Introduction
Colon cancer is the third most commonly diagnosed
cancer in males and the second most commonly diagnosed in females
worldwide (1). Overall ~75% of
patients are diagnosed with stage I–III colon cancer, at which
curative resection can be performed (2). Although the use of adjuvant
chemotherapy (AC) in patients with stage III colon cancer is widely
recognized, whether AC is recommended or not for stage II patients
should be considered on an individual basis (3).
Carcinoembryonic antigen (CEA) is a commonly
examined low-cost biomarker for colon cancer (4–6).
Margalit et al (2) analyzed
45,449 patients with stage I–II colon cancer and determined that
preoperative (pre)-CEA could be a potential prognostic factor.
Furthermore, postoperative (post)-CEA has recently been identified
as a reliable prognostic factor. Konishi et al (7) reported that post-CEA, and not
pre-CEA, is an important prognostic factor for patients with stage
I–III colon cancer. Auclin et al (8) performed a post-hoc analysis of the
Multicenter International Study of
Oxaliplatin/Fluorouracil/Leucovorin in the Adjuvant Treatment of
Colon Cancer (MOSAIC) trial, which indicated that high post-CEA is
an independent prognostic factor in patients with stage II colon
cancer. Furthermore this study reported that the addition of
oxaliplatin to fluorouracil and leucovorin as AC is only a benefit
to stage II patients with a high risk (T4, tumor perforation, or
<12 examined lymph nodes) and high post-CEA levels. Patients
with <10 ng/ml post-CEA were included in this previous study and
all patients received AC.
However, AC is usually prescribed for patients with
stage II colon cancer using the guidelines published by the
American Society of Clinical Oncology (ASCO), the National
Comprehensive Cancer Network (NCCN) and the European Society of
Medical Oncology (ESMO), which do not include post-CEA levels among
the high-risk criteria (3,9–11).
However, this may be because, to the best of our knowledge, there
are no studies assessing high post-CEA as an independent risk
factor of recurrence in patients with stage II colon cancer without
AC.
Therefore, the aim of the present study was to
assess post-CEA in patients with stage II colon cancer for which
the significance of AC is unknown.
Materials and methods
Study population
The present study included patients with stage II
primary colon cancer who underwent curative surgery, with
appropriate lymphadenectomy, at the institute between January 2007
and December 2016. Patients with cancer in other organs were
excluded. All patients provided informed consent and patient
anonymity was preserved. The present study was approved by the
ethics committee at the institution (Approval number: 15144-6).
Definitions
Pre-CEA was defined as the last CEA value examined
before surgery and post-CEA was defined as the first CEA value
examined after surgery. The CEA value was considered high when it
was ≥5.0 ng/ml, which had previously been determined as a cut-off
value (7,12).
Data collection
Clinicopathological and demographic data, including
sex, age, BMI, pre- and post-CEA values, tumor location, bowel
obstructions caused by a tumor, tumor histology, pathological T/N
stage, and lymphovascular invasion, were collected retrospectively.
The right side of the colon was defined as ascending and
transverse, whereas the left side of the colon was defined as
descending and sigmoid. Furthermore, retrospective data were
acquired about the surgical procedure (laparoscopic or open
surgery), the number of harvested lymph nodes, postoperative
complications (Clavien-Dindo grade ≥3) and AC.
Statistical analysis
Demographic data are presented as the absolute count
and proportion of patients, the mean ± SD, or the median and
interquartile range (IQR). An unpaired Student's t-test was used
for comparing quantitative variables, and Pearson χ2
test or Fisher's exact test was used to compare categorical data
depending on sample size. The association between different
factors, including pre-CEA or post-CEA and 3-year overall survival
(OS) or 3-year relapse-free survival (RFS), was assessed using
Kaplan-Meier survival analysis followed by the log-rank test.
Bonferroni correction was used to adjust the P-values for
statistical comparison tests among more than two groups, including
for the statistical comparison of survival curves. The RFS was
defined as the time between surgery and relapse, second colon
occurrence, or death, whichever occurred first. Patients without
relapse, second colon cancer, or death were recorded at the last
date of their follow-up. The OS was defined as the time between
surgery and death from any cause. Patients who survived were
recorded at the last date of their follow-up. The relationship of
demographic, clinicopathological, and therapeutic factors to
survival was assessed using the univariate Cox proportional-hazards
model. Risk factors with P<0.05 in univariate analyses and
certain high-risk factors reported by ASCO, NCCN, or ESMO, were
included in the multivariate Cox regression model. The factors
included in the multivariate analysis were determined based on
Akaike's Information Criterion (13). These data are presented as the
hazard ratio (HR) and 95% confidence interval (CI). All data
analysis was performed using JMP Pro 14.1.0 (JMP Statistical
Discovery, LLC). P-values were two-sided. P<0.05 was considered
to indicate a statistically significant difference.
Results
Patient clinicopathological
characteristics
In the present study there were 207 patients with
stage II primary colon cancer who underwent surgery between January
2007 and December 2016. In total 8 patients with cancer in other
organs and 10 patients who underwent AC were excluded, which
resulted in 189 patients being analyzed in the present study
(Fig. S1). The clinicopathological
characteristics of all the patients are presented in Table I. Moreover, 89.9% of the patients
underwent laparoscopic surgery, 37.7% of the patients had high
pre-CEA levels and 9% had high post-CEA levels. The median (IQR)
follow-up was 5.0 (3.6–5.6) years. Furthermore, 5% of the patients
had postoperative complications (Clavien-Dindo grade ≥3),
consisting of seven anastomotic leakages, one ureteral stricture,
one intraperitoneal hemorrhage and one bowel obstruction.
 | Table I.Clinicopathological characteristics of
patients (stage II; n=199). |
Table I.
Clinicopathological characteristics of
patients (stage II; n=199).
| Characteristics | Value |
|---|
| Sex, n (%) |
|
|
Female | 94 (47.2) |
|
Male | 105 (52.8) |
| Mean age, years
(SD) | 67.9 (11.4) |
| Age, n (%) |
|
| <75
years | 146 (73.4) |
| ≥75
years | 53 (26.6) |
| Mean body mass
index (SD) | 22.3 (3.3) |
| Tumor location, n
(%) |
|
|
Right-sided colon | 94 (47.2) |
|
Left-sided colon | 105 (52.8) |
| Surgical procedure,
n (%) |
|
|
Open | 22 (11.1) |
|
Laparoscopic | 177 (88.9) |
| Histology, n
(%) |
|
| tub1,
tub2 | 179 (89.9) |
| por,
muc | 20 (10.1) |
| pT stage, n
(%) |
|
| T3 | 190 (95.5) |
| T4 | 9 (4.5) |
| Lymphovascular
invasion, n (%) |
|
|
Yes | 134 (67.3) |
| No | 65 (32.7) |
| Harvested lymph
nodes, n (%) |
|
|
<12 | 42 (21.1) |
|
≥12 | 157 (78.9) |
| Bowel obstruction,
n (%) |
|
|
Yes | 9 (4.5) |
| No | 190 (95.5) |
| Postoperative
complications, n (%) |
|
|
Yes | 10 (5.0) |
| No | 189 (95.0) |
| Adjuvant
chemotherapy, n (%) |
|
|
Yes | 10 (5.0) |
| No | 189 (95.0) |
| Preoperative CEA, n
(%) |
|
| <5
ng/ml | 124 (62.3) |
| ≥5
ng/ml | 75 (37.7) |
| Postoperative CEA,
n (%) |
|
| <5
ng/ml | 181 (91.0) |
| ≥5
ng/ml | 18 (9.0) |
| Median time between
surgery and | 36 (27–93) |
| CEA measurement,
days (IQR) |
|
| Median follow-up,
years (IQR) | 5.0 (3.6-5.6) |
High post-CEA is associated with a
worse prognosis
Patients were divided into the following three
groups: i) Normal pre-CEA; ii) normalized (high pre-CEA and normal
post-CEA); and iii) high post-CEA. The 3-year RFS rates of these
three groups were 85.6, 91.4 and 60.0%, respectively. A statistical
comparison of the 3-year RFS of these three groups demonstrated
that there was no significant difference between the 122 patients
in the normal pre-CEA group and the 59 patients in the normalized
group. However, the 3-year RFS of the 18 patients in the high
post-CEA group was significantly worse compared with the normal
pre-CEA group (P=0.011) and the normalized group (P=0.003)
(Fig. 1A). The 3-year OS rates of
these groups were 96.7, 94.8 and 77.8%, respectively (Fig. 1B). A statistical comparison of the
3-year OS of these three groups demonstrated that there was no
significant difference between the normal pre-CEA group and the
normalized group. However, the OS in the high post-CEA group was
significantly worse compared with the high pre-CEA group (P=0.003)
and was also markedly worse compared with the normalized group
(P=0.072). These results indicated that post-CEA levels, not
pre-CEA levels, may be an optimal potential prognostic factor for
3-year RFS and OS of patients with stage II colon cancer.
High post-CEA patients have a worse
prognosis compared with normal post-CEA patients
The clinicopathological characteristics of high
post-CEA patients and normal post-CEA patients are presented in
Table II. The results
demonstrated that there were no significant differences between
these two groups. The relationship between post-CEA status and
survival of patients with stage II colon cancer was determined
using Kaplan-Meier survival curves (Fig. 2). The results demonstrated that
patients with high post-CEA levels had significantly worse 3-year
RFS or 3-year OS compared with patients in the normal post-CEA
group (RFS, 60.0 vs. 87.5%, P<0.001; and HR, 77.8 vs. 96.1%,
P<0.001, respectively).
 | Table II.Clinicopathological characteristics
of patients in the high and normal post-CEA groups. |
Table II.
Clinicopathological characteristics
of patients in the high and normal post-CEA groups.
|
Characteristics | High post-CEA
(n=18) | Normal post-CEA
(n=181) | P-value |
|---|
| Sex, n (%) |
|
|
|
|
Male | 9 (50.0) | 96 (53.0) | 0.806 |
|
Female | 9 (50.0) | 85 (47.0) |
|
| Age, n (%) |
|
|
|
|
≥75 | 6 (33.3) | 47 (26.0) | 0.500 |
| <75
years | 12 (66.7) | 134 (74.0) |
|
| Mean body mass
index (SD) | 21.3 (3.6) | 22.4 (3.3) | 0.192 |
| Tumor location, n
(%) |
|
|
|
|
Right-sided colon | 9 (50.0) | 85 (47.0) | 0.806 |
|
Left-sided colon | 9 (50.0) | 96 (53.0) |
|
| Surgical procedure,
n (%) |
|
|
|
|
Open | 1 (5.6) | 21 (11.6) | 0.699a |
|
Laparoscopic | 17 (94.4) | 160 (88.4) |
|
| Histology, n
(%) |
|
|
|
| por,
muc | 2 (11.1) | 18 (9.9) | 0.699a |
| tub1,
tub2 | 16 (88.9) | 163 (90.1) |
|
| pT stage, n
(%) |
|
|
|
| T4 | 1 (5.6) | 8 (4.4) | 0.582a |
| T3 | 17 (94.4) | 173 (95.6) |
|
| Lymphovascular
invasion, n (%) |
|
|
|
|
Yes | 12 (66.7) | 122 (67.4) | 0.949 |
| No | 6 (33.3) | 59 (32.6) |
|
| Harvested lymph
nodes, n (%) |
|
|
|
|
<12 | 2 (11.1) | 40 (22.1) | 0.373a |
|
≥12 | 16 (88.9) | 141 (77.9) |
|
| Bowel obstruction,
n (%) |
|
|
|
|
Yes | 1 (5.6) | 8 (4.4) | 0.582a |
| No | 17 (94.4) | 173 (95.6) |
|
| Postoperative
complications, n (%) |
|
|
|
|
Yes | 1 (5.6) | 9 (5.0) |
>0.990a |
| No | 17 (94.4) | 172 (95.0) |
|
| Adjuvant
chemotherapy, n (%) |
|
|
|
|
Yes | 1 (5.6) | 9 (5.0) |
>0.990a |
| No | 17 (94.4) | 172 (95.0) |
|
| Median time between
surgery and CEA measurement, days (IQR) | 28 (22–131) | 37 (28–92) | 0.512 |
High post-CEA levels are an
independent risk factor
Univariate analysis demonstrated that an age of ≥75
years, pT4 stage and high post-CEA levels were significant risk
factors for RFS (Table III).
Multivariate analysis demonstrated that high post-CEA levels
remained a significant independent risk factor for worse RFS (HR,
4.47; 95% CI, 1.83-10.96; P=0.001).
 | Table III.Univariate and multivariate analysis
of risk factors for relapse-free survival (n=199). |
Table III.
Univariate and multivariate analysis
of risk factors for relapse-free survival (n=199).
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Sex
(male/female) | 1.53
(0.72-3.23) | 0.269 | 1.62
(0.76-3.44) | 0.212 |
| Age (≥75/<75
years) | 2.14
(1.02-4.48) | 0.044 | 1.82
(0.86-3.85) | 0.116 |
|
Left-sided/right-sided | 1.32
(0.63-2.77) | 0.458 |
|
|
| Obstruction
(+/-) | 1.63
(0.39-6.86) | 0.505 |
|
|
| Histology (por,
muc/tub1, tub2) | 0.66
(0.16-2.78) | 0.573 |
|
|
| pT stage
(T4/T3) | 3.41
(1.03-11.29) | 0.045 | 2.92
(0.87-9.78) | 0.083 |
| Lymphovascular
invasion (+/-) | 0.95
(0.44-2.04) | 0.888 |
|
|
| Harvested lymph
nodes (<12/≥12) | 1.89
(0.86-4.16) | 0.112 | 2.20
(0.97-4.98) | 0.059 |
| Surgical procedure
(open/laparoscopic) | 1.81
(0.69-4.74) | 0.228 |
|
|
| Postoperative CEA
(≥5/<5 ng/ml) | 3.96
(1.69-9.29) | 0.001 | 4.47
(1.83-10.96) | 0.001 |
| Adjuvant
chemotherapy (+/-) | 2.34
(0.71-7.72) | 0.164 |
|
|
Furthermore, univariate analysis revealed that an
age of ≥75 years, pT4 stage, and high post-CEA levels were
significant risk factors for OS (Table
SI). Multivariate analysis demonstrated that high post-CEA
remained a significant independent risk factor for worse OS (HR,
5.62; 95% CI, 1.62-19.53; P=0.007).
High post-CEA levels are an
independent risk factor for patients without AC
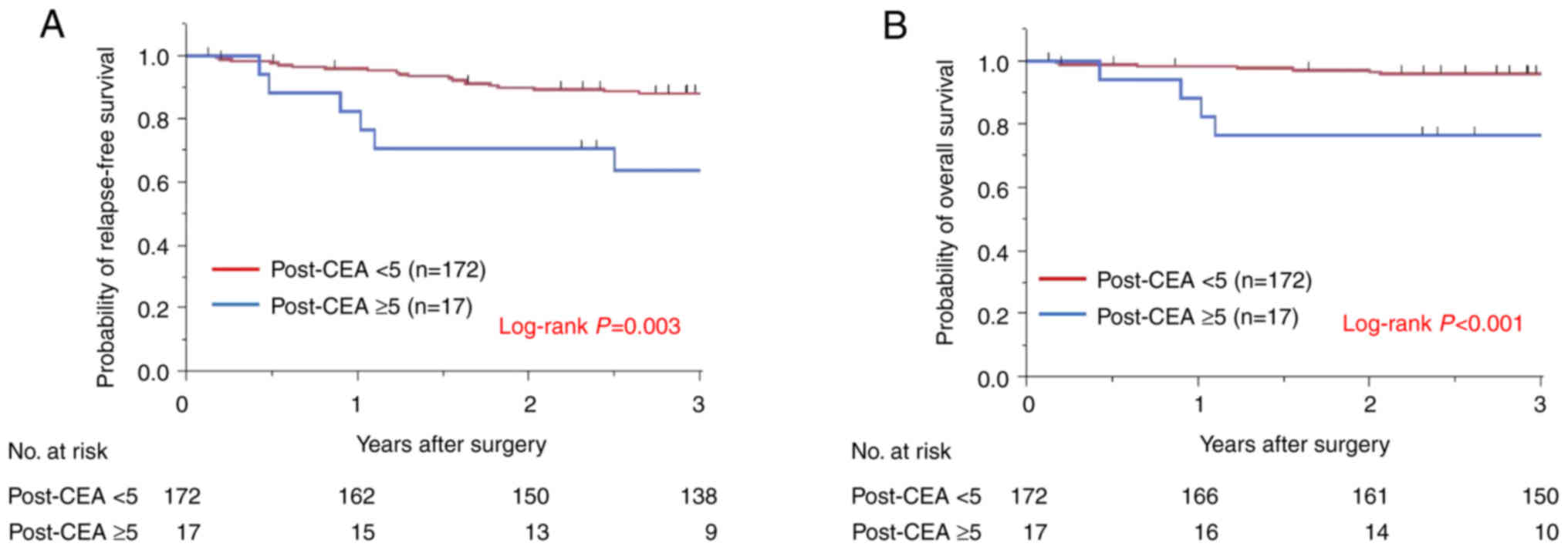
Subsequently the significance of post-CEA as a
prognostic factor for patients with stage II colon cancer who did
not undergo AC, were assessed. The 3-year RFS was determined to be
85.9% and the 3-year OS was 94.1%. Overall 19 patients had high
post-CEA levels. The relationship between post-CEA levels and
patient survival without AC was determined (Fig. 3). Kaplan-Meier survival curves
demonstrated that patients with high post-CEA levels had
significantly worse RFS or OS compared with the normal post-CEA
group (RFS, 63.5 vs. 88.0%, P=0.003; and OS, 76.5 vs. 96.8%,
P<0.001, respectively).
Univariate analysis revealed that in males, an age
of ≥75 years, pT4 stage, with a number of harvested lymph nodes
<12 and high post-CEA levels were significant risk factors for
RFS (Table IV). Multivariate
analysis demonstrated that high post-CEA remained a significant
independent risk factor for a worse RFS (HR, 3.98; 95% CI,
1.48-10.70; P=0.006).
 | Table IV.Univariate and multivariate analysis
for relapse-free survival among patients without adjuvant
chemotherapy (n=189). |
Table IV.
Univariate and multivariate analysis
for relapse-free survival among patients without adjuvant
chemotherapy (n=189).
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Sex
(male/female) | 2.09
(0.91-4.80) | 0.084 | 2.45
(1.05-5.73) | 0.039 |
| Age (≥75/<75
years) | 2.43
(1.13-5.26) | 0.024 | 1.83
(0.82-4.07) | 0.138 |
|
Left-sided/right-sided | 1.30
(0.60-2.84) | 0.504 |
|
|
| Obstruction
(+/-) | 1.74
(0.41-7.37) | 0.452 |
|
|
| Histology (por,
muc/tub1, tub2) | 0.39
(0.05-2.87) | 0.354 |
|
|
| pT stage
(T4/T3) | 7.83
(2.33-26.38) | <0.001 | 6.10
(1.67-22.33) | 0.006 |
| Lymphovascular
invasion (+/-) | 1.01
(0.45-2.27) | 0.979 |
|
|
| Harvested lymph
nodes (<12/≥12) | 2.27
(1.01-5.09) | 0.047 | 2.86
(1.22-6.71) | 0.016 |
| Surgical procedure
(open/laparoscopic) | 2.23
(0.84-5.92) | 0.107 |
|
|
| Postoperative CEA
(≥5/<5 ng/ml) | 3.58
(1.44-8.93) | 0.006 | 3.98
(1.48-10.70) | 0.006 |
Furthermore, univariate analysis revealed that an
age of ≥75 years, pT4 stage, and high post-CEA levels were
significant risk factors for OS (Table
SII). Multivariate analysis demonstrated that high post-CEA
levels remained a significant independent risk factor for a worse
OS (HR, 5.43; 95% CI, 1.55-18.98; P=0.008).
Discussion
To the best of our knowledge this is the first study
that has demonstrated that high post-CEA levels, more than high
pre-CEA levels, may be an independent risk factor for patients with
stage II primary colon cancer who do not undergo AC following
curative resection. These results strongly suggested that high
post-CEA is a potential indicator of AC for stage II colon cancer
following surgery.
Previous studies have demonstrated that pre-CEA can
be a risk factor for the recurrence of stage II colon cancer
(2,14). In the present study the 3-year RFS
and OS of high pre-CEA patients were not significantly different
from those of low pre-CEA patients when the CEA level normalized
following surgery. However, the 3-year RFS and OS of high post-CEA
patients were significantly worse than those of normal post-CEA
patients. This result is consistent with a previous report of 1,027
patients with stage I–III colon cancer, but this previous study did
not determine the significance of post-CEA for stage II colon
cancer (7). Other studies have
also demonstrated that post-CEA is a high-risk factor for stage II
colon cancer. Lin et al (15) reported that high post-CEA patients
had a worse 5-year RFS compared with normal post-CEA patients.
However, multivariate analysis for patients with stage II colon
cancer was not performed as part of this previous study. Tsai et
al (12) reported that high
post-CEA can be a risk factor for early relapse (within 12 months
following surgery) in patients with stage II colon cancer, but
multivariate analysis was not performed and 46.1% of the patients
underwent AC. As previously stated, a post-hoc analysis of the
MOSAIC trial demonstrated that patients with high post-CEA have a
50% increased risk of death or recurrence compared with those who
have low post-CEA levels determined via multivariate analysis,
although all patients received AC (8). Therefore, to the best of our
knowledge the present study was the first to have demonstrated the
significance of high post-CEA as an indicator of AC for stage II
colon cancer.
The decision to perform AC should be made cautiously
because it can cause adverse effects in patients (16–18).
Therefore, it is essential to precisely assess a patients risk
factors for relapse. Previous studies have reported that
circulating tumor cells and circulating tumor DNA are potential
indicators of a high risk of recurrence for stage II–III colorectal
cancer (19–22). However, detection of circulating
tumor cells and DNA requires special techniques, equipment and is
expensive. Therefore, these tests are not yet commonly used to
examine patients with colon cancer. The CEA value, on the other
hand, is commonly determined perioperatively and at low cost.
Furthermore, because CEA half life is reported to be 3–7 days
(23) its levels can normalize
within a few weeks following complete tumor resection. Therefore,
if the CEA level does not normalize after apparent curative
resection this may indicate that there are some residual
micrometastases. The 3-year RFS of high post-CEA patients in the
present study was worse than that in the post hoc analysis of the
MOSAIC study, whereby all patients underwent AC, and the cutoff CEA
value in the present study was more severe (high post-CEA was
defined as ≥5 ng/ml) (8). Taken
together, these results indicated that high post-CEA levels may be
a good indicator for AC or more intensive follow-up treatment than
patients with normal CEA levels. However, this should be validated
by a multicenter prospective trial.
In the present study multivariate analyses for
patients without AC revealed that being male was an independent
risk factor, which is consistent with previous reports (8,24).
However, the underlying reason for this remains to be investigated.
Pathological T4 stage and the number of harvested lymph nodes
(<12) were also independent risk factors for relapse and are
well-known high-risk stage II indicators as suggested by the ASCO,
ESMO and NCCN.
The present study has several limitations. First, it
is a retrospective study conducted in one institute. The sample
size of 189 patients without AC was considered to be sufficient
because the minimum sample size was 65 patients, which was
determined using an α-error value of 0.05 and statistical power of
0.8 (25). Moreover, there were no
significant differences in clinicopathological characteristics
between the high and normal post-CEA groups without any
adjustments. Second, the interval between surgery and the first CEA
examination after operation was not controlled. Given that AC
should be performed within 8 weeks (26,27),
post-CEA levels must be examined within 8 weeks after surgery at
the latest. As the present study included high post-CEA patients
whose CEA values were obtained more than 8 weeks following
resection, there is a possibility that the CEA values were once
less than 5 ng/ml after surgery and later exceeded the cutoff.
However, it is also possible that the CEA values remained high
after surgery; the median value of this interval was 36 days, which
is within 8 weeks. In order to deal with this limitation, a
well-designed prospective study should be performed in the future.
Third, no data was included on smoking history, a factor that is
associated with higher CEA levels, and other factors which can also
have an affect, such as gastritis, diverticulitis, pancreatitis,
liver diseases, diabetes and inflammatory bowel disease (28–30).
Therefore, these false-positive-CEA-related factors should be
considered when using the post-CEA value as a high-risk indicator.
Despite these limitations, the present study, to the best of our
knowledge, is the first report to analyze patients without AC and
to demonstrate that the post-CEA value could be a potential
indicator of AC for patients with stage II colon cancer.
In conclusion, the present study demonstrated the
importance of high post-CEA as a prognostic factor for stage II
colon cancer. Even though it is not included in the major
guidelines, data from previous multicenter and large-scale studies
support these findings. On the basis of the present study, a
prospective multicenter study with more patients should be
performed to validate the significance of the post-CEA value.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YM was responsible for the conceptualization, the
acquisition and analysis of data, interpretation of data,
methodology, project administration, resources, validation,
visualization and writing of the first draft of the manuscript. YM
and HT confirm the authenticity of all the raw data. HT, YD and HE
were responsible for the conceptualization, the acquisition and
analysis of data, methodology, project administration, resources,
supervision, validation, visualization and reviewing and editing of
the manuscript. AA and HI performed the acquisition and analysis of
data and helped with validation and visualization. MF was also
involved in the acquisition and analysis of data, and validation.
YS, TH, SF and NM were responsible for the interpretation of data,
methodology, project administration, resources, validation,
visualization and reviewing and editing of the manuscript. TO was
responsible for the interpretation of data, methodology, project
administration, resources, validation and visualization. MU, CM, HY
and TM were responsible for the interpretation of data,
methodology, project administration, resources, supervision,
validation, visualization and the reviewing and editing of the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All patients provided written informed consent and
patient anonymity was preserved. The present study was approved by
the Ethics Board of Osaka University Hospital (Suita, Osaka,
Japan).
Patient consent for publication
Not applicable.
Competing interests
TM was supported by donations from Kinshukai Medical
Corporation. The other authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CEA
|
carcinoembryonic antigen
|
|
pre
|
preoperative
|
|
post
|
postoperative
|
|
AC
|
adjuvant chemotherapy
|
|
ASCO
|
American Society of Clinical
Oncology
|
|
NCCN
|
National Comprehensive Cancer
Network
|
|
ESMO
|
European Society of Medical
Oncology
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Margalit O, Mamtani R, Yang YX, Reiss KA,
Golan T, Halpern N, Aderka D, Giantonio B, Shacham-Shmueli E and
Boursi B: Assessing the prognostic value of carcinoembryonic
antigen levels in stage I and II colon cancer. Eur J Cancer.
94:1–5. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kannarkatt J, Joseph J, Kurniali PC,
Al-Janadi A and Hrinczenko B: Adjuvant Chemotherapy for Stage II
Colon Cancer: A Clinical Dilemma. J Oncol Pract. 13:233–241. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gold P and Freedman SO: Demonstration of
tumor-specific antigens in human colonic carcinomata by
immunological tolerance and absorption techniques. J Exp Med.
121:439–462. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takagawa R, Fujii S, Ohta M, Nagano Y,
Kunisaki C, Yamagishi S, Osada S, Ichikawa Y and Shimada H:
Preoperative serum carcinoembryonic antigen level as a predictive
factor of recurrence after curative resection of colorectal cancer.
Ann Surg Oncol. 15:3433–3439. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Araujo RL, Gönen M, Allen P, DeMatteo R,
Kingham P, Jarnagin W, D'Angelica M and Fong Y: Positive
postoperative CEA is a strong predictor of recurrence for patients
after resection for colorectal liver metastases. Ann Surg Oncol.
22:3087–3093. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Konishi T, Shimada Y, Hsu M, Tufts L,
Jimenez-Rodriguez R, Cercek A, Yaeger R, Saltz L, Smith JJ, Nash
GM, et al: Association of preoperative and postoperative serum
carcinoembryonic antigen and colon cancer outcome. JAMA Oncol.
4:309–315. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Auclin E, André T, Taieb J, Banzi M, Van
Laethem JL, Tabernero J, Hickish T, de Gramont A and Vernerey D:
Association of post-operative CEA with survival and oxaliplatin
benefit in patients with stage II colon cancer: A post hoc analysis
of the MOSAIC trial. Br J Cancer. 121:312–317. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Benson AB III, Schrag D, Somerfield MR,
Cohen AM, Figueredo AT, Flynn PJ, Krzyzanowska MK, Maroun J,
McAllister P, Van Cutsem E, et al: American Society of Clinical
Oncology recommendations on adjuvant chemotherapy for stage II
colon cancer. J Clin Oncol. 22:3408–3419. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Labianca R, Nordlinger B, Beretta GD,
Mosconi S, Mandalà M, Cervantes A and Arnold D; ESMO Guidelines
Working Group, : Early colon cancer: ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 24
(Suppl 6):vi64–vi72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
National Comprehensive Cancer Network, .
Colon Cancer (Version 2.2017). https://www2.tri-kobe.org/nccn/guideline/colorectal/english/colon.pdfAugust
25–2020
|
|
12
|
Tsai HL, Huang CW, Chen CW, Yeh YS, Ma CJ
and Wang JY: Survival in resected stage II colorectal cancer is
dependent on tumor depth, vascular invasion, postoperative CEA
level, and the number of examined lymph nodes. World J Surg.
40:1002–1009. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Akaike H: Information theory and an
extension of the maximum likelihood principle. 2nd International
Symposium on Information Theory, Tsahkadsor, Armenia, USSR,
September 2–8, 1971. Petrov BN and Caski F: Akadémiai Kiadó;
Budapest: pp. 267–281. 1973
|
|
14
|
Kim CW, Yoon YS, Park IJ, Lim SB, Yu CS
and Kim JC: Elevation of preoperative s-CEA concentration in stage
IIA colorectal cancer can also be a high risk factor for stage II
patients. Ann Surg Oncol. 20:2914–2920. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin JK, Lin CC, Yang SH, Wang HS, Jiang
JK, Lan YT, Lin TC, Li AF, Chen WS and Chang SC: Early
postoperative CEA level is a better prognostic indicator than is
preoperative CEA level in predicting prognosis of patients with
curable colorectal cancer. Int J Colorectal Dis. 26:1135–1141.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Akagi T and Inomata M: Essential advances
in surgical and adjuvant therapies for colorectal cancer 2018–2019.
Ann Gastroenterol Surg. 4:39–46. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gelibter AJ, Caponnetto S, Urbano F,
Emiliani A, Scagnoli S, Sirgiovanni G, Napoli VM and Cortesi E:
Adjuvant chemotherapy in resected colon cancer: When, how and how
long? Surg Oncol. 30:100–107. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsuda T, Yamashita K, Hasegawa H,
Oshikiri T, Hosono M, Higashino N, Yamamoto M, Matsuda Y, Kanaji S,
Nakamura T, et al: Recent updates in the surgical treatment of
colorectal cancer. Ann Gastroenterol Surg. 2:129–136. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peach G, Kim C, Zacharakis E, Purkayastha
S and Ziprin P: Prognostic significance of circulating tumour cells
following surgical resection of colorectal cancers: A systematic
review. Br J Cancer. 102:1327–1334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu CY, Uen YH, Tsai HL, Chuang SC, Hou MF,
Wu DC, Juo SH, Lin SR and Wang JY: Molecular detection of
persistent postoperative circulating tumour cells in stages II and
III colon cancer patients via multiple blood sampling: Prognostic
significance of detection for early relapse. Br J Cancer.
104:1178–1184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lecomte T, Berger A, Zinzindohoué F,
Micard S, Landi B, Blons H, Beaune P, Cugnenc PH and Laurent-Puig
P: Detection of free-circulating tumor-associated DNA in plasma of
colorectal cancer patients and its association with prognosis. Int
J Cancer. 100:542–548. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tie J, Wang Y, Tomasetti C, Li L, Springer
S, Kinde I, Silliman N, Tacey M, Wong HL, Christie M, et al:
Circulating tumor DNA analysis detects minimal residual disease and
predicts recurrence in patients with stage II colon cancer. Sci
Transl Med. 8:346ra922016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yakabe T, Nakafusa Y, Sumi K, Miyoshi A,
Kitajima Y, Sato S, Noshiro H and Miyazaki K: Clinical significance
of CEA and CA19-9 in postoperative follow-up of colorectal cancer.
Ann Surg Oncol. 17:2349–2356. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Burdy G, Panis Y, Alves A, Nemeth J,
Lavergne-Slove A and Valleur P: Identifying patients with T3-T4
node-negative colon cancer at high risk of recurrence. Dis Colon
Rectum. 44:1682–1688. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Freedman LS: Tables of the number of
patients required in clinical trials using the logrank test. Stat
Med. 1:121–129. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Biagi JJ, Raphael MJ, Mackillop WJ, Kong
W, King WD and Booth CM: Association between time to initiation of
adjuvant chemotherapy and survival in colorectal cancer: A
systematic review and meta-analysis. JAMA. 305:2335–2342. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Des Guetz G, Nicolas P, Perret GY, Morere
JF and Uzzan B: Does delaying adjuvant chemotherapy after curative
surgery for colorectal cancer impair survival? A meta-analysis. Eur
J Cancer. 46:1049–1055. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alexander JC, Silverman NA and Chretien
PB: Effect of age and cigarette smoking on carcinoembryonic antigen
levels. JAMA. 235:1975–1979. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Clinical practice guidelines for the use
of tumor markers in breast and colorectal cancer. Adopted on May
17, 1996 by the American Society of Clinical Oncology. J Clin
Oncol. 14:2843–2877. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Litvak A, Cercek A, Segal N, Reidy-Lagunes
D, Stadler ZK, Yaeger RD, Kemeny NE, Weiser MR, Pessin MS and Saltz
L: False-positive elevations of carcinoembryonic antigen in
patients with a history of resected colorectal cancer. J Natl Compr
Canc Netw. 12:907–913. 2014. View Article : Google Scholar : PubMed/NCBI
|