Introduction
Pulmonary embolism (PE), which is the obstruction of
the pulmonary arteries, is a part of venous thromboembolism (VTE)
along with deep vein thrombosis (DVT). Globally, PE represents the
third most frequent cause of cardiovascular-related mortality,
following stroke and myocardial infarction (1). The incidence of PE is similar in the
USA and Europe, and it is estimated to be ~300,000 to 600,000 cases
annually (1). There are several
well-recognized genetic mutations responsible for the increased
risk of PE (2). Major acquired
predisposing factors include post-operative conditions, pregnancy,
cancer and an advanced age (3).
The strong association between cancer and VTE is
known, and cancer patients present a 6–7-fold greater risk of
undergoing a thrombotic event compared to the general population.
PE is a notable cause of morbidity and mortality in this group
(4). The real prevalence of PE in
patients with malignancy is probably underestimated (5). Of note, approximately half of the
cases of PE among cancer patients were incidentally diagnosed by
imaging. Advances in radiological techniques may have contributed
to this fact (5).
The majority of cancer patients present with the
upregulation of the coagulation cascade, and increased platelet
activation and aggregation. The coagulation activation state in
these patients appears to have a multifactorial underlying
mechanism. Tumor cells may express prothrombotic molecules and may
produce enzymes such as cysteine proteases, which directly result
in clotting by activating factor X and produce physiological tissue
factor, which is related to the activation of the extrinsic pathway
of blood coagulation. Additionally, tumor cells can indirectly
contribute to clotting by secreting cytokines that act on
endothelial cells and mononuclear cells, thus stimulating the
production of prothrombotic molecules (6).
It has been reported that patients with active
cancer who have undergone surgery, particularly in the abdomen or
pelvis, are subject to a higher risk of developing PE, which is
affected by age, the presence of obesity, duration of the surgical
procedure, long recovery times, radiotherapy and systemic therapy.
In addition, chemotherapy and hormone therapy can induce both
venous and arterial thrombosis. Furthermore, factors influencing
the incidence of PE in cancer patients include the type of cancer,
as well as the stage, type and duration of chemotherapy, the
response to therapy, nutritional status, an individual's mobility,
and liver and kidney functional status (7).
In Greece, few studies have reported data associated
with pulmonary embolism in cancer patients (8,9). The
aim of the present study was to illustrate the clinical
characteristics, laboratory findings, radiology features and
outcomes of individuals with malignancy who developed PE, collected
from an anticancer hospital in Greece. The present study was
designed in order to identify possible additional predisposing
factors for PE among cancer patients and potential biomarkers
indicative of PE, particularly in asymptomatic cancer patients.
Patients and methods
Study design
The design of the present study was cross-sectional.
The present study obtained approval from the Institutional Board of
Agios Savvas Hospital (protocol no. 8034/1-12-18). The study was in
line with the declaration of Helsinki in 1995 (revised in Edinburgh
2000). This research involved adult cancer patients who visited
Agios Savvas Anticancer Hospital (Athens, Greece) and who were
diagnosed with PE by imaging with computed tomography (CT)
pulmonary angiography (CTPA). Another inclusion criterion was a
Miller index point score ≥1, which indicates either the obstruction
of a segmental artery or at least a moderate reduction in the
peripheral perfusion of a lung zone (10). The exclusion criteria were evidence
of previous PE, inconclusive findings due to poor imaging quality
and multiple primary malignancy sites. The aim of the present study
was to record the clinical, radiological and laboratory data of
these patients and to associate these with the occurrence of PE.
The data collection took place at Agios Savvas Anticancer Hospital
from January, 2019 to January, 2020. The patients were also
followed-up on outcomes and for the detection of PE recurrence.
Data analysis was performed with the use of a comprehensive
statistical analysis software.
Participants and data collection
The study participants had active cancer or suffered
from cancer over the last decade and were in follow-up. Imaging
diagnosis of PE was confirmed by a CTPA scan, performed using a
64-slice CT scanner (Philips Ingenuity Core 64, Integrity Medical
Systems, Inc.), in accordance with the dedicated protocol, with the
use of 80–100 ml iodinated intravenous contrast agent (350 mg/ml).
CT images were evaluated by experienced chest radiologists who
specifically searched for the presence of contrast filling defects
within the pulmonary arterial tree down to a sub-segmental level.
Findings consistent with acute PE are a complete filling defect
(vessel size normal or dilated, eccentric filling defect with the
acute angle with the artery wall, central filling defect surrounded
by contrast, ‘polo-mint sign’ (in cross-section), which is central
filling defect surrounded by contrast circumferentially and
‘railway track sign’ (along the long axis of the vessel). Findings
consistent with old PE are a complete filling defect (vessel size
normal or smaller than adjacent patent vessel), and a peripheral,
crescent-shaped defect with the obtuse angle with the artery wall
and web or flap (linear defect) (11). The patients were classified
according to the most proximal site of occlusion as having central
PE (main trunk, main pulmonary arteries and lobar branches) or
peripheral PE (segmental and subsegmental branches). Unilateral or
bilateral embolus cite were also noted.
For all patients, the following data were collected:
i) Demographics (age and sex); ii) comorbidities (diabetes
mellitus, arterial hypertension, history of smoking, depression,
coronary artery disease); iii) data concerning cancer: Type of
cancer, time interval between cancer diagnosis and the occurrence
of PE, type of received therapy (surgery, chemotherapy,
radiotherapy, or a combination) and the presence of metastases; iv)
clinical signs and symptoms: Tachypnea, fever, chest pain,
precordial pain, lower limb edema, fatigue, arterial pressure value
and the number of patients with an incidental diagnosis of PE
(asymptomatic); v) predisposing factors for PE development:
Performance status, hospitalization, immobility, the presence of
central venous catheter, history and type of chemotherapy
administration over the past month, medical history of PE or VTE or
receiving anticoagulants for any another reason; vi) laboratory
data: a) Complete blood count: White blood cell, hemoglobin (Hb),
hematocrit (Ht) and platelet count (PLT count); b) coagulation
testing: Prothrombin time, partial thromboplastin time,
international normalized ratio, fibrinogen and D-dimer levels; c)
biochemical parameters: Levels of blood urea nitrogen, creatinine,
total proteins, albumin; d) serum levels of tumor markers:
Carcinoembryonic antigen (CEA), CA 125, CA 19-9; e) inflammatory
markers: C-reactive protein (CRP) and procalcitonin (PCT); and f)
data from blood gases analysis: pH, partial pressure of oxygen,
partial pressure of carbon dioxide, lactic acid and oxygen
saturation; vii) radiological findings: a) CTPA: Location of
obstructed branches of pulmonary arteries, the presence of pleural
effusion and the presence of pulmonary metastases; b)
echocardiography: Ejection fraction, dilation of right ventricle;
and c) ultrasonography of the lower extremity veins: Venous
thrombosis, venous insufficiency; viii) electrocardiography (ECG)
findings: Basic rhythm, heart rate, the presence of abnormal
findings; ix) type of therapy received for PE, outcome and
re-occurrence of PE over a follow-up period of 6 months.
Statistical analysis
Data entry and analysis were performed using the
SPSS statistical software (version 13.0; SPSS, Inc.). Categorical
variables were summarized as the number (percentage) and continuous
variables as the mean (standard deviation). The normal distribution
of variables was assessed using the Kolmogorov-Smirnov test.
Normally distributed variables were compared using an independent
samples Student's unpaired t-test. A value of P<0.05 was
considered to indicate a statistically significant difference. The
authors consider that the statistical analysis was successful as
the data collected were of good quality with no missing records and
the analysis was conducted with rigorous responses to the research
questions.
Results
A total of 60 cancer patients with a confirmed
diagnosis of PE by CTPA were enrolled in the present study. As
regards the study demographics, the majority of the cancer patients
were males (38/60, 63.3%). The mean age of the patients was
61.1±7.1 years. In total, 42 patients had comorbidities. The most
common comorbidity was arterial hypertension (16/10, 26.7%), while
12 patients (12/60, 20%) were active smokers (Table I).
 | Table I.Characteristics of the study
population and cancer-related data. |
Table I.
Characteristics of the study
population and cancer-related data.
| Parameter | No. of
patients | Percentage |
|---|
| Sex |
|
|
|
Male | 38 | 63.3 |
|
Female | 22 | 36.7 |
| Smoking status
(active smokers) |
|
|
|
Yes | 12 | 20 |
| No | 48 | 80 |
| Comorbidities |
|
|
|
Arterial hypertension | 16 | 26.7 |
|
Diabetes mellitus | 6 | 10 |
|
Coronary artery disease | 4 |
6.7 |
|
Depression | 4 |
6.7 |
| No
comorbidities | 18 | 30 |
| Type of cancer |
|
|
| Lung
cancer | 16 | 26.7 |
|
Gastrointestinal cancer | 14 | 23.3 |
|
Pancreatic | 4 |
6.7 |
|
Stomach | 2 |
3.3 |
|
Rectal | 2 |
3.3 |
| Large
bowel | 2 |
3.3 |
|
Appendix | 2 |
3.3 |
|
Cholangiocarcinoma | 2 |
3.3 |
|
Breast | 12 | 20 |
|
Renal | 6 | 10 |
|
Nasal | 2 |
3.3 |
| Unknown
primary | 6 | 10 |
|
Ovarian | 2 |
3.3 |
|
Endometrial | 2 |
3.3 |
| Time interval
between cancer diagnosis and PE occurrence |
|
|
| ≤6
months | 38 | 63.3 |
| 1
month | 12 | 20 |
| 2
months | 8 | 13.3 |
| 5
months | 6 | 10 |
| 6
months | 12 | 20 |
| >6
months | 22 | 36.7 |
| 6–12
months | 12 | 20 |
| 13–24
months | 4 |
6.7 |
| 25–36
months | 0 |
0.0 |
| 37–48
months | 2 |
3.3 |
| 49–60
months | 2 |
3.3 |
| 61–72
months | 2 |
3.3 |
| Patients who
developed PE in the first year from the time of cancer
diagnosis | 50 |
3.3 |
| Type of therapy
received |
|
|
|
Chemotherapy | 22 | 36.7 |
|
Surgery | 6 | 10 |
|
Chemotherapy + surgery | 14 | 23.3 |
|
Chemotherapy +
radiotherapy | 4 |
6.7 |
|
Chemotherapy + surgery +
radiotherapy | 6 | 10 |
|
None | 8 | 13.3 |
| Presence of
metastases |
|
|
|
Yes | 38 | 63.3 |
| No | 22 | 36.7 |
Concerning the cancer-related data, the most common
type of cancer was lung cancer (16/60, 26.7%), followed by breast
cancer (12/60, 20%), renal cancer (6/60, 10%) and cancer of unknown
primary, under investigation (6/60, 10%). The mean time interval
between cancer diagnosis and the occurrence PE was >6 months in
22 patients (22/60, 36.7%) and <6 months in 38 patients (38/60,
63.3%), while 50 patients (50/60, 83.3%) developed PE in the first
year from the time of cancer diagnosis. In total, 22 (22/60, 36.7%)
had only received chemotherapy, 14 patients (14/60, 23.3%) had
undergone surgical resection of the tumor and had received
chemotherapy, 6 patients (6/60, 10%) had undergone surgical
resection of the tumor and had received chemotherapy and radiation,
and 4 patients (4/60, 6.7%) had received chemotherapy and
radiation, while 8 patients (8/60, 13.3%) had not received any
therapy. As regards the presence of metastases, the majority of
patients had metastases at the time of PE occurrence (38/60, 63.3%)
(Table I).
More specifically, 4 patients with lung cancer were
at stage IIA (T2BN0M0), 2 patients with appendix cancer were at
stage IIIA (T2N1M0) and IIIB (T3N1M0), respectively, 2 patients
with renal cancer were at stage II (T2N0M0 and T3N0M0), 2 patients
with breast cancer were at stage IB [T2N0M0, grade 3, human
epidermal growth factor receptor 2 (HER2)-negative, estrogen
receptor (ER)-positive and progesterone receptor (PR)-positive; and
T3N2M0, grade 2, HER20positive, ER-positive and PR-positive], 1
patient with breast cancer was at stage IIB (T3N2M0, grade 2,
HER2-negative, ER-negative and PR-negative), 1 patient with rectal
cancer was at stage IIA (T4aN0M0), 1 patient with rectal cancer was
at stage IIB (T4bN0M0), 1 patient with cholangiocarcinoma was at
stage IIIA (T3N0M0), 1 patient with endometrial cancer was at stage
II (T2N0M0) and 1 patient with endometrial cancer was at stage IIIB
(T3bN0M0). All the other cancer patients were at stage IV (data not
shown).
A total of 38 patients (38/60, 63.3%) were
symptomatic, while in 22 patients (22/60, 36.7%), PE was an
incidental finding. Among the asymptomatic patients (out of the
total number of patients), 8 patients (8/60, 13.4%) were
hospitalized and 14 patients (14/60, 23.3%) were outpatients who
visited the hospital for investigation, follow-up, chemotherapy
administration or to undergo surgery. Among the asymptomatic
patients, the majority of the cancer patients were females (16/22,
72.7%). Also among the asymptomatic patients, the most common type
of cancer observed was breast cancer (6/22, 27.3%) and of unknown
primary (6/22, 27.3%), and the majority of patients had metastases
at the time of PE occurrence (14/22, 63.3%) (data not shown).
Among the symptomatic individuals, the most common
symptom was dyspnea (30/38, 78.9%), followed by fever (12/38,
31.5%) and chest pain (8/38, 21%). A total of 14 patients presented
with signs of lower limb edema (14/38, 36.8%). The majority of
patients (40/60, 66.7%) had an arterial pressure value within the
normal range (Table II).
 | Table II.Symptoms, signs and arterial pressure
values of the study population, predisposing factors for PE, types
of receiving chemotherapy and anticoagulants. |
Table II.
Symptoms, signs and arterial pressure
values of the study population, predisposing factors for PE, types
of receiving chemotherapy and anticoagulants.
| Parameter | No. of
patients | Percentage |
|---|
| Symptomatic
patients | 38/60 | 63.3 |
|
Dyspnea | 30/38 | 78.9 |
|
Fever | 12/38 | 31.5 |
| Chest
pain | 8/38 | 21 |
|
Tachypnea | 6/38 | 15.8 |
|
Fatigue | 6/38 | 15.8 |
|
Precordial pain | 2/38 |
5.3 |
| Lower
limb edema | 14/38 | 36.8 |
| Arterial
hypertension (normal range, 90–130 mmHg) |
|
|
|
Normal | 40 | 66.7 |
| >130
mmHg | 14 | 23.3 |
| <90
mmHg | 6 | 10 |
| Asymptomatic
patients | 22/60 | 36.7 |
|
Incidental finding in
outpatients | 14/22 | 63.3 |
| Visit
for investigation | 2/22 | 9 |
| Visit
for follow-up | 4/22 | 18 |
| Visit
for chemotherapy | 6/22 | 27.3 |
| Visit
for surgery | 2/22 | 9 |
| Predisposing
factors for PE |
|
|
|
Performance status 1,2 | 30 | 50 |
|
Performance status 3,4 | 30 | 50 |
|
Immobility >7 days | 16 | 26.7 |
|
Hospitalization | 24 | 40 |
| Central
venous catheter | 4 |
6.7 |
| History
of DVT | 6 | 10 |
| History
of PE | 2 |
3.3 |
|
Chemotherapy received the last
month | 36 | 60 |
| Type of
chemotherapya |
|
|
|
Platinum-based | 24 | 40 |
|
Cisplatin | 8 | 13.3 |
|
Carboplatin | 8 | 13.3 |
|
Oxaliplatin | 8 | 13.3 |
|
Abraxane | 2 |
3.3 |
|
Doxorubicin | 2 |
3.3 |
|
Tamoxifen | 2 |
3.3 |
|
Lonsurf | 2 |
3.3 |
|
Letrozole | 2 |
3.3 |
|
Carbozanitib | 2 |
3.3 |
| No
chemotherapy | 24 | 40 |
| Anticoagulants | 12 | 20 |
| LMWH
(prophylactic) | 10 | 16.7 |
| LMWH
(therapeutic) + acetylsalicylic acid | 2 |
3.3 |
| Not
receiving anticoagulants | 48 | 80 |
|
Never | 46 | 76.7 |
|
Discontinuation 5 days prior
to PE (clopidogrel) | 2 |
3.3 |
After analyzing the predisposing factors for PE, the
most common factor was chemotherapy administration over the past
month (36/60, 60%). The most common type of chemotherapy used was
platinum-based chemotherapy (24/60, 40%). As regards the use of
anticoagulants, 48 patients (48/60, 80%) were not receiving
anticoagulants, 10 patients (10/60, 16.7%) were receiving low
molecular weight heparin (LMWH) and 2 patients (2/60, 3.3%) were
receiving a combination of LMWH and acetylsalicylic acid due to
known arterial thrombosis (Table
II).
All the patients presented with lower than normal
values of Hb, Ht and oxygen saturation and greater than normal
D-dimer levels. A large number of patients presented with greater
than normal values of fibrinogen (44/60, 73.3%), CRP (46/60,
76.7%), PCT (48/60, 80%), pH (52/60, 86.66%) and lactic acid
(44/60, 73.3%) (Table III).
 | Table III.Laboratory findings of the study
population. |
Table III.
Laboratory findings of the study
population.
| Laboratory
parameter | No. of patients
(n=60) | Percentage |
|---|
| WBC (normal range,
4–10×103/µl) |
|
|
|
Normal | 24 | 40 |
|
>10×103/µl | 28 | 46.7 |
|
<4×103/µl | 8 | 13.3 |
| PLT count (normal
range, 150–450×103/µl) |
|
|
|
Normal | 42 | 70 |
|
>450×103/µl | 14 | 23.3 |
|
<150×103/µl | 4 |
6.7 |
| Hb (normal range,
14–18 g/dl) |
|
|
| <14
g/dl | 60 | 100 |
| Ht (normal range,
38–48 %) |
|
|
|
<38% | 60 | 100 |
| PT (normal range,
11–14 sec) |
|
|
|
Normal | 60 | 100 |
| APTT (normal range,
20–40 sec) |
|
|
|
Normal | 58 | 96.7 |
| >40
sec | 2 |
3.3 |
| INR (normal range,
0.8-1.25 sec) |
|
|
|
Normal | 52 | 86.7 |
|
>1.25 sec | 8 | 13.3 |
| Fibrinogen (normal
range, 200–400 mg/dl) |
|
|
|
Normal | 16 | 26.7 |
| >400
mg/dl | 44 | 73.3 |
| D-Dimer (normal,
<0.5 µg/ml) |
|
|
| >0.5
µg/ml) | 60 | 100 |
| Blood urea nitrogen
(normal range, 15–45 mg/dl) |
|
|
|
Normal | 54 | 90 |
| >45
mg/dl | 6 | 10 |
| Creatinine (normal
range, 0.6-1.40 mg/dl) |
|
|
|
Normal | 44 | 73.3 |
|
>1.40 mg/dl | 2 |
3.3 |
| <0.6
mg/dl | 14 | 23.6 |
| Total proteins
(normal range, 6.2-8.5 g/dl) |
|
|
|
Normal | 40 | 66.7 |
| <6.2
g/dl | 20 | 33.3 |
| Albumin (normal
range, 3.5-5.2 g/dl) |
|
|
|
Normal | 40 | 66.7 |
| <3.5
g/dl | 20 |
3.3 |
| CRP (normal, <6
mg/l) |
|
|
|
Normal | 14 | 23.3 |
| >6
mg/l | 46 | 76.7 |
| PCT (normal,
<0.05 ng/ml) |
|
|
|
Normal | 12 | 20 |
|
>0.05 ng/ml | 48 | 80 |
| No. of elevated
serum tumor markers |
|
|
| 1 | 22 | 45.8 |
| 2 | 6 | 12.5 |
| 3 | 6 | 12.5 |
| pH (normal range,
7.35-7.45) |
|
|
|
Normal | 8 | 13.3 |
|
>7.45 | 52 | 86.7 |
|
<7.35 | 0 | 0 |
| pO2
(normal range, 75–100 mmHg) |
|
|
|
Normal | 10 | 16.7 |
| >100
mmHg | 2 |
3.3 |
| <75
mmHg | 48 | 80 |
| pCO2
(normal range, 35–45 mmHg) |
|
|
|
Normal | 34 | 56.7 |
| >45
mmHg | 20 | 33.3 |
| <35
mmHg | 6 | 10 |
| Lactic acid (normal
range, 0.5-2 mmol/l) |
|
|
|
Normal | 16 | 26.7 |
| >2
mmol/l | 44 | 73.3 |
| Oxygen saturation
(normal range, 95–100%) |
|
|
|
Normal | 0 | 0 |
|
<95% | 60 | 100 |
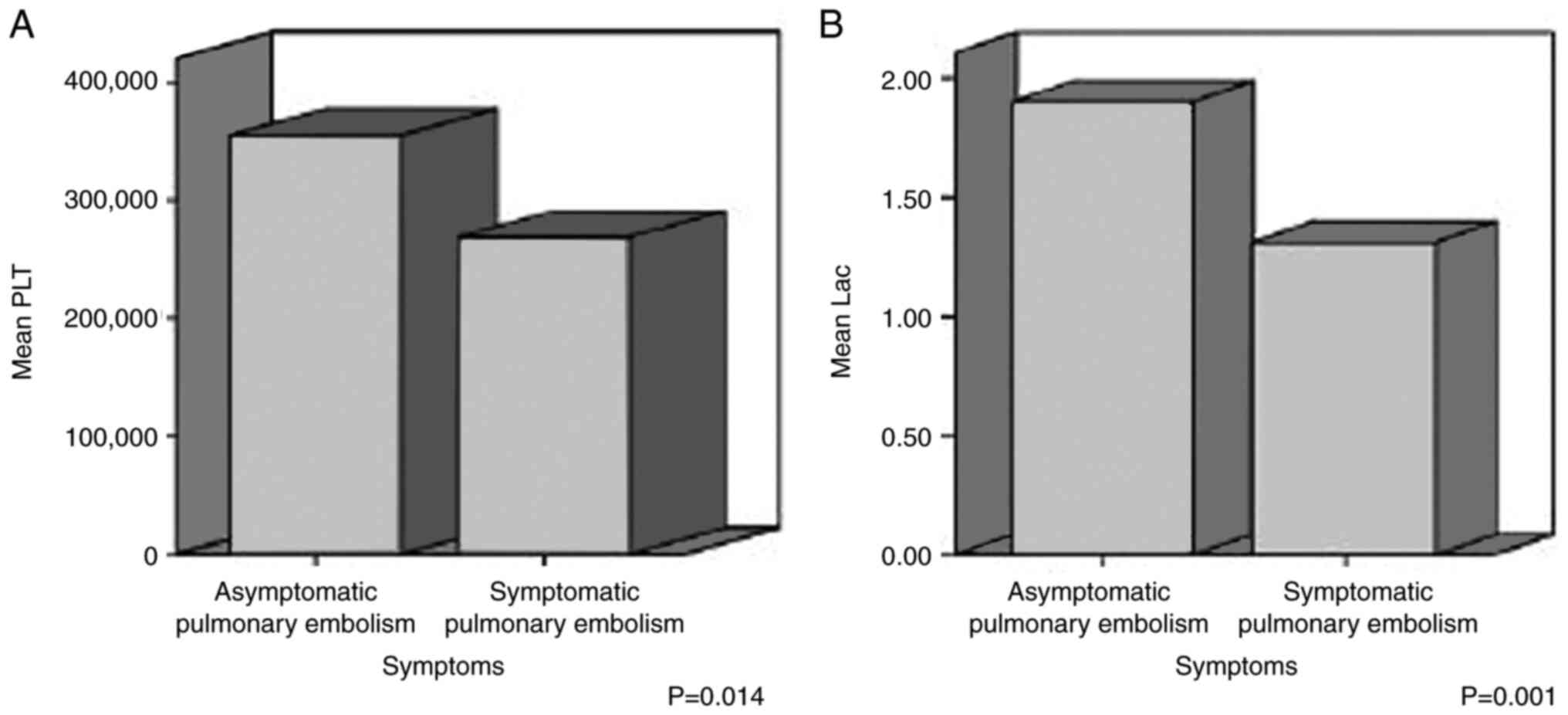
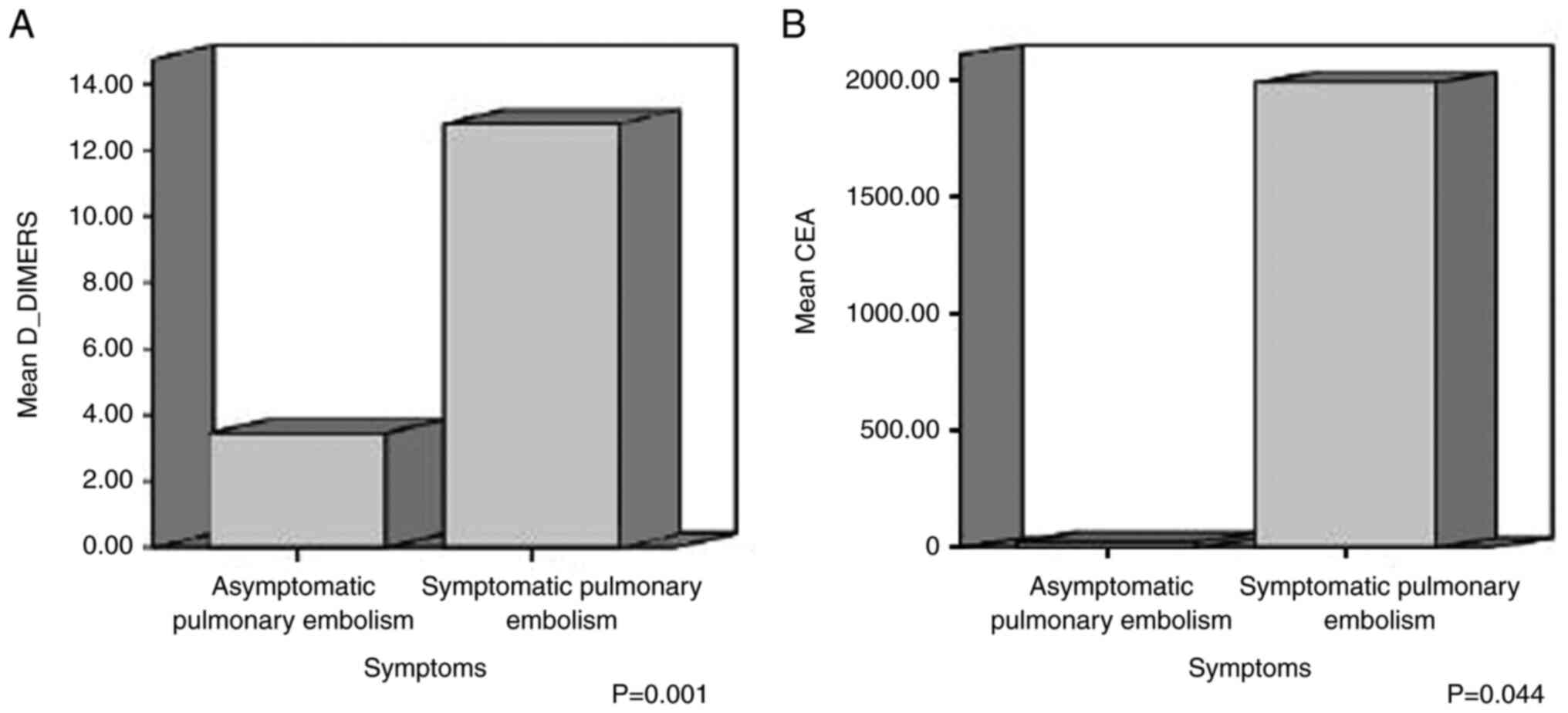
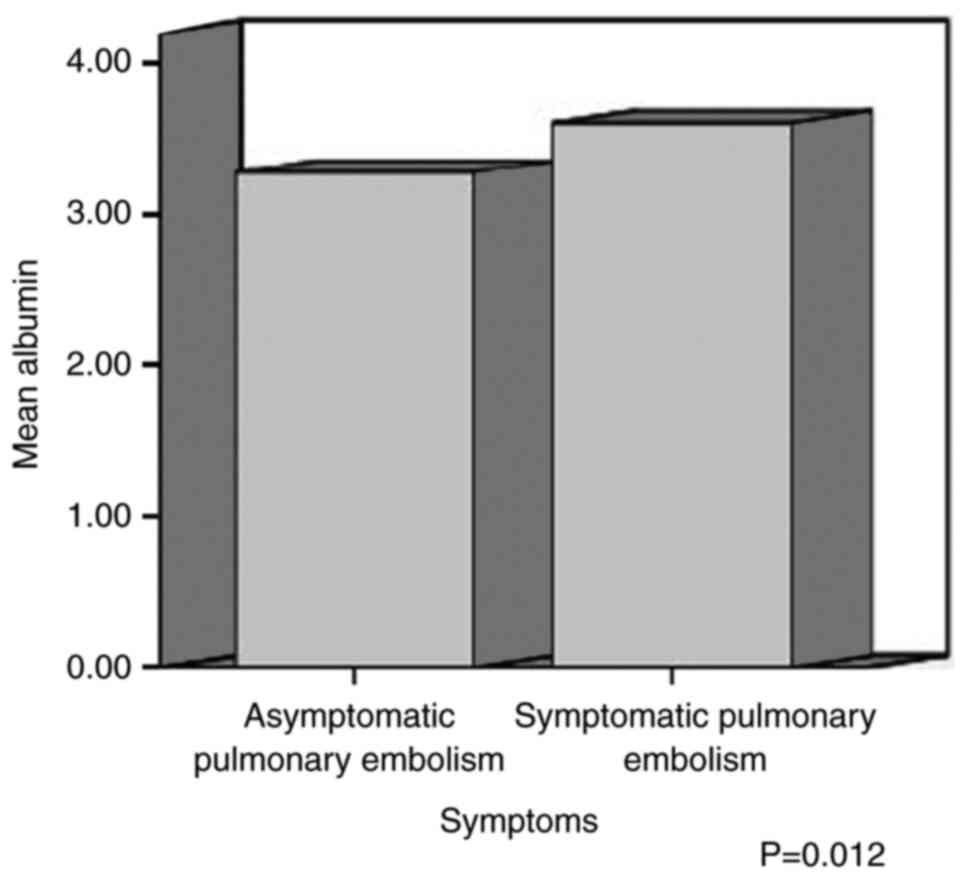
The mean value of the PLT count was 268.64±128.89
×103/µl in symptomatic patients and 355.46±134.58
×103/µl in asymptomatic patients; the mean value of
D-dimer was 12.78±10.81 µg/ml in symptomatic patients and 3.43±2.06
µg/ml in asymptomatic patients; the mean value of serum albumin was
3.61±0.37 g/dl in symptomatic patients and 3.28±0.55 g/dl in
asymptomatic patients; the mean value of serum CEA was
1988.60±4313.63 µg/l in symptomatic patients and 22.793±48.84 µg/l
in asymptomatic patients; and the mean value of lactic acid was
1.31±0.37 mmol/l in symptomatic patients and 1.9±0.59 mmol/l in
asymptomatic patients. The results of analysis using independent
t-tests are presented in Table
SI. In the Levene's test, when the significance level (sig) was
>0.05, the P-value in the first row in the table for each
parameter was taken into account and when the significance level
(sig) was <0.05, the P-value in the second row in the table for
each parameter was taken into account. There was a statistically
significant difference in the mean values of the PLT count,
D-dimer, albumin, CEA and lactic acid between the symptomatic and
asymptomatic cancer patients with PE (P<0.05) (Table IV, and Fig. 1 , Fig.
2 and Fig. 3).
 | Table IV.Laboratory parameters with
statistically significant difference between symptomatic and
asymptomatic cancer patients. |
Table IV.
Laboratory parameters with
statistically significant difference between symptomatic and
asymptomatic cancer patients.
| Laboratory
parameter | Mean value
(SD) | P-value |
|---|
| PLT count
(×103/µl) |
|
|
|
Symptomatic | 268.64
(128.89) | 0.014 |
|
Asymptomatic | 355.46
(134.58) |
|
| D-dimer
(µg/ml) |
|
|
|
Symptomatic | 12.78 (10.81) | 0.001 |
|
Asymptomatic | 3.43 (2.06) |
|
| Albumin (g/dl) |
|
|
|
Symptomatic | 3.61 (0.37) | 0.012 |
|
Asymptomatic | 3.28 (0.55) |
|
| CEA (µg/l) |
|
|
|
Symptomatic | 1,988.60
(4,313.63) | 0.044 |
|
Asymptomatic | 22.79 (48.84) |
|
| Lactic acid
(mmol/l) |
|
|
|
Symptomatic | 1.31 (0.37) | 0.001 |
|
Asymptomatic | 1.9
(0.597) |
|
The majority of the cancer patients developed
central PE (44/60, 73.3%). In total, 16 patients (16/60, 26.7%) had
thrombosis on ultrasonography of lower extremity veins, while 8
patients (8/60, 13.3%) had a dilated right ventricle on
echocardiography (Table V).
 | Table V.CTPA, ultrasonography of the lower
extremity veins, echocardiography and electrocardiography
findings. |
Table V.
CTPA, ultrasonography of the lower
extremity veins, echocardiography and electrocardiography
findings.
| CTPA | No. of
patients | Percentage |
|---|
| Location of
obstructed branches of pulmonary arteries |
|
|
|
Central | 44 | 73.3 |
| Μain
pulmonary arteries and lobar branches | 44 | 73.3 |
|
Lateral | 24 | 40 |
|
Bilateral | 20 | 33.3 |
|
Peripheral | 16 | 26.3 |
|
Segmental branches | 14 | 23.3 |
|
Subsegmental branches | 2 |
3.3 |
| Pleural
effusion | 20 | 33.3 |
|
Pulmonary metastases | 20 | 33.3 |
| Ultrasonography of
the lower extremity veins |
|
|
|
Thrombosis | 16 | 26.7 |
|
Symptomatic | 14 | 23.4 |
|
Asymptomatic | 2 |
3.3 |
| Venous
insufficiency | 2 |
3.3 |
| No
abnormal findings | 42 | 70 |
|
Echocardiography |
|
|
| Normal
EF | 60 | 100 |
|
Dilation of right
ventricle | 8 | 13.3 |
| Electrocardiogram
findings |
|
|
| Sinus
rhythm | 60 | 100 |
|
RBBB | 6 | 10 |
| Sinus
tachycardia | 34 | 56.7 |
| Normal
rhythm (60–100 pbm) | 26 | 43.3 |
In addition, 34 patients (34/60, 56.67%) presented
with sinus tachycardia on the ECG and 6 patients (6/60, 10%)
presented with right bundle branch block (Table V). As regards outcomes, 8 patients
(8/60, 13.3%) succumbed during hospitalization, and during the
follow-up period of 6 months none of the remaining patients had a
relapse of PE and all survived (Table
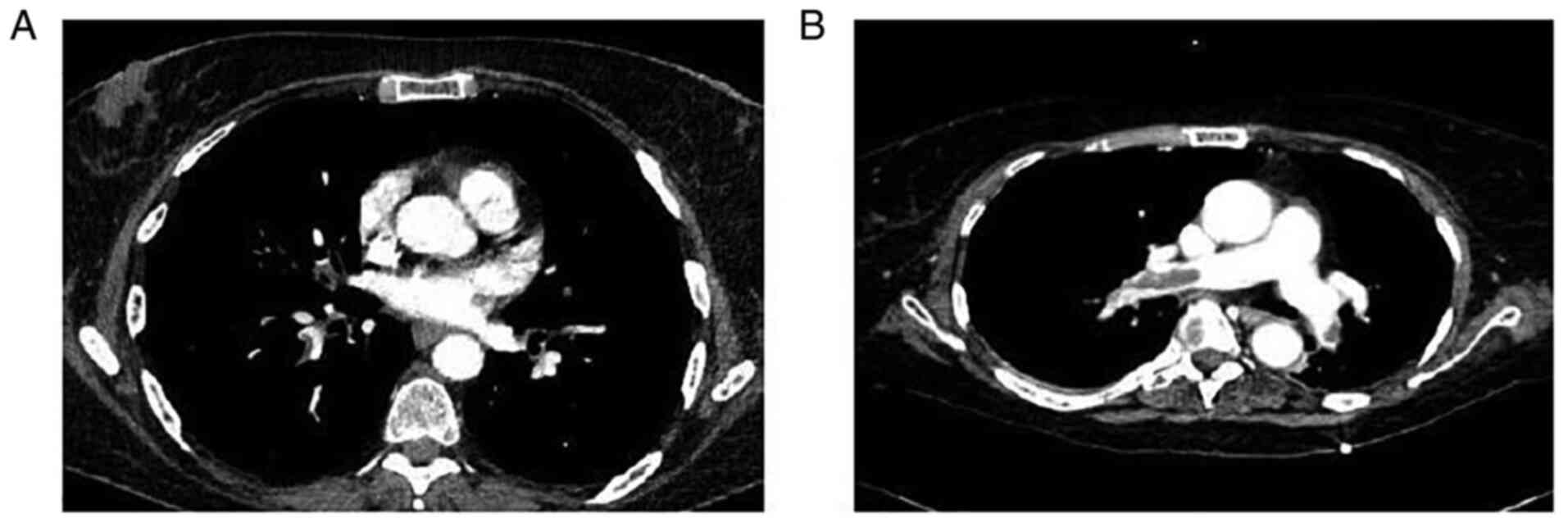
VI). Representative images of PE are illustrated in Fig. 4.
 | Table VI.Type of anticoagulation received for
the treatment of pulmonary embolism and outcomes. |
Table VI.
Type of anticoagulation received for
the treatment of pulmonary embolism and outcomes.
| Therapy and
outcome | No. of
patients | Percentage |
|---|
| Anticoagulation
therapy received during hospitalization |
|
|
|
Tinzaparin | 44 | 73.3 |
|
Enoxaparin | 16 | 26.7 |
| Mortality during
hospitalization | 8 | 13.3 |
|
Tinzaparin | 8 | 13.3 |
|
Enoxaparin | 0 | 0 |
| Anticoagulation
therapy received following discharge |
|
|
|
Tinzaparin | 34 | 65.4 |
|
Enoxaparin | 16 | 30.8 |
|
DOAC | 2 |
3.8 |
| Follow-up | 0 | 0 |
|
Relapse | 0 | 0 |
|
Mortality | 0 | 0 |
Discussion
According to the results of the present study, the
majority of the cancer patients were male, with the vast number of
these patients being female in the asymptomatic group. The most
common type of cancer was lung cancer, with the majority of the
cases of PE occurring within the first year of cancer diagnosis,
while the majority had already had metastases. The majority of the
cancer patients had received chemotherapy over the past month, were
not receiving anticoagulants and had central obstruction of
pulmonary arteries. These factors may be considered by clinicians
as additional predisposing factors for the development of PE. In
addition, the present study found that 36.7% of the patients had
asymptomatic PE. This finding indicates that clinicians need to be
aware of this frequent complication in cancer patients, even in the
absence of clinical symptoms.
Aleem et al (12), in their study on cancer patients
who developed PE, found that the majority of the patients had
symptomatic thrombosis, developed PE the during the first year
after diagnosis and were at an advanced stage of cancer at the time
of diagnosis. According to another study by Ohashi et al
(13), the most common type of
cancer associated with the occurrence of PE was pancreatic cancer
and the majority of the patients were at an advanced stage when
diagnosed with PE. Furthermore, Meyer et al (14), in their study on cancer patients
with PE, found that 3,36% had asymptomatic PE. The most common type
of cancer was prostate cancer, followed by hepatobiliary carcinoma
and pancreatic cancer (14). In
another study by Silva et al (4), it was found that the majority of the
cancer patients who developed PE were female and the most common
types of cancer were colorectal and lung cancer, most of which had
metastases or had received chemotherapy. In the same study, PE was
an incidental finding in 69.4% of the patients (4).
In their study, Myat Moe et al (15) found that the incidence of
asymptomatic PE among cancer patients was low (1.6%); the majority
of patients were female and the most common types of cancer
observed in these patients were lung, breast and colorectal cancer,
which is most likely due to the frequency of imaging (15). Furthermore, in the study by
Abdel-Razeq et al (16), it
was demonstrated that the most frequent types of cancer in cancer
patients with asymptomatic PE were gastric, lung, colorectal and
lymphomas. Similar to the findings of the present study the
majority of the asymptomatic patients were female and most of the
patients (77%) had already developed metastases at the time of PE
diagnosis (16). In addition, in a
review article by van Es et al (17), the reported incidence of incidental
PE in cancer patients was 1–5%. This finding is in contrast to the
results of the present study.
Another notable finding of the present was a
statistically significant difference in the mean values of PLT
counts, D-dimer, albumin, CEA and lactic acid between the
symptomatic and asymptomatic cancer patients with PE, with greater
values of PLT counts and lactic acid, and lower values of D-dimer,
CEA and albumin observed in asymptomatic cancer patients. These
parameters may guide clinicians to suspect PE even in asymptomatic
patients.
To date, several PE clinical scoring systems are
used to calculate the pretest probability of PE. Among the most
common scoring systems are the PERC score, the Wells score and the
Geneva score (18–20). The PERC score suggests that when a
patient is <50 years of age, has a pulse <100 bpm, an oxygen
saturation >94%, no unilateral leg swelling, no hemoptysis, no
recent surgery and no oral hormone use, the pretest probability of
PE is likely to be very low (18).
The Wells score is used to guide additional investigations and
management using medical history data, including a history of
cancer and clinicals signs of VTE to determine whether PE is likely
or unlikely (19). In addition,
the Geneva score is used to calculate the pretest probability of PE
by using patient risk factors, such as an age >65 years,
surgery, previous DVT and a history of cancer, and clinical signs
and symptoms (20).
CEA has been reported to be associated with an
increased risk of developing VTE in patients with pancreatic,
colorectal and ovarian cancer (21), and is related to PE in patients
with lung cancer, with a positive correlation with D-dimer values
(22). To the best of our
knowledge, the present study is the first to describe low levels of
CEA as a potential biomarker for detecting PE in asymptomatic
cancer patients.
Lactic acid has been reported to be associated with
a high risk of mortality and adverse outcomes among patients with
PE (23), and an increased
in-hospital mortality in patients with acute PE (24). Furthermore, lactic acid has been
linked to a greater risk of short-term mortality in patients with
PE with a low-intermediate risk, independent of other gas-analytic
parameters (25). In a recent
study, Ząbczyk et al (26)
reported that increased lactic acid levels were associated with
increased neutrophil extracellular trap (NET) formation and
prothrombotic fibrin clot features, with impaired plasma
fibrinolytic potential in patients with acute PE. However, cancer
patients were excluded from that study (26). Although there are several reports
regarding the role of lactic acid in patients with PE, the present
study is the first, to our knowledge, to mention elevated lactic
acid levels as a possible indicator of asymptomatic PE among cancer
patients.
Low levels of serum albumin have been shown to be
associated with massive PE (27)
and an increased risk of VTE development in acutely ill
hospitalized patients (28).
Moreover, decreased serum albumin levels have been found to be
significantly associated with an increased risk of VTE and
mortality in cancer patients (29). Of note, Li et al (30) reported that low serum levels of
albumin were independently associated with the development of
asymptomatic PE. According to the present study, low levels of
serum albumin may be a potential biomarker for detecting PE among
asymptomatic cancer patients.
In their study on cancer patients, Ali et al
(31) found that cancer patients
with asymptomatic PE had increased D-dimer levels similar to those
found among cancer patients with symptomatic PE, indicating that
elevated D-dimer levels should raise the suspicion of PE in
asymptomatic cancer patients. In the present study, D-dimer levels
were significantly lower in asymptomatic cancer patients with PE as
compared to symptomatic patients. The inverse association of
D-dimer levels with PLT counts may be explained by the local
consumption of platelets due to a thrombotic state (32). According to the present study,
another potential biomarker for detecting PE among asymptomatic
cancer patients is the increased PLT count.
In the present study, the in-hospital mortality rate
was 13.3%, while during a follow-up period of 6 months, there was
no relapse or mortality observed in the patients. In the study by
Silva et al (4), the
mortality rate at 30 days associated with PE in cancer patients was
7.5%. In another study, the reported overall 30-day mortality rate
in a large cohort of cancer patients with PE was 14% (33). Furthermore, a mortality rate of
22.1% was reported in a study on cancer patients with PE at the end
of follow-up period (34).
To the best of our knowledge, the present study is
to one of a limited number of studies investigating the
characteristics and outcomes of cancer patients who developed PE in
Greece. The strong point of the study was its cross-sectional
design, accompanied by reliable follow-up and outcome data.
However, the study has some limitations. One limitation of the
research is the relatively small sample size of the patients. In
addition, it is based on data from a single center that do not
allow the generalization of conclusions. Thus, larger prospective
studies, conducted in multiple cancer hospitals, are needed for
better evaluation of the results.
In conclusion, the majority of the cancer patients
who developed PE were male. The most common type of cancer observed
was lung cancer, with the vast number of cases of PE occurring
within the first year from cancer diagnosis, while the majority of
the patients had already developed metastases. The majority of the
cancer patients had received chemotherapy over in past month, were
not receiving anticoagulants and had central obstruction of
pulmonary arteries. A large proportion had asymptomatic PE.
Clinicians may consider these factors as additional predisposing
factors for the development of PE. A great proportion had
asymptomatic PE. This finding suggests that even in the absence of
clinical signs and symptoms, doctors need to be aware of this
common consequence in cancer patients. The in-hospital mortality
rate was 13.3% and no relapse or mortality were noted during the
follow-up period of these patients. Increased levels of lactic acid
and increased number of PLTs, as well as low serum levels of CEA,
albumin and D-dimer, may be potential biomarkers for asymptomatic
PE among cancer patients. These parameters may guide oncologists to
suspect PE even in asymptomatic patients.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SC, VEG and MM conceptualized the study. CD, PS, NT
and PP obtained the data and prepared the tables. EG, PG and DT
obtained the data and prepared the figures. AG, GAL, AT were
involved in the design of the study and prepared the draft of the
manuscript. VEG and SC wrote and prepared the draft of the
manuscript. DAS and GK analyzed the data and provided critical
revisions. VEG and SC confirm the authenticity of all the raw data.
All authors contributed to manuscript revision and have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Ethical approval for the present study was obtained
from the Research Ethics Committee of Agios Savvas Hospital
(protocol no. 8034/1-12-18). The study was in line with the
declaration of Helsinki in 1995 (as revised in Edinburgh 2000).
Written informed was obtained from all the patients prior to
enrollment.
Patient consent for publication
Written informed was obtained from the patients for
publication of the data. A copy of the written consent is available
for review by the Editor-in-Chief of this journal on request.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The author authors declare that they have no competing
interests.
References
|
1
|
Essien EO, Rali P and Mathai SC: Pulmonary
embolism. Med Clin North Am. 103:549–564. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blom JW, Doggen CJ, Osanto S and Rosendaal
FR: Malignancies, prothrombotic mutations, and the risk of venous
thrombosis. JAMA. 293:715–722. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goldhaber SZ: Risk factors for venous
thromboembolism. J Am Coll Cardiol. 56:1–7. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Silva P, Rosales M, Milheiro MJ and Santos
LL: Pulmonary embolism in ambulatory oncologic patients. Acta Med
Port. 28:463–468. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gladish GW, Choe DH, Marom EM, Sabloff BS,
Broemeling LD and Munden RF: Incidental pulmonary emboli in
oncology patients: Prevalence, CT evaluation, and natural history.
Radiology. 240:246–255. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abdol Razak NB, Jones G, Bhandari M,
Berndt MC and Metharom P: Cancer-associated thrombosis: An overview
of mechanisms, risk factors, and treatment. Cancers (Basel).
10:3802018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bloom J, Doggen C and Rosendaal F: The
risk of venous thrombosis in cancer patients with or without the
factor V Leiden mutation. Haemostasis. 31:732001.
|
|
8
|
Tsoukalas N, Tsapakidis K, Galanopoulos M,
Karamitrousis E, Kamposioras K and Tolia M: Real world data
regarding the management of cancer-associated thrombosis. Curr Opin
Oncol. 32:289–294. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Anagnostopoulos I, Lagou S, Spanorriga MK,
Tavernaraki K, Poulakou G, Syrigos KN and Thanos L: Epidemiology
and diagnosis of pulmonary embolism in lung cancer patients: Is
there a role for age adjusted D-dimers cutoff? J Thromb
Thrombolysis. 49:572–577. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miller GA, Sutton GC, Kerr IH, Gibson RV
and Honey M: Comparison of streptokinase and heparin in treatment
of isolated acute massive pulmonary embolism. Br Med J. 2:681–684.
1971. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krilokuva I: Pulmonary embolism (acute or
chronic). J Respir Dis Med. 2:1–3. 2019.
|
|
12
|
Aleem A, Al Diab AR, Alsaleh K, Algahtani
F, Alsaeed E, Iqbal Z and El-Sherkawy MS: Frequency, clinical
pattern and outcome of thrombosis in cancer patients in Saudi
Arabia. Asian Pac J Cancer Prev. 13:1311–1315. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ohashi Y, Ikeda M, Kunitoh H, Sasako M,
Okusaka T, Mukai H, Fujiwara K, Nakamura M, Oba MS, Kimura T, et
al: Venous thromboembolism in cancer patients: Report of baseline
data from the multicentre, prospective cancer-VTE Registry. Jpn J
Clin Oncol. 50:1246–1253. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meyer HJ, Wienke A and Surov A: Incidental
pulmonary embolism in oncologic patients-a systematic review and
meta-analysis. Support Care Cancer. 29:1293–1302. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Myat Moe MM and Redla S: Incidental
pulmonary embolism in oncology patients with current macroscopic
malignancy: Incidence in different tumour type and impact of
delayed treatment on survival outcome. Br J Radiol.
91:201708062018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abdel-Razeq HN, Mansour AH and Ismael YM:
Incidental pulmonary embolism in cancer patients: Clinical
characteristics and outcome-a comprehensive cancer center
experience. Vasc Health Risk Manag. 7:153–158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van Es N, Bleker SM and Di Nisio M:
Cancer-associated unsuspected pulmonary embolism. Thromb Res. 133
(Suppl 2):S172–S178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kline JA, Mitchell AM, Kabrhel C, Richman
PB and Courtney DM: Clinical criteria to prevent unnecessary
diagnostic testing in emergency department patients with suspected
pulmonary embolism. J Thromb Haemost. 2:1247–1255. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Douma RA, Gibson NS, Gerdes VE, Büller HR,
Wells PS, Perrier A and Le Gal G: Validity and clinical utility of
the simplified Wells rule for assessing clinical probability for
the exclusion of pulmonary embolism. Thromb Haemost. 101:197–200.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Klok FA, Mos IC, Nijkeuter M, Righini M,
Perrier A, Le Gal G and Huisman MV: Simplification of the revised
Geneva score for assessing clinical probability of pulmonary
embolism. Arch Intern Med. 168:2131–2136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Awkar N, Amireh S, Rai S, Shaaban H, Guron
G and Maroules M: Association between level of tumor markers and
development of VTE in patients with pancreatic, colorectal and
ovarian Ca: Retrospective case-control study in two community
hospitals. Pathol Oncol Res. 24:283–287. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiong W, Zhao Y, Xu M, Guo J, Pudasaini B,
Wu X and Liu J: The relationship between tumor markers and
pulmonary embolism in lung cancer. Oncotarget. 8:41412–41421. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vanni S, Viviani G, Baioni M, Pepe G,
Nazerian P, Socci F, Bartolucci M, Bartolini M and Grifoni S:
Prognostic value of plasma lactate levels among patients with acute
pulmonary embolism: The thrombo-embolism lactate outcome study. Ann
Emerg Med. 61:330–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vanni S, Socci F, Pepe G, Nazerian P,
Viviani G, Baioni M, Conti A and Grifoni S: High plasma lactate
levels are associated with increased risk of in-hospital mortality
in patients with pulmonary embolism. Acad Emerg Med. 18:830–835.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Galić K, Pravdić D, Prskalo Z, Kukulj S,
Starčević B and Vukojević M: Prognostic value of lactates in
relation to gas analysis and acid-base status in patients with
pulmonary embolism. Croat Med J. 59:149–155. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ząbczyk M, Natorska J, Janion-Sadowska A,
Malinowski KP, Janion M and Undas A: Elevated lactate levels in
acute pulmonary embolism are associated with prothrombotic fibrin
clot properties: Contribution of NETs formation. J Clin Med.
9:9532020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Omar HR, Mirsaeidi M, Rashad R, Hassaballa
H, Enten G, Helal E, Mangar D and Camporesi EM: Association of
serum albumin and severity of pulmonary embolism. Medicina
(Kaunas). 56:262020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chi G, Gibson CM, Liu Y, Hernandez AF,
Hull RD, Cohen AT, Harrington RA and Goldhaber SZ: Inverse
relationship of serum albumin to the risk of venous thromboembolism
among acutely ill hospitalized patients: Analysis from the APEX
trial. Am J Hematol. 94:21–28. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Königsbrügge O, Posch F, Riedl J, Reitter
EM, Zielinski C, Pabinger I and Ay C: Association between decreased
serum albumin with risk of venous thromboembolism and mortality in
cancer patients. Oncologist. 21:252–257. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li G, Li Y and Ma S: Lung cancer
complicated with asymptomatic pulmonary embolism: Clinical analysis
of 84 patients. Technol Cancer Res Treat. 16:1130–1135. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ali S, Dilday E, Tagawa S, Akhtar NH,
Liebman HA, Razavi P, Rochanda L, Quinn DI, Seaton K and O'Connell
CL: D-dimer levels among cancer patients with unsuspected pulmonary
embolism: Clinical correlates and relevance. Blood. 120:11542012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Greenberg CS: The role of D-dimer testing
in clinical hematology and oncology. Clin Adv Hematol Oncol.
15:580–583. 2017.PubMed/NCBI
|
|
33
|
Font C, Carmona-Bayonas A, Beato C, Reig
Ò, Sáez A, Jiménez-Fonseca P, Plasencia JM, Calvo-Temprano D,
Sanchez M, Benegas M, et al: Clinical features and short-term
outcomes of cancer patients with suspected and unsuspected
pulmonary embolism: The EPIPHANY study. Eur Respir J.
49:16002822017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang H, Xu X, Pu C and Li L: Clinical
characteristics and prognosis of cancer patients with venous
thromboembolism. J Can Res Ther. 15:344–349. 2019.PubMed/NCBI
|