Introduction
Triple-negative breast cancer (TNBC) is the most
aggressive subtype of breast cancer and characterized by a lack of
expression of estrogen and progesterone receptors and human
epidermal growth factor receptor 2 (HER2) (1,2). The
Nottingham Prognostic Index, tumor size, Ki-67 labelling index (LI)
and lymph node status are recognized as useful prognostic factors;
however, these factors are not specific for TNBC (3,4).
Thus, a novel prognostic marker for patients with TNBC is
needed.
Adipophilin (ADP), also referred to as perilipin 2,
is a lipid-associated protein that coats the surface of
intracytoplasmic lipid droplets and modulates lipolysis within the
cells (5–7). ADP has been reported to be related
with some non-neoplastic conditions, such as steatosis of the liver
and diabetes (6,8). In addition, several studies show that
ADP expression in tumor cells is associated with poor prognosis for
some types of carcinomas, including lung (9) and pancreatic ductal adenocarcinomas
(10). The present authors
recently demonstrated ADP expression as an independent poor
prognostic indicator for patients with TNBC (11), with ADP expression in the tumor
cells of resected TNBC specimens (defined as >30% of the
neoplastic cells) observed in 23.0% of patients with TNBC. In
addition, TNBC patients with ADP-positive tumors exhibit poorer
relapse-free survival (RFS) as compared with those with
ADP-negative tumors, with multivariate analysis revealing ADP as an
independent poor prognostic marker (11).
Initial treatment plans for patients with breast
cancer are typically decided based on the analysis of biopsy
specimens. As the results are derived from operative specimens of
patients with TNBC, the prognostic significance of ADP expression
in biopsy specimens and the relationship of ADP expression between
operative and biopsy specimens must be clarified. The aim of the
present study was to analyze the prognostic significance of ADP
expression using preoperative biopsy specimens from patients with
TNBC and to compare the ADP-expression status between biopsy and
operative specimens.
Materials and methods
Patient selection
The present study selected 165 consecutive patients
with TNBC who underwent surgical resection at the Department of
Surgery of Kansai Medical University Hospital between January 2006
and December 2018. Patients diagnosed with invasive breast
carcinoma of no special type according to the recent World Health
Organization Classification of Breast Tumors (12) were selected. Patients for whom
biopsy specimens were unavailable were excluded from the study. In
addition, patients with a special type of invasive carcinoma were
excluded from the present study, as each special type of carcinoma
has unique clinicopathological features. Ultimately, 102 patients
with TNBC were included in the present study. The patient cohort
overlapped with those of our previous studies (11,13,14).
The prognostic significance of ADP expression was previously
analyzed in tissue microarrays using operative specimens from
patients with TNBC (11). The
present study included information from our previous study
regarding the ADP-expression status of operative specimens
(11). Additionally, the authors
previously examined the relationship between clinicopathological
features and PD-L1-positive cancer-associated fibroblasts (13) and the immune-checkpoint protein
CD155 (14) in patients with TNBC
using tissue microarrays of operative specimens. However, the
content of the present study does not overlap with that of the
previous two studies.
This retrospective single-institution study was
conducted in accordance with the principles of the Declaration of
Helsinki and the study protocol was approved by the Institutional
Review Board of Kansai Medical University Hospital (approval no.
2019234). Informed consent was obtained from patients using the
opt-out method because of the retrospective design of the study and
because there was no risk to the participants. In addition, the
present study does not include minors. Information regarding this
study, such as the inclusion criteria and opportunity to opt out,
is provided on the institutional website
(kmu.ac.jp/hirakata/hospital/2671t800000136cd-att/a1582783269511.pdf).
Histopathologic analysis
Surgically resected and biopsy specimens were fixed
with 10% formalin at room temperature (24–48 h), sectioned,
dehydrated by ethanol and xylene at room temperature, embedded in
paraffin (60°C), and stained with hematoxylin and eosin (5 min
each) at room temperature. All histopathological diagnoses were
independently evaluated by more than two experienced diagnostic
pathologists. The TNM Classification of Malignant Tumors (8th
edition;
legeforeningen.no/contentassets/201604933ce448e888a101ab969a4205/tnm-classification-of-malignant-tumours-8th-edition.pdf)
was used and histopathological grading was based on the Nottingham
histological grade (15).
According to a meta-analysis of patients with TNBC, the Ki-67 LI
was considered high at ≥40% (16).
The response following neoadjuvant chemotherapy (NAC) was assessed
based on the Miller-Payne grading system established by Ogston
et al (17).
Tissue microarray
Hematoxylin and eosin-stained slides were evaluated
to select regions of the resected specimens that were most
morphologically representative of carcinoma. A total of three
tissue cores with diameters of 2 mm were punched out from the
paraffin-embedded blocks for each patient. The tissue cores were
then arrayed in recipient paraffin blocks for analysis. These
specimens were also used in our previous studies (11,13,14).
Immunohistochemistry
Immunohistochemical analyses were performed using a
Discovery ULTRA automated immunohistochemistry staining system
(Roche Diagnostics). A mouse monoclonal antibody against ADP
(1:100; cat. no. AP125; Progen Biotechnik GmbH) was used as the
primary antibody. Human sebaceous gland tissues were used as
positive controls for ADP staining. All biopsy specimens and tumor
microarrays were evaluated for ADP levels. At least two researchers
independently evaluated the immunohistochemical staining results.
These procedures were similar to those previously described
(11). To determine the cut-off
value for ADP expression, analyses were performed using positive
cut-off values of 1, 5, 10, 20 and 30%.
Statistical analyses
All analyses were performed using SPSS software
(v.27.0; IBM Corp.). Correlations between two groups were
determined using Fisher's exact test for categorical variables and
the Mann-Whitney U test for continuous variables. RFS was evaluated
using Kaplan-Meier analysis and log-rank tests were used to compare
the two groups. The Cox proportional hazards model was used to
examine the correlation between clinicopathological parameters and
survival. Statistical significance was set at P≤0.05.
Results
Clinicopathological features
The present study included 102 women with TNBC.
Table I summarizes the
clinicopathological features of the study cohort. The median age at
the time of initial diagnosis was 63 years (range: 31–93 years).
All patients were diagnosed with TNBC based on biopsy results and
all tumors were invasive carcinomas of no special type. The median
clinical tumor diameter was 20 mm (range: 0–100 mm). Two patients
were initially diagnosed with ductal carcinoma in situ
(non-invasive ductal carcinoma); thus, the cohort included a
clinical tumor diameter of 0 mm. Patients were clinically staged as
0 (2 patients), I (41 patients), IIA (28 patients), IIB (23
patients), IIIA (3 patients), IIIB (4 patients) and IIIC (1
patient). A total of 21 patients (20.6%) experienced relapse
involving distant metastasis and none experienced local
recurrence.
 | Table I.Clinical characteristics of patients
with triple-negative breast cancer. |
Table I.
Clinical characteristics of patients
with triple-negative breast cancer.
| Factors | n | % |
|---|
| Total | 102 |
|
| Age median (range)
(years) |
|
|
| 63
(31–93) |
|
|
| Menopause status |
|
|
|
Premenopausal | 16 | 15.7 |
|
Postmenopausal | 83 | 81.4 |
|
Unknown | 3 | 2.9 |
| Tumor size median
(range) (clinical: mm) |
|
|
| 20
(0–100) |
|
|
| Lymph node status
(clinical) |
|
|
|
Positive | 38 | 37.3 |
|
Negative | 64 | 62.7 |
| Clinical stage |
|
|
| 0 | 2 | 2.0 |
| I | 41 | 40.2 |
| IIA | 28 | 27.5 |
| IIB | 23 | 22.5 |
| IIIA | 3 | 2.9 |
| IIIB | 4 | 3.9 |
| IIIC | 1 | 1.0 |
| Ki-67 LI (biopsy
specimen) |
|
|
| High | 69 | 67.6 |
| Low | 33 | 32.4 |
| Adjuvant
chemotherapy |
|
|
|
Preoperation
(anthracycline-based, taxane-based regimens) | 47 | 46.1 |
|
Post-operation
(anthracycline-based, taxane-based regimens (22 patients),
anthracycline-based regimens (3 patients) and fluoropyridine (6
patients) | 31 | 30.4 |
| Not
performed | 22 | 21.6 |
|
Undetermined | 2 | 2.0 |
As shown in Table
I, NAC was administered to 47 patients (46.1%) and adjuvant
chemotherapy to 31 patients (30.4%). No NAC or adjuvant
chemotherapy was administered to 22 patients (21.6%). An overview
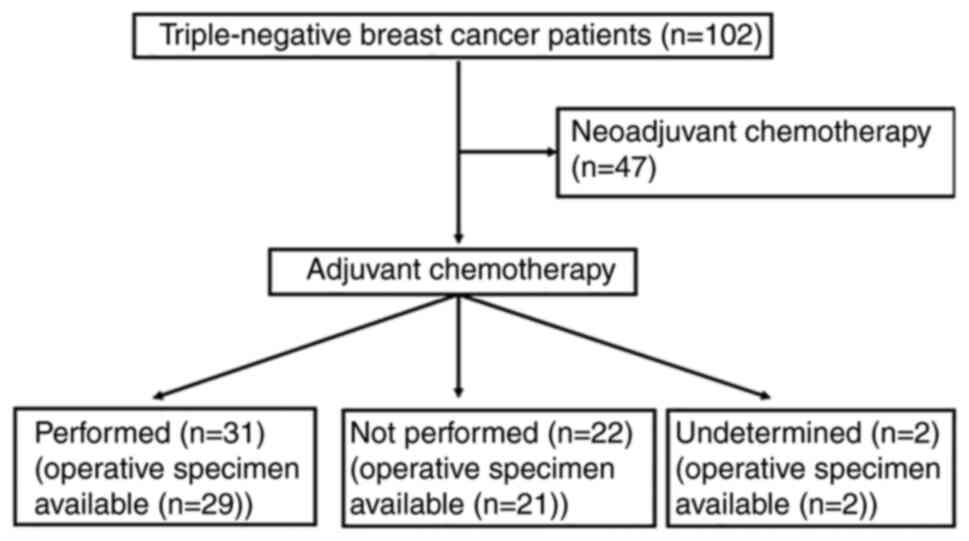
of the study cohort is summarized in Fig. 1 and subclassification of the
clinicopathological characteristics of the NAC and non-NAC groups
is shown in Table II. Tumor size
and lymph node status were clinically evaluated in the NAC group
and pathologically evaluated in the non-NAC group.
 | Table II.Subclassification of clinical
characteristics of patients with triple-negative breast cancer. |
Table II.
Subclassification of clinical
characteristics of patients with triple-negative breast cancer.
| Factors (NAC
group) | n | % |
|---|
| Total | 47 |
|
| Age median (range)
(years) | 53 (31–77) |
|
| Menopause
status |
|
|
|
Premenopausal | 14 | 29.8 |
|
Postmenopausal | 30 | 63.8 |
|
Unknown | 3 | 6.4 |
| Tumor size median
(range) (clinical: mm) | 23 (4–100) |
|
| Lymph node status
(clinical) |
|
|
|
Positive | 22 | 46.8 |
|
Negative | 25 | 53.2 |
| Clinical stage |
|
|
| I | 15 | 31.9 |
|
IIA | 14 | 29.8 |
|
IIB | 11 | 23.4 |
|
IIIA | 3 | 6.4 |
|
IIIB | 3 | 6.4 |
|
IIIC | 1 | 2.1 |
| Ki-67 LI (biopsy
specimen) |
|
|
|
High | 34 | 72.3 |
|
Low | 13 | 27.7 |
| Miller-Payne
grading |
|
|
| 1 | 13 | 27.7 |
| 2 | 9 | 19.1 |
| 3 | 2 | 4.3 |
| 4 | 5 | 10.6 |
| 5 | 18 | 38.3 |
| Factors (Non-NAC
group) |
|
|
|
Total | 55 |
|
| Age median (range)
(years) | 69 (31–93) |
|
| Menopause
status |
|
|
|
Premenopausal | 2 | 3.6 |
|
Postmenopausal | 53 | 96.4 |
| Tumor size median
(range) (clinical: mm) | 20 (2–55) |
|
| Pathological
stage |
|
|
| I | 24 | 43.6 |
|
IIA | 19 | 34.5 |
|
IIB | 4 | 7.3 |
|
IIIA | 4 | 7.3 |
|
IIIB | 3 | 5.5 |
|
IIIC | 1 | 1.8 |
| Lymph node
status |
|
|
|
Positive | 30 | 54.5 |
|
Negative | 14 | 25.5 |
| Not
tested | 11 | 20.0 |
| Nottingham
histological grade |
|
|
| 1 | 2 | 3.6 |
| 2 | 26 | 47.3 |
| 3 | 27 | 49.1 |
| Ki-67 LI (biopsy
specimen) |
|
|
|
High | 35 | 63.6 |
|
Low | 20 | 36.4 |
| Adjuvant
chemotherapy |
|
|
|
Performed | 31 | 56.4 |
| Not
performed | 22 | 40.0 |
|
Undetermined | 2 | 3.6 |
Chemotherapy regimens were selected based on the
treatment criteria (Table I). All
patients in the NAC group and 22 patients (71%) in the adjuvant
chemotherapy group were administered sequential anthracycline-based
and taxane-based regimens. A total of three patients in the
adjuvant chemotherapy group (10%) were administered only
anthracycline-based regimens and six patients in the adjuvant
chemotherapy group (19%) were administered oral fluoropyridine
therapy. The anthracycline-based regimens included EC (epirubicin,
100 mg/m2; and cyclophosphamide, 500 mg/m2),
AC (doxorubicin, 60 mg/m2; and cyclophosphamide, 600
mg/m2) and FEC (epirubicin, 100 mg/m2;
cyclophosphamide, 500 mg/m2; and 5-fluorouracil, 500
mg/m2). Chemotherapy was administered every 2 to 3 weeks
for four cycles. Taxane-based regimens included docetaxel at a dose
of 70 mg/m2 every 3 weeks for four cycles or paclitaxel
at a weekly dose of 80 mg/m2 for 12 doses with scheduled
rest. Fluoropyrimidine regimens included oral uracil-tegafur (300
mg/m2) daily for 2 years and oral S-1 (100 mg/day) on a
21-day cycle of 14 consecutive days dosing with 7 days off, which
was repeated for 1 year.
Correlation between ADP expression in
biopsy specimens and postoperative RFS in patients without NAC or
adjuvant chemotherapy
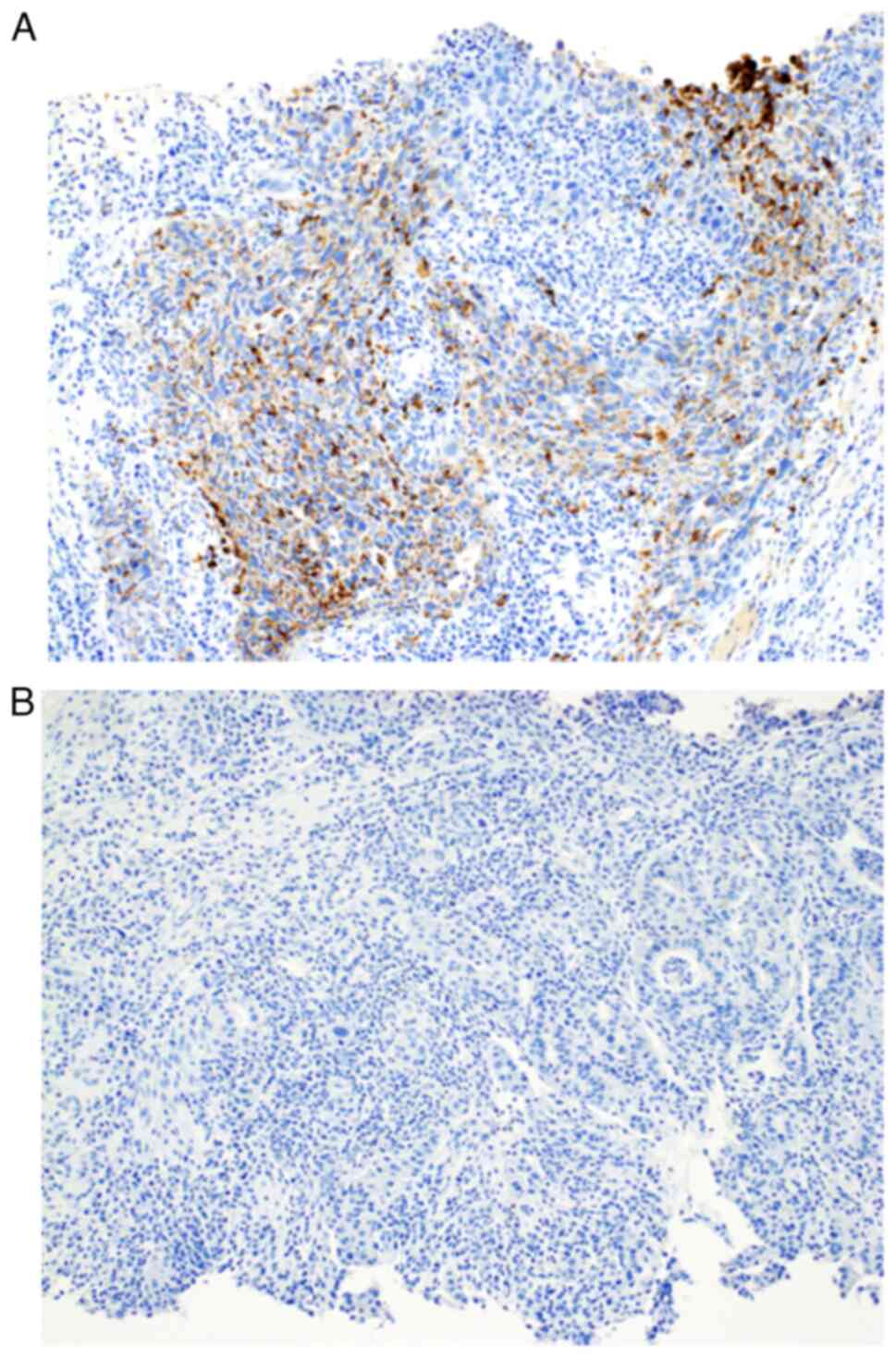
Typical positive and negative immunohistochemical
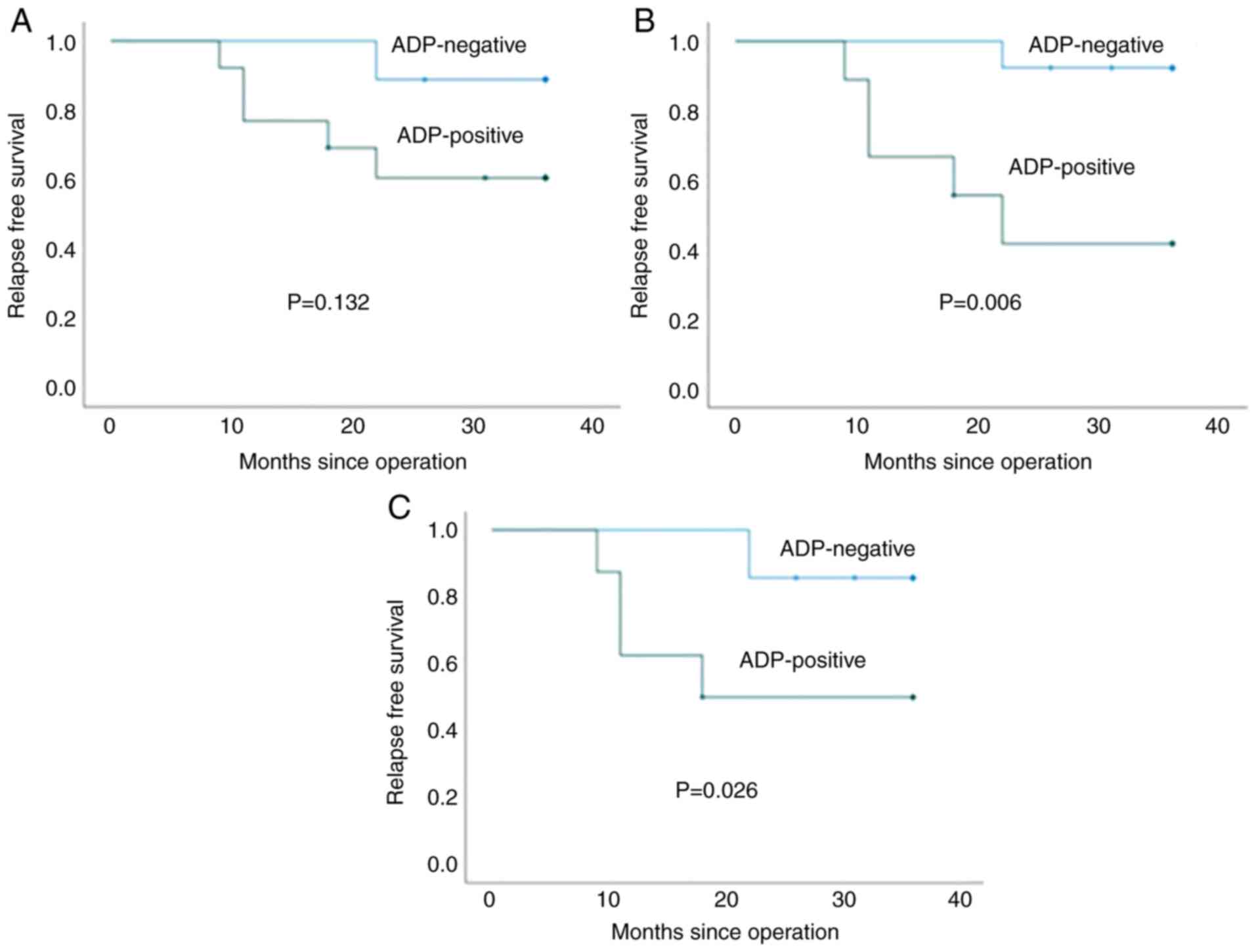
staining of biopsy specimens for ADP are shown in Fig. 2. To evaluate the optimal cut-off
value of ADP expression in the biopsy specimens, the relationship
between ADP-expression values and RFS in patients who were not
administered NAC or adjuvant chemotherapy was analyzed. Cut-off
values of 5, 10, 20 and 30% were significantly associated with RFS
(P=0.006, 0.006, 0.006 and 0.026, respectively), whereas a cut-off
value of 1% was not significantly associated with RFS (P=0.132;
Fig. 3). Based on these findings
and because the cut-off value for operative specimens in our
previous study was set at 30% (11), a cut-off value of 30% was used for
subsequent analyses.
Correlation between
clinicopathological factors and ADP expression in biopsy
specimens
The correlation between clinicopathological factors
and ADP expression in biopsy specimens (cut-off value: 30%) is
summarized in Table III. Among
the entire cohort, including the NAC and non-NAC groups, ADP
expression was observed in biopsy specimens from 35.3% of patients
(36/102), whereas biopsy specimens from the remaining 64.7% of
patients (66/102) were ADP-negative. The presence of lymph node
metastasis based on clinical evaluation was significantly higher in
ADP-negative patients compared with that in ADP-positive patients
(P=0.031) and a high Ki-67 LI was associated with ADP-positivity
(P<0.001). However, tumor size and clinical stage were not
significantly associated with ADP expression.
 | Table III.Correlation between
clinicopathological factors and ADP expression in biopsy
specimens. |
Table III.
Correlation between
clinicopathological factors and ADP expression in biopsy
specimens.
| Factors | ADP-positive
(n=36) | ADP-negative
(n=66) | P-value |
|---|
| Age (years; median
± SD) | 57±16 | 64±14 | 0.038a |
| Menopause
status |
|
|
|
|
Premenopausal | 9 | 7 | 0.085 |
|
Postmenopausal | 26 | 57 |
|
|
Unknown | 1 | 2 |
|
| Tumor size
(clinical: mm) |
|
|
|
|
≤20 | 18 | 35 | 0.837 |
|
>20 | 18 | 31 |
|
| Clinical stage |
|
|
|
| 0 + I +
II | 34 | 60 | 0.709 |
|
III | 2 | 6 |
|
| Lymph node status
(clinical) |
|
|
|
|
Positive | 8 | 30 | 0.031a |
|
Negative | 28 | 36 |
|
| Ki-67 LI |
|
|
|
|
High | 32 | 37 |
<0.001a |
|
Low | 4 | 29 |
|
ADP expression was observed in 34% (16/47) of
patients in the NAC group and 36.4% (20/55) of patients in the
non-NAC group (Tables IV and
V). In the NAC group, ADP
expression was significantly associated with the clinically
evaluated absence of lymph node metastasis (P=0.007) and high Ki-67
LI (P=0.004) but not with tumor size, clinical stage, or the effect
of NAC (Miller-Payne grading). In the non-NAC group, ADP expression
was significantly associated with histological grade (P=0.026) and
a high Ki-67 LI (P=0.019) but not with pathologically evaluated
lymph node metastasis or tumor size. In the early stage of disease
for patients with TNBC (clinical or pathological stages 0, I, or
II), ADP expression was significantly associated with absence of
lymph node metastasis by clinical assessment in all patient groups
(P=0.022; Table VI). This trend
was also observed in the NAC group (P=0.005) but not in the non-NAC
group (P=0.759).
 | Table IV.Correlation between
clinicopathological factors (NAC group) and ADP expression in
biopsy specimens . |
Table IV.
Correlation between
clinicopathological factors (NAC group) and ADP expression in
biopsy specimens .
| Factors | ADP-positive
(n=16) | ADP-negative
(n=31) | P-value |
|---|
| Age (years; median
± SD) | 50±13 | 57±14 | 0.082 |
| Menopause
status |
|
|
|
|
Premenopausal | 7 | 7 | 0.177 |
|
Postmenopausal | 8 | 22 |
|
|
Unknown | 1 | 2 |
|
| Tumor size
(clinical: mm) |
|
|
|
|
≤20 | 7 | 14 | 1.000 |
|
>20 | 9 | 17 |
|
| Clinical stage |
|
|
|
| I +
II | 14 | 26 | 1.000 |
|
III | 2 | 5 |
|
| Lymph node status
(clinical) |
|
|
|
|
Positive | 3 | 19 | 0.007a |
|
Negative | 13 | 12 |
|
| Ki-67 LI |
|
|
|
|
High | 15 | 19 | 0.004a |
|
Low | 1 | 12 |
|
| Miller-Payne
grading |
|
|
|
|
1+2 | 6 | 16 | 0.538 |
|
3+4+5 | 10 | 15 |
|
 | Table V.Correlation between
clinicopathological factors (non-NAC group) and ADP expression in
biopsy specimens. |
Table V.
Correlation between
clinicopathological factors (non-NAC group) and ADP expression in
biopsy specimens.
| Factors | ADP-positive
(n=20) | ADP-negative
(n=35) | P-value |
|---|
| Age (years; median
± SD) | 63±17 | 70±12 | 0.156 |
| Menopause
status |
|
|
|
|
Premenopausal | 2 | 0 | 0.128 |
|
Postmenopausal | 18 | 35 |
|
| Tumor size
(pathological: mm) |
|
|
|
|
≤20 | 13 | 16 | 0.262 |
|
>20 | 7 | 19 |
|
| Pathological
stage |
|
|
|
| I +
II | 18 | 29 | 0.696 |
|
III | 2 | 6 |
|
| Lymph node status
(pathological) |
|
|
|
|
Positive | 5 | 9 | 0.534 |
|
Negative | 14 | 16 |
|
| Not
tested | 1 | 10 |
|
| Nottingham
histological grade |
|
|
|
|
1+2 | 6 | 22 | 0.026a |
| 3 | 14 | 13 |
|
| Ki-67 LI |
|
|
|
|
High | 17 | 18 | 0.019a |
|
Low | 3 | 17 |
|
| Adjuvant
chemotherapy |
|
|
|
|
Performed | 11 | 20 | 1.000 |
| Not
performed | 8 | 14 |
|
|
Undetermined | 1 | 1 |
|
 | Table VI.Correlation between lymph node
metastasis and ADP expression in early-stage patients. |
Table VI.
Correlation between lymph node
metastasis and ADP expression in early-stage patients.
| Factors | ADP-positive
(n=16) | ADP-negative
(n=31) | P-value |
|---|
| Lymph node status
(clinical) |
|
|
|
|
Positive | 6 | 25 | 0.022a |
|
Negative | 28 | 35 |
|
| Factors (NAC
group) | ADP-positive
(n=14) | ADP-negative
(n=26) |
|
| Lymph node status
(clinical) |
|
|
|
|
Positive | 1 | 14 | 0.005a |
|
Negative | 13 | 12 |
|
| Factors (Non-NAC
group) | ADP-positive
(n=20) | ADP-negative
(n=34) |
|
| Lymph node status
(clinical) |
|
|
|
|
Positive | 5 | 11 | 0.759 |
|
Negative | 15 | 23 |
|
| Factors (Non-NAC
group) | ADP-positive
(n=17) | ADP-negative
(n=20) |
|
| Lymph node status
(pathological) |
|
|
|
|
Positive | 3 | 4 | 1.000 |
|
Negative | 14 | 16 |
|
Association of ADP expression in
biopsy specimens and postoperative RFS
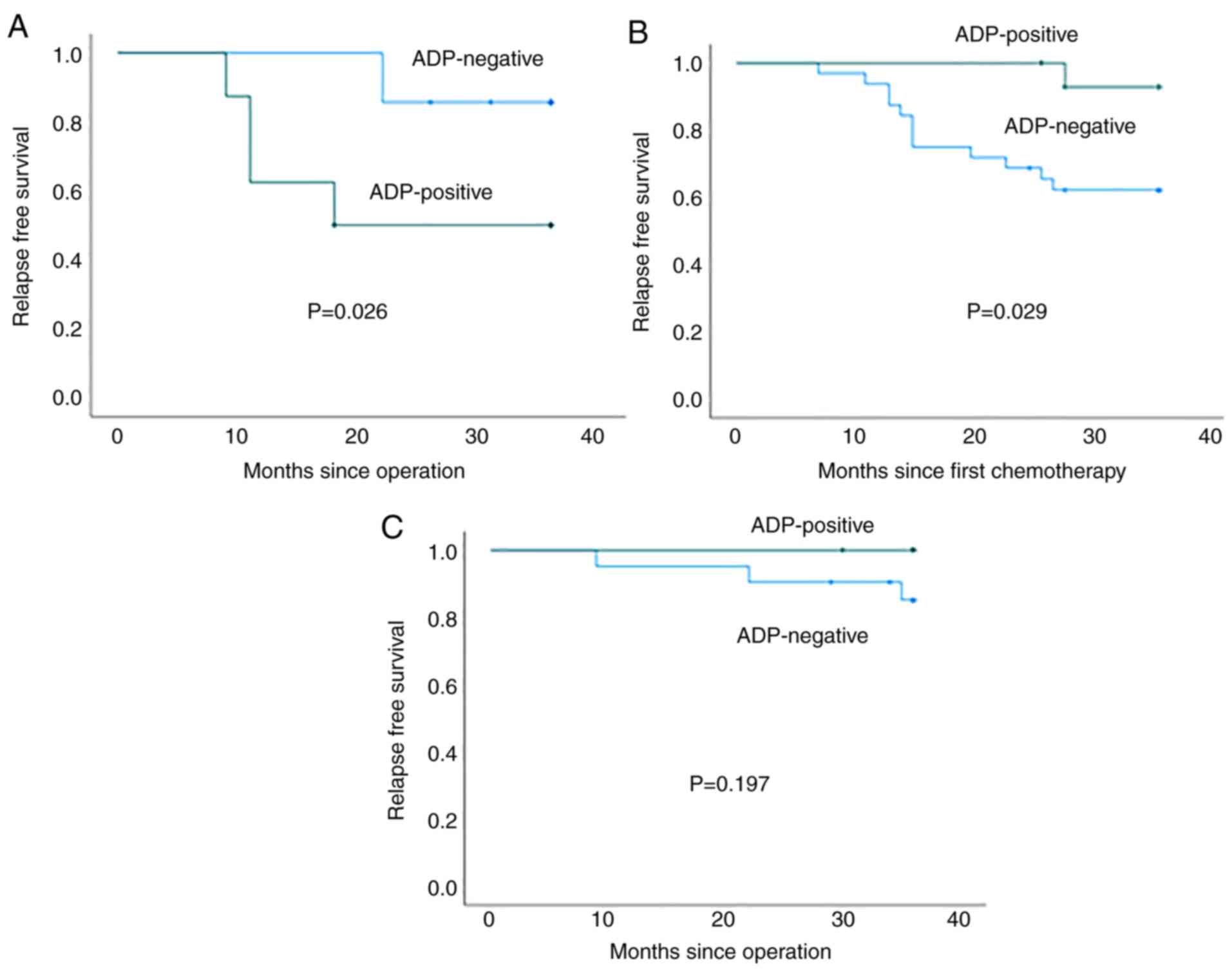
Fig. 4 shows the
RFS curves for ADP-positive and -negative patients. As described,
ADP expression in biopsy specimens was significantly associated
with a poor RFS in patients who were not administered NAC or
adjuvant chemotherapy (P=0.026; Fig.
4A). By contrast, the RFS of ADP-negative patients was
significantly poorer as compared with that of ADP-positive patients
in the NAC group (P=0.029; Fig.
4B). ADP expression was not significantly associated with the
RFS of patients administered adjuvant chemotherapy (P=0.197;
Fig. 4C).
Fig. 5 shows the
RFS curves of ADP expression and Ki-67 LI for biopsy specimens of
patients who were not administered NAC or adjuvant chemotherapy.
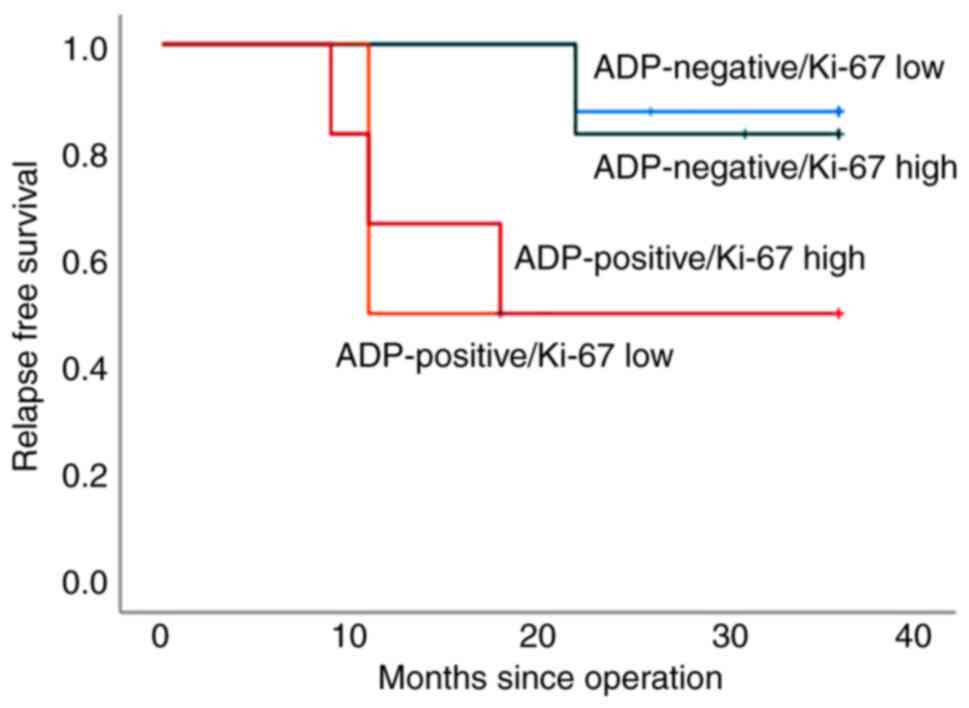
There was no difference in RFS between patients with high and low
Ki-67 LI values among both ADP-negative and -positive patients
(P=0.832 and P=0.979, respectively).
Prognostic potential of ADP expression
in biopsy specimens from patients without NAC or adjuvant
chemotherapy
Univariate analysis was then performed to determine
the association between clinicopathological factors and RFS in
patients who were not treated with NAC or adjuvant chemotherapy
(Table VII). Only ADP expression
was significantly associated with a poor RFS (hazard ratio, 5.630;
95% confidence interval, 1–31.72; P=0.05), whereas tumor size,
lymph node status, Ki-67 LI and histological grade were not.
 | Table VII.Univariate analysis of RFS in
patients without NAC and adjuvant chemotherapy. |
Table VII.
Univariate analysis of RFS in
patients without NAC and adjuvant chemotherapy.
| Variables | HR | 95% CI | P-value |
|---|
| Tumor size
(mm) | 1.831 | 0.335-10.000 | 0.485 |
| 20 <
vs. ≤ 20 |
|
|
|
| Lymph node
status | 5.154 | 0.570-46.65 | 0.145 |
|
Positive vs. negative |
|
|
|
| Nottingham
histological grade | 1.567 | 0.315-7.793 | 0.583 |
| 3 vs.
1+2 |
|
|
|
| Ki-67 LI | 1.776 | 0.325-9.697 | 0.507 |
| High
vs. low |
|
|
|
| ADP expression | 5.630 | 1.000-31.720 | 0.050a |
|
Positive vs. Negative |
|
|
|
Correlation of ADP expression in
biopsy and operative specimens
Not including the operative specimens from patients
treated with NAC, both biopsy and operative specimens were
available for 52 patients in the current study cohort. This
included 29 patients who had been treated with adjuvant
chemotherapy, 21 patients without adjuvant chemotherapy and two
patients for whom the performance of adjuvant chemotherapy was not
determined (Fig. 1). Table VII shows the correlation of ADP
expression in biopsy and operative specimens according to a cut-off
value of 30% for expression. The concordance rate was 73.1% and
Cohen's kappa coefficient was 0.385, indicating fair agreement
(P=0.003).
Discussion
The present study clearly demonstrated that ADP
expression in biopsy specimens was a poor prognostic factor in
patients with TNBC and consistent with its expression in operative
specimens (11). Using an optimal
cut-off value of 30%, ADP expression in biopsy specimens was
significantly associated with higher Ki-67 LI. Finally, fair
agreement was observed in ADP expression between biopsy and
surgical specimens.
Biopsy specimens provide critical information for
deciding the treatment strategy for patients with breast cancer,
including the expression status of hormone receptors and HER2.
Furthermore, histological grade and Ki-67 LI are well-known
prognostic indicators (3,4); however, these indicators are not
specific for TNBC. Using immunohistochemical staining and operative
specimens of TNBC, the present authors previously demonstrated that
ADP expression is an independent poor prognostic factor in patients
with TNBC (9). The present study
examined the prognostic role of ADP expression in preoperative
biopsy specimens of patients with TNBC. First, the optimal cut-off
value of ADP expression in biopsy specimens was determined by
analyzing the relationship between ADP expression and RFS in
patients who had not been treated with NAC or adjuvant
chemotherapy. These patients were chosen because they were not
influenced by chemotherapy and would likely demonstrate the direct
effect of ADP expression. Cut-off values of 5, 10, 20 and 30% were
significantly associated with RFS. The cut-off value of ADP
expression was set at 30% for subsequent analyses, as it was
significantly associated with RFS and used as the cut-off value in
our previous study evaluating operative specimens of TNBC (11). In general, a cut-off value of 30%
ADP expression can be used to predict the RFS of patients with TNBC
using either biopsy or operative specimens. Other studies showed
ADP expression as a significant poor prognostic indicator in some
types of carcinomas, including lung adenocarcinoma (9) and pancreatic ductal adenocarcinoma
(10). However, these results were
derived using only operative specimens from carcinomas (9,10).
Therefore, the present study was the first to analyze the
prognostic significance of ADP expression in biopsy specimens. In
addition, the cut-off value of ADP expression might differ among
the several types of carcinoma. For instance, in lung
adenocarcinoma (9) and pancreatic
ductal adenocarcinoma (10) it was
set at 5%.
The prognostic significance of ADP expression was
subsequently analyzed in biopsy specimens of patients with TNBC
treated with NAC or adjuvant chemotherapy. Interestingly, ADP
expression was a significantly worse indicator of RFS in patients
who did not receive NAC or adjuvant chemotherapy. However, among
the NAC group, ADP-positive patients showed a significantly better
RFS compared with ADP-negative patients. ADP expression was not a
significant factor in patients administered adjuvant chemotherapy.
These results suggested that ADP-positive patients exhibit better
chemotherapy responsiveness as compared with ADP-negative patients;
however, the histological NAC effect (Miller-Payne grading) did not
differ significantly between ADP-positive and -negative patients.
This may have been related to the fact that Ki-67 LI in the current
study cohort was significantly higher in ADP-positive patients
compared with ADP-negative patients. This trend was also observed
in another report on breast cancer (18). It was hypothesized that NAC could
control ADP-positive highly proliferative carcinoma cells to some
degree, but some of these cells might show chemoresistance. These
carcinoma cells showing chemoresistance might influence the effect
of adjuvant chemotherapy. Additional studies are needed to clarify
the relationship between ADP expression and chemotherapeutic
effectiveness.
Notably, neither a low nor high Ki-67 LI
significantly associated with RFS among ADP-positive or -negative
patients who were not administered NAC or adjuvant chemotherapy.
This indicated that ADP is a superior prognostic marker as compared
with Ki-67LI in patients with TNBC (11) and a result consistent with ADP
expression in operative specimens from patients with TNBC.
Accordingly, ADP expression in both biopsy and operative specimens
may be a superior prognostic marker in patients with TNBC.
As discussed in our previous study (11), the mechanism of ADP expression in
TNBC leading to poor prognosis remains to be elucidated. ADP
expression in TNBC reflects the upregulation of lipid metabolism
and lipid synthesis is associated with TNBC growth (19). As Ki-67 LI was significantly higher
in ADP-positive patients in the cohort of the present study as
compared with that in ADP-negative patients [a finding consistent
with a previous study (18)], ADP
expression was associated with higher proliferative activity of
TNBC neoplastic cells. Intracytoplasmic metabolism, including lipid
metabolism and amino acid metabolism, may differ in ADP-positive
TNBC as compared with ADP-negative TNBC. Additional studies are
needed to clarify the mechanism of ADP expression in TNBC and
determine the metabolic differences between ADP-positive and
-negative TNBC.
There are several limitations to the present study.
First, this was a retrospective, single-institute study. Although
it evaluated >100 patients with TNBC, the subgroups, such as
patients who had not been treated with NAC or adjuvant
chemotherapy, were relatively small, which may have led to
selection bias. Therefore, additional studies of a large number of
patients with TNBC must be performed to verify the prognostic
significance of ADP expression in patients with TNBC with or
without NAC or adjuvant chemotherapy. Second, the results
demonstrated that ADP expression was significantly associated with
the clinically evaluated absence of lymph node metastasis in the
entire cohort and the NAC subgroup. Lymph node metastasis was
evaluated clinically because NAC influences the status of lymph
node metastasis. Although ADP expression in the biopsy specimens
was a significant poor prognostic marker, lymph node metastasis is
considered to be a poor prognostic factor. Thus, ADP expression in
the biopsy specimens might be a more useful marker compared with
the clinically evaluated lymph node status because the evaluation
of the presence of lymph node metastasis can depend on the
observer. Third, the prognostic significance of ADP expression in
both biopsy and operative specimens for patients with hormone
receptor-positive or HER2-positive breast cancer remains unresolved
and requires further analysis.
In conclusion, these results clearly demonstrated
that ADP expression in biopsy specimens is a poor prognostic factor
in patients with TNBC. Furthermore, ADP expression in biopsy
specimens was significantly associated with a higher Ki-67 LI and
might be associated with chemotherapeutic effectiveness.
Accordingly, additional studies are needed to establish new
preoperative treatment strategies, including the evaluation of ADP
expression in biopsy specimens.
Acknowledgements
Not applicable.
Funding
This study was supported in part by AMED (grant no.
JP21lm0203006 to MI), the Osaka Community Foundation 2020 (to MI)
and research grants D1 (to MI) and D2 (to KY) from Kansai Medical
University.
Availability of data and materials
All data generated and analyzed in this study are
included in this published article.
Authors' contributions
KY and MI were responsible for the conception and
design of the study. KY and MI performed immunohistochemical
analyses. KY, MI, HY, KT, MS and TS performed acquisition and
analysis of data. KY and MI confirm the authenticity of all the raw
data and KY and MI drafted the manuscript, tables and figures. All
authors reviewed and approved the final manuscript.
Ethics approval and consent to
participate
This study was conducted in accordance with the
Declaration of Helsinki and the study protocol was approved by the
institutional review board of the Kansai Medical University
Hospital (protocol no. 2019234).
Consent for publication
The need for informed consent was waived because of
the retrospective design of the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cleator S, Heller W and Coombes RC:
Triple-negative breast cancer: Therapeutic options. Lancet Oncol.
8:235–244. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dent R, Trudeau M, Pritchard KI, Hanna WM,
Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P and Narod SA:
Triple-negative breast cancer: Clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rakha EA, El-Sayed ME, Green AR, Lee AH,
Robertson JF and Ellis IO: Prognostic markers in triple-negative
breast cancer. Cancer. 109:25–32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Keam B, Im SA, Lee KH, Han SW, Oh DY, Kim
JH, Lee SH, Han W, Kim DW, Kim TY, et al: Ki-67 can be used for
further classification of triple negative breast cancer into two
subtypes with different response and prognosis. Breast Cancer Res.
13:R222011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bickel PE, Tansey JT and Welte MA: PAT
proteins, an ancient family of lipid droplet proteins that regulate
cellular lipid stores. Biochim Biophys Acta. 1791:419–440. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sztalryd C and Kimmel AR: Perilipins:
Lipid droplet coat proteins adapted for tissue-specific energy
storage and utilization, and lipid cytoprotection. Biochimie.
96:96–101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sztalryd C and Brasaemle DL: The perilipin
family of lipid droplet proteins: Gatekeepers of intracellular
lipolysis. Biochim Biophys Acta Mol Cell Biol Lipids.
1862:1221–1232. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nitulescu GM, Van De Venter M, Nitulescu
G, Ungurianu A, Juzenas P, Peng Q, Olaru OT, Grădinaru D, Tsatsakis
A, Tsoukalas D, et al: The Akt pathway in oncology therapy and
beyond (Review). Int J Oncol. 53:2319–2331. 2018.PubMed/NCBI
|
|
9
|
Fujimoto M, Yoshizawa A, Sumiyoshi S,
Sonobe M, Menju T, Hirata M, Momose M, Date H and Haga H:
Adipophilin expression in lung adenocarcinoma is associated with
apocrine-like features and poor clinical prognosis: An
immunohistochemical study of 328 cases. Histopathology. 70:232–241.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hashimoto Y, Ishida M, Ryota H, Yamamoto
T, Kosaka H, Hirooka S, Yamaki S, Kotsuka M, Matsui Y, Yanagimoto
H, et al: Adipophilin expression is an indicator of poor prognosis
in patients with pancreatic ductal adenocarcinoma: An
immunohistochemical analysis. Pancreatology. 19:443–448. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoshikawa K, Ishida M, Yanai H, Tsuta K,
Sekimoto M and Sugie T: Adipophilin expression is an independent
marker for poor prognosis of patients with triple-negative breast
cancer: An immunohistochemical study. PLoS One. 15:e02425632020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rakha EA, Allison KH, Bu H, Ellis IO,
Foschini MP, Horii R, et al: Invasive breast carcinoma of no
special type. WHO Classification of Tumours. 5th edition. Breast
Tumours IARC; Lyon: pp. 102–109. 2019
|
|
13
|
Yoshikawa K, Ishida M, Yanai H, Tsuta K,
Sekimoto M and Sugie T: Prognostic significance of PD-L1-positive
cancer-associated fibroblasts in patients with triple-negative
breast cancer. BMC Cancer. 21:2392021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoshikawa K, Ishida M, Yanai H, Tsuta K,
Sekimoto M and Sugie T: Immunohistochemical analysis of CD155
expression in triple-negative breast cancer patients. PLoS One.
16:e02531762021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I. The value of histological
grade in breast cancer: Experience from a large study with
long-term follow-up. Histopathology. 19:403–410. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu Q, Ma G, Deng Y, Luo W, Zhao Y, Li W
and Zhou Q: Prognostic value of Ki-67 in patients with resected
triple-negative breast cancer: A meta-analysis. Front Oncol.
9:10682019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ogston KN, Miller ID, Payne S, Hutcheon
AW, Sarkar TK, Smith I, Schofield A and Heys SD: A new histological
grading system to assess response of breast cancers to primary
chemotherapy: Prognostic significance and survival. Breast.
12:320–327. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuniyoshi S, Miki Y, Sasaki A, Iwabuchi E,
Ono K, Onodera Y, Hirakawa H, Ishida T, Yoshimi N and Sasano H: The
significance of lipid accumulation in breast carcinoma cells
through perilipin 2 and its clinicopathological significance.
Pathol Int. 69:463–471. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pucer A, Brglez V, Payré C, Pungerčar J,
Lambeau G and Petan T: Group X secreted phospholipase A(2) induces
lipid droplet formation and prolongs breast cancer cell survival.
Mol Cancer. 12:1112013. View Article : Google Scholar : PubMed/NCBI
|