|
1
|
Tan S, Li D and Zhu X: Cancer immunotherapy: Pros, cons and beyond. Biomed Pharmacother. 124:1098212020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yakirevich E, Sabo E, Lavie O, Mazareb S, Spagnoli GC and Resnick MB: Expression of the MAGE-A4 and NY-ESO-1 cancer-testis antigens in serous ovarian neoplasms. Clin Cancer Res. 9:6453–6460. 2003.PubMed/NCBI
|
|
3
|
Scanlan MJ, Simpson AJ and Old LJ: The cancer/testis genes: Review, standardization, and commentary. Cancer Immun. 4:12004.PubMed/NCBI
|
|
4
|
Liu Y, Wen L, Ma L, Kang Y, Liu KY, Huang XJ, Ruan GR and Lu J: MAGE genes: Prognostic indicators in AL amyloidosis patients. J Cell Mol Med. 23:5672–5678. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Simpson AJ, Caballero OL, Jungbluth A, Chen YT and Old LJ: Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 5:615–625. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sanderson JP, Crowley DJ, Wiedermann GE, Quinn LL, Crossland KL, Tunbridge HM, Cornforth TV, Barnes CS, Ahmed T, Howe K, et al: Preclinical evaluation of an affinity-enhanced MAGE-A4-specific T-cell receptor for adoptive T-cell therapy. Oncoimmunology. 9:16823812019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
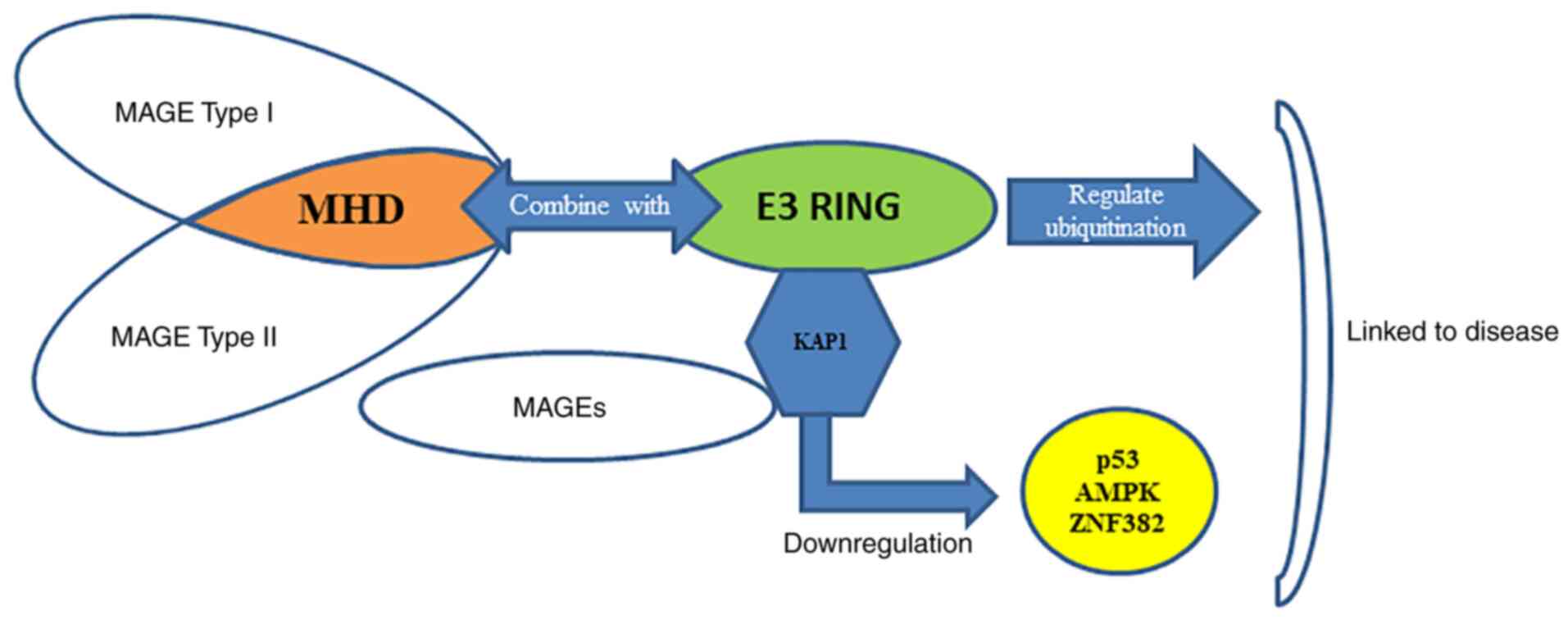
Lee AK and Potts PR: A comprehensive guide to the MAGE family of ubiquitin ligases. J Mol Biol. 429:1114–1142. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu S, Zhao Y, Xu Y, Sang M, Zhao R, Gu L and Shan B: The clinical significance of methylation of MAGE-A1 and-A3 promoters and expression of DNA methyltransferase in patients with laryngeal squamous cell carcinoma. Am J Otolaryngol. 41:1023182020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
De Donato M, Peters SO, Hussain T, Rodulfo H, Thomas BN, Babar ME and Imumorin IG: Molecular evolution of type II MAGE genes from ancestral MAGED2 gene and their phylogenetic resolution of basal mammalian clades. Mamm Genome. 28:443–454. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barker PA and Salehi A: The MAGE proteins: Emerging roles in cell cycle progression, apoptosis, and neurogenetic disease. J Neurosci Res. 67:705–712. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Doyle JM, Gao J, Wang J, Yang M and Potts PR: MAGE-RING protein complexes comprise a family of E3 ubiquitin ligases. Mol Cell. 39:963–974. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Feng Y, Gao J and Yang M: When MAGE meets RING: Insights into biological functions of MAGE proteins. Protein Cell. 2:7–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pineda CT, Ramanathan S, Fon Tacer K, Weon JL, Potts MB, Ou YH, White MA and Potts PR: Degradation of AMPK by a cancer-specific ubiquitin ligase. Cell. 160:715–728. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mao Y, Fan W, Hu H, Zhang L, Michel J, Wu Y, Wang J, Jia L, Tang X, Xu L, et al: MAGE-A1 in lung adenocarcinoma as a promising target of chimeric antigen receptor T cells. J Hematol Oncol. 12:1062019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kerkar SP, Wang ZF, Lasota J, Park T, Patel K, Groh E, Rosenberg SA and Miettinen MM: MAGE-a is more highly expressed than NY-ESO-1 in a systematic immunohistochemical analysis of 3668 cases. J Immunother. 39:181–187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hao YH, Doyle JM, Ramanathan S, Gomez TS, Jia D, Xu M, Chen ZJ, Billadeau DD, Rosen MK and Potts PR: Regulation of WASH-dependent actin polymerization and protein trafficking by ubiquitination. Cell. 152:1051–1064. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Taniura H, Kobayashi M and Yoshikawa K: Functional domains of necdin for protein-protein interaction, nuclear matrix targeting, and cell growth suppression. J Cell Biochem. 94:804–815. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen YC, Hsu WL, Chiu CY, Liao JW, Chang CC and Chang SC: Expression of MAGE-A restricted to testis and ovary or to various cancers in dogs. Vet Immunol Immunopathol. 153:26–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fon Tacer K, Montoya MC, Oatley MJ, Lord T, Oatley JM, Klein J, Ravichandran R, Tillman H, Kim M, Connelly JP, et al: MAGE cancer-testis antigens protect the mammalian germline under environmental stress. Sci Adv. 5:eaav48322019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mouri A, Sasaki A, Watanabe K, Sogawa C, Kitayama S, Mamiya T, Miyamoto Y, Yamada K, Noda Y and Nabeshima T: MAGE-D1 regulates expression of depression-like behavior through serotonin transporter ubiquitylation. J Neurosci. 32:4562–4580. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saenko V, Rogounovitch T, Shimizu-Yoshida Y, Abrosimov A, Lushnikov E, Roumiantsev P, Matsumoto N, Nakashima M, Meirmanov S, Ohtsuru A, et al: Novel tumorigenic rearrangement, delta rfp/ret, in a papillary thyroid carcinoma from externally irradiated patient. Mutat Res. 527:81–90. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cai X, Srivastava S, Sun Y, Li Z, Wu H, Zuvela-Jelaska L, Li J, Salamon RS, Backer JM and Skolnik EY: Tripartite motif containing protein 27 negatively regulates CD4 T cells by ubiquitinating and inhibiting the class II PI3K-C2β. Proc Natl Acad Sci USA. 108:20072–20077. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van den Elsen GA, Tobben L, Ahmed AI, Verkes RJ, Kramers C, Marijnissen RM, Olde Rikkert MG and van der Marck MA: Effects of tetrahydrocannabinol on balance and gait in patients with dementia: A randomised controlled crossover trial. J Psychopharmacol. 31:184–191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carias KV, Zoeteman M, Seewald A, Sanderson MR, Bischof JM and Wevrick R: A MAGEL2-deubiquitinase complex modulates the ubiquitination of circadian rhythm protein CRY1. PLoS One. 15:e02308742020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Faktor J, Pjechová M, Hernychová L and Vojtěšek B: Protein ubiquitination research in oncology. Klin Onkol. 32 (Suppl 3):S56–S64. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Swatek KN and Komander D: Ubiquitin modifications. Cell Res. 26:399–422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shaid S, Brandts CH, Serve H and Dikic I: Ubiquitination and selective autophagy. Cell Death Differ. 20:21–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mattiroli F and Penengo L: Histone ubiquitination: An integrative signaling platform in genome stability. Trends Genet. 37:566–581. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Krützfeldt M, Ellis M, Weekes DB, Bull JJ, Eilers M, Vivanco MD, Sellers WR and Mittnacht S: Selective ablation of retinoblastoma protein function by the RET finger protein. Mol Cell. 18:213–224. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zoumpoulidou G, Broceño C, Li H, Bird D, Thomas G and Mittnacht S: Role of the tripartite motif protein 27 in cancer development. J Natl Cancer Inst. 104:941–952. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Scott KL, Kabbarah O, Liang MC, Ivanova E, Anagnostou V, Wu J, Dhakal S, Wu M, Chen S, Feinberg T, et al: GOLPH3 modulates mTOR signalling and rapamycin sensitivity in cancer. Nature. 459:1085–1090. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kozakova L, Vondrova L, Stejskal K, Charalabous P, Kolesar P, Lehmann AR, Uldrijan S, Sanderson CM, Zdrahal Z and Palecek JJ: The melanoma-associated antigen 1 (MAGEA1) protein stimulates the E3 ubiquitin-ligase activity of TRIM31 within a TRIM31-MAGEA1-NSE4 complex. Cell Cycle. 14:920–930. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zech T, Calaminus SD, Caswell P, Spence HJ, Carnell M, Insall RH, Norman J and Machesky LM: The Arp2/3 activator WASH regulates α5β1-integrin-mediated invasive migration. J Cell Sci. 124:3753–3759. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Small SA: Retromer sorting: A pathogenic pathway in late-onset Alzheimer disease. Arch Neurol. 65:323–328. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Blalock EM, Geddes JW, Chen KC, Porter NM, Markesbery WR and Landfield PW: Incipient Alzheimer's disease: Microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proc Natl Acad Sci USA. 101:2173–2178. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Osterlund C, Töhönen V, Forslund KO and Nordqvist K: Mage-b4, a novel melanoma antigen (MAGE) gene specifically expressed during germ cell differentiation. Cancer Res. 60:1054–1061. 2000.PubMed/NCBI
|
|
37
|
Gjerstorff MF, Harkness L, Kassem M, Frandsen U, Nielsen O, Lutterodt M, Møllgård K and Ditzel HJ: Distinct GAGE and MAGE-A expression during early human development indicate specific roles in lineage differentiation. Hum Reprod. 23:2194–2201. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hao YH, Fountain MD Jr, Fon Tacer K, Xia F, Bi W, Kang SH, Patel A, Rosenfeld JA, Le Caignec C, Isidor B, et al: USP7 acts as a molecular rheostat to promote WASH-dependent endosomal protein recycling and is mutated in a human neurodevelopmental disorder. Mol Cell. 59:956–969. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang J, Teng JL, Zhao D, Ge P, Li B, Woo PC and Liu CH: The ubiquitin ligase TRIM27 functions as a host restriction factor antagonized by mycobacterium tuberculosis PtpA during mycobacterial infection. Sci Rep. 6:348272016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang D, Wang J, Ding N, Li Y, Yang Y, Fang X and Zhao H: MAGE-A1 promotes melanoma proliferation and migration through C-JUN activation. Biochem Biophys Res Commun. 473:959–965. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sang M, Gu L, Yin D, Liu F, Lian Y, Zhang X, Liu S, Huang W, Wu Y and Shan B: MAGE-A family expression is correlated with poor survival of patients with lung adenocarcinoma: A retrospective clinical study based on tissue microarray. J Clin Pathol. 70:533–540. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sang M, Gu L, Liu F, Lian Y, Yin D, Fan X, Ding C, Huang W, Liu S and Shan B: Prognostic significance of MAGE-A11 in esophageal squamous cell carcinoma and identification of related genes based on DNA microarray. Arch Med Res. 47:151–161. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tacer KF and Potts PR: Cellular and disease functions of the Prader-Willi syndrome gene MAGEL2. Biochem J. 474:2177–2190. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xiao TZ, Bhatia N, Urrutia R, Lomberk GA, Simpson A and Longley BJ: MAGE I transcription factors regulate KAP1 and KRAB domain zinc finger transcription factor mediated gene repression. PLoS One. 6:e237472011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang G, Fu Y, Lu X, Wang M, Dong H and Li Q: miR-34a regulates the chemosensitivity of retinoblastoma cells via modulation of MAGE-A/p53 signaling. Int J Oncol. 54:177–187. 2019.PubMed/NCBI
|
|
46
|
Borden KL: RING domains: Master builders of molecular scaffolds? J Mol Biol. 295:1103–1112. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jackson PK, Eldridge AG, Freed E, Furstenthal L, Hsu JY, Kaiser BK and Reimann JD: The lore of the RINGs: Substrate recognition and catalysis by ubiquitin ligases. Trends Cell Biol. 10:429–439. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lupo A, Cesaro E, Montano G, Zurlo D, Izzo P and Costanzo P: KRAB-zinc finger proteins: A repressor family displaying multiple biological functions. Curr Genomics. 14:268–278. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Santos J and Gil J: TRIM28/KAP1 regulates senescence. Immunol Lett. 162:281–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lian Y, Meng L, Ding P and Sang M: Epigenetic regulation of MAGE family in human cancer progression-DNA methylation, histone modification, and non-coding RNAs. Clin Epigenetics. 10:1152018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ladelfa MF, Peche LY, Toledo MF, Laiseca JE, Schneider C and Monte M: Tumor-specific MAGE proteins as regulators of p53 function. Cancer Lett. 325:11–17. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gure AO, Chua R, Williamson B, Gonen M, Ferrera CA, Gnjatic S, Ritter G, Simpson AJ, Chen YT, Old LJ and Altorki NK: Cancer-testis genes are coordinately expressed and are markers of poor outcome in non-small cell lung cancer. Clin Cancer Res. 11:8055–8062. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang S, Zhai X, Wang G, Feng J, Zhu H, Xu L, Mao G and Huang J: High expression of MAGE-A9 in tumor and stromal cells of non-small cell lung cancer was correlated with patient poor survival. Int J Clin Exp Pathol. 8:541–550. 2015.PubMed/NCBI
|
|
54
|
Carling D: AMPK signalling in health and disease. Curr Opin Cell Biol. 45:31–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yang F, Zhou X, Miao X, Zhang T, Hang X, Tie R, Liu N, Tian F, Wang F and Yuan J: MAGEC2, an epithelial-mesenchymal transition inducer, is associated with breast cancer metastasis. Breast Cancer Res Treat. 145:23–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Daudi S, Eng KH, Mhawech-Fauceglia P, Morrison C, Miliotto A, Beck A, Matsuzaki J, Tsuji T, Groman A, Gnjatic S, et al: Expression and immune responses to MAGE antigens predict survival in epithelial ovarian cancer. PLoS One. 9:e1040992014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wischnewski F, Friese O, Pantel K and Schwarzenbach H: Methyl-CpG binding domain proteins and their involvement in the regulation of the MAGE-A1, MAGE-A2, MAGE-A3, and MAGE-A12 gene promoters. Mol Cancer Res. 5:749–759. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Xu Y, Wang C, Zhang Y, Jia L and Huang J: Overexpression of MAGE-A9 is predictive of poor prognosis in epithelial ovarian cancer. Sci Rep. 5:121042015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jeon CH, Kim IH and Chae HD: Prognostic value of genetic detection using CEA and MAGE in peritoneal washes with gastric carcinoma after curative resection: Result of a 3-year follow-up. Medicine (Baltimore). 93:e832014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gu X, Fu M, Ge Z, Zhan F, Ding Y, Ni H, Zhang W, Zhu Y, Tang X, Xiong L, et al: High expression of MAGE-A9 correlates with unfavorable survival in hepatocellular carcinoma. Sci Rep. 4:66252014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hardie DG, Ross FA and Hawley SA: AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 13:251–262. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
White E: Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer. 12:401–410. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Karpf AR, Bai S, James SR, Mohler JL and Wilson EM: Increased expression of androgen receptor coregulator MAGE-11 in prostate cancer by DNA hypomethylation and cyclic AMP. Mol Cancer Res. 7:523–535. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kondo T, Zhu X, Asa SL and Ezzat S: The cancer/testis antigen melanoma-associated antigen-A3/A6 is a novel target of fibroblast growth factor receptor 2-IIIb through histone H3 modifications in thyroid cancer. Clin Cancer Res. 13:4713–4720. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yang B, Wu J, Maddodi N, Ma Y, Setaluri V and Longley BJ: Epigenetic control of MAGE gene expression by the KIT tyrosine kinase. J Invest Dermatol. 127:2123–2128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kouhara H, Hadari YR, Spivak-Kroizman T, Schilling J, Bar-Sagi D, Lax I and Schlessinger J: A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell. 89:693–702. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Weber J, Salgaller M, Samid D, Johnson B, Herlyn M, Lassam N, Treisman J and Rosenberg SA: Expression of the MAGE-1 tumor antigen is up-regulated by the demethylating agent 5-aza-2′-deoxycytidine. Cancer Res. 54:1766–1771. 1994.PubMed/NCBI
|
|
68
|
Vatolin S, Abdullaev Z, Pack SD, Flanagan PT, Custer M, Loukinov DI, Pugacheva E, Hong JA, Morse H III, Schrump DS, et al: Conditional expression of the CTCF-paralogous transcriptional factor BORIS in normal cells results in demethylation and derepression of MAGE-A1 and reactivation of other cancer-testis genes. Cancer Res. 65:7751–7762. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Schwarzenbach H, Eichelser C, Steinbach B, Tadewaldt J, Pantel K, Lobanenkov V and Loukinov D: Differential regulation of MAGE-A1 promoter activity by BORIS and Sp1, both interacting with the TATA binding protein. BMC Cancer. 14:7962014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Colemon A, Harris TM and Ramanathan S: DNA hypomethylation drives changes in MAGE-A gene expression resulting in alteration of proliferative status of cells. Genes Environ. 42:242020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Gu L, Sang M, Li J, Liu F, Wu Y, Liu S, Wang P and Shan B: Expression and prognostic significance of MAGE-A11 and transcription factors (SP1,TFCP2 and ZEB1) in ESCC tissues. Pathol Res Pract. 215:1524462019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Chen A, Santana AL, Doudican N, Roudiani N, Laursen K, Therrien JP, Lee J, Felsen D and Carucci JA: MAGE-A3 is a prognostic biomarker for poor clinical outcome in cutaneous squamous cell carcinoma with perineural invasion via modulation of cell proliferation. PLoS One. 15:e02415512020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Bai S, He B and Wilson EM: Melanoma antigen gene protein MAGE-11 regulates androgen receptor function by modulating the interdomain interaction. Mol Cell Biol. 25:1238–1257. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Li XF, Ren P, Shen WZ, Jin X and Zhang J: The expression, modulation and use of cancer-testis antigens as potential biomarkers for cancer immunotherapy. Am J Transl Res. 12:7002–7019. 2020.PubMed/NCBI
|
|
75
|
Bai S and Wilson EM: Epidermal-growth-factor-dependent phosphorylation and ubiquitinylation of MAGE-11 regulates its interaction with the androgen receptor. Mol Cell Biol. 28:1947–1963. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Aprelikova O, Pandolfi S, Tackett S, Ferreira M, Salnikow K, Ward Y, Risinger JI, Barrett JC and Niederhuber J: Melanoma antigen-11 inhibits the hypoxia-inducible factor prolyl hydroxylase 2 and activates hypoxic response. Cancer Res. 69:616–624. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
James SR, Cedeno CD, Sharma A, Zhang W, Mohler JL, Odunsi K, Wilson EM and Karpf AR: DNA methylation and nucleosome occupancy regulate the cancer germline antigen gene MAGEA11. Epigenetics. 8:849–863. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Krüger S, Ola V, Feller AC, Fischer D and Friedrich M: Expression of cancer-testis antigen CT7 (MAGE-C1) in breast cancer: An immunohistochemical study with emphasis on prognostic utility. Pathol Oncol Res. 13:91–96. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Bai S, Grossman G, Yuan L, Lessey BA, French FS, Young SL and Wilson EM: Hormone control and expression of androgen receptor coregulator MAGE-11 in human endometrium during the window of receptivity to embryo implantation. Mol Hum Reprod. 14:107–116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wilson EM: Androgen receptor molecular biology and potential targets in prostate cancer. Ther Adv Urol. 2:105–117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Askew EB, Bai S, Hnat AT, Minges JT and Wilson EM: Melanoma antigen gene protein-A11 (MAGE-11) F-box links the androgen receptor NH2-terminal transactivation domain to p160 coactivators. J Biol Chem. 284:34793–34808. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Su S, Minges JT, Grossman G, Blackwelder AJ, Mohler JL and Wilson EM: Proto-oncogene activity of melanoma antigen-A11 (MAGE-A11) regulates retinoblastoma-related p107 and E2F1 proteins. J Biol Chem. 288:24809–24824. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yin B, Zeng Y, Liu G, Wang X, Wang P and Song Y: MAGE-A3 is highly expressed in a cancer stem cell-like side population of bladder cancer cells. Int J Clin Exp Pathol. 7:2934–2941. 2014.PubMed/NCBI
|
|
84
|
Wienand K and Shires K: The use of MAGE C1 and flow cytometry to determine the malignant cell type in multiple myeloma. PLoS One. 10:e01207342015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Atanackovic D, Luetkens T, Hildebrandt Y, Arfsten J, Bartels K, Horn C, Stahl T, Cao Y, Zander AR, Bokemeyer C and Kröger N: Longitudinal analysis and prognostic effect of cancer-testis antigen expression in multiple myeloma. Clin Cancer Res. 15:1343–1352. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Chen X, Wang L, Liu J, Huang L, Yang L, Gao Q, Shi X, Li J, Li F, Zhang Z, et al: Expression and prognostic relevance of MAGE-A3 and MAGE-C2 in non-small cell lung cancer. Oncol Lett. 13:1609–1618. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yang B, O'Herrin SM, Wu J, Reagan-Shaw S, Ma Y, Bhat KM, Gravekamp C, Setaluri V, Peters N, Hoffmann FM, et al: MAGE-A, mMage-b, and MAGE-C proteins form complexes with KAP1 and suppress p53-dependent apoptosis in MAGE-positive cell lines. Cancer Res. 67:9954–9962. 2007. View Article : Google Scholar : PubMed/NCBI
|