Introduction
Female breast cancer is a common malignancy globally
(1). Triple-negative breast cancer
(TNBC), making up 12–17% of cancers of the breast, is defined by
the absence of receptors for oestrogen, progesterone, and human
epidermal growth factor receptor 2 (HER2) on the tumour cells
(2–4). TNBC is the most aggressive form of
breast cancer, and rates of recurrence, distant metastasis, and
mortality are significantly higher than for other types of breast
cancer (2,3). Part of the reason for this may be a
more limited range of treatment options for TNBC than for these
other types of breast cancer.
Over the last decade, cancer immunotherapy has
become established as a highly effective treatment modality for
certain cancers (5). Immune
checkpoint blockade (ICB) has achieved remarkable clinical results
in some patients with malignant melanoma, renal cancer, non-small
cell lung cancer, and other solid tumours (6). Currently, treatment with antibodies
to programmed death-ligand 1 (PD-L1) or programmed death-1 (PD-1)
is the mainstay of ICB, and has also been investigated for TNBC,
with some degree of success. Thus, the IMpassion 130 trial
(NCT02425891) used anti-PD-L1 (atezolizumab) together with
nab-paclitaxel as first-line treatment for advanced or metastatic
TNBC and reported that this combination was superior to
nab-paclitaxel alone (7).
Additionally, KEYNOTE-355 investigated the efficacy of anti-PD-1
(pembrolizumab) combined with chemotherapy (nab-paclitaxel;
paclitaxel; or gemcitabine plus carboplatin) and reported increased
progression-free survival of TNBC patients relative to chemotherapy
alone (8). Nonetheless, only
20–58% of TNBC tumours express PD-L1, making it likely that many
TNBC patients will not experience any clinical benefit from ICB
directed to this molecule (9–15).
Also, repetitive administration can result in resistance to ICB, as
with chemotherapy (16). Hence,
there is an urgent unmet medical need for novel treatment targets
in TNBC.
Potential ICB targets other than PD-L1 and PD-1 may
also be considered for application in TNBC. One of these,
immunoglobulin superfamily member, T-cell immunoglobulin mucin-3
(TIM-3), is a checkpoint molecule present on many different immune
cells, including dendritic cells, macrophages, and T cells
(17–20). TIM-3 mediates suppressive activity
after binding a variety of different ligands, including
phosphatidylserine, CEACAM-1, and galectin-9 (17,21,22).
The latter is one of the family of h-galactoside-binding proteins
which is over-expressed by many tumours; its binding to TIM-3 on T
cell surface results in cytotoxic T cell suppression via an
autocrine pathway (23–29). It has therefore been hypothesized
that either or both TIM-3 and galectin-9 could represent novel
therapeutic targets (30–32). However, the prognostic significance
of these two molecules has not been unequivocally established,
because their high expression has been reported to associate with
either a better or worse prognosis, depending on the specific
tumour entity (33–38). In the case of TNBC, TIM-3 or
galectin-9 expression has been associated with certain
clinicopathological features and with prognostic significance
(34,38) but to the best of our knowledge, no
studies to date have examined the relationship between TIM-3 in
combination with one of its ligands, galectin-9. Therefore, we
explored correlations between TIM-3 and galectin-9 expression in
TNBC by immunohistochemistry, and investigated their impact on
patient prognosis and clinicopathological features.
Materials and methods
Patient selection
Patients with TNBC undergoing surgical resection at
the Department of Surgery of the Kansai Medical University Hospital
between January 2006 and December 2018 were enrolled. Patients
receiving neoadjuvant chemotherapy, known to influence TIM-3 and
galectin-9 expression, were excluded. Inclusion criteria were
invasive breast carcinoma of no special type according to the
recent World Health Organization Classification of Breast Tumors
(39), but those with special
types were excluded because each of these has different
clinicopathological features. Finally, 62 TNBC patients were
included. This cohort is essentially identical to that described in
our previous studies (40–43). To date, in this cohort, we have
analysed associations between adipophilin expression and prognosis
(40), as well as the prognostic
impact of PD-L1 expression by cancer-associated fibroblasts
(41), and relationships between
PD-L1 and the expression of the immune checkpoint protein CD155
(42). We have also compared three
different PD-L1 assays in patients with TNBC using
immunohistochemistry (43). The
focus of the current study was to determine the prognostic
significance of TIM3 and galectin-9 expression in this same cohort
of TNBC patients.
This is a retrospective single-centre study
conducted in accordance with the principles of the Declaration of
Helsinki. The study protocol was approved by the Institutional
Review Board of Kansai Medical University Hospital (Approval
#2019041). Because of the retrospective study design, informed
consent was obtained using the opt-out method, there being no risk
to the participants. Information on the study, including the
inclusion criteria and the opportunity to opt-out, was made
available on the institutional website (https://www.kmu.ac.jp/hirakata/hospital/2671t800000136cd-att/a1565060399005.pdf).
Histopathology
All histopathological diagnoses were independently
evaluated by at least two experienced diagnostic pathologists,
using the TNM Classification of Malignant Tumors, 8th Ed. Grading
followed the Nottingham scale (44). Dichotomization of the Ki-67
labelling index (LI) was set as high at ≥40% and low at <40%,
following a meta-analysis of patients with TNBC (45). Stromal tumour-infiltrating
lymphocytes (TILs) were identified using haematoxylin and eosin
staining and were considered lymphocyte-predominant breast cancer
(LPBC) at ≥60% and non-LPBC at <59%, according to TIL Working
Group guidelines (46,47).
Tissue microarrays
Regions most morphologically representative of
carcinoma were selected by H&E staining of the slides, and for
every patient, three 2 mm-diameter tissue cores were punched out of
the paraffin-embedded blocks.
Immunohistochemistry
Immunohistochemistry used the Discovery ULTRA System
(Roche Diagnostics, Basel, Switzerland) according to the
manufacturer's instructions. Antibodies were as follows: TIM-3
(rabbit monoclonal antibody, D5D5R, Cell Signaling Technology,
Danvers, MA, USA; diluted 1:200); galectin-9 (mouse monoclonal
antibody, ab153673, 1G3, Abcam plc, Cambridge, UK; diluted 1:200).
At least of two researchers independently evaluated the
immunohistochemistry results. TIM-3-positivity was defined as
membrane staining of any intensity on ≥1% of TILs (36). Galectin-9-positivity was defined as
membrane staining of any intensity on ≥1% of tumour cells (48). The patient was classified as having
TIM-3- or galectin-9-positive tumour when one or more cores from
the same individual were positive according to this definition.
Statistical analysis
We used SPSS Statistics 27.0 (IBM, Armonk, NY, USA)
for all analyses. Correlations between two groups were calculated
using Fisher's exact test for categorical variables and
Mann-Whitney U testing for continuous variables. Relapse-free
survival (RFS) was determined using the Kaplan-Meier method, with
log-rank testing. Cox proportional hazards modelling was used to
estimate relationships between clinicopathological parameters and
survival. Statistical significance was set at P<0.05.
Results
Patients
The cohort of 62 women with TNBC studied here is the
same as described earlier (43).
Their clinicopathological characteristics were presented in the
previous publication (43).
Briefly, median age at initial diagnosis was 68 years (range,
31–93); the diagnosis of TNBC relied on biopsy results. Patients
with invasive carcinoma of no special type were selected (see
Materials and Methods). Median tumour diameter was 21 mm (range,
2–55 mm). Median follow-up was 58 months (range, 11–173 months).
Eleven (17.7%) patients relapsed, all with distant metastases.
There were no local recurrences. Nine patients (14.5%) died of
their disease.
Correlations between galectin-9 or
TIM-3 expression and clinicopathological factors
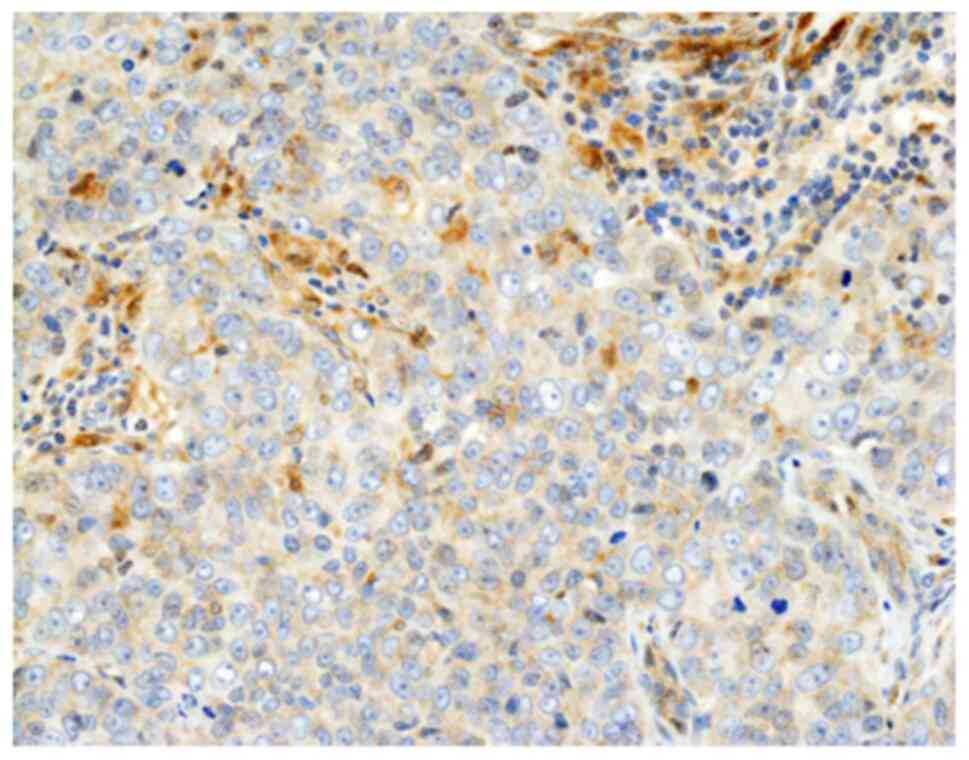
Of the 62 patients, 49 (79.0%) were classified as
galectin-9-positive (Fig. 1).
Table I presents associations
between galectin-9-positivity and clinicopathological factors. The
use of adjuvant chemotherapy was associated with galectin-9
expression (P=0.040), but not with any other factors, including age
and menopausal status. There were also no associations between
galectin-9-positivity and the clinicopathological factors staging,
Nottingham histological grade, lymph node status, lymphovascular
invasion, Ki-67 LI, or stromal TILs.
 | Table I.Association between
clinicopathological factors and galectin-9 expression. |
Table I.
Association between
clinicopathological factors and galectin-9 expression.
| Factors | Galectin-9-positive
(n=49) | Galectin-9-negative
(n=13) | P-value |
|---|
| Median age ± SD,
years | 64±15 | 72±13 | 0.115 |
| Menopausal status,
n |
|
|
|
|
Premenopausal | 9 | 0 | 0.184 |
|
Postmenopausal | 39 | 13 |
|
|
Unknown | 1 | 0 |
|
| Tumour size, n |
|
|
|
| ≤20
mm | 26 | 5 | 0.534 |
| >20
mm | 23 | 8 |
|
| Pathological stage,
n |
|
|
|
|
I+II | 45 | 9 | 0.052 |
|
III | 4 | 4 |
|
| Lymph node status,
n |
|
|
|
|
Positive | 9 | 7 | 0.075 |
|
Negative | 26 | 5 |
|
| Not
tested | 14 | 1 |
|
| Lymphatic invasion,
n |
|
|
|
|
Positive | 41 | 12 | 0.670 |
|
Negative | 8 | 1 |
|
| Venous invasion,
n |
|
|
|
|
Positive | 27 | 10 | 0.210 |
|
Negative | 22 | 3 |
|
| Nottingham
histological grade, n |
|
|
|
|
1+2 | 22 | 8 | 0.357 |
| 3 | 27 | 5 |
|
| Ki-67 labeling
index, n |
|
|
|
| High
(≥40%) | 28 | 9 | 0.506 |
| Low
(<40%) | 18 | 3 |
|
| Not
tested | 3 | 1 |
|
| Stromal TILs,
n |
|
|
|
|
LPBC | 16 | 3 | 0.737 |
|
Non-LPBC | 33 | 10 |
|
| Adjuvant
chemotherapy, n |
|
|
|
|
Performed | 31 | 3 | 0.040 |
| Not
performed | 17 | 8 |
|
|
Undetermined | 1 | 2 |
|
Regarding TIM-3 expression, 30 patients (48.4%) were
classified as TIM-3-positive (Fig.
2). Table II depicts
associations between TIM-3 expression and clinicopathological
factors in this cohort. Larger tumour size was associated with
TIM-3-negativity (P<0.001), whereas LPBC correlated with
TIM-3-positivity (P=0.013). However, there were no associations
between TIM-3 expression and the clinicopathological factors,
including age, menopausal status, administration of adjuvant
chemotherapy, staging, Nottingham histological grade, lymph node
status, lymphovascular invasion, or Ki-67 LI.
 | Table II.Association between
clinicopathological factors and TIM-3 expression. |
Table II.
Association between
clinicopathological factors and TIM-3 expression.
| Factors | TIM-3-positive
(n=30) | TIM-3-negative
(n=32) | P-value |
|---|
| Median age ± SD,
years | 63±15 | 67±14 | 0.313 |
| Menopausal status,
n |
|
|
|
|
Premenopausal | 5 | 4 | 0.724 |
|
Postmenopausal | 24 | 28 |
|
|
Unknown | 1 | 0 |
|
| Tumour size, n |
|
|
|
| ≤20
mm | 22 | 9 | <0.001 |
| >20
mm | 8 | 23 |
|
| Pathological stage,
n |
|
|
|
|
I+II | 29 | 25 | 0.054 |
|
III | 1 | 7 |
|
| Lymph node status,
n |
|
|
|
|
Positive | 6 | 8 | 0.752 |
|
Negative | 17 | 16 |
|
| Not
tested | 7 | 8 |
|
| Lymphatic invasion,
n |
|
|
|
|
Positive | 23 | 30 | 0.077 |
|
Negative | 7 | 2 |
|
| Venous invasion,
n |
|
|
|
|
Positive | 15 | 22 | 0.195 |
|
Negative | 15 | 10 |
|
| Nottingham
histological grade, n |
|
|
|
|
1+2 | 15 | 15 | >0.999 |
| 3 | 15 | 17 |
|
| Ki-67 labeling
index, n |
|
|
|
| High
(≥40%) | 16 | 9 | 0.062 |
| Low
(<40%) | 12 | 21 |
|
| Not
tested | 2 | 2 |
|
| Stromal TILs,
n |
|
|
|
|
LPBC | 14 | 5 | 0.013 |
|
Non-LPBC | 16 | 27 |
|
| Adjuvant
chemotherapy, n |
|
|
|
|
Performed | 19 | 15 | 0.295 |
| Not
performed | 10 | 15 |
|
|
Undetermined | 1 | 2 |
|
Correlations between galectin-9 or
TIM-3 expression and relapse-free survival after surgery
RFS was superior for galectin-9-positive relative to
-negative patients (60-vs.-44 months, P=0.027) as shown in Fig. 3A. For TIM-3-positive-vs.-negative
patients, these values were 63 and 54 months (Fig. 3B, P=0.007).
Impact of positivity for both
galectin-9 and TIM-3 on clinicopathological features
Correlations between galectin-9- and
TIM-3-positivity are shown in Table
III, indicating a lack of association between galectin-9 and
TIM-3 expression (P=0.548). Eight patients (12.9%) were both
galectin-9- and TIM-3-negative (double negative), and 25 (40.3%)
were positive for both (double-positive). The remaining 29 patients
were either galectin-9- or TIM-3-single-positive.
 | Table III.Association between galectin-9 and
TIM-3 expression. |
Table III.
Association between galectin-9 and
TIM-3 expression.
|
| TIM-3 |
|---|
|
|
|
|---|
| Galectin-9 | Positive, n | Negative, n |
|---|
| Positive | 25 | 24 |
| Negative | 5 | 8 |
Table IV
summarizes correlations between the galectin-9/TIM-3
double-negative group and clinicopathological factors in the
present cohort. Only larger tumour size and higher Ki-67 LI
correlated with double-negative status (P=0.029 and 0.020,
respectively) but there were no associations with age, menopausal
status, presence of adjuvant chemotherapy, staging, Nottingham
histological grade, lymph node status, lymphovascular invasion, or
stromal TILs.
 | Table IV.Association between
clinicopathological factors and galectin-9 and TIM-3
expression. |
Table IV.
Association between
clinicopathological factors and galectin-9 and TIM-3
expression.
| Factors | Galectin-9 and
TIM-3-negative (n=8) | Galectin-9 and/or
TIM-3-positive (n=54) | P-value |
|---|
| Median age ± SD,
years | 72±10 | 64±15 | 0.166 |
| Menopausal status,
n |
|
|
|
|
Premenopausal | 0 | 9 | 0.590 |
|
Postmenopausal | 8 | 44 |
|
|
Unknown | 0 | 1 |
|
| Tumour size, n |
|
|
|
| ≤20
mm | 1 | 30 | 0.029 |
| >20
mm | 7 | 24 |
|
| Pathological stage,
n |
|
|
|
|
I+II | 5 | 49 | 0.059 |
|
III | 3 | 5 |
|
| Lymph node status,
n |
|
|
|
|
Positive | 3 | 11 | 0.670 |
|
Negative | 5 | 28 |
|
| Not
tested | 0 | 15 |
|
| Lymphatic invasion,
n |
|
|
|
|
Positive | 8 | 45 | 0.580 |
|
Negative | 0 | 9 |
|
| Venous invasion,
n |
|
|
|
|
Positive | 7 | 30 | 0.128 |
|
Negative | 1 | 24 |
|
| Nottingham
histological grade, n |
|
|
|
|
1+2 | 4 | 26 | >0.999 |
| 3 | 4 | 28 |
|
| Ki-67 labeling
index, n |
|
|
|
| High
(≥40%) | 7 | 30 | 0.020 |
| Low
(<40%) | 1 | 20 |
|
| Not
tested | 0 | 4 |
|
| Stromal TILs,
n |
|
|
|
|
LPBC | 1 | 18 | 0.416 |
|
Non-LPBC | 7 | 36 |
|
| Adjuvant
chemotherapy, n |
|
|
|
|
Performed | 3 | 31 | 0.691 |
| Not
performed | 3 | 22 |
|
|
Undetermined | 2 | 1 |
|
Correlation between galectin-9 and
TIM-3 combined expression and relapse-free survival after
surgery
Fig. 3C shows RFS
for double-negative, double-positive or single-positive patients.
The median RFS time of galectin-9/TIM-3-double-positive,
galectin-9-positive/TIM-3-negative,
galectin-9-negative/TIM-3-positive, and galectin-9/TIM3-double
negative patients was 64, 57, 61, and 42 months, respectively.
Thus, double-positive patients had a better prognosis, and
double-negative patients had the worst prognosis (P=0.011).
Prognostic significance of galectin-9
and TIM-3 expression
According to univariate analysis, the presence of
lymph node metastasis (P=0.004), galectin-9 negativity (P=0.039),
TIM-3 negativity (P=0.029), and galectin-9/TIM-3 double-negativity
(P=0.020) were each significantly correlated with poor RFS
(Table V). Multivariate Cox
proportional hazards analyses showed that galectin-9/TIM-3
double-negativity was an independent predictor of poor prognosis
[hazard ratio (HR) 3.627, 95% confidence interval (CI) 1.037-12.68;
P=0.044] (Table V). Additionally,
lymph node metastasis was an independent risk factor for poor RFS
(HR 5.925, 95% CI 1.555-22.58, P=0.009).
 | Table V.Univariate and multivariate analyses
of relapse-free survival of patients with triple-negative breast
cancer. |
Table V.
Univariate and multivariate analyses
of relapse-free survival of patients with triple-negative breast
cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Tumor size (>20
vs. ≤20 mm) | 2.660 | 0.706-10.03 | 0.148 |
|
|
|
| Lymph node status
(positive vs. negative) | 6.891 | 1.825-26.02 | 0.004 | 5.925 | 1.555-22.58 | 0.009 |
| Nottingham
histological grade (3 vs. 1+2) | 1.829 | 0.535-6.256 | 0.336 |
|
|
|
| Ki-67 labeling
index (high vs. low) | 1.497 | 0.387-5.793 | 0.559 |
|
|
|
| Stromal TILs (LPBC
vs. non-LPBC) | 0.470 | 0.101-2.175 | 0.334 |
|
|
|
| Adjuvant
chemotherapy (performed vs. not performed) | 0.358 | 0.104-1.225 | 0.102 |
|
|
|
| Galectin-9
(negative vs. positive) | 3.508 | 1.068-11.52 | 0.039 | 2.736 | 0.809-9.253 | 0.106 |
| TIM-3 (negative vs.
positive) | 9.888 | 1.265-77.27 | 0.029 | 7.141 | 0.905-56.33 | 0.062 |
| Galectin-9 and
TIM-3 (double negative vs. others) | 4.321 | 1.260-14.82 | 0.020 | 3.627 | 1.037-12.68 | 0.044 |
Discussion
Recently, the importance of TIM-3 in cancer
immunology has been increasingly recognized due to its role as a
checkpoint receptor inhibiting cytotoxic T cells (30–32).
A previous meta-analysis implicated TIM-3 expression as an
independent risk factor predicting poor overall survival (OS), but
not cancer-specific and disease-free survival, in different
malignant tumours (49). It was
hypothesized that interactions of TIM-3 with its ligands, including
galectin-9, results in the inhibition of both T cell responses and
natural killer cell-mediated tumour cell cytotoxicity, resulting in
the dampening of anti-tumour immunity and thence tumour escape
(17). TIM-3 is believed to be
expressed by exhausted T cells, the presence of which is associated
with poor prognosis in several different cancers (49,50).
In contrast, as mentioned above, TIM-3-positivity was associated
with more favourable OS in patients with TNBC (38,49),
although this conclusion is based only three studies which
investigated whether TIM-3 expression predicted prognosis in TNBC.
Table VI presents details of
these previous studies, together with the current study (36–38).
According to our results, TIM-3 expression on TILs from TNBC
correlates with a more favourable prognosis. The reason for
differences in the prognostic relevance of TIM-3 expression for
TNBC as opposed to other cancer entities remains to be established.
In this context, Burugu et al (37) suspected that it might reflect a
more potent recognition of cancer cells by the immune system. The
immune response of TILs expressing TIM-3 to tumour cells might be
different in the tumour microenvironment of TNBC compared to that
of other cancers. Therefore, additional studies examining the
molecular mechanisms underlying the immune response of TILs
expressing TIM-3 to carcinoma cells in TNBC are needed in order to
explain this difference.
 | Table VI.Summary of the relationship between
TIM-3 expression and prognosis of patients with triple-negative
breast cancer. |
Table VI.
Summary of the relationship between
TIM-3 expression and prognosis of patients with triple-negative
breast cancer.
| First author/s,
year | Patients, n | Prognosis | (Refs.) |
|---|
| Cabioglu et
al, 2021 | 61 | No prognostic
significance was noted (using operative specimens after neoadjuvant
chemotherapy), although TIM-3 expression was associated with a
worse chemotherapy response | (36) |
| Burugu et
al, 2018 | 387 | TIM-3 expression
was associated with good disease-free and overall survival | (37) |
| Byun et al,
2018 | 109 | TIM-3 expression
was associated with good cancer-specific survival | (38) |
| Present study | 62 | TIM-3 expression
was associated with good relapse-free survival | - |
Additionally, some other associations between
clinicopathological features of TIM-3 expression in TNBC patients
have been reported by other investigators. One study included 30
TNBC patients, reporting that TIM-3 expression by TILs correlated
significantly with the presence of lymph node metastasis and a
higher Ki-67 LI, but its prognostic significance was not discussed
(51). It may be important to note
that TIM-3 expression is more frequent in TNBC than in other forms
of breast cancer (51,52) and is also associated with higher
PD-L1/PD-1 expression (37,38).
Furthermore, consistent with the results presented here, it has
also been noted that TIM-3-positivity is associated with the
presence of abundant TILs (38).
Here, we have also demonstrated that negativity for
the TIM-3 ligand galectin-9 is a poor prognostic factor, but this
association lost significance in the multivariate analysis.
However, we did find that TIM-3/galectin-9-double-negativity
remains significantly predictive of poor prognosis in such a
multivariate analysis. Galectin-9 on tumour cells is also a key
protein that negatively regulates T cell function, leading to
suppression of anti-cancer immune surveillance (21,26,27,53).
Using breast cancer cell lines it was found that galectin-9
expression was associated with the suppression of anti-cancer
immune surveillance (53).
However, similar to our findings, some studies reported that
galectin-9 expression predicts a favourable prognosis in breast
cancer (34,54). Nonetheless, it must be recognized
that there is a discrepancy between the generally reported
immunosuppressive function of galectin-9 and its opposite
prognostic significance in TNBC. Although galectin-9 ligation of
TIM-3 pathway induces dysfunction of TILs [for example, in
hepatocellular carcinoma (55)],
here we found that it was the double-negativity for TIM-3 and
galectin-9 that predicted a poor prognosis whereas positivity for
both was associated with a more favourable course. The functional
role of galectin-9 and TIM-3 in anti-tumour responses might be
different in TNBC than in some other types of cancer. It is clear
that the TIM-3/galectin-9 pathway can suppress cytotoxic T cells
and NK cells and protect the tumour (16), but it is also known that the
presence of galectin-9 on breast cancer cells increases the
strength of cell-cell interactions. This could thus prevent
metastasis or at least reduce the metastatic potential of the
tumour (56). As such, the outcome
might be more favourable when breast tumour cells express
galectin-9. To resolve this issue, additional molecular studies are
needed, especially for TIM-3/galectin-9 double-negativity. Better
understanding of the oncoimmunology of TNBC will hopefully lead to
improved prognosis.
Some limitations of the present study must be
recognized, including a relatively small sample size. Thus,
additional studies with a larger number of participants must be
performed. Second, this study used tissue microarrays to evaluate
immunohistochemical staining for TIM-3 and galectin-9. This might
have led to a bias in evaluating the expression of these proteins,
despite the fact that we selected the most morphologically
representative regions from the patients. Third, TIM-3 can be
glycosylated, and glycosylated TIM-3 has a weaker ability to bind
galectin-9 (57). Moreover,
galectin-9 has three isoforms (58). This study analysed TIM-3 and
galectin-9 expression by immunohistochemical methods using
monoclonal antibodies which may have different specificities. Thus,
the galectin-9 antibody used in this study reacts with all three
isoforms of galectin-9 (59), so
positivity for galectin-9 may reflect different isoforms of
galectin-9. Although this method is versatile, further studies
using Western blotting conducted on fresh tumour samples could
provide further information on the roles of glycosylated TIM-3
and/or the different galectin-9 isoforms. Fourth, chemotherapy
and/or ICB might influence the expression of TIM-3 and/or
galectin-9. Anthracycline and taxane upregulate galectin-9
expression in some types of cancer cells (60)-although there were no patients
receiving neoadjuvant chemotherapy in the present study.
Nonetheless, additional studies should determine whether these
drugs influence the expression of TIM-3 and/or galectin-9 in
TNBC.
In conclusion, this study documented that TIM-3 or
galectin-9 positivity predicts a more favourable prognosis in TNBC
patients, in particular when the TILs are TIM-3-positive and the
tumour is galectin-9-positive. Generally, the TIM-3/galectin-9
pathway is thought to suppress anti-cancer immunosurveillance, but
here we reveal a positive influence on TNBC prognosis. However, the
molecular mechanisms underlying the difference between TNBC and
other cancers in this respect remain unclear and further analyses
are needed to resolve this issue. This could contribute to improved
therapy for patients with TNBC.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by AMED (grant no.
JP21lm0203006), the Osaka Community Foundation 2020, and research
grants D1 and D2 from Kansai Medical University.
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
KY and MI conceived and designed the study. KY and
MI were involved in immunohistochemical analyses. KY, MI, HY, KT,
MS and TS acquired and analysed data. KY and MI drafted the
manuscript, tables and figures. KY and MI confirm the authenticity
of all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki, and the study protocol was approved by
the Institutional Review Board of the Kansai Medical University
Hospital (protocol no. 2019041; Hirakata, Japan). All data are
completely anonymized. The Institutional Review Board waived the
requirement of informed consent due to the retrospective design of
the study, with no risk of identity exposure for the patients. The
present study did not include any minors.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cleator S, Heller W and Coombes RC:
Triple-negative breast cancer: Therapeutic options. Lancet Oncol.
8:235–244. 2007. View Article : Google Scholar
|
|
3
|
Dent R, Trudeau M, Pritchard KI, Hanna WM,
Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P and Narod SA:
Triple-negative breast cancer: Clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kakimi K, Karasaki T, Matsushita H and
Sugie T: Advances in personalized cancer immunotherapy. Breast
Cancer. 24:16–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sharma P and Allison JP: The future of
immune checkpoint therapy. Science. 348:56–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schmid P, Adams S, Rugo HS, Schneeweiss A,
Barrios CH, Iwata H, Diéras V, Hegg R, Im SA, Shaw Wright G, et al:
Atezolizumab and nab-paclitaxel in advanced triple-negative breast
cancer. N Engl J Med. 379:2108–2121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cortes J, Cescon DW, Rugo HS, Nowecki Z,
Im SA, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Holgado E, et
al: Pembrolizumab plus chemotherapy versus placebo plus
chemotherapy for previously untreated locally recurrent inoperable
or metastatic triple-negative breast cancer (KEYNOTE-355): A
randomised, placebo-controlled, double-blind, phase 3 clinical
trial. Lancet. 396:1817–1828. 2020. View Article : Google Scholar
|
|
9
|
Mittendorf EA, Philips AV, Meric-Bernstam
F, Qiao N, Wu Y, Harrington S, Su X, Wang Y, Gonzalez-Angulo AM,
Akcakanat A, et al: PD-L1 expression in triple-negative breast
cancer. Cancer Immunol Res. 2:361–370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tung N, Garber JE, Hacker MR, Torous V,
Freeman GJ, Poles E, Rodig S, Alexander B, Lee L, Collins LC and
Schnitt SJ: Prevalence and predictors of androgen receptor and
programmed death-ligand 1 in BRCA1-associated and sporadic
triple-negative breast cancer. NPJ Breast Cancer. 2:160022016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ali HR, Glont SE, Blows FM, Provenzano E,
Dawson SJ, Liu B, Hiller L, Dunn J, Poole CJ, Bowden S, et al:
PD-L1 protein expression in breast cancer is rare, enriched in
basal-like tumours and associated with infiltrating lymphocytes.
Ann Oncol. 26:1488–1493. 2015. View Article : Google Scholar
|
|
12
|
Wang C, Zhu H, Zhou Y, Mao F, Lin Y, Pan
B, Zhang X, Xu Q, Huang X and Sun Q: Prognostic value of PD-L1 in
breast cancer: A meta-analysis. Breast J. 23:436–443. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dill EA, Gru AA, Atkins KA, Friedman LA,
Moore ME, Bullock TN, Cross JV, Dillon PM and Mills AM: PD-L1
expression and intratumoral heterogeneity across breast cancer
subtypes and stages: An assessment of 245 primary and 40 metastatic
tumors. Am J Surg Pathol. 41:334–342. 2017. View Article : Google Scholar
|
|
14
|
Mori H, Kubo M, Yamaguchi R, Nishimura R,
Osako T, Arima N, Okumura Y, Okido M, Yamada M, Kai M, et al: The
combination of PD-L1 expression and decreased tumor-infiltrating
lymphocytes is associated with a poor prognosis in triple-negative
breast cancer. Oncotarget. 8:15584–15592. 2017. View Article : Google Scholar
|
|
15
|
Li Z, Dong P, Ren M, Song Y, Qian X, Yang
Y, Li S, Zhang X and Liu F: PD-L1 expression is associated with
tumor FOXP3 (+) regulatory T-cell infiltration of breast cancer and
poor prognosis of patient. J Cancer. 7:784–793. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gong B, Kiyotani K, Sakata S, Nagano S,
Kumehara S, Baba S, Besse B, Yanagitani N, Friboulet L, Nishio M,
et al: Secreted PD-L1 variants mediate resistance to PD-L1 blockade
therapy in non-small cell lung cancer. J Exp Med. 216:982–1000.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wolf Y, Anderson AC and Kuchroo VK: TIM3
comes of age as an inhibitory receptor. Nat Rev Immunol.
20:173–185. 2020. View Article : Google Scholar
|
|
18
|
Monney L, Sabatos CA, Gaglia JL, Ryu A,
Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel
RA, et al: Th1-specific cell surface protein Tim-3 regulates
macrophage activation and severity of an autoimmune disease.
Nature. 415:536–541. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
De Mingo Pulido A, Gardner A, Hiebler S,
Soliman H, Rugo HS, Krummel MF, Coussens LM and Ruffell B: TIM-3
regulates CD103+ dendritic cell function and response to
chemotherapy in breast cancer. Cancer Cell. 33:60–74.e6. 2018.
View Article : Google Scholar
|
|
20
|
Yan W, Liu X, Ma H, Zhang H, Song X, Gao
L, Liang X and Ma C: Tim-3 fosters HCC development by enhancing
TGF-β-mediated alternative activation of macrophages. Gut.
64:1593–1604. 2015. View Article : Google Scholar
|
|
21
|
Zhu C, Anderson AC, Schubart A, Xiong H,
Imitola J, Khoury SJ, Zheng XX, Strom TB and Kuchroo VK: The Tim-3
ligand galectin-9 negatively regulates T helper type 1 immunity.
Nat Immunol. 6:1245–1252. 2005. View
Article : Google Scholar
|
|
22
|
Sabatos-Peyton CA, Nevin J, Brock A,
Venable JD, Tan DJ, Kassam N, Xu F, Taraszka J, Wesemann L, Pertel
T, et al: Blockade of Tim-3 binding to phosphatidylserine and
CEACAM1 is a shared feature of anti-Tim-3 antibodies that have
functional efficacy. Oncoimmunology. 7:e13856902018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gitt MA and Barondes SH: Evidence that a
human soluble beta-galactoside-binding lectin is encoded by a
family of genes. Proc Natl Acad Sci USA. 83:7603–7607. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Paroutaud P, Levi G, Teichberg VI and
Strosberg AD: Extensive amino acid sequence homologies between
animal lectins. Proc Natl Acad Sci USA. 84:6345–6348. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Caron M, Bladier D and Joubert R: Soluble
galactoside-binding vertebrate lectins: A protein family with
common properties. Int J Biochem. 22:1379–1385. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kikushige Y, Miyamoto T, Yuda J,
Jabbarzadeh-Tabrizi S, Shima T, Takayanagi S, Niiro H, Yurino A,
Miyawaki K, Takenaka K, et al: A TIM-3/Gal-9 autocrine stimulatory
loop drives self-renewal of human myeloid leukemia stem cells and
leukemic progression. Cell Stem Cell. 17:341–352. 2015. View Article : Google Scholar
|
|
27
|
Gonçalves Silva I, Yasinska IM, Sakhnevych
SS, Fiedler W, Wellbrock J, Bardelli M, Varani L, Hussain R,
Siligardi G, Ceccone G, et al: The Tim-3-galectin-9 secretory
pathway is involved in the immune escape of human acute myeloid
leukemia cells. EBioMedicine. 22:44–57. 2017. View Article : Google Scholar
|
|
28
|
Sakhnevych SS, Yasinska IM, Bratt AM,
Benlaouer O, Gonçalves Silva I, Hussain R, Siligardi G, Fiedler W,
Wellbrock J, Gibbs BF, et al: Cortisol facilitates the immune
escape of human acute myeloid leukemia cells by inducing
latrophilin 1 expression. Cell Mol Immunol. 15:994–997. 2018.
View Article : Google Scholar
|
|
29
|
Gonçalves Silva I, Rüegg L, Gibbs BF,
Bardelli M, Fruehwirth A, Varani L, Berger SM, Fasler-Kan E and
Sumbayev VV: The immune receptor Tim-3 acts as a trafficker in a
Tim-3/galectin-9 autocrine loop in human myeloid leukaemia cells.
Oncoimmunology. 5:e11955352016. View Article : Google Scholar
|
|
30
|
Sharma P, Hu-Lieskovan S, Wargo JA and
Ribas A: Primary adaptive and acquired resistance to cancer
immunotherapy. Cell. 168:707–723. 2017. View Article : Google Scholar
|
|
31
|
Yang R, Sun L, Li CF, Wang YH, Yao J, Li
H, Yan M, Chang WC, Hsu JM, Cha JH, et al: Galectin-9 interacts
with PD-1 and TIM-3 to regulate T cell death and is a target for
cancer immunotherapy. Nat Commun. 12:8322021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cong Y, Liu J, Chen G and Qiao G: The
emerging role of T-cell immunoglobulin Mucin-3 in breast cancer: A
promising target for immunotherapy. Front Oncol. 11:7232382021.
View Article : Google Scholar
|
|
33
|
Jikuya R, Kishida T, Sakaguchi M, Yokose
T, Yasui M, Hashizume A, Tatenuma T, Mizuno N, Muraoka K, Umemoto
S, et al: Galectin-9 expression as a poor prognostic factor in
patients with renal cell carcinoma. Cancer Immunol Immunother.
69:2041–205. 2015. View Article : Google Scholar
|
|
34
|
Irie A, Yamauchi A, Kontani K, Kihara M,
Liu D, Shirato Y, Seki M, Nishi N, Nakamura T, Yokomise H, et al:
Galectin-9 as a prognostic factor with antimetastatic potential in
breast cancer. Clin Cancer Res. 11:2962–2968. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen H, Wang M, Weng T, Wei Y, Liu C, Yang
L, Ren K, Tang Y, Tang Z and Gou X: The prognostic and
clinicopathological significance of Tim-3 and PD-1 expression in
the prognosis of upper urinary tract urothelial carcinoma. Urol
Oncol. 39:743–753. 2021. View Article : Google Scholar
|
|
36
|
Cabioglu N, Onder S, Oner G, Karatay H,
Tukenmez M, Muslumanoglu M, İgci A, Eralp Y, Aydiner A, Saip P, et
al: TIM3 expression on TILs is associated with poor response to
neoadjuvant chemotherapy in patients with locally advanced
triple-negative breast cancer. BMC Cancer. 21:3572021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Burugu S, Gao D, Leung S, Chia SK and
Nielsen TO: TIM-3 expression in breast cancer. Oncoimmunology.
7:e15021282018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Byun KD, Hwang HJ, Park KJ, Kim MC, Cho
SH, Ju MH, Lee JH and Jeong JS: T-cell immunoglobulin mucin 3
expression on tumor infiltrating lymphocytes as a positive
prognosticator in triple-negative breast cancer. J Breast Cancer.
21:406–414. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rakha EA, Allison KH, Bu H, Ellis IO,
Foschini MP, Horii R, et al: Invasive breast carcinoma of no
special type. WHO classification of tumours: Breast tumours. 5th
edition. Volume 2. IARC; Lyon: pp. 102–109. 2019
|
|
40
|
Yoshikawa K, Ishida M, Yanai H, Tsuta K,
Sekimoto M and Sugie T: Adipophilin expression is an independent
marker for poor prognosis of patients with triple-negative breast
cancer: An immunohistochemical study. PLoS One. 15:e02425632020.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yoshikawa K, Ishida M, Yanai H, Tsuta K,
Sekimoto M and Sugie T: Prognostic significance of PD-L1-positive
cancer-associated fibroblasts in patients with triple-negative
breast cancer. BMC Cancer. 21:2392021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yoshikawa K, Ishida M, Yanai H, Tsuta K,
Sekimoto M and Sugie T: Immunohistochemical analysis of CD155
expression in triple-negative breast cancer patients. PLoS One.
16:e02531762021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yoshikawa K, Ishida M, Yanai H, Tsuta K,
Sekimoto M and Sugie T: Immunohistochemical comparison of three
programmed death-ligand 1 (PD-L1) assays in triple-negative breast
cancer. PLoS One. 16:e02578602021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I. The value of histological
grade in breast cancer: Experience from a large study with
long-term follow-up. Histopathology. 19:403–410. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu Q, Ma G, Deng Y, Luo W, Zhao Y, Li W
and Zhou Q: Prognostic value of Ki-67 in patients with resected
triple-negative breast cancer: A meta-analysis. Front Oncol.
9:10682019. View Article : Google Scholar
|
|
46
|
Salgado R, Denkert C, Demaria S, Sirtaine
N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL,
Penault-Llorca F, et al: The evaluation of tumor-infiltrating
lymphocytes (TILs) in breast cancer: Recommendations by an
International TILs Working Group 2014. Ann Oncol. 26:259–271. 2015.
View Article : Google Scholar
|
|
47
|
Denkert C, von Minckwitz G, Darb-Esfahani
S, Lederer B, Heppner BI, Weber KE, Budczies J, Huober J, Klauschen
F, Furlanetto J, et al: Tumour-infiltrating lymphocytes and
prognosis in different subtypes of breast cancer: A pooled analysis
of 3771 patients treated with neoadjuvant therapy. Lancet Oncol.
19:40–50. 2018. View Article : Google Scholar
|
|
48
|
Sideras K, Biermann K, Verheij J,
Takkenberg BR, Mancham S, Hansen BE, Schutz HM, de Man RA,
Sprengers D, Buschow SI, et al: PD-L1, galectin-9 and
CD8+ tumor-infiltrating lymphocytes are associated with
survival in hepatocellular carcinoma. Oncoimmunology.
6:e12733092017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zang K, Hui L, Wang M, Huang Y, Zhu X and
Yao B: TIM-3 as a Prognostic marker and a potential immunotherapy
target in human malignant tumors: A meta-analysis and
bioinformatics validation. Front Oncol. 11:5793512021. View Article : Google Scholar
|
|
50
|
Saleh R, Toor SM and Elkord E: Targeting
TIM-3 in solid tumors: Innovations in the preclinical and
translational realm and therapeutic potential. Expert Opin Ther
Targets. 24:1251–1262. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang H, Xiang R, Wu B, Li J and Luo G:
T-cell immunoglobulin mucin-3 expression in invasive ductal breast
carcinoma: Clinicopathological correlations and association with
tumor infiltration by cytotoxic lymphocytes. Mol Clin Oncol.
7:557–563. 2017. View Article : Google Scholar
|
|
52
|
Solinas C, Garaud S, De Silva P, Boisson
A, Van den Eynden G, de Wind A, Risso P, Rodrigues Vitória J,
Richard F, Migliori E, et al: Immune checkpoint molecules on
tumor-infiltrating lymphocytes and their association with tertiary
lymphoid structures in human breast cancer. Front Immunol.
8:14122017. View Article : Google Scholar
|
|
53
|
Yasinska IM, Sakhnevych SS, Pavlova L, Teo
Hansen Selnø A, Teuscher Abeleira AM, Benlaouer O, Gonçalves Silva
I, Mosimann M, Varani L, Bardelli M, et al: The Tim-3-Galectin-9
pathway and its regulatory mechanisms in human breast cancer. Front
Immunol. 10:15942019. View Article : Google Scholar
|
|
54
|
Yamauchi A, Kontani K, Kihara M, Nishi N,
Yokomise H and Hirashima M: Galectin-9, a novel prognostic factor
with antimetastatic potential in breast cancer. Breast J. 12 (5
Suppl 2):S196–S200. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X,
Liu J, Shi L, Liu C, Wang G and Zou W: Tim-3/galectin-9 signaling
pathway mediates T-cell dysfunction and predicts poor prognosis in
patients with hepatitis B virus-associated hepatocellular
carcinoma. Hepatology. 56:1342–1351. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang ZY, Dong JH, Chen YW, Wang XQ, Li
CH, Wang J, Wang GQ, Li HL and Wang XD: Galectin-9 acts as a
prognostic factor with antimetastatic potential in hepatocellular
carcinoma. Asian Pac J Cancer Prev. 13:2503–2509. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lee MJ, Heo YM, Hong SH, Kim K and Park S:
The binding properties of glycosylated and non-glycosylated Tim-3
molecules on CD4CD25 T cells. Immune Netw. 9:58–63. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sato M, Nishi N, Shoji H, Seki M,
Hashidate T, Hirabayashi J, Kasai Ki K, Hata Y, Suzuki S, Hirashima
M and Nakamura T: Functional analysis of the carbohydrate
recognition domains and a linker peptide of galectin-9 as to
eosinophil chemoattractant activity. Glycobiology. 12:191–197.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Barjon C, Niki T, Vérillaud B, Opolon P,
Bedossa P, Hirashima M, Blanchin S, Wassef M, Rosen HR, Jimenez AS,
et al: A novel monoclonal antibody for detection of galectin-9 in
tissue sections: Application to human tissues infected by oncogenic
viruses. Infect Agent Cancer. 7:162012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yoon HK, Kim TH, Park S, Jung H, Quan X,
Park SJ, Han J and Lee A: Effect of anthracycline and taxane on the
expression of programmed cell death ligand-1 and galectin-9 in
triple-negative breast cancer. Pathol Res Pract. 214:1626–1631.
2018. View Article : Google Scholar
|