pan Y:
RNA-Seq analysis of peripheral blood mononuclear cells reveals
unique transcriptional signatures associated with radiotherapy
response of nasopharyngeal carcinoma and prognosis of head and neck
cancer. Cancer Biol Ther. 21:139–146. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baidoo KE, Yong K and Brechbiel MW:
Molecular pathways: Targeted α-particle radiation therapy. Clin
Cancer Res. 19:530–537. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Srinivas US, Tan BWQ, Vellayappan BA and
Jeyasekharan AD: ROS and the DNA damage response in cancer. Redox
Biol. 25:1010842019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee AWM, Ng WT, Chan JYW, Corry J, Mäkitie
A, Mendenhall WM, Rinaldo A, Rodrigo JP, Saba NF, Strojan P, et al:
Management of locally recurrent nasopharyngeal carcinoma. Cancer
Treat Rev. 79:1018902019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu T, Ding W, Ji X, Ao X, Liu Y, Yu W and
Wang J: Molecular mechanisms of ferroptosis and its role in cancer
therapy. J Cell Mol Med. 23:4900–4912. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Friedmann Angeli JP, Schneider M, Proneth
B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch
A, Eggenhofer E, et al: Inactivation of the ferroptosis regulator
Gpx4 triggers acute renal failure in mice. Nat Cell Biol.
16:1180–1191. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Raven EP, Lu PH, Tishler TA, Heydari P and
Bartzokis G: Increased iron levels and decreased tissue integrity
in hippocampus of Alzheimer's disease detected in vivo with
magnetic resonance imaging. J Alzheimers Dis. 37:127–136. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang S, Cao B, Zhang J, Feng Y, Wang L,
Chen X, Su H, Liao S, Liu J, Yan J and Liang B: Induction of
ferroptosis in human nasopharyngeal cancer cells by cucurbitacin B:
molecular mechanism and therapeutic potential. Cell Death Dis.
12:2372021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Z, Chen L, Chen C, Zhou Y, Hu D, Yang
J, Chen Y, Zhuo W, Mao M, Zhang X, et al: Targeting ferroptosis in
breast cancer. Biomark Res. 8:582020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun
X, Kang R and Tang D: Ferroptosis: Process and function. Cell Death
Differ. 23:369–379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kazan HH, Urfali-Mamatoglu C and Gunduz U:
Iron metabolism and drug resistance in cancer. Biometals.
30:629–641. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He YJ, Liu XY, Xing L, Wan X, Chang X and
Jiang HL: Fenton reaction-independent ferroptosis therapy via
glutathione and iron redox couple sequentially triggered lipid
peroxide generator. Biomaterials. 241:1199112020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Torti SV and Torti FM: Iron and cancer:
More ore to be mined. Nat Rev Cancer. 13:342–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang C, Zhang X, Yang M and Dong X:
Recent progress in ferroptosis inducers for cancer therapy. Adv
Mater. 31:e19041972019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin LS, Song J, Song L, Ke K, Liu Y, Zhou
Z, Shen Z, Li J, Yang Z, Tang W and Niu G: Simultaneous fenton-like
ion delivery and glutathione depletion by MnO2-based
nanoagent to enhance chemodynamic therapy. Angew Chem Int Ed Engl.
57:4902–4906. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang H, Lin D, Yu Q, Li Z, Lenahan C, Dong
Y, Wei Q and Shao A: A promising future of ferroptosis in tumor
therapy. Front Cell Dev Biol. 9:6291502021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB, et al: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stockwell BR, Jiang X and Gu W: Emerging
mechanisms and disease relevance of ferroptosis. Trends Cell Biol.
30:478–490. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sato H, Tamba M, Ishii T and Bannai S:
Cloning and expression of a plasma membrane cystine/glutamate
exchange transporter composed of two distinct proteins. J Biol
Chem. 274:11455–11458. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lo M, Ling V, Wang YZ and Gout PW: The
xc-cystine/glutamate antiporter: A mediator of pancreatic cancer
growth with a role in drug resistance. Br J Cancer. 99:464–472.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao M, Yi J, Zhu J, Minikes AM, Monian P,
Thompson CB and Jiang X: Role of mitochondria in ferroptosis. Mol
Cell. 73:354–363.e3. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang D, Chen X, Kang R and Kroemer G:
Ferroptosis: Molecular mechanisms and health implications. Cell
Res. 31:107–125. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yagoda N, von Rechenberg M, Zaganjor E,
Bauer AJ, Yang WS, Fridman DJ, Wolpaw AJ, Smukste I, Peltier JM,
Boniface J, et al: RAS-RAF-MEK-dependent oxidative cell death
involving voltage-dependent anion channels. Nature. 447:864–868.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang WH, Huang Z, Wu J, Ding CC, Murphy SK
and Chi JT: A TAZ-ANGPTL4-NOX2 axis regulates ferroptotic cell
death and chemoresistance in epithelial ovarian cancer. Mol Cancer
Res. 18:79–90. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lang X, Green MD, Wang W, Yu J, Choi JE,
Jiang L, Liao P, Zhou J, Zhang Q, Dow A, et al: Radiotherapy and
immunotherapy promote tumoral lipid oxidation and ferroptosis via
synergistic repression of SLC7A11. Cancer Discov. 9:1673–1685.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lei G, Mao C, Yan Y, Zhuang L and Gan B:
Ferroptosis, radiotherapy, and combination therapeutic strategies.
Protein Cell. Apr 23–2021.(Epub ahead of print). View Article : Google Scholar
|
|
32
|
Ye LF, Chaudhary KR, Zandkarimi F, Harken
AD, Kinslow CJ, Upadhyayula PS, Dovas A, Higgins DM, Tan H, Zhang
Y, et al: Radiation-induced lipid peroxidation triggers ferroptosis
and synergizes with ferroptosis inducers. ACS Chem Biol.
15:469–484. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lei G, Zhang Y, Koppula P, Liu X, Zhang J,
Lin SH, Ajani JA, Xiao Q, Liao Z, Wang H and Gan B: The role of
ferroptosis in ionizing radiation-induced cell death and tumor
suppression. Cell Res. 30:146–162. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xie L, Song X, Yu J, Guo W, Wei L, Liu Y
and Wang X: Solute carrier protein family may involve in
radiation-induced radioresistance of non-small cell lung cancer. J
Cancer Res Clin Oncol. 137:1739–1747. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
McDonald JT, Kim K, Norris AJ, Vlashi E,
Phillips TM, Lagadec C, Della Donna L, Ratikan J, Szelag H, Hlatky
L and McBride WH: Ionizing radiation activates the Nrf2 antioxidant
response. Cancer Res. 70:8886–8895. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zong Y, Feng S, Cheng J, Yu C and Lu G:
Up-regulated ATF4 expression increases cell sensitivity to
apoptosis in response to radiation. Cell Physiol Biochem.
41:784–794. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Koppula P, Zhuang L and Gan B: Cystine
transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient
dependency, and cancer therapy. Protein Cell. 12:599–620. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Maier P, Hartmann L, Wenz F and Herskind
C: Cellular pathways in response to ionizing radiation and their
targetability for tumor radiosensitization. Int J Mol Sci.
17:1022016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stracker TH, Roig I, Knobel PA and
Marjanović M: The ATM signaling network in development and disease.
Front Genet. 4:372013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sanli T, Steinberg GR, Singh G and
Tsakiridis T: AMP-activated protein kinase (AMPK) beyond
metabolism: Anovel genomic stress sensor participating in the DNA
damage response pathway. Cancer Biol Ther. 15:156–169. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Degterev A and Yuan J: Expansion and
evolution of cell death programmes. Nat Rev Mol Cell Biol.
9:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gudkov AV and Komarova EA: The role of p53
in determining sensitivity to radiotherapy. Nat Rev Cancer.
3:117–129. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kirtonia A, Sethi G and Garg M: The
multifaceted role of reactive oxygen species in tumorigenesis. Cell
Mol Life Sci. 77:4459–4483. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Halliwell B and Cross CE: Oxygen-derived
species: Their relation to human disease and environmental stress.
Environ Health Perspect. 102 (Suppl 10):S5–S12. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Grivennikova VG and Vinogradov AD:
Generation of superoxide by the mitochondrial Complex I. Biochim
Biophys Acta. 1757:553–561. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hentze MW, Muckenthaler MU, Galy B and
Camaschella C: Two to tango: Regulation of mammalian iron
metabolism. Cell. 142:24–38. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gutteridge JM and Halliwell B: Iron
toxicity and oxygen radicals. Baillieres Clin Haematol. 2:195–256.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Doll S and Conrad M: Iron and ferroptosis:
A still ill-defined liaison. IUBMB Life. 69:423–434. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Andrews NC: Disorders of iron metabolism.
N Engl J Med. 341:1986–1995. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li B, Liao Z, Mo Y, Zhao W, Zhou X, Xiao
X, Cui W, Feng G, Zhong S, Liang Y, et al: Inactivation of
3-hydroxybutyrate dehydrogenase type 2 promotes proliferation and
metastasis of nasopharyngeal carcinoma by iron retention. Br J
Cancer. 122:102–110. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xu Y, Wang Q, Li X, Chen Y and Xu G:
Itraconazole attenuates the stemness of nasopharyngeal carcinoma
cells via triggering ferroptosis. Environ Toxicol. 36:257–266.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Andrews NC: Forging a field: The golden
age of iron biology. Blood. 112:219–230. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ivanov SD, Semenov AL, Kovan'ko EG and
Yamshanov VA: Effects of iron ions and iron chelation on the
efficiency of experimental radiotherapy of animals with gliomas.
Bull Exp Biol Med. 158:800–803. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh
HJ III, Kang R and Tang D: Autophagy promotes ferroptosis by
degradation of ferritin. Autophagy. 12:1425–1428. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Park E and Chung SW: ROS-mediated
autophagy increases intracellular iron levels and ferroptosis by
ferritin and transferrin receptor regulation. Cell Death Dis.
10:8222019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hong X, Roh W, Sullivan RJ, Wong KHK,
Wittner BS, Guo H, Dubash TD, Sade-Feldman M, Wesley B, Horwitz E,
et al: The lipogenic regulator SREBP2 induces transferrin in
circulating melanoma cells and suppresses ferroptosis. Cancer
Discov. 11:678–695. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Feng H, Schorpp K, Jin J, Yozwiak CE,
Hoffstrom BG, Decker AM, Rajbhandari P, Stokes ME, Bender HG, Csuka
JM, et al: Transferrin receptor is a specific ferroptosis marker.
Cell Rep. 30:3411–3423.e7. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yang WS and Stockwell BR: Synthetic lethal
screening identifies compounds activating iron-dependent,
nonapoptotic cell death in oncogenic-RAS-harboring cancer cells.
Chem Biol. 15:234–245. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Guagnozzi D, Severi C, Ialongo P, Viscido
A, Patrizi F, Testino G, Vannella L, Labriola R, Strom R and
Caprilli R: Ferritin as a simple indicator of iron deficiency in
anemic IBD patients. Inflamm Bowel Dis. 12:150–151. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ma BB, Leungm SF, Hui EP, Mo F, Kwan WH,
Zee B, Yuen J and Chan AT: Prospective validation of serum CYFRA
21-1, beta-2-microglobulin, and ferritin levels as prognostic
markers in patients with nonmetastatic nasopharyngeal carcinoma
undergoing radiotherapy. Cancer. 101:776–781. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chen X, Long X, Liang Z, Lei H, Li L, Qu S
and Zhu X: Higher N stage and serum ferritin, but lower serum
albumin levels are associated with distant metastasis and poor
survival in patients with nasopharyngeal carcinoma following
intensity-modulated radiotherapy. Oncotarget. 8:73177–73186. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang W, Fan S, Zou G, Shi L, Zeng Z, Ma
J, Zhou Y, Li X, Zhang X, Li X, et al: Lactotransferrin could be a
novel independent molecular prognosticator of nasopharyngeal
carcinoma. Tumour Biol. 36:675–683. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhou Y, Zeng Z, Zhang W, Xiong W, Wu M,
Tan Y, Yi W, Xiao L, Li X, Huang C, et al: Lactotransferrin: A
candidate tumor suppressor-deficient expression in human
nasopharyngeal carcinoma and inhibition of NPC cell proliferation
by modulating the mitogen-activated protein kinase pathway. Int J
Cancer. 123:2065–2072. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhang H, Feng X, Liu W, Jiang X, Shan W,
Huang C, Yi H, Zhu B, Zhou W, Wang L, et al: Underlying mechanisms
for LTF inactivation and its functional analysis in nasopharyngeal
carcinoma cell lines. J Cell Biochem. 112:1832–1843. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Deng M, Zhang W, Tang H, Ye Q, Liao Q,
Zhou Y, Wu M, Xiong W, Zheng Y, Guo X, et al: Lactotransferrin acts
as a tumor suppressor in nasopharyngeal carcinoma by repressing AKT
through multiple mechanisms. Oncogene. 32:4273–4283. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Song M, Bode AM, Dong Z and Lee MH: AKT as
a therapeutic target for cancer. Cancer Res. 79:1019–1031. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Dodson M, Castro-Portuguez R and Zhang DD:
NRF2 plays a critical role in mitigating lipid peroxidation and
ferroptosis. Redox Biol. 23:1011072019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Qiang Z, Dong H, Xia Y, Chai D, Hu R and
Jiang H: Nrf2 and STAT3 alleviates ferroptosis-mediated IIR-ALI by
regulating SLC7A11. Oxid Med Cell Longev. 2020:51469822020.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R
and Tang D: Activation of the p62-Keap1-NRF2 pathway protects
against ferroptosis in hepatocellular carcinoma cells. Hepatology.
63:173–184. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Cloer EW, Goldfarb D, Schrank TP, Weissman
BE and Major MB: NRF2 activation in cancer: From DNA to protein.
Cancer Res. 79:889–898. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Huang W, Shi G, Yong Z, Li J, Qiu J, Cao
Y, Zhao Y and Yuan L: Downregulation of RKIP promotes
radioresistance of nasopharyngeal carcinoma by activating NRF2/NQO1
axis via downregulating miR-450b-5p. Cell Death Dis. 11:5042020.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhou J, Ding J, Ma X, Zhang M, Huo Z, Yao
Y, Li D and Wang Z: The NRF2/KEAP1 pathway modulates nasopharyngeal
carcinoma cell radiosensitivity via ROS elimination. Onco Targets
Ther. 13:9113–9122. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhang G, Wang W, Yao C, Ren J, Zhang S and
Han M: Salinomycin overcomes radioresistance in nasopharyngeal
carcinoma cells by inhibiting Nrf2 level and promoting ROS
generation. Biomed Pharmacother. 91:147–154. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yun SM, Kim YS and Hur DY: LMP1 and 2A
induce the expression of Nrf2 through Akt signaling pathway in
Epstein-Barr virus-transformed B cells. Transl Oncol. 12:775–783.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hsu JL, Chou JW, Chen TF, Hsu JT, Su FY,
Lan JL, Wu PC, Hu CM, Lee EY and Lee WH: Glutathione peroxidase 8
negatively regulates caspase-4/11 to protect against colitis. EMBO
Mol Med. 12:e93862020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Koeberle SC, Gollowitzer A, Laoukili J,
Kranenburg O, Werz O, Koeberle A and Kipp AP: Distinct and
overlapping functions of glutathione peroxidases 1 and 2 in
limiting NF-κB-driven inflammation through redox-active mechanisms.
Redox Biol. 28:1013882020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Maiorino M, Conrad M and Ursini F: GPx4,
lipid peroxidation, and cell death: Discoveries, rediscoveries, and
open issues. Antioxid Redox Signal. 29:61–74. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Nunes SC and Serpa J: Glutathione in
ovarian cancer: A Double-edged sword. Int J Mol Sci. 19:18822018.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Meng DF, Guo LL, Peng LX, Zheng LS, Xie P,
Mei Y, Li CZ, Peng XS, Lang YH, Liu ZJ, et al: Antioxidants
suppress radiation-induced apoptosis via inhibiting MAPK pathway in
nasopharyngeal carcinoma cells. Biochem Biophys Res Commun.
527:770–777. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Song X, Xie Y, Kang R, Hou W, Sun X,
Epperly MW, Greenberger JS and Tang D: FANCD2 protects against bone
marrow injury from ferroptosis. Biochem Biophys Res Commun.
480:443–449. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Bao Y, Feng H, Zhao F, Zhang L, Xu S,
Zhang C, Zhao C and Qin G: FANCD2 knockdown with shRNA interference
enhances the ionizing radiation sensitivity of nasopharyngeal
carcinoma CNE-2 cells. Neoplasma. 68:40–52. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Xu S, Zhao F, Liang Z, Feng H, Bao Y, Xu
W, Zhao C and Qin G: Expression of FANCD2 is associated with
prognosis in patients with nasopharyngeal carcinoma. Int J Clin Exp
Pathol. 12:3465–3473. 2019.PubMed/NCBI
|
|
83
|
Suttner DM and Dennery PA: Reversal of
HO-1 related cytoprotection with increased expression is due to
reactive iron. FASEB J. 13:1800–1809. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Adedoyin O, Boddu R, Traylor A, Lever JM,
Bolisetty S, George JF and Agarwal A: Heme oxygenase-1 mitigates
ferroptosis in renal proximal tubule cells. Am J Physiol Renal
Physiol. 314:F702–F714. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Shi L and Fang J: Implication of heme
oxygenase-1 in the sensitivity of nasopharyngeal carcinomas to
radiotherapy. J Exp Clin Cancer Res. 27:132008. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Jiang L, Kon N, Li T, Wang SJ, Su T,
Hibshoosh H, Baer R and Gu W: Ferroptosis as a p53-mediated
activity during tumour suppression. Nature. 520:57–62. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wang SJ, Li D, Ou Y, Jiang L, Chen Y, Zhao
Y and Gu W: Acetylation is crucial for p53-mediated ferroptosis and
tumor suppression. Cell Rep. 17:366–373. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Xie Y, Zhu S, Song X, Sun X, Fan Y, Liu J,
Zhong M, Yuan H, Zhang L, Billiar TR, et al: The tumor suppressor
p53 limits Ferroptosis by blocking DPP4 activity. Cell Rep.
20:1692–1704. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wang Z, Mao JW, Liu GY, Wang FG, Ju ZS,
Zhou D and Wang RY: MicroRNA-372 enhances radiosensitivity while
inhibiting cell invasion and metastasis in nasopharyngeal carcinoma
through activating the PBK-dependent p53 signaling pathway. Cancer
Med. 8:712–728. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ma WS, Ma JG and Xing LN: Efficacy and
safety of recombinant human adenovirus p53 combined with
chemoradiotherapy in the treatment of recurrent nasopharyngeal
carcinoma. Anticancer Drugs. 28:230–236. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Song X, Zhu S, Chen P, Hou W, Wen Q, Liu
J, Xie Y, Liu J, Klionsky DJ, Kroemer G, et al: AMPK-mediated BECN1
phosphorylation promotes ferroptosis by directly blocking System
Xc− activity. Curr Biol. 28:2388–2399.e5. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kang R, Zhu S, Zeh HJ, Klionsky DJ and
Tang D: BECN1 is a new driver of ferroptosis. Autophagy.
14:2173–2175. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wan XB, Fan XJ, Chen MY, Xiang J, Huang
PY, Guo L, Wu XY, Xu J, Long ZJ, Zhao Y, et al: Elevated Beclin 1
expression is correlated with HIF-1alpha in predicting poor
prognosis of nasopharyngeal carcinoma. Autophagy. 6:395–404. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Wang Y, Chen W, Lian J, Zhang H, Yu B,
Zhang M, Wei F, Wu J, Jiang J, Jia Y, et al: The lncRNA PVT1
regulates nasopharyngeal carcinoma cell proliferation via
activating the KAT2A acetyltransferase and stabilizing HIF-1α. Cell
Death Differ. 27:695–710. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Majidinia M, Karimian A, Alemi F, Yousefi
B and Safa A: Targeting miRNAs by polyphenols: Novel therapeutic
strategy for aging. Biochem Pharmacol. 173:1136882020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ding C, Ding X, Zheng J, Wang B, Li Y,
Xiang H, Dou M, Qiao Y, Tian P and Xue W: miR-182-5p and
miR-378a-3p regulate ferroptosis in I/R-induced renal injury. Cell
Death Dis. 11:9292020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Deng SH, Wu DM, Li L, Liu T, Zhang T, Li
J, Yu Y, He M, Zhao YY, Han R and Xu Y: miR-324-3p reverses
cisplatin resistance by inducing GPX4-mediated ferroptosis in lung
adenocarcinoma cell line A549. Biochem Biophys Res Commun.
549:54–60. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Xiao FJ, Zhang D, Wu Y, Jia QH, Zhang L,
Li YX, Yang YF, Wang H, Wu CT and Wang LS: miRNA-17-92 protects
endothelial cells from erastin-induced ferroptosis through
targeting the A20-ACSL4 axis. Biochem Biophys Res Commun.
515:448–454. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Ma LL, Liang L, Zhou D and Wang SW: Tumor
suppressor miR-424-5p abrogates ferroptosis in ovarian cancer
through targeting ACSL4. Neoplasma. 68:165–173. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Tomita K, Fukumoto M, Itoh K, Kuwahara Y,
Igarashi K, Nagasawa T, Suzuki M, Kurimasa A and Sato T: MiR-7-5p
is a key factor that controls radioresistance via intracellular
Fe2+ content in clinically relevant radioresistant
cells. Biochem Biophys Res Commun. 518:712–718. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zhang K, Wu L, Zhang P, Luo M, Du J, Gao
T, O'Connell D, Wang G, Wang H and Yang Y: miR-9 regulates
ferroptosis by targeting glutamic-oxaloacetic transaminase GOT1 in
melanoma. Mol Carcinog. 57:1566–1576. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Luo M, Wu L, Zhang K, Wang H, Zhang T,
Gutierrez L, O'Connell D, Zhang P, Li Y, Gao T, et al: miR-137
regulates ferroptosis by targeting glutamine transporter SLC1A5 in
melanoma. Cell Death Differ. 25:1457–1472. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Qi YF, Yang Y, Zhang Y, Liu S, Luo B and
Liu W: Down regulation of lactotransferrin enhanced
radio-sensitivity of nasopharyngeal carcinoma. Comput Biol Chem.
90:1074262021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
He W, Jin H, Liu Q and Sun Q: miR-182-5p
contributes to radioresistance in nasopharyngeal carcinoma by
regulating BNIP3 expression. Mol Med Rep. 23:1302021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zhang Y, Zheng L, Lin S, Liu Y, Wang Y and
Gao F: MiR-124 enhances cell radiosensitivity by targeting PDCD6 in
nasopharyngeal carcinoma. Int J Clin Exp Pathol. 10:11461–11470.
2017.PubMed/NCBI
|
|
106
|
Tian Y, Tian Y, Tu Y, Zhang G, Zeng X, Lin
J, Ai M, Mao Z, Zheng R and Yuan Y: microRNA-124 inhibits stem-like
properties and enhances radiosensitivity in nasopharyngeal
carcinoma cells via direct repression of expression of JAMA. J Cell
Mol Med. 24:9533–9544. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Chen L, Zhou H and Guan Z: CircRNA_000543
knockdown sensitizes nasopharyngeal carcinoma to irradiation by
targeting miR-9/platelet-derived growth factor receptor B axis.
Biochem Biophys Res Commun. 512:786–792. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Hu JL, He GY, Lan XL, Zeng ZC, Guan J,
Ding Y, Qian XL, Liao WT, Ding YQ and Liang L: Inhibition of
ATG12-mediated autophagy by miR-214 enhances radiosensitivity in
colorectal cancer. Oncogenesis. 7:162018. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Han JB, Huang ML, Li F, Yang R, Chen SM
and Tao ZZ: MiR-214 mediates cell proliferation and apoptosis of
nasopharyngeal carcinoma through targeting both WWOX and PTEN.
Cancer Biother Radiopharm. 35:615–625. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhang ZC, Li YY, Wang HY, Fu S, Wang XP,
Zeng MS, Zeng YX and Shao JY: Knockdown of miR-214 promotes
apoptosis and inhibits cell proliferation in nasopharyngeal
carcinoma. PLoS One. 9:e861492014. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
He J, Tang Y and Tian Y: MicroRNA-214
promotes proliferation and inhibits apoptosis via targeting Bax in
nasopharyngeal carcinoma cells. Mol Med Rep. 12:6286–6292. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Deng M, Ye Q, Qin Z, Zheng Y, He W, Tang
H, Zhou Y, Xiong W, Zhou M, Li X, et al: miR-214 promotes
tumorigenesis by targeting lactotransferrin in nasopharyngeal
carcinoma. Tumour Biol. 34:1793–1800. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Deng X, Ma L, Wu M, Zhang G, Jin C, Guo Y
and Liu R: miR-124 radiosensitizes human glioma cells by targeting
CDK4. J Neurooncol. 114:263–274. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Luo Y, Wang J, Wang F, Liu X, Lu J, Yu X,
Ma X, Peng X and Li X: Foxq1 promotes metastasis of nasopharyngeal
carcinoma by inducing vasculogenic mimicry via the EGFR signaling
pathway. Cell Death Dis. 12:4112021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Lu J, Xu F and Lu H: LncRNA PVT1 regulates
ferroptosis through miR-214-mediated TFR1 and p53. Life Sci.
260:1183052020. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Bao WD, Zhou XT, Zhou LT, Wang F, Yin X,
Lu Y, Zhu LQ and Liu D: Targeting miR-124/Ferroportin signaling
ameliorated neuronal cell death through inhibiting apoptosis and
ferroptosis in aged intracerebral hemorrhage murine model. Aging
Cell. 19:e132352020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Li Y, Chen F, Chen J, Chan S, He Y, Liu W
and Zhang G: Disulfiram/Copper induces antitumor activity against
both nasopharyngeal cancer cells and cancer-associated fibroblasts
through ROS/MAPK and Ferroptosis pathways. Cancers (Basel).
12:1382020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
He X, Yao Q, Fan D, Duan L, You Y, Liang
W, Zhou Z, Teng S, Liang Z, Hall DD, et al: Cephalosporin
antibiotics specifically and selectively target nasopharyngeal
carcinoma through HMOX1-induced ferroptosis. Life Sci.
277:1194572021. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Shibata Y, Yasui H, Higashikawa K,
Miyamoto N and Kuge Y: Erastin, a ferroptosis-inducing agent,
sensitized cancer cells to X-ray irradiation via glutathione
starvation in vitro and in vivo. PLoS One. 14:e02259312019.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Li M, Wang X, Lu S, He C, Wang C, Wang L,
Wang X, Ge P and Song D: Erastin triggers autophagic death of
breast cancer cells by increasing intracellular iron levels. Oncol
Lett. 20:572020.PubMed/NCBI
|
|
121
|
Tam SY, Wu VW and Law HK: Influence of
autophagy on the efficacy of radiotherapy. Radiat Oncol. 12:572017.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Mou Y, Wang J, Wu J, He D, Zhang C, Duan C
and Li B: Ferroptosis, a new form of cell death: opportunities and
challenges in cancer. J Hematol Oncol. 12:342019. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Friedmann Angeli JP, Krysko DV and Conrad
M: Ferroptosis at the crossroads of cancer-acquired drug resistance
and immune evasion. Nat Rev Cancer. 19:405–414. 2019. View Article : Google Scholar : PubMed/NCBI
|