Introduction
The majority of head and neck squamous cell
carcinomas (HNSCC) is causally linked to enhanced tobacco and
alcohol consumption of individuals which especially holds true for
cancers of the larynx and hypopharynx and HPV-negative
oropharyngeal carcinomas (1).
Pronounced in Asia and East Africa, oral cancer might also be
caused by betel nut chewing (2).
Carcinogenesis of the lingual and specifically palatine tonsils,
however, is to a substantial proportion linked to infections with
human papillomaviruses (HPV) (3–6).
30–90% of tonsillar SCC (TSCC) can be attributed to HPV16
infections with the infection rate diversity depending on the
geographical region the tested patients live in (7,8). It
is well established that, among others, patients with HPV-driven
TSCC show significantly better survival rates and are predominantly
non-smokers (9–11). Analysis of the association between
HPV infection and smoking of the here described study population
has confirmed that HPV-negative and HPV-positive patients are
smokers vs. non-smokers, respectively, reaching strong statistical
significance (for details on the latter and on further
epidemiologic data see Ref. 12).
The reason for this reciprocal correlation between HPV-status and
smoking is up to date only poorly understood.
Previously analyzing altogether 928 tissue samples
from 892 patients in retrospective studies (13 and references
therein), we have consistently shown a significant link between a)
smoking habit, b) expression levels of the human secretory
leucocyte protease inhibitor (SLPI) and Annexin A2 (AnxA2), and c)
HPV infections in various SCCs and also benign tonsillar lesions
(12). Basically, for these two
proteins it is described, that SLPI as a serine protease inhibitor
potently inhibits (among others) neutrophil elastase and thereby
protects skin and mucosa from proteolysis. Its antiviral activity
is mediated by affecting the host cells rather than the virus
itself. AnxA2 is involved in various cell functions, among others
endocytosis and exocytosis [for both see References listed in
(13)]. Data from meanwhile 892
patients with various diseases of either the head and neck or
anogenital region analyzed for smoking habit, expression levels of
SLPI and AnxA2, and HPV infection status of the investigated
tissues prompted us to formulate the following hypothesis: Smoking
leads to increased SLPI and AnxA2 expression in mucosal tissues
with significant SLPI excess. SLPI binds to AnxA2, which
consecutively inhibits the binding of HPV, if present, to AnxA2.
HPV binding to AnxA2 is crucial for successful HPV infection of
mucosal cells. Conversely, in non-smokers with significantly higher
levels of AnxA2 compared to SLPI, HPV can bind more readily to
unoccupied-non-SLPI-bound-AnxA2, and successful infection of cells
is likely. This hypothesis is supported by experimental evidence
provided by U.S. American groups regarding the binding capacity of
SLPI and HPV to AnxA2 (14–16)
and by other groups describing elevated SLPI expression in smokers
(17,18). Moreover, similar observations as
described above have been made by Ma and coworkers (19) already in 2004 and later on by
others (20,21) in terms of infections with HIV. Only
recently, we published a summary of six preceding retrospective
studies highlighting the aforementioned hypothesis (13).
Here, for the first time in a prospective study
design the association of smoking habit, SLPI- and AnxA2-expression
as well as HPV infection of 215 patients with benign and malignant
diseases of the palatine tonsils have been investigated. Moreover,
it was tested whether or not SLPI- and AnxA2-gene expression levels
measured in sputum or tonsillar swabs are suitable as surrogate
marker for SLPI- and AnxA2-gene expression in the respective
tonsillar tissue of the same individual. The latter was prompted by
studies reporting on 73% oral gargle/tumor biopsy HPV agreement in
oropharyngeal cancer patients (22). This gave rise to hope for superior
agreement ratios for SLPI and AnxA2 in different biomaterial e.g.
tonsillar tissue, swab and/or sputum since SLPI and AnxA2 are
intrinsic proteins, i.e. produced by mucosal cells, instead of
extrinsic factors such as viral infections.
Materials and methods
Study design
In a prospective, consecutive setting, patients with
tonsillar squamous cell carcinoma (TSCC) or non-neoplastic
tonsillar lesions, namely tonsillar hyperplasia (H) or chronic or
recurrent tonsillitis (CRT), were enrolled between February 2016
and April 2018 when treated at the ENT departments of the
university hospitals of Kiel, Rostock or Oldenburg or in the ENT
department of the Asklepios-Clinic, Hamburg-Harburg, Germany. In
the study presented the main focus was to study benign vs.
malignant tonsillar lesions. Since it is described that
p16INK4A and SLPI might be elevated by inflammation as
might be the case in patients with chronic or recurrent tonsillitis
(23,24) we additionally enrolled patients
with tonsillar hyperplasia, a tonsillar lesion not showing
high-grade signs of inflammation. All patients were asked about
their smoking habit and their age was recorded. Each patient was
asked to deliver a sputum sample directly pre-operatively, which
was immediately taken to the laboratories of the respective
clinics. For later isolation of DNA and RNA from the sputum, the
sputum sample was supplemented with 2 ml nucleic acid stabilizer
(AmpTec). All samples were stored at room temperature until nucleic
acid extraction, which was performed in the Kiel laboratories.
Intraoperatively, patient material was extracted in different ways
depending on the diagnosis: In patients with benign tonsillar
lesions tonsils of both sides were swabbed and resected; in
TSCC-patients only the affected tonsil was swabbed and resected.
From each tonsil that was eligible for analysis, 2 swabs were taken
with the Buccalyse swabs (Isohelix). One swab was used to extract
DNA, the other swab was transferred in 700 µl of the
above-mentioned stabilizer (AmpTec) to extract RNA. All swabs were
stored at room temperature until nucleic acid extraction in Kiel;
samples were once every fortnight send to Kiel. Tissue specimens
(~1 cm3) of each tonsil was shock frozen in liquid
nitrogen and stored at −80°C until nucleic acid extraction in Kiel.
A second piece of tissue was transferred to formalin for
immunohistochemistry. All samples were obtained following informed
written consent and approval by the local Ethics Committee (D
429/14).
Nucleic acid extraction
DNA and RNA from all sputum samples and RNA from the
tonsillar swabs was extracted using the ExpressArt Mag RNA+DNAready
kit (AmpTec), according to the manufactures protocol. DNA of the
tonsillar swabs was isolated using the Buccalyse DNA Release kit
(Isohelix), according to the manufactures protocol. To isolate DNA
and RNA from the tissue samples, 20–30 mg tissues were transferred
into a Precellys® ceramic-Kit 1,4 mm tube (VWR
International), containing 600 µl RLT-buffer part of the AllPrep
DNA/RNA Mini Kit (Qiagen). The tubes were transferred into the
Precellys® tissue homogenizer and were homogenized using
the program 1 × 5,000 for 30 sec. Afterwards the lysates were
centrifuged in an Eppendorf centrifuge 5417R at maximum speed for 3
min at 4°C. DNA and RNA was isolated from the supernatants using
the AllPrep DNA/RNA mini kit (Qiagen) according to the
manufacture's protocol. All RNA samples were stored at −80°C and
all DNA sample at −20°C until further analysis. Nucleic
acid-quantity and -quality was assessed using the NanoDrop 1000
(PeqLab) and the Tapestation 2200 (Agilent), respectively.
cDNA synthesis and SLPI and AnxA2 gene
expression
RNA (200 ng) was transcribed into cDNA (TR-cDNA
synthesis kit; AmpTec) under the following reaction conditions: 30
min at 16°C, 30 min at 42°C, 5 min at 85°C, using a Biometra T1
cycler (Analytik Jena; Jena Germany) followed by 5 min storage on
ice. RT-qPCR was performed as described, previously (25) using the following primers: SLPI
Forward: 3′-AATGCCTGGATCCTGTTGAC-5′; SLPI Reverse:
3′-AAAGGACCTGGACCACACAG-5′; AnxA2 Forward:
3′-AACCGACGAGGACTCTCTCA-5′; AnxA2 Reverse:
3′-CGCTGATCCACTTGGGAACAT-5′ (26).
RT-qPCR using a Rotorgene 3000 (Corbett, LTF) was performed
amplifying 2.5 µl each of the abovementioned cDNA (=10 ng RNA)
under the following PCR conditions: 10 min initial denaturation at
95°C, followed by 40 cycles: 20 sec denaturation at 95°C, annealing
20 sec at 60°C and elongation: 20 sec at 72°C followed by melt
curve analysis from 60°C to 95°C ramping: 5°/sec. Primers for the
housekeeping genes 18S rRNA (P-030126), β-actin (P030124) and
b-2-microglobulin (B2M; P-030127) were purchased from Promolgene
(with catalogue numbers given in parenthesis) and used according to
the manufacturer's protocol. RT-qPCR data were analyzed according
to the ΔΔCt method (26) using the
mean Ct value of the housekeeping genes. Fold changes of expression
levels were calculated using the following equations: 2^(ΔCt
HPV-positive-ΔCt HPV-negative) for decreases in HPV-related gene
expression; 1/2^(ΔCt HPV-positive-ΔCt HPV-negative) for increases
in HPV-related gene expression; 1/2^(ΔCt active/former smoker-ΔCt
never smoker) for increases in smoking-related gene expression;
2^(ΔCt AnxA2-ΔCt SLPI) for negative AnxA2/SLPI ratios and 1/2^(ΔCt
AnxA2-ΔCt SLPI) for positive AnxA2/SLPI ratios (26).
SLPI immunohistochemistry
Paraffin-embedded tissue specimens were cut into 2
µm sections and stained for SLPI. Immunostaining was performed and
evaluated according to Cordes et al (27). In brief: Paraffin-embedded tissue
specimens were deparaffinized, and rehydrated, followed by
heat-induced epitope retrieval. Methanol containing 1% hydrogen
peroxide was used to block the endogenous peroxidase. Sections were
blocked with the corresponding pre-immune serum for 15 min and
incubated for 1 h with monoclonal primary antibody directed against
SLPI (LifeSpan BioSciences) followed by incubation with a
biotin-conjugated rabbit anti-mouse IgG secondary antibody (Dako)
at room temperature for 30 min. After washing with tris-buffered
saline, a labelled peroxidase complex system (ABC-Vectorstain;
Dako) was used to visualize all immune reactions. To asses SLPI
protein levels, 300 cells in at least five areas was analyzed (×200
magnification). A mean percentage of positive cells were determined
and cases were assigned to one of the following categories:
negative (−) <5%, weak (+) 5–30%, moderate (++) 31–75% and
strong (+++) >75%.
HPV detection
HPV DNA detection was performed by PCR amplifying 50
ng DNA per sample using the primers GP5+/GP6+, as described
previously (28). DNA integrity
was analyzed using genomic B2M primers (Promolgene; P-030127)
according to the manufacturer's protocol. Additionally, a positive
control (a synthetic oligonucleotide of the HPV L1 gene, covered by
the GP5+/GP6+ primers; Eurofins; Ebersberg Germany) was amplified
in the GP5+/GP6+ PCRs. Amplification products were sequenced by
Sanger sequencing and alignments were obtained from the GenBank
online BLAST server (http://blast.ncbi.nlm.nih.gov/Blast.cgi). A
representative Sanger sequencing trace of a HPV16-positive cancer
tissue sample is shown in Fig.
S1.
Statistical analyses
Firstly, demographic differences between the patient
cohorts suffering from either tonsillar hyperplasia (H) or chronic
or recurrent tonsillitis (CRT), were analyzed using Fisher's exact
tests. Next the demographic characteristics of the cancer patients
and of all patients with non-neoplastic lesions (H and CRT) were
compared using Fisher's exact tests. Student's t-test was performed
to assess age-related differences first between the H and CRT
patients and later between all patients with benign lesions and the
TSCC patients. Fisher's exact test was performed relating SLPI
protein expression to HPV-positivity and smoking habit. For
analysis of the RT-qPCR data ΔCt values [Ct value of the target
genes (SLPI and AnxA2) corrected for mean Ct values of the
housekeeping genes (18S rRNA, β-actin B2M)] were used. Since data
were, as confirmed by Kolmogorov-Smirnov test, non-parametric
Mann-Whitney- and Kruskal-Wallis test was performed to analyze
differences between 2 and more than 2 groups, respectively. All
tests were performed using SPSS 20.0; P-values ≤0.05 were
considered statistically significant, confirmed by Bonferroni
post-testing for multiple comparisons where appropriate.
Results
Patient demographics
A total of 215 patients was enrolled in the study.
52 patients (age: 63.03±8.99 years) were treated for cancer of the
palatine tonsil [(TSCC) 9/52 (17.2%) never smokers, 19/52 (36.5%)
active smokers, and 22/52 (42.3%) had given up smoking at least two
years before diagnosis; in two (3.8%) cases, data regarding smoking
habit were missing]. Further 163 patients (age 25.69±15.10 years)
with benign tonsillar lesions were enrolled. [97/163 (59.5%) never
smokers; 63/163 (38.7%) active smokers; two patients (1.2%) had
given up smoking at least 2 years prior to diagnosis; for one case
(0.6%) no data regarding the smoking habit was available]. Patients
with benign tonsillar lesions were comprised of 56 patients (age:
29.30±21.47 years) suffering from tonsillar hyperplasia [(H) 31/56
(55.4%) never smokers; 23/56 (31.1%) active smokes; one patient
(1.8%) had given up smoking at least 2 years prior to diagnosis and
for one case (1.8%) no data regarding the smoking habit was
available]. 107 patients (age: 24.05±10.78 years) were treated for
non-neoplastic chronic or recurrent tonsillitis [(CRT) 66/107
(61.7%) never smokers; 40/107 (37.4%) active smokers; one patient
(0.9%) had given up smoking at least 2 years prior to diagnosis;
age is in all cases given as mean ± standard deviation]. No
significant differences between patient demographics were observed
between CRT and H patients (Table
I). As expected TSCC patients are older and this group is
comprised of significantly fewer never smokers but also more former
smokers when compared to the CRT and H patients. Given the
different disease entities studied, the number of HPV-positive
cases is significantly higher in the TCSS patients (Table I). The two cases with benign
tonsillar lesions who had given up smoking were, due to too small
numbers, excluded from further analysis regarding the effect of
smoking on SLPI- and AnxA2-expression.
 | Table I.Patient demographics (n=215). |
Table I.
Patient demographics (n=215).
|
| Cohort | Significance |
|---|
|
|
|
|
|---|
| Variable | CRT (n=107) | H (n=56) | All benign
(n=163)c | TSCC (n=52) | CRT vs. H | All benign vs.
TSCC |
|---|
| Age (years) | 24.05±10.78 | 29.30±21.47 | 25.68±15.10 | 63.03±8.99 | n.s. | P<0.0001 |
| Smoking status at
time of diagnosisa |
|
|
|
| n.s | P<0.0001 |
|
Active | 40 (37.4) | 23 (31.1) | 63 (38.7) | 19 (36.5) |
|
|
|
Never | 66 (61.7) | 31 (55.4) | 97 (59.5) | 9 (17.2) |
|
|
|
Formerb | 1 (0.9) | 1 (1.8) | 2 (1.2) | 22 (42.3) |
|
|
| HPV DNA |
|
|
|
| n.s | P<0.0001 |
|
Negative | 95 (88.8) | 45 (80.4) | 140 (85.9) | 31 (59.6) |
|
|
|
Positive | 12 (11.2) | 11 (19.6) | 23 (14.1) | 21 (40.4) |
|
|
| SLPI IHC |
|
|
|
| n.s | n.s |
|
Negative/weak | 62 (57.9) | 36 (64.3) | 98 (60.1) | 31 (59.6) |
|
|
|
Moderate/strong | 45 (42.1) | 20 (35.7) | 65 (39.9) | 21 (40.4) |
|
|
HPV DNA-status in tonsillar
tissue
The tonsils of 23/163 (14.1%) patients treated for
benign tonsillar lesions were HPV DNA-positive [12/107 (11.2%) CRT-
and 11/56 (19.6%) H-patients]. Of the 52 TSCC-patients, 21/52
(40.4%) were HPV DNA-positive. Given the underlying hypothesis that
SLPI expression levels influence the initial HPV cell-entry in
human mucosa, we focused on the presence of exclusively HPV DNA in
the present study. Since in this context it is not important
whether or not HPV cell-entry finally results in an inactive or
active HPV infection of the tonsils, direct or indirect markers of
HPV activity were neglected here. These HPV-related results along
with further details on the study population are described
elsewhere (12, and are briefly summarized in Table I).
SLPI and AnxA2 gene expression
As shown in Fig. 1
smoking resulted in tonsillar tissue, swabs and sputum of
TSCC-patients (Fig. 1A-C) and
patients with benign tonsillar lesions (Fig. 1D-F) in significantly increased SLPI
gene expression levels. In TSCC-patients who quit smoking at least
2 years prior to diagnosis (former smoker) SLPI gene expression was
still significantly higher than in never smokers albeit not as high
as in still active smokers; approx. 2.5-fold increase in former and
3.5 to 5.2-fold increase in active smokers (Fig. 1A-C). Analyzing patients with begin
lesions separated by disease type, it can be seen that in
CRT-patients (Fig. 1G-I) smoking
resulted in similarly increased SLPI gene expression levels as seen
in TSCC-patients. In H-patients (Fig.
1J-L), the smoking related increase in SLPI gene expression was
less pronounced and was only significantly increased in tonsillar
tissue, while gene expression in swabs and sputum of these patients
was not significantly altered. Smoking resulted in only
insignificantly increased AnxA2 levels, in all patient groups and
biomaterial analyzed (Fig.
1A-L).
Next, the effect of HPV on SLPI and AnxA2 gene
expression was tested. SLPI gene expression was significantly
decreased in all HPV-positive samples (tissue, swab and sputum) in
TSCC-patients and in patients with benign lesion, when analyzing
the latter as one group. AnxA2 gene expression, on the other hand,
was significantly increased in HPV-positive when compared to
HPV-negative samples of these patient groups (Fig. 2A-F). Analyzing H- and CRT-patients
separately, it can be seen that in CRT-patients (Fig. 2G-I) HPV-positivity resulted in
similarly decreased SLPI gene expression levels as already seen in
TSCC-patients. AnxA2 gene expression in CRT-patients was
significantly increased in HPV-positive samples when compared to
HPV-negative ones. In H-patients, on the other hand, neither SLPI
nor AnxA2 gene expression was significantly different between
HPV-positive and HPV-negative samples, irrespective of the
biomaterial analyzed (Fig. 2J-L).
It should, however, be mentioned that SLPI gene expression in all
HPV-positive biomaterials of H-patients was decreased, albeit not
significantly. Likewise, AnxA2 levels of HPV-positive biomaterials
of H-patients showed small but insignificant increases in
comparison to HPV-negative materials (Fig. 2J-L).
To determine the relation between SLPI and AnxA2
gene expression, the fold change of AnxA2 gene expression in
relation to SLPI gene expression was calculated (for mathematical
details see Material & Methods and Legend to Fig. 3). The biomaterial of all never
smoking patients had significantly more AnxA2 than SLPI (Fig. 3A-L). In active and former smokers,
with the exception of the tonsillar swabs of TSCC-patients who
reported to have stopped smoking at least 2 years prior to
diagnosis, AnxA2 and SLPI gene expression were not significantly
different. HPV-negativity resulted in all biomaterials, with the
exception of the swabs obtained from H-patients, in significantly
reduced AnxA2 gene expression in comparison to SLPI gene expression
(Fig. 3A-L). It should, however,
be noted that AnxA2 gene expression in comparison to SLPI gene
expression in tonsillar swabs of H-patients was also reduced,
albeit not significantly. In all HPV-positive biomaterials with the
exception of the sputum samples obtained from H-patients (Fig. 3L), AnxA2 gene expression was
significantly increased in comparison to SLPI gene expression
(Fig. 3A-K).
 | Figure 3.Comparison of AnxA2- and SLPI-gene
expression in tonsillar tissue, tonsillar swabs and the sputum of
patients with malignant and benign tonsillar lesions. Relationship
between AnxA2 and SLPI gene expression in (A) tissue (B) swab and
(C) sputum samples dependent on the smoking habit and HPV status of
patients with TSCC patients. Relationship between AnxA2 and SLPI
gene expression in (D) tissue (E) swab and (F) sputum samples
dependent on the smoking habit and HPV status of all patients with
benign tonsillar lesions (CRT and H). Relationship between AnxA2
and SLPI gene expression in (G) tissue (H) swab and (I) sputum
samples dependent on the smoking habit and HPV status of patients
with CRT patients. Relationship between AnxA2 and SLPI gene
expression in (J) tissue (K) swab and (L) sputum samples dependent
on the smoking habit and HPV status of patients with H. Fold-change
expression of AnxA2 in relation to SLPI gene expression was
calculated using the following equations: 2^(ΔCt AnxA2-ΔCt SLPI)
for negative AnxA2/SLPI ratios and 1/2^(ΔCt AnxA2-ΔCt SLPI) for
positive AnxA2/SLPI ratios (26).
To compare the AnxA2 and SLPI gene expression levels, SLP1 gene
expression for each group [never smoker, active smoker and former
smoker (TSCC patients only) or never and active smoker;,
HPV− and HPV+] was set as ‘1’. The SLPI data
are not displayed. Former smokers had stopped smoking at least 2
years prior to diagnosis (only TSCC). *P<0.05, **P<0.001 and
***P<0.0001 AnxA2 vs. SLPI gene expression. AnxA2, Annexin A2;
CRT, chronic or recurrent tonsillitis; H, tonsillar hyperplasia;
HPV, human papilloma virus; SLPI, secretory leucocyte protease
inhibitor; TSCC, tonsillar SCC. |
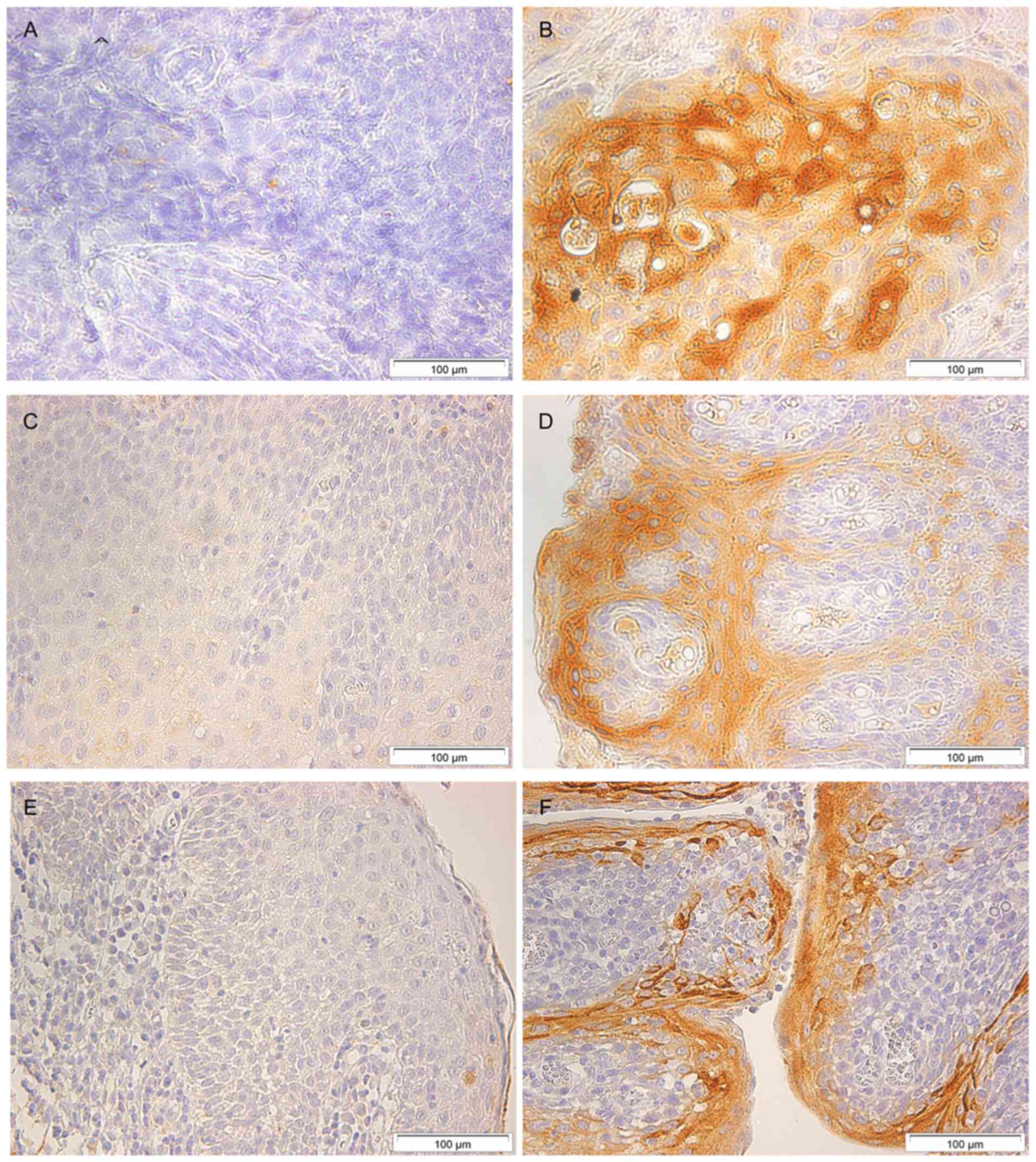
SLPI protein expression
To corroborate tonsillar SLPI gene expression levels
by protein levels, the SLPI protein expression of the tonsils was
measured by means of immunohistochemistry. Representative cases for
negative (<5% cells were stained) and strong (>75% cells were
stained) SLPI staining in tonsillar tissue of patients with TSCC,
patients with chronic or recurrent tonsillitis, and patients with
tonsillar hyperplasia are shown in Fig. 4. Immunohistochemical analysis could
be performed on all 215 samples; the results relating SLPI protein
expression to smoking habit are presented in Table II. In the tonsillar tissue of
TSCC-patients, a significant correlation between SLPI–IHC and
smoking habit could be found; P=0.0022. Similarly, in the benign
samples, when analyzed as one group, a significant correlation
between SLPI protein expression and smoking habit could be found
(P=0.0009). Analyzing the two subgroups of benign lesion,
separately, reveled a significant correlation between SLPI–IHC and
smoking habit for CRT-but not for H-patients (P=0.0022 and
P>0.05 respectively; Table
II).
 | Table II.Relationship between SLPI
immunohistochemistry and the smoking status of patients. |
Table II.
Relationship between SLPI
immunohistochemistry and the smoking status of patients.
|
| Smoking status |
|
|---|
|
|
|
|
|---|
| Pathology | Active | Never | Former | P-value |
|---|
| TSCC SLPI–IHC |
|
|
| 0.0022 |
|
Negative/weak | 6 (12.0) | 9 (18.0) | 14 (28.0) |
|
|
Moderate/strong | 13 (26.0) | 0 (0.0) | 8 (16.0) |
|
| CRT + H
SLPI–IHC |
|
|
| 0.0009 |
|
Negative/weak | 27 (16.9) | 68 (42.5) | N/A |
|
|
Moderate/strong | 36 (22.5) | 29 (18.1) | N/A |
|
| CRT SLPI–IHC |
|
|
| 0.0022 |
|
Negative/weak | 15 (14.1) | 46 (43.4) | N/A |
|
|
Moderate/strong | 25 (23.6) | 20 (18.9) | N/A |
|
| H SLPI–IHC |
|
|
| n.s. |
|
Negative/weak | 12 (22.1) | 22 (40.8) | N/A |
|
|
Moderate/strong | 11 (20.4) | 9 (16.7) | N/A |
|
The correlation between HPV DNA-status and SLPI
protein expression was also calculated and the results are
presented in Table III. In the
majority of patients with HPV-positive tonsillar tissue the
tonsillar tissue was characterized by negative/weak SLPI–IHC
staining, while HPV-negative tissue showed a relative even
distribution between negative/weak and moderate/strong SLPI–IHC
staining, resulting in significant correlations in TSCC-patients
and patients with benign tonsillar lesions (P<0.0001 and
P=0.0209, respectively; Table
III). Analyzing the different subgroups of benign lesion
separately reveled a significant correlation between SLPI–IHC and
HPV DNA-status for CRT- but not for H-patients (P=0.001 and
P>0.05 respectively; Table
III).
 | Table III.Relationship between SLPI
immunohistochemistry and tonsillar HPV DNA status. |
Table III.
Relationship between SLPI
immunohistochemistry and tonsillar HPV DNA status.
|
| HPV-DNA |
|---|
|
|
|
|---|
| Pathology | Positive | Negative | P-value |
|---|
| TSCC SLPI–IHC |
|
| <0.0001 |
|
Negative/weak | 20 (38.5) | 11 (21.1) |
|
|
Moderate/strong | 1 (1.9) | 20 (38.5) |
|
| CRT+H SLPI–IHC |
|
| 0.0209 |
|
Negative/weak | 19 (11.6) | 79 (48.5) |
|
|
Moderate/strong | 4 (2.5) | 61 (37.4) |
|
| CRT SLPI–IHC |
|
| 0.001 |
|
Negative/weak | 12 (11.2) | 50 (46.7) |
|
|
Moderate/strong | 0 (0.0) | 45 (42.1) |
|
| H SLPI–IHC |
|
| n.s. |
|
Negative/weak | 7 (12.5) | 29 (51.8) |
|
|
Moderate/strong | 4 (7.1) | 16 (28.6) |
|
Correlating SLPI and AnxA2 expression
in the different biomaterials analyzed
To assess the validity of SLPI and AnxA2 gene
expression in tonsillar swabs and/or sputum samples as surrogate
marker for SLPI and AnxA2 gene expression in tonsillar tissue we
focused on those samples where we previously found incongruent HPV
DNA-results in the analyzed biomaterials (12). Among the 52 patients with TSCC 40
patients showed concordant results in all biomaterials analyzed.
The remaining 12 cases are depicted in Table IV. In 97/107 patients with CRT and
in 48/56 patients with H concordant results in all biomaterials
analysed were obtained. The remaining 10/107 CRT and 8/56 H, where
differences between the tissue HPV-status, the sputum and swab
results were detected are presented in Table V. Of note: cases 9–12 in Table IV as well as cases 5–10 and 14–18
in Table V were characterized by
HPV-negative tissue but HPV-positive sputum/swab samples. These
samples were reanalyzed by re-extracting DNA and RNA from the
tissues. DNA was analyzed again by performing GP5+/GP6+ PCRs and
RNA was transcribed into cDNA. RT-qPCR was performed using HPV16
and HPV18 specific primers, in case of the TSCC cases and HPV 6, 11
and 18 specific primers in case of the CRT and H cases, depending
on the HPV types found in the corresponding HPV-positive samples,
assuming that in these cases, as in all other cases, all HPV
positive materials would have been affected with the same HPV type.
However, no HPV-DNA or HPV-RNA could be detected in these tissues,
despite positive signals for the positive controls. Furthermore, in
Tables IV and V AnxA2 gene expression in relation to
SLPI gene expression is shown. All cases are characterized by the
same AnxA2/SLPI ratio in tonsillar swabs, sputum and the respective
tissue samples.
 | Table IV.Ratio of AnxA2/SLPI gene expression
levels in patients with tonsillar SCC that exhibit differing HPV
results in different biomaterials. |
Table IV.
Ratio of AnxA2/SLPI gene expression
levels in patients with tonsillar SCC that exhibit differing HPV
results in different biomaterials.
|
| Tonsillar
tissue | Tonsillar swab | Sputum |
|---|
|
|
|
|
|
|---|
| Case | HPV | SLPI–IHC | AnxA2/SLPI | HPV | AnxA2/SLPI | HPV | AnxA2/SLPI |
|---|
| 1 | 16 | -/+ | +5.23 | 16 | +4.58 | - | +5.32 |
| 2 | 16 | -/+ | +5.64 | 16 | +5.03 | 16 | +5.63 |
| 3 | 16 | -/+ | +4.86 | 16 | +4.34 | 16 | +5.10 |
| 4 | 16 | -/+ | +5.98 | 16 | +5.14 | 16 | +5.71 |
| 5 | 18 | -/+ | +4.86 | 18 | +4.56 | - | +5.13 |
| 6 | 18 | -/+ | +5.32 | - | +4.94 | - | +5.41 |
| 7 | 16 | -/+ | +5.74 | - | +5.08 | - | +5.68 |
| 8 | 18 | -/+ | +5.50 | 18 | +4.98 | 18 | +5.58 |
| 9 | - | ++/+++ | −2.90 | 18 | −2.51 | 18 | −2.33 |
| 10 | - | ++/+++ | −2.93 | 16 | −2.53 | 16 | −2.40 |
| 11 | - | ++/+++ | −2.87 | 16 | −2.48 | 16 | −2.11 |
| 12 | - | ++/+++ | −3.13 | 16 | −2.58 | - | −2.41 |
 | Table V.Ratio of AnxA2/SLPI gene expression
in patients with CRT and H with different HPV results in different
biomaterials. |
Table V.
Ratio of AnxA2/SLPI gene expression
in patients with CRT and H with different HPV results in different
biomaterials.
|
|
| Tonsillar
tissue | Tonsillar swab |
|
|
|---|
|
|
|
|
|
|
|
|---|
|
|
| Right | Left | Right | Left | Sputum |
|---|
|
|
|
|
|
|
|
|
|---|
| Case | Pathology | HPV | SLPI–IHC | AnxA2/SLPI | HPV | SLPI-HPV | AnxA2/IHC | HPV | AnxA2/SLPI | HPV | AnxA2/SLPI | HPV | AnxA2/SLPI |
|---|
| 1 | CRT | 6 | -/+ | +4.44 | 6 | -/+ | +4.29 | - | +3.77 | - | +3.92 | 6 | +3.48 |
| 2 | CRT | 6 | -/+ | +4.32 | 6 | -/+ | +4.23 | 6 | +3.71 | 6 | +3.76 | - | +3.39 |
| 3 | CRT | 11 | -/+ | +3.56 | 11 | -/+ | +3.03 | - | +3.71 | 11 | +3.39 | 11 | +2.73 |
| 4 | CRT | 11 | -/+ | +3.58 | 11 | -/+ | +3.88 | 11 | +3.86 | 11 | +3.63 | - | +3.18 |
| 5 | CRT | - | ++/+++ | −4.03 | - | ++/+++ | −4.10 | 6 | −3.45 | 6 | −3.58 | 6 | −2.83 |
| 6 | CRT | - | ++/+++ | −3.87 | - | ++/+++ | −3.95 | 6 | −3.14 | 6 | −2.99 | 6 | −2.50 |
| 7 | CRT | - | ++/+++ | −3.71 | - | ++/+++ | −3.81 | 6 | −2.97 | 6 | −2.91 | 6 | −2.38 |
| 8 | CRT | - | ++/+++ | −3.93 | - | ++/+++ | −3.97 | 6 | −3.18 | 6 | −3.05 | 6 | −2.57 |
| 9 | CRT | - | ++/+++ | −3.95 | - | ++/+++ | −4.01 | 11 | −3.25 | 11 | −3.28 | 11 | −2.57 |
| 10 | CRT | - | ++/+++ | −4.03 | - | ++/+++ | −4.05 | 11 | −3.25 | 11 | −3.39 | 11 | −2.64 |
| 11 | H | 11 | -/+ | +2.39 | 11 | -/+ | +2.16 | 11 | +2.09 | - | +1.91 | 11 | +2.06 |
| 12 | H | 18 | -/+ | +2.25 | 18 | -/+ | +2.14 | 18 | +1.95 | 18 | +1.89 | - | +2.03 |
| 13 | H | 6 | -/+ | +2.10 | 6 | -/+ | +2.06 | 6 | +1.93 | 6 | +1.78 | - | +2.02 |
| 14 | H | - | ++/+++ | −2.03 | - | ++/+++ | −2.08 | 11 | −2.02 | 11 | −2.07 | 11 | −2.19 |
| 15 | H | - | ++/+++ | −1.99 | - | ++/+++ | −1.97 | 6 | −1.85 | 6 | −1.96 | - | −1.95 |
| 16 | H | - | ++/+++ | −1.96 | - | ++/+++ | −1.92 | 11 | −1.72 | 11 | −1.67 | - | −1.82 |
| 17 | H | - | ++/+++ | −2.03 | - | ++/+++ | −2.04 | 11 | −1.97 | 11 | −2.03 | - | −2.04 |
| 18 | H | - | ++/+++ | −2.00 | - | ++/+++ | −1.99 | 18 | −1.96 | 18 | −1.99 | - | −2.00 |
Discussion
This prospective study investigating 215 patients
confirms the significant association between (a) smoking, (b) SLPI-
and AnxA2-expression and (c) HPV infection in benign and malignant
tonsillar lesions as we have previously shown in various, yet,
retrospective studies (13). For
TSCC- and CRT-cases both, i) the correlation between a positive
smoking habit and high SLPI-expression levels and vice versa as
well as ii) the correlation between high SLPI-expression and fewer
HPV infections and vice versa, show strong statistical
significance. However, this is not the case in H-cases, although of
the 11 HPV-positive cases fittingly 8 are non-smokers and 7 of
these show negative/weak SLPI staining (Ref. 13 and Tab
3). Possibly with n=54, the number of investigated H-cases is
not sufficient for statistical analysis to reach significance.
Moreover, chronic inflammation which is present in TSCC and CRT,
yet, to a lesser extent in H, might be additionally involved in the
interaction of the here investigated parameters (17,18).
Among the benign cases 29/163 show strong staining for SLPI in
immunohistochemistry even among never smokers. The latter has not
been detected in a single case among never smokers of the
TSCC-group. Thus, inflammation cannot be ruled out to trigger
tissue expression of SLPI and AnxA2 in non-malignant tonsils.
Perhaps, the comparably small number of H-cases accompanied by
inflammation-induced SLPI expression is responsible for the lack of
significance correlating smoking, SLPI/AnxA2 expression and HPV
infection in H-cases. However, analyzing all cases with benign
tonsillar lesions as one group showed similar results as seen in
the TSCC-patients.
As specifically depicted by the tables and figures
in the results section, the here performed analysis affirms the
positive correlation between a positive smoking history with
elevated SLPI- and AnxA2-expression (and vice versa) with yet a
significant surplus of SLPI (13).
Furthermore, HPV DNA-presence in the investigated tissue specimens
is associated with low SLPI-expression, a positive AnxA2/SLPI ratio
and vice versa. These results were, as already mentioned, expected
due to comparable results obtained from 6 retrospective studies on
altogether 892 patients performed, previously (13). With the prospective validation of
the results of these 6 previous studies, in total now adding up to
1.107 investigated patients, epidemiologic aspects of the here
tested parameters and correlations seem to be described
sufficiently, at least for respective disease entities of the head
and neck. While own studies on mechanistic aspects in terms of HPV
transfection assays in the presence and absence of SLPI are
ongoing, we encourage others to test the findings described by us
in different geographical regions of the world and for other
neoplastic and non-neoplastic HPV-associated disease entities.
Data derived from the present study population on
HPV-epidemiology and on the validity of the detection methods used
(12) showed i) a 14%
dis-agreement of HPV- and p16INK4A-status among the
various tested biomaterials and ii) with 26% an even higher
mis-match ratio between p16INK4A-staining and the true
HPV-status (HPV-DNA and HPV-RNA positive) of the TSCC-cases
analyzed (12). However, analyzing
SLPI- and AnxA2-gene expression, sputum and tonsillar swabs
precisely match the results of SLPI- and AnxA2-gene expression
levels measured in the respective tonsillar tissue specimens. The
latter instance represents the most intriguing result of the
present study. The fact that analysis of the AnxA2/SLPI ratio in
sputum or oral swabs precisely predict the expression ratio in the
respective tonsillar tissue appears promising. As shown in Tables III and IV hardly any differences can be found
between the AnxA2/SLPI ratio in the different biomaterials, hence
one can argue that sputum might be the better biomaterial to
analyze since sputum samples are often easier obtainable than
tonsillar swabs.
According to the presented results, AnxA2/SLPI
ratios determined in sputum and tonsillar swabs resemble the ratio
in the respective tonsillar tissue to an extent that both sputum
and swab analysis qualify as a surrogate marker for the situation
in the tonsillar tissue. There seem to be two measures that could
possibly derive from the knowledge of the AnxA2/SLPI ratio in
tonsils of a person determined by the surrogate sputum or tonsillar
swab: i) It could be discussed whether or not persons with a
positive AnxA2/SLPI ratio should possibly be tonsillectomized,
precautionary. This notion is supported by the finding that within
this study population out of in total 163 patients with benign
tonsillar lesions 23 (14.1%) were HPV-positive, five (21.7%) of
these also being positive for HPV mRNA, classifying these HPV
infections as active ones (12).
Moreover, three of these five HPV RNA-positive cases showed
HPV16-infections classified as a high-risk virus type with a high
potential that infection will progress to carcinogenesis. The
latter two facts proof that active high-risk HPV infections in
non-malignant tonsils exist and it might be assumed that in these
cases progression to carcinogenesis would have been likely if the
patients were not tonsillectomized prior to that possible
progression. The idea that tonsillectomy might prevent from TSCC is
supported by a nation-wide cohort study in Sweden comparing cancer
incidence in a tonsillectomy cohort (n=225.718) with Swedens
general population. The authors detected a reduced risk of
tonsillar cancer in the tonsillectomy cohort but unrelated to other
oropharyngeal or other head and neck cancers (29). The cases with active HPV16
infection in the non-malignant tonsils, indeed, showed a positive
AnxA2/SLPI ratio in tonsillar swab and sputum samples in the
present study which identifies them at risk for an HPV infection.
However, it must be critically noted that tonsillectomies are not
without danger due to possible complications, in particular
postoperative secondary bleeding with sometimes even fatal outcome
(30). Focusing on the here
investigated patients with benign tonsillar lesions by
tonsillectomy on the basis of a positive AnxA2/SLPI ratio 87%
(20/23) would have been overtreated, however, it would possibly
have saved 13% (3/23) of patients from developing tonsillar cancer.
Since the latter seems intriguing, more epidemiologic data are
required to further provide a risk-benefit assessment before
tonsillectomy can be recommended solely based on AnxA2/SLPI ratios
in a cancer prevention scenario. ii) A second measure that could
possibly derive from the AnxA2/SLPI levels of the tonsillar swab
and sputum samples is HPV vaccination of individuals with positive
AnxA2/SLPI ratios, since these are at higher risk to be
successfully infected by HPV. The latter would be particularly
interesting for economically disadvantaged developing countries
(31–33), which could use the AnxA2/SLPI
analysis to identify a subgroup of the population as such a group
most likely to benefit from HPV vaccination. Thereby, the
relatively cost-intensive vaccines could be better targeted, which
should positively influence the cost-effectiveness ratio in the
countries concerned. Following our results, one could argue to
include only non-smokers in vaccination strategies, as they are
associated with lower SLPI levels. However, there are also smokers
with low and non-smokers with high SLPI levels. Fittingly, Carpén
and coworkers (34) found that
smoking and alcohol consumption is also common in patients with
HPV-positive, p16INK4A-positive oropharyngeal
carcinomas, as we also have observed in comparable study
populations investigated, previously (7,10).
Therefore, stratifying by smoking habit of individuals would not be
precise enough for a vaccination campaign. Indeed, we believe that
this second aspect is worth further evaluation. Since at present
HPV vaccination is predominantly targeted to prevent cervical
cancer, it might be of interest that we previously found similar
associations between SLPI, AnxA2 und tissue HPV-status when
analyzing 99 vulvar carcinomas (35). Based on these findings, analysis of
AnxA2/SLPI ratio of cervical swabs might also be suitable as a
surrogate marker for the situation in the cervical tissue, hence
identifying women who might be at risk developing cervical cancers
and thereby contribute to better targeted HPV vaccination
strategies.
A limitation of the study is the rather small number
of patients with tonsillar hyperplasia which is due to the fact
that the vast majority of tonsils histopathologicaly show to some
degree inflammation. We aimed to enroll H-patients as
‘counterparts’ to CRT-patients (lower versus higher degree of
inflammation) but were unable to enroll more than the here analyzed
56 patients with tonsillar hyperplasia. The above discussed
measures in case of a positive AnxA2/SLPI ratio is significantly
supported by all data except for analysis of H-cases, separately.
Focusing on H-cases, data show at least a to the other entities´
results comparable trend (decreased and increased changes in gene
expression levels as seen in CRT- and TSCC-patients), yet, without
significance. Significance also in H-cases possibly would have been
reached if a larger number of H-patients could have been
enrolled.
In conclusion, the data prospectively collected here
confirm the previous retrospective results and thus underline the
plausibility of the hypothesis put forward. According to this
hypothesis, smoking leads to enhanced SLPI expression, which in
turn prevents HPV from binding to cellular AnxA2. However, binding
of HPV to AnxA2 appears to be essential for successful HPV
cell-entry and, thus, active infection prompting carcinogenesis.
Most interestingly, SLPI- and AnxA2-gene expression levels measured
in tonsillar swabs and sputum accurately reflect the expression
status of both genes in tonsillar tissue. Accordingly, the
AnxA2/SLPI ratio in sputum and/or tonsillar swab might be used to
possibly identify patients which could be subjected to either
tonsillectomy or HPV vaccination, thereby making HPV-associated
carcinogenesis less likely.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank the Institute of
Clinical Molecular Biology in Kiel for providing Sanger sequencing
as supported in part by the DFG (German Research Foundation)
Clusters of Excellence ‘Inflammation at Interfaces’ and ‘Future
Ocean’. The authors would also like to thank Mrs. T. Naujoks and
Mrs. C. Noack (Institute of Clinical Molecular Biology, Kiel) for
their technical support. The authors further thank Mrs. G. Scherer,
Mrs. H. Clasen and Mrs. M. Kunz (Department of Otorhinolaryngology,
Head and Neck Surgery, University Clinic Schleswig-Holstein) for
their technical assistance with immunohistochemistry and
RNA-isolation, cDNA synthesis and PCR, respectively.
Funding
The present study was supported by a grant from the Deutsche
Krebshilfe (German Cancer Aid; grant no. 111777).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MH and ESQ designed, supervised and/or performed the
experiments. ESQ analyzed the data, generated the figures and wrote
the first draft of the manuscript. ML and PA made substantial
contributions to data acquisition. MH and ML performed plausibility
checks and reviewed and edited the first draft, which was further
edited and later approved for publication by all authors. AH, AK,
FH and RM provided patient data and material, and performed or
supervised the initial experimental sampling procedures before
samples were sent to Kiel. The laboratories in Kiel are part of the
Department of Otorhinolaryngology, Head and Neck Surgery,
University Clinic Schleswig-Holstein, directed by PA. MH was the
principal investigator and acquired the project funding. MH and ESQ
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of the Medical Faculty of the
Christian-Albrechts-University of Kiel, Germany (approval no. D
429/14). Informed consent was obtained from all subjects involved
in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Leemans CR, Snijders PJ and Brakenhoff RH:
The molecular landscape of head and neck cancer. Nat Rev Cancer.
18:269–282. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hu YJ, Chen J, Zhong WS, Ling TY, Jian XC,
Lu RH, Tang ZG and Tao L: Trend analysis of Betel Nut-associated
oral cancer and health Burden in China. Chin J Dent Res. 20:69–78.
2017.PubMed/NCBI
|
|
3
|
Gillison ML, Koch WM, Capone RB, Spafford
M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, et
al: Evidence for a causal association between human papillomavirus
and a subset of head and neck cancers. J Natl Cancer Inst.
92:709–720. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Syrjänen S: Human papillomavirus (HPV) in
head and neck cancer. J Clin Virol. 32 (Suppl 1):S59–S66. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O'Rorke MA, Ellison MV, Murray LJ, Moran
M, James J and Anderson LA: Human papillomavirus related head and
neck cancer survival: A systematic review and meta-analysis. Oral
Oncol. 48:1191–1201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sabatini ME and Chiocca S: Human
papillomavirus as a driver of head and neck cancers. Br J Cancer.
122:306–314. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quabius ES, Haag J, Kühnel A, Henry H,
Hoffmann AS, Görögh T, Hedderich J, Evert M, Beule AG, Maune S, et
al: Geographical and anatomical influences on human papillomavirus
prevalence diversity in head and neck squamous cell carcinoma in
Germany. Int J Oncol. 46:414–422. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ang KK, Harris J, Wheeler R, Weber R,
Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C,
et al: Human papillomavirus and survival of patients with
oropharyngeal cancer. N Engl J Med. 363:24–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Anantharaman D, Abedi-Ardekani B, Beachler
DC, Gheit T, Olshan AF, Wisniewski K, Wunsch-Filho V, Toporcov TN,
Tajara EH, Levi JE, et al: Geographic heterogeneity in the
prevalence of human papillomavirus in head and neck cancer. Int J
Cancer. 140:1968–1975. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lassen P, Lacas B, Pignon JP, Trotti A,
Zackrisson B, Zhang Q, Overgaard J and Blanchard P; MARCH
Collaborative Group, : Prognostic impact of HPV-associated
p16-expression and smoking status on outcomes following
radiotherapy for oropharyngeal cancer: The MARCH-HPV project.
Radiother Oncol. 126:107–115. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hoffmann M, Quabius ES, Tribius S,
Gebhardt S, Görögh T, Hedderich J, Huber K, Dunst J and Ambrosch P:
Influence of HPV-status on survival of patients with tonsillar
carcinomas (TSCC) treated by CO2-laser surgery plus risk adapted
therapy-A 10 year retrospective single centre study. Cancer Lett.
413:59–68. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Quabius ES, Tribius S, Heinrichs A, Haaser
D, Kühnel A, Laudien M, Hoppe F, Mlynski R, Ambrosch P and Hoffmann
M: HPV DNA/RNA detection in various oral and oropharyngeal
biomaterials identifies active HPV infections also in
non-neoplastic tonsils. Transl Oncol. 14:1010022021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoffmann M, Quabius ES, Fabian A, Laudien
M and Ambrosch P: The interaction of smoking habit, SLPI and AnxA2
in HPV associated head and neck and other cancers. Cancer Treat Res
Commun. 26:1002992021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Woodham AW, Da Silva DM, Skeate JG, Raff
AB, Ambroso MR, Brand HE, Isas JM, Langen R and Kast WM: The
S100A10 subunit of the Annexin A2 heterotetramer facilitates
L2-mediated human papillomavirus infection. PLoS One. 7:e435192012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dziduszko A and Ozbun MA: Annexin A2 and
S100A10 regulate human papillomavirus type 16 entry and
intracellular trafficking in human keratinocytes. J Virol.
87:7502–7515. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Taylor JR, Fernandez DJ, Thornton SM,
Skeate JG, Lühen KP, Da Silva DM, Langen R and Kast WM:
Heterotetrameric Annexin A2/S100A10 (A2t) is essential for
oncogenic human papillomavirus trafficking and capsid disassembly,
and protects virions from lysosomal degradation. Sci Rep.
8:116422018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bouloukaki I, Tsiligianni IG, Tsoumakidou
M, Mitrouska I, Prokopakis EP, Mavroudi I, Siafakas NM and Tzanakis
N: Sputum and nasal lavage lung-specific biomarkers before and
after smoking cessation. BMC Pulm Med. 11:352011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meyer M, Bauer RN, Letang BD, Brighton L,
Thompson E, Simmen RC, Bonner J and Jaspers I: Regulation and
activity of secretory leukoprotease inhibitor (SLPI) is altered in
smokers. Am J Physiol Lung Cell Mol Physiol. 306:L269–L276. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma G, Greenwell-Wild T, Lei K, Jin W,
Swisher J, Hardegen N, Wild CT and Wahl SM: Secretory leukocyte
protease inhibitor binds to annexin II, a cofactor for macrophage
HIV-1 infection. J Exp Med. 200:1337–1346. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Drannik AG, Henrick BM and Rosenthal KL:
War and Peace between WAP and HIV: Role of SLPI, Trappin-2, Elafin
and Ps20 in susceptibility to HIV infection. Biochem Soc Trans.
39:1427–1432. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Woodham AW, Sanna AM, Taylor JR, Skeate
JG, Da Silva DM, Dekker LV and Kast WM: Annexin A2 antibodies but
not inhibitors of the Annexin A2 heterotetramer impair productive
HIV-1 infection of macrophages in vitro. Virol J. 13:1872016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Martin-Gomez L, Fulp WJ, Schell MJ, Sirak
B, Abrahamsen M, Isaacs-Soriano KA, Lorincz A, Wenig B, Chung CH,
Caudell JJ and Giuliano AR: Oral gargle-tumor biopsy human
papillomavirus (HPV) agreement and associated factors among
oropharyngeal squamous cell carcinoma (OPSCC) cases. Oral Oncol.
92:85–91. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Majchrzak Gorecka M, Majewski P, Grygier
B, Murzyn K and Cichy J: Secretory leukocyte protease inhibitor
(SLPI), a multi-functional protein in the host defense response.
Cytokine Growth Factor Rev. 28:79–93. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Klingenberg B, Hafkamp HC, Haesevoets A,
Manni JJ, Slootweg PJ, Weissenborn SJ, Klussmann JP and Speel EJ:
p16 INK4A overexpression is frequently detected in tumour free
tonsil tissue without association with HPV. Histopathology.
56:957–967. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Quabius ES, Ossenkop L, Harder S and Kern
M: Dental implants stimulate expression of interleukin-8 and its
receptor in human blood-an in vitro approach. J Biomed Mater Res B
Appl Biomater. 100:1283–1288. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cordes C, Häsler R, Werner C, Görögh T,
Röcken C, Hebebrand L, Kast WM, Hoffmann M, Schreiber S and
Ambrosch P: The level of secretory leukocyte protease inhibitor is
decreased in metastatic head and neck squamous cell carcinoma. Int
J Oncol. 39:185–191. 2011.PubMed/NCBI
|
|
28
|
Remmerbach TW, Brinckmann UG, Hemprich A,
Chekol M, Kühndel K and Liebert UG: PCR detection of human
papillomavirus of the Mucosa: Comparison between MY09/11 and
GP5+/6+ primer sets. J Clin Virol. 30:302–308. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chaturvedi AK, Song H, Rosenberg P,
Ramqvist T, Anderson WF, Munck-Wikland E, Ye W and Dalianis T:
Tonsillectomy and incidence of oropharyngeal cancers. Cancer
Epidemiol Biomarkers Prev. 25:944–950. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Windfuhr JP, Schloendorff G, Sesterhenn
AM, Prescher A and Kremer B: A devastating outcome after
adenoidectomy and tonsillectomy: Ideas for improved prevention and
management. Otolaryngol Head Neck Surg. 140:191–196. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gallagher KE, LaMontagne DS and
Watson-Jones D: Status of HPV vaccine introduction and barriers to
country uptake. Vaccine. 36:4761–4767. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jit M, Brisson M, Portnoy A and Hutubessy
R: Cost-effectiveness of female human papillomavirus vaccination in
179 countries: A PRIME modelling study. Lancet Glob Health.
2:e406–e414. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ng SS, Hutubessy R and Chaiyakunapruk N:
Systematic review of cost-effectiveness studies of human
papillomavirus (HPV) vaccination: 9-Valent vaccine, gender-neutral
and multiple age cohort vaccination. Vaccine. 36:2529–2544. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Carpén T, Sjöblom A, Lundberg M, Haglund
C, Markkola A, Syrjänen S, Tarkkanen J, Mäkitie A, Hagström J and
Mattila P: Presenting symptoms and clinical findings in
HPV-positive and HPV-negative oropharyngeal cancer patients. Acta
Otolaryngol. 138:513–518. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Quabius ES, Loehr J, Haaser D, Günther V,
Maass N, Röcken C, Mathiak M, Alkatout I and Hoffmann M:
Smoking-induced SLPI expression hinders HPV infections also in
squamous cell carcinomas of the Vulva. Transl Oncol. 12:36–42.
2019. View Article : Google Scholar : PubMed/NCBI
|