Introduction
Colorectal cancer (CRC) is one of the most common
causes of cancer-related mortality and remains a significant
challenge for oncological therapy. CRC was the 3rd most common type
of cancer worldwide following lung and breast cancer in 2020
(1). Analysis of epidemiological
trends has indicated that the incidence rate of colon cancer is
constantly increasing. According to the World Health Organization
(WHO) data for 2018, the estimated number of cases of CRC worldwide
was 1,849,518 (1–3). Despite medical developments, the
number of cases of CRC is estimated to increase by 679,280 in the
world by the year 2030 (1). The
5-year survival rate largely depends on the stage of CRC. It is
estimated to be 90% in patients with stage I cancer and 15% in
patients with metastatic CRC (4).
The increasing trend in the number of patients with CRC motivates
continued research on prognostic factors that may be evaluated
during routine histopathological examinations. There are several
types of CRC. Adenocarcinoma accounts for 90% of all CRC cases.
Other less common histological types include squamous cell, spindle
cell, neuroendocrine and undifferentiated carcinoma (5).
Histopathological evaluation plays a key role in the
diagnosis of CRC. In addition to primary diagnosis, a
histopathological examination provides information on the stage of
the disease, vascular-lymphatic invasion and/or prognostic
parameters in CRC (5).
Consequently, pathological analysis provides the information
necessary to select a therapy and assess patient prognosis. The
tumor microenvironment, which can be assessed in a
histopathological specimen, is another important element. It is
mainly created by inflammatory cells. Tumor-related local
inflammation is regarded as a novel prognostic parameter (6,7)
Neutrophils represent one of the main types of inflammatory cells,
and these cells have multifaceted functions by releasing various
effector molecules, cytotoxic mediators and cytokines (8). The histopathological assessment of
inflammatory cells may have predictive value in CRC. The role of
inflammation in colon carcinogenesis has been the subject of
numerous studies (9,10). Researchers have demonstrated the
effects of proinflammatory agents (factors produced by inflammatory
cells) on the development of CRC or on the regulation of genes
associated with carcinogenesis (9). The presence of intratumoral
neutrophils is considered to indicate a poorer prognosis (10).
Necrosis is a common phenomenon in CRC. It is
observed both during tumor development and in patients following
treatment. Tumor necrosis may be the result of rapid tumor growth
and may reflect the level of hypoxia (11,12).
Its presence is relatively simple to assess in histopathological
slides (13) Thus far, the
incidence of necrosis has been associated with a high tumor grade
and with a serrated histology (11). Recent studies have indicated the
association between the type of molecular CRC and necrosis
(14,15). The aim of the present study was to
define the role of necrosis and neutrophil infiltration in tumor
tissue, and to assess its clinical significance.
Patients and methods
Study population
Specimens from 160 patients, who had been surgically
treated for CRC, were examined. Patients with CRC received surgery
at the Department of Oncological Surgery, in the Comprehensive
Cancer Center of Bialystok (Poland) between April 2014 and December
2016. The study was designed with no restrictive inclusion and
exclusion criteria to obtain a sample reflecting a wide
representation of patients with CRC. The 160 patients consisted of
96 males and 64 females, with a mean age of 67.5 years (range,
32–86 years). The study protocol was reviewed and approved by the
Local Ethics Committee at the Medical University of Bialystok
(approval no. APK.002.164.2020; Poland).
Clinical and laboratory
characteristics of the study group
The majority of the patients presented with similar
symptoms, including abdominal pain, anemia, rectal bleeding,
constipation, diarrhea, vomiting and anorexia. In addition,
patients received treatment for hypertension (n=45), type II
diabetes (n=12), osteoarthritis (n=3) and coronary heart disease
(n=7). However, none of the patients received any anti-inflammatory
therapy. All patients underwent routine diagnostic tests, including
basic diagnostic laboratory tests (morphological tests and lipid
profiles), electrocardiography, spirometry, arterial blood
gasometry test, X-rays and computerized chest tomography. The
clinical stage of CRC was evaluated according to the
tumor-node-metastasis (TNM) classification (16). Prior to surgery, patients with
tumors identified in other sites had not received any
anti-inflammatory or immunosuppressive therapy.
Patients diagnosed with neoplasms in the rectum
received pre-operative therapy (n=53). They received radiotherapy
(n=39), chemotherapy (n=7) or radio-chemotherapy (n=7) and were
treated with a dose of 25 Gy in fractions of 5 Gy for 1 week in the
pelvic area. The response to pre-operative therapy was determined
according to the Response Evaluation Criteria in Solid Tumors
(17). Stable disease (SD) was
observed in 26 patients, while partial response (PR) was observed
in 27 patients.
Blood samples were obtained within 3 days prior to
and following surgery. The differential white blood cell count
(WBC) was determined using an XN-1000 automated hematology analyzer
(Sysmex Co.). The combined neutrophil-to-lymphocyte ratio (NLR) and
platelet-to-lymphocyte ratio (NLR-PLR status) was calculated as
previously described by Hirahara et al (18) and Jakubowska et al (19). Themonocyte-to-lymphocyte ratio
(MLR) and platelet (PLT)-NLR status were calculated as previously
described (19). Cancer biomarkers
[carcinoembryonic antigen (CEA) and CA19-9] were analyzed using a
Cobas 6000 analyzer (Roche).
The inclusion criteria were as follows: i)
Pathologically confirmed CRC; ii) treatment with radical resection;
iii) patients had not received any anti-inflammatory therapy. The
exclusion criteria were as follows: i) Incomplete
clinicopathological and follow-up data; ii) the presence of
hematological disorders, such as anemia; and iii) evidence of an
autoimmune disease.
Tissue samples
Tissues obtained from surgery were fixed in 4%
buffered formalin for 24–72 h at room temperature. Small sections
of tissue were embedded in paraffin. Sections (4-µm-thick) were cut
from the paraffin blocks and stained with hematoxylin and eosin
(H&E; cat. no. 468802128; POCH S.A.; Avantor Performance
Materials Poland) at room temperature for 4 min according to the
manufacturer's protocol. The slides were deparaffinized in an oven
at 60°C for 5 min. Subsequently, the slides were washed with xylene
(three washes, 10 min each) and rehydrated in a graded ethanol
series (100, 95, 85 and 75%, 1 min at each concentration). A
routine histopathological evaluation of the slides was performed in
accordance with the recommendation from the WHO (20). The type of tumor growth, tumor
size, histological type, the percentage of mucinous components, the
grade of malignancy and the TNM stage were determined by
pathologists (MK and K.L.) who were blinded to the clinical
information. Venous, lymphatic and perineural invasions of cancer
cells were also analyzed. The characteristic features of lymph node
invasion were examined, including the number of resected and
invaded lymph nodes, the presence of micro- and macro-metastases,
the invasion of the pouch lymph node, the presence of distant
metastases, and the size of metastases. The presence, number and
size of the deposits of cancer cells were also assessed (21).
Histopathological analysis of
intratumoral and stromal tumor-associated neutrophils (TANs) in the
primary tumor mass
In the present study, the intratumoral TANs
(intraTANs) and stromal TANs (stromaTANs) in CRC tissues were
assessed. The analysis was performed by two independent
pathologists, blinded to patient clinical information, treatment
regimen and outcomes. Morphologically, neutrophils are recognized
as polymorphonuclear cells with segmented nuclei that possess
clumped chromatin, eosinophilic cytoplasm and pink granules
(22). IntraTANs were determined
according to the modified classification described in the study by
Harbaum et al (23).
Neutrophils were assessed in four H&E-stained slides under a
light microscope (magnification, ×400; Leica DM6 B, KAWA.SKA, Sp. z
o.o.; Leica Microsystems, Inc.). They were counted and scored as
the ‘low’ group [absent or <10 cells/high power field (HPF)],
‘moderate’ group (10–50 cells/HPF) and ‘high’ group (>50
cells/HPF).
The analysis of stromaTANs was performed as
previously described (24).
Briefly, TANs were assessed at the invasive front and in the tumor
center under a light microscope at a high-power magnification
(×400). The cells were counted and quantified as a percentage of
all the cells examined. Neutrophils were divided into two groups as
follows: ‘Low’ (0–20% neutrophils) and ‘high’ (>21%
neutrophils).
Histopathological examination of
necrosis
The degree of tissue necrosis in the center of the
tumor (in the core region of the tumor) was classified using a
modified version of the criteria described by Väyrynen et al
(11) and Gao et al
(25). Tumor necrosis was defined
as an area with increased eosinophilia, neutrophilia and nuclear
shrinkage, and the fragmentation and disappearance of cells in the
tumor stroma. Intraluminal neutrophilic inflammatory infiltrate was
excluded from the evolution of the necrosis tumor and classified as
intraTANs. The areas of tumor necrosis were assessed
semi-quantitatively and graded as follows: 1, ‘Absent’ (none); 2,
‘focal’ (<10% of tumor area); 3, ‘moderate’ (10–30%); or 4,
‘extensive’ (>30%). For analysis, the study group was divided
into two subgroups as follows: i) The ‘low’ group (absent or focal
necrosis); and ii) the ‘high’ group (moderate or extensive
necrosis).
Combined parametric value
In the present study, the combination of
intratumoral neutrophils, stromaTANs in the primary tumor mass and
necrosis was also examined. The study group was divided into four
groups as follows: Group 1 (low intraTANs, low stromaTANs and low
necrosis); group 2 (low intraTANs, high stromaTANs and high
necrosis); group 3 (high intraTANs, low stromaTANs and low
necrosis); and group 4 (high intraTANs, high stromaTANs and high
necrosis).
Follow-up data
Patients were followed up annually for 2–5 years.
They were monitored by measuring CEA and CA19-9 levels, a physical
examination, colonoscopy or/and radiological imaging, including
computerized tomography of the chest, abdomen and pelvis, bone
scan, and positron emission tomography scans. Local and distant
recurrences were defined as pathological evidence of the spread of
tumors in the region of anastomosis (local recurrence) and/or
present outside of the primary tumor at other sites, such as the
liver, lungs, bones, brain (distant recurrence) and confirmed by
the aforementioned techniques.
Statistical analysis
Statistical analysis was performed using the
STATISTICA software version 13.0 (StatSoft). Numerical data were
analyzed using a χ2 test. To analysis small number of
cases (≤5), we use the Fisher's exact test. Comparisons amongst
multiple groups were analyzed using one-way ANOVA followed by
Tukey's post hoc test. The disease-free survival (DFS) time was
calculated as the duration between the date of diagnosis and the
date of disease progression, including local or distant relapse.
The DFS rate was calculated using the Kaplan-Meier method and
survival curves were compared using log-rank tests. Prognostic
factors were assessed using univariate and multivariate analyses
(Cox proportional hazard regression model). P<0.05 was
considered to indicate a statistically significant difference.
Results
Characteristics of the study
group
Overall, 12.5% of the patients (20/160) had a
primary tumor on the right side of the colon, 10% (16/160) in the
transverse colon and 9.375% (15/160) on the left side of the colon;
18.125% (29/160) had CRC in the sigmoid colon and 51.25% (82/160)
in the rectum. Among the 160 tumors, 81.25% (130/160) were
classified as adenocarcinoma and 18.75% (30/160) were classified as
adenocarcinoma with mucosal component. According to the TNM
classification, 45.625% of the patients (73/160) had stage Ior II
cancer, and 54.375% of the patients (87/160) had stage III or IV
cancer. The majority of the tumors were moderately differentiated
(G2) (N=148; 92.5%), and the remaining tumors were poorly
differentiated (G3) (N=12; 7.5%). Lymph node metastases were
present in 49.375% of the patients (79/160) and distal metastases
were present in 10.625% of the patients (17/160). Pre-operative
treatment was performed in 53 cases.
Analysis of intraTAN infiltration,
stromaTAN infiltration and tumor necrosis in patients with CRC
IntraTANs extensively infiltrated the tumor tissue
in the majority of cases (n=82), while weak and moderate
inflammation were observed in 43 and 35 cases, respectively.
StromaTANs in the core region of the tumor tissue mainly exhibited
a low cell infiltration (n=104, Fig.
1). Similar to stromaTANs, the majority of the patients in the
study group exhibited low levels of necrosis in tissue (n=105;
Fig. 2). The analysis of the
combined parametric value indicated the majority of CRC cases were
in groups 3 (high intraTANs, low stromaTANs and low necrosis; n=53)
and 4 (high intraTANs, high stromaTANs and high necrosis; n=51),
where the tissue had a large amount of intraTANs (Fig. 3). Fig.
4 demonstrates the distribution of intraTANs and stromaTANs in
the center of the tumor mass, necrosis and combined parametric
value.
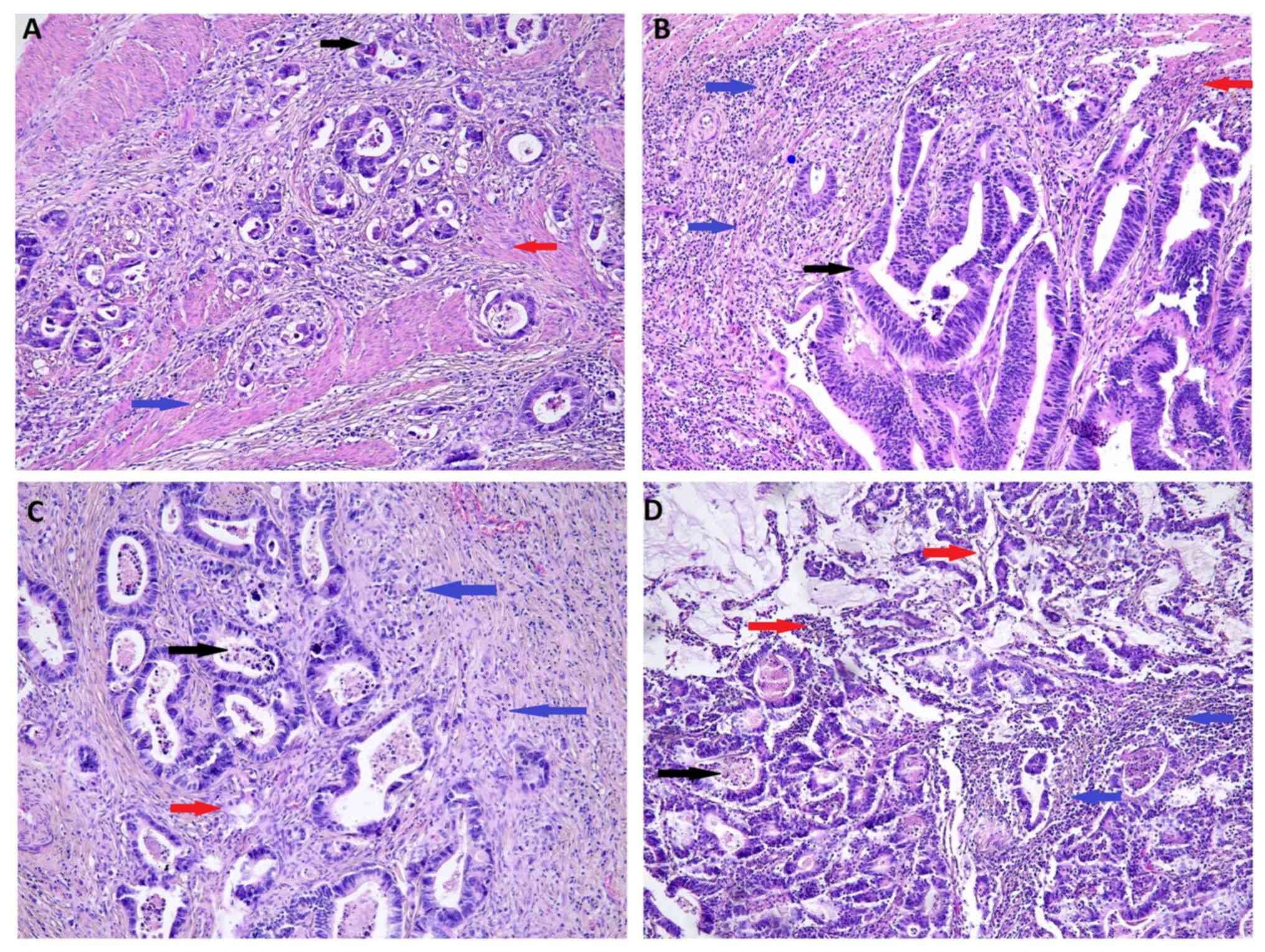 | Figure 3.Representative examples of the
combined parametric value in hematoxylin and eosin-stained CRC
tissue. Histological findings in resected CRC tissues from patients
with (A) low levels of intraTANs, low levels of stromaTANs and a
low degree of necrosis (group 1) (B) low levels of intraTANs, high
levels of stromaTANs and a high degree of necrosis (group 2) (C)
high levels of intraTANs, low levels of stromaTANs and a low degree
of necrosis (group 3), and (D) high levels of intraTANs, high
levels of stromaTANs and a high degree of necrosis (group 4).
magnification, ×100. IntraTANS (black arrow), stromaTANs (blue
arrow), necrosis (red arrow). CRC, colorectal cancer; intraTANs,
intratumoral tumor-associated neutrophils; stromaTANs, stomal
tumor-associated neutrophils. |
 | Figure 4.Distribution of (A) intraTANs and (B)
stromaTANs in the center of the tumor mass, (C) necrosis, and (D)
their combined parametric value. Stromal TANs scored as low group
(0–20% neutrophils) and high group (more than 21% neutrophils).
Necrosis scored as low group (absent or focal necrosis) and high
group (moderate or extensive necrosis). Group 1, low levels of
intraTANs, low levels of stroma TANs and a low degree of necrosis;
group 2, low levels of intraTANs, high levels of stromaTANs and a
high degree of necrosis; group 3, high levels of intraTANs, low
levels of stromaTANs and a low degree of necrosis; group 4, high
levels of intraTANs, high levels of stromaTANs and a high degree of
necrosis. |
Association between intraTAN
infiltration, stromaTAN infiltration or tumor necrosis and the
clinicopathological features of patients with CRC
IntraTANs were associated with the number of
resected lymph nodes (P=0.004), fibrosis (P=0.032) and
pre-operative treatment (P=0.015) (Table I). StromaTANs were associated with
lymph node metastasis (P=0.049) and tumor deposits (P=0.041)
(Table II). Necrosis was
associated with venous (P=0.003), lymphatic (P=0.007) and
perineural (P=0.015) invasion, as well as with lymph node
metastasis (P=0.033), the number of invaded lymph nodes (P=0.012)
and lymph node pouch invasion (P=0.043) (Table III). The combined parametric
value was associated with pT stage (P=0.049), and with venous
(P=0.034) and lymphatic (P=0.026) invasion (Table IV).
 | Table I.Association between intraTAN
infiltration and clinicopathological features in patients with
colorectal cancer (n=160). |
Table I.
Association between intraTAN
infiltration and clinicopathological features in patients with
colorectal cancer (n=160).
|
| IntraTAN
infiltration |
|---|
|
|
|
|---|
| Clinicopathological
variable | Low, <10
cells/HPF | Moderate, 10–50
cells/HPF | High, >50
cells/HPF | P-value |
|---|
| Age, years |
|
|
|
|
|
<60 | 11 | 13 | 16 | 0.756 |
|
>60 | 29 | 44 | 47 |
|
| Sex |
|
|
|
|
|
Female | 13 | 18 | 33 | 0.132 |
|
Male | 27 | 39 | 30 |
|
| Location |
|
|
|
|
|
Rightside | 3 | 8 | 9 | 0.899 |
|
Transverse | 3 | 2 | 11 |
|
|
Leftside | 2 | 2 | 11 |
|
|
Sigmoid | 1 | 13 | 15 |
|
|
Rectum | 29 | 26 | 27 |
|
| Tumor growth |
|
|
|
|
|
Expanding | 34 | 48 | 51 | 0.670 |
|
Infiltrate | 6 | 9 | 12 |
|
| Tumor size, cm |
|
|
|
|
|
<2.5 | 7 | 10 | 10 | 0.672 |
|
2.5-5.0 | 25 | 40 | 41 |
|
|
>5.0 | 8 | 7 | 12 |
|
| Histological
type |
|
|
|
|
|
Mucinous | 8 | 9 | 13 | 0.825 |
|
Adenocarcinoma | 32 | 48 | 50 |
|
| Percentage of
mucinous component, % |
|
|
|
|
|
10-30 | 2 | 4 | 9 | 0.971 |
|
30-50 | 5 | 5 | 5 |
|
| TNM stage |
|
|
|
|
|
I+II | 16 | 26 | 31 | 0.702 |
|
III+IV | 24 | 29 | 34 |
|
| Grade
ofmalignancies |
|
|
|
|
| 2 | 37 | 54 | 57 | 0.763 |
| 3 | 3 | 3 | 6 |
|
| pT stage |
|
|
|
|
|
1+2 | 18 | 22 | 25 | 0.122 |
|
3+4 | 22 | 35 | 38 |
|
| Venous
invasion |
|
|
|
|
|
Absent | 31 | 36 | 46 | 0.712 |
|
Present | 9 | 21 | 16 |
|
| Lymphatic
invasion |
|
|
|
|
|
Absent | 32 | 41 | 48 | 0.805 |
|
Present | 8 | 16 | 14 |
|
| Perineural
invasion |
|
|
|
|
|
Absent | 37 | 52 | 54 | 0.780 |
|
Present | 3 | 5 | 9 |
|
| Lymph
nodemetastasis |
|
|
|
|
|
Absent | 21 | 34 | 26 | 0.752 |
|
Present | 19 | 23 | 37 |
|
| Number of resected
lymph nodes |
|
|
|
|
|
<5 | 7 | 4 | 2 | 0.035 |
|
5-10 | 12 | 10 | 7 |
|
|
≥10 | 21 | 43 | 38 |
|
| Number of invaded
lymph nodes |
|
|
|
|
|
<5 | 13 | 17 | 21 | 0.922 |
| ≥5 | 6 | 6 | 16 |
|
| Lymph node pouch
invasion |
|
|
|
|
|
Absent | 25 | 9 | 8 | 0.957 |
|
Present | 15 | 18 | 2 |
|
| Distant
metastasis |
|
|
|
|
|
Absent | 37 | 49 | 57 | 0.949 |
|
Present | 3 | 8 | 6 |
|
| Tumor Deposits |
|
|
|
|
|
Absent | 29 | 48 | 56 | 0.160 |
|
Present | 10 | 9 | 8 |
|
| Tumor budding |
|
|
|
|
|
Absent | 24 | 34 | 36 | 0.572 |
|
Present | 16 | 23 | 27 |
|
| Necrosis |
|
|
|
|
|
Absent | 10 | 17 | 18 | 0.942 |
|
Focal | 7 | 27 | 27 |
|
|
Moderate | 14 | 8 | 14 |
|
|
Extensive | 9 | 5 | 4 |
|
| Fibrosis |
|
|
|
|
|
Absent | 1 | 7 | 3 | 0.034 |
|
Focal | 19 | 28 | 25 |
|
|
Moderate | 11 | 14 | 18 |
|
|
Extensive | 9 | 8 | 17 |
|
| Crohn's-like
aggregates |
|
|
|
|
|
Absent | 34 | 37 | 42 | 0.271 |
|
Present | 6 | 20 | 16 |
|
| Response to
neoadjuvant treatment |
|
|
|
|
| SD | 10 | 5 | 1 | 0.517 |
| PR | 12 | 9 | 17 |
|
| Pre-operative
treatment |
|
|
|
|
|
Yes | 12 | 11 | 31 | 0.016 |
| No | 28 | 46 | 30 |
|
 | Table II.Association between stromaTANs and
clinicopathological features in patients with colorectal cancer
(n=160).a |
Table II.
Association between stromaTANs and
clinicopathological features in patients with colorectal cancer
(n=160).a
|
| StromaTANs in the
center of tumor |
|---|
|
|
|
|---|
| Clinicopathological
feature | Low | High | P-value |
|---|
| Age, years |
|
|
|
|
<60 | 35 | 5 | 0.602 |
|
>60 | 109 | 11 |
|
| Sex |
|
|
|
|
Female | 58 | 6 | 0.862 |
|
Male | 86 | 10 |
|
| Location |
|
|
|
|
Rightside | 15 | 5 | 0.320 |
|
Transverse | 11 | 3 |
|
|
Leftside | 14 | 1 |
|
|
Sigmoid | 28 | 1 |
|
|
Rectum | 78 | 4 |
|
| Tumor growth |
|
|
|
|
Expanding | 121 | 12 | 0.494 |
|
Infiltrate | 23 | 4 |
|
| Tumor size, cm |
|
|
|
|
<2.5 | 23 | 4 | 0.892 |
|
2.5-5.0 | 100 | 6 |
|
|
>5.0 | 21 | 6 |
|
| Histological
type |
|
|
|
|
Mucinous | 18 | 12 | 0.982 |
|
Adenocarcinoma | 126 | 4 |
|
| Percentage of
mucinous component, % |
|
|
|
|
10-30 | 14 | 1 | 0.302 |
|
30-50 | 11 | 4 |
|
| TNM stage |
|
|
|
|
I+II | 65 | 8 | 0.383 |
|
III+IV | 79 | 8 |
|
| Grade
ofmalignancies |
|
|
|
| 2 | 133 | 15 | 0.852 |
| 3 | 11 | 1 |
|
| pT stage |
|
|
|
|
I+II | 61 | 4 | 0.622 |
|
III+IV | 83 | 12 |
|
| Venous
invasion |
|
|
|
|
Absent | 105 | 8 | 0.193 |
|
Present | 38 | 8 |
|
| Lymphatic
invasion |
|
|
|
|
Absent | 110 | 11 | 0.415 |
|
Present | 33 | 5 |
|
| Perineural
invasion |
|
|
|
|
Absent | 127 | 16 | 0.833 |
|
Present | 17 | 0 |
|
| Lymph
nodemetastasis |
|
|
|
|
Absent | 72 | 9 | 0.047 |
|
Present | 72 | 7 |
|
| Number of resected
lymph nodes |
|
|
|
|
<5 | 11 | 2 | 0.142 |
|
5-10 | 27 | 2 |
|
|
≥10 | 90 | 12 |
|
| Number of invaded
lymph nodes |
|
|
|
|
<5 | 44 | 7 | 0.736 |
| ≥5 | 28 | 0 |
|
| Lymph node pouch
invasion |
|
|
|
|
Absent | 31 | 10 | 0.887 |
|
Present | 33 | 6 |
|
| Distant
metastasis |
|
|
|
|
Absent | 128 | 15 | 0.252 |
|
Present | 16 | 1 |
|
| Tumor deposits |
|
|
|
|
Absent | 118 | 15 | 0.052 |
|
Present | 26 | 1 |
|
| Tumor budding |
|
|
|
|
Absent | 85 | 9 | 0.130 |
|
Present | 59 | 7 |
|
| Necrosis |
|
|
|
|
Absent | 44 | 1 | 0.319 |
|
Focal | 56 | 5 |
|
|
Moderate | 30 | 6 |
|
|
Extensive | 14 | 4 |
|
| Fibrosis |
|
|
|
|
Absent | 10 | 1 | 0.742 |
|
Focal | 64 | 8 |
|
|
Moderate | 39 | 4 |
|
|
Extensive | 31 | 3 |
|
| Crohn's-like
aggregates |
|
|
|
|
Absent | 100 | 13 | 0.083 |
|
Present | 39 | 3 |
|
| Response to
neoadjuvant treatment |
|
|
|
| SD | 15 | 1 | 0.973 |
| PR | 35 | 3 |
|
| Pre-operative
treatment |
|
|
|
|
Yes | 45 | 9 | 0.252 |
| No | 101 | 5 |
|
 | Table III.Association between necrosis and
clinicopathological features in patients with colorectal cancer
(n=160).a |
Table III.
Association between necrosis and
clinicopathological features in patients with colorectal cancer
(n=160).a
|
| Necrosis |
|---|
|
|
|
|---|
| Clinicopathological
feature | Low | High | P-value |
|---|
| Age, years |
|
|
|
|
<60 | 25 | 15 | 0.623 |
|
>60 | 67 | 53 |
|
| Sex |
|
|
|
|
Female | 40 | 24 | 0.212 |
|
Male | 53 | 43 |
|
| Location |
|
|
|
|
Right-side | 9 | 11 | 0.552 |
|
Transverse | 4 | 10 |
|
|
Left-side | 6 | 11 |
|
|
Sigmoid | 6 | 23 |
|
|
Rectum | 46 | 36 |
|
| Tumor growth |
|
|
|
|
Expanding | 75 | 58 | 0.422 |
|
Infiltrate | 18 | 9 |
|
| Tumor size, cm |
|
|
|
|
<2.5 | 17 | 10 | 0.499 |
|
2.5-5.0 | 57 | 49 |
|
|
>5.0 | 19 | 8 |
|
| Histological
type |
|
|
|
|
Mucinous | 19 | 11 | 0.946 |
|
Adenocarcinoma | 74 | 56 |
|
| Percentage of
mucinous component, % |
|
|
|
|
10-30 | 5 | 10 | 0.715 |
|
30-50 | 14 | 1 |
|
| TNM stage |
|
|
|
|
I+II | 50 | 23 | 0.069 |
|
III+IV | 43 | 44 |
|
| Grade
ofmalignancies |
|
|
|
| 2 | 88 | 60 | 0.532 |
| 3 | 5 | 7 |
|
| pT stage |
|
|
|
|
1+2 | 38 | 27 | 0.822 |
|
3+4 | 55 | 40 |
|
| Venous
invasion |
|
|
|
|
Absent | 71 | 42 | 0.002 |
|
Present | 22 | 24 |
|
| Lymphatic
invasion |
|
|
|
|
Absent | 75 | 46 | 0.006 |
|
Present | 18 | 20 |
|
| Perineural
invasion |
|
|
|
|
Absent | 88 | 55 | 0.011 |
|
Present | 5 | 12 |
|
| Lymph node
metastasis |
|
|
|
|
Absent | 60 | 21 | 0.031 |
|
Present | 33 | 46 |
|
| Number of resected
lymph nodes |
|
|
|
|
<5 | 8 | 5 | 0.682 |
|
5-10 | 17 | 12 |
|
|
≥10 | 68 | 34 |
|
| Number of invaded
lymph nodes |
|
|
|
|
<5 | 23 | 28 | 0.011 |
| ≥5 | 10 | 18 |
|
| Lymph node pouch
invasion |
|
|
|
|
Absent | 14 | 27 | 0.040 |
|
Present | 27 | 12 |
|
| Distant
metastasis |
|
|
|
|
Absent | 83 | 60 | 0.622 |
|
Present | 10 | 7 |
|
| Tumor deposits |
|
|
|
|
Absent | 79 | 54 |
|
|
Present | 13 | 14 | 0.087 |
| Tumor budding |
|
|
|
|
Absent | 56 | 38 | 0.432 |
|
Present | 37 | 29 |
|
| Fibrosis |
|
|
|
|
Absent | 7 | 4 | 0.822 |
|
Focal | 43 | 29 |
|
|
Moderate | 25 | 18 |
|
|
Extensive | 18 | 16 |
|
| Crohn's-like
aggregates |
|
|
|
|
Absent | 64 | 49 | 0.815 |
|
Present | 29 | 13 |
|
| Response to
neoadjuvant treatment |
|
|
|
| SD | 8 | 8 | 0.521 |
| PR | 9 | 29 |
|
| Pre-operative
treatment |
|
|
|
|
Yes | 16 | 38 | 0.682 |
| No | 76 | 30 |
|
 | Table IV.Association between intraTAN
infiltration, stromaTAN infiltration and tumor necrosis and
clinicopathological features in patients with colorectal cancer
(n=160).a |
Table IV.
Association between intraTAN
infiltration, stromaTAN infiltration and tumor necrosis and
clinicopathological features in patients with colorectal cancer
(n=160).a
|
| Group number |
|
|---|
|
|
|
|
|---|
| Clinicopathological
variable | 1 | 2 | 3 | 4 | P-value |
|---|
| Age, years |
|
|
|
|
|
|
<60 | 7 | 9 | 14 | 10 | 0.584 |
|
>60 | 18 | 18 | 41 | 43 |
|
| Sex |
|
|
|
|
|
|
Female | 12 | 8 | 27 | 17 | 0.257 |
|
Male | 13 | 19 | 28 | 36 |
|
| Location |
|
|
|
|
|
|
Rightside | 3 | 2 | 5 | 10 | 0.500 |
|
Transverse | 1 | 5 | 1 | 7 |
|
|
Leftside | 2 | 1 | 10 | 2 |
|
|
Sigmoid | 2 | 2 | 23 | 2 |
|
|
Rectum | 17 | 15 | 23 | 27 |
|
| Tumor growth |
|
|
|
|
|
|
Expanding | 21 | 22 | 47 | 43 | 0.796 |
|
Infiltrate | 4 | 5 | 8 | 10 |
|
| Tumor size, cm |
|
|
|
|
|
|
<2.5 | 5 | 5 | 7 | 10 | 0.437 |
|
2.5-5.0 | 13 | 18 | 43 | 36 |
|
|
>5.0 | 7 | 4 | 6 | 7 |
|
| Histological
type |
|
|
|
|
|
|
Mucinous | 5 | 7 | 12 | 8 | 0.433 |
|
Adenocarcinoma | 20 | 20 | 45 | 45 |
|
| Percentage of
mucinous component, % |
|
|
|
|
|
|
10-30 | 0 | 3 | 8 | 4 | 0.094 |
|
30-50 | 5 | 4 | 2 | 4 |
|
| TNM stage |
|
|
|
|
|
|
I+II | 12 | 13 | 29 | 19 | 0.462 |
|
III+IV | 13 | 14 | 26 | 34 |
|
| Grade
ofmalignancies |
|
|
|
|
|
| 2 | 23 | 26 | 49 | 50 | 0.566 |
| 3 | 2 | 1 | 6 | 3 |
|
| pT stage |
|
|
|
|
|
|
1+2 | 9 | 14 | 19 | 23 | 0.049 |
|
3+4 | 16 | 13 | 36 | 30 |
|
| Venous
invasion |
|
|
|
|
|
|
Absent | 20 | 16 | 47 | 30 | 0.034 |
|
Present | 9 | 11 | 3 | 23 |
|
| Lymphatic
invasion |
|
|
|
|
|
|
Absent | 21 | 18 | 48 | 34 | 0.026 |
|
Present | 4 | 9 | 6 | 19 |
|
| Perineural
invasion |
|
|
|
|
|
|
Absent | 24 | 24 | 50 | 45 | 0.362 |
|
Present | 1 | 3 | 5 | 8 |
|
| Lymph
nodemetastasis |
|
|
|
|
|
|
Absent | 15 | 16 | 24 | 26 | 0.511 |
|
Present | 10 | 11 | 31 | 27 |
|
| Number of resected
lymph nodes |
|
|
|
|
|
|
<5 | 3 | 5 | 0 | 4 | 0.254 |
|
5-10 | 3 | 9 | 8 | 9 |
|
|
≥10 | 19 | 13 | 31 | 39 |
|
| Number of invaded
lymph nodes |
|
|
|
|
|
|
<5 | 7 | 6 | 16 | 22 | 0.256 |
| ≥5 | 3 | 5 | 15 | 5 |
|
| Lymph node pouch
invasion |
|
|
|
|
|
|
Absent | 16 | 19 | 1 | 5 | 0.511 |
|
Present | 9 | 8 | 0 | 22 |
|
| Distant
metastasis |
|
|
|
|
|
|
Absent | 23 | 24 | 49 | 47 | 0.678 |
|
Present | 2 | 3 | 6 | 6 |
|
| Tumor deposits |
|
|
|
|
|
|
Absent | 19 | 21 | 48 | 45 | 0.328 |
|
Present | 5 | 6 | 8 | 8 |
|
| Tumor budding |
|
|
|
|
|
|
Absent | 15 | 15 | 33 | 31 | 0.998 |
|
Present | 10 | 12 | 22 | 22 |
|
| Necrosis |
|
|
|
|
|
|
Absent | 7 | 7 | 17 | 14 | 0.023 |
|
Focal | 6 | 5 | 24 | 26 |
|
|
Moderate | 5 | 13 | 7 | 11 |
|
|
Extensive | 7 | 2 | 7 | 2 |
|
| Fibrosis |
|
|
|
|
|
|
Absent | 1 | 1 | 4 | 5 | 0.459 |
|
Focal | 10 | 6 | 35 | 21 |
|
|
Moderate | 7 | 5 | 15 | 16 |
|
|
Extensive | 7 | 0 | 16 | 11 |
|
| Crohn's-like
aggregates |
|
|
|
|
|
|
Absent | 20 | 20 | 37 | 36 | 0.156 |
|
Present | 5 | 6 | 14 | 17 |
|
| Response to
neoadjuvant treatment |
|
|
|
|
|
| SD | 3 | 1 | 7 | 5 | 0.852 |
| PR | 2 | 5 | 26 | 5 |
|
| Pre-operative
treatment |
|
|
|
|
|
|
Yes | 4 | 10 | 31 | 9 | 0.442 |
| No | 21 | 17 | 25 | 43 |
|
Association between intraTAN
infiltration, stromaTAN infiltration or tumor necrosis and the
hematological parameters of patients with CRC
IntraTANs were found to be associated with
lymphocyte count following surgery (P=0.046) and serum CEA level
prior to surgery (P=0.042) (Table
SI). The analysis of stromaTANs and hematological parameters
did not reveal a significant association (Table SII). The degree of necrosis was
associated with hematological parameters measured following
surgery, such as WBC (P=0.030), neutrophil count (P=0.011), the
NLR-PLR status (P=0.038), the PLT-NLR status (P=0.030) and serum
CEA level following surgery (P=0.011). Furthermore, necrosis was
associated with MLR, measured in pre-operative whole blood samples
(P=0.023; Table SIII). The
combined parametric value was significantly associated with serum
CEA levels prior to surgery (P=0.029) (Table SIV).
Prognostic value of intraTANs,
stromaTANs and necrosisin patients with CRC, and their combined
parametric value
To investigate the association between intraTANs,
stromaTANs and necrosis and prognosis, and their combined
parametric value in patients with CRC, the survival curves of 3-
and 5-year DFS time in all the patients were determined usingthe
Kaplan-Meier method. The median of the 3- and 5-year DFS was 11.6
and 27.6 months, respectively. Patients with low levels of
intraTANs survived ~10.9 months (3-year DFS time) and 28.2 months
(5-year DFS time) compared with that in patients with moderate and
strong levels ofintraTANs [(10.9 and 12.4 months for 3-year DFS
time, respectively and 26.0 and 29.1 months for5-year DFS time,
respectively). The results revealed that patients in the low
stromaTANs level group exhibited significantly longer 3- and 5-year
DFS rates compared with that in patients in the high stromaTANs
level group (P=0.061 and 0.162, respectively). The mean 3- and
5-year DFS rates were 11.6 and 29.0 months in the high necrosis
group, and 9.85 and 23.8 months, in the low necrosis group,
respectively. The analysis of the combined parametric value
indicated that patients in groups 1 (low intraTANs, low stromaTANs
and low necrosis), 3 (high intraTANs, low stromaTANs and low
necrosis) and 4 (high intraTANs, high stromaTANs and high necrosis)
had significantly longer 3-year DFS rates compared with that
inpatients in group 2 (low intraTANs, high stromaTANs and high
necrosis) (P=0.027). However, the analysis of 5-DFS rate did not
confirm this trend (P=0.895) (Fig.
5).
 | Figure 5.Effect of intraTANs, stromaTANs,
necrosis and their combined parametric value on the DFS time in
patients with CRC. Kaplan-Meier plots of the 3- and 5-year DFS
times based on (A and B) intraTANs, (C and D) stromal TANs, (E and
F) necrosis, and (G and H) their combined triple parametric value,
respectively. Group 1, low levels of intraTANs, low levels of
stroma TANs and a low degree of necrosis; group 2, low levels of
intraTANs, high levels of stromaTANs and a high degree of necrosis;
group 3, high levels of intraTANs, low levels of stromaTANs and a
low degree of necrosis; group 4, high levels of intraTANs, high
levels of stromaTANs and a high degree of necrosis. IntraTANs,
intratumoral tumor-associated neutrophils; stromaTANs, stomal
tumor-associated neutrophils. IntraTANs scored as low group (absent
or <10 cells/HPF), moderate group (10–50 cells/HPF) and high
group (>50 cells/HPF). Stromal TANs scored as low group (0–20%
neutrophils) and high group (more than 21% neutrophils). Necrosis
scored as low group (absent or focal necrosis) and high group
(moderate or extensive necrosis). |
To compare the prognostic value of intraTANs,
stromaTANs, necrosis and their combined parametric value with other
histopathological parameters, univariate and multivariate analyses
were performed (Tables V and
VI). Thefactors found to be
predictive of 3-year DFS times in univariate Cox regression
analysis included tumor growth [hazard ratio (HR), 2.070; 95%
confidence interval (CI), 1.837-3.808; P=0.040], tumor budding (HR,
1.932; 95% CI,-1.036-0.613; P=0.049) and
intraTANs/stromaTANs/necrosis (HR, 1.577; 95% CI, 1.372-3.032;
P=0.014). According to multivariate Cox proportional model, tumor
growth (HR, 1.925; 95% CI, 1.145-3.420; P=0.003) and
intraTANs/stromaTANs/necrosis (HR, 1.344; 95% CI, 1.235-3.015;
P=0.028) were independent factors of 3-year DFS time in patients
with CRC. However, other factors such as age, sex, tumor size, TNM
stage, histopathological type, grade of malignancies, venous
invasion, metastasis to the lymphatic vessels and perineural
spaces, lymph node involvement, distant metastasis, tumor deposits,
tumor budding, intraTANs, stromaTANs and their combined parametric
value were not significant in univariate and multivariate analyses
of the 5-year DFS times in patients with CRC.
 | Table V.Prognostic factors for 3-year
disease-free survival time in patients with colorectal cancer. |
Table V.
Prognostic factors for 3-year
disease-free survival time in patients with colorectal cancer.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | HR (95%CI) | P-value | HR (95%CI) | P-value |
|---|
| Age, years (≤60 vs.
≥60) | 0.649
(1.048–1.597) | 0.512 | - | - |
| Sex (female vs.
male) | 0.822
(1.198–1.465) | 0.414 | - | - |
| Tumor growth
(expanding vs. infiltrate) | 2.070
(1.837–3.808) | 0.040 | 1.925
(1.145–3.420) | 0.003a |
| Tumor size, cm
<2.5 vs. 2.5–5 vs. >5 | 0.19.1
(0.264–1.225) | 0.829 | - | - |
| TNM stage (I–II vs.
III–IV) | −1.491
(−0.801–0.514) | 0.121 | - | - |
| Adenocarcinoma type
(non-muc vs. partim mucin) | −0.062
(−0.120–1.738) | 0.944 | - | - |
| Grade of malignancy
(2 vs. 3) | 1.639
(2.715–4.442) | 0.104 | - | - |
| Pre-operative
treatment (yes vs. no) | −2.044
(−4.677–2.286) | 0.443 | - | - |
| pT stage (1–2 vs.
3–4) | 1.331
(1.490–2.125) | 0.156 | - | - |
| Venous invasion
(yes vs. no) | 0.046
(−0.885–3.135) | 0.777 | - | - |
| Lymphatic invasion
(yes vs. no) | 0.596
(−0.288–3.434) | 0.933 | - | - |
| Perineural invasion
(yes vs. no) | 0.578
(1.266–2.728) | 0.643 | - | - |
| Number of removed
lymph nodes (<5 vs. 5–10 vs. <10) | 1.458
(1.017–1.762) | 0.566 | - | - |
| Lymph node
metastasis (yes vs. no) | 1.623
(1.250–1.964) | 0.848 | - | - |
| Type of lymph node
metastasis (micro vs. macro) | 1.244
(0.442–1.144) | 0.700 | - | - |
| Number of
metastatic lymph nodes (<5 vs. >5) | 1.556
(2.653–3.778) | 0.160 | - | - |
| Lymph node pouch
invasion (yes vs. no) | −0.249
(−0.963–2.496) | 0.701 | - | - |
| Distant metastasis
(yes vs. no) | 0.079
(−0.395–2.083) | 0.937 | - | - |
| Distant metastasis
size, mm (<10 vs. >10) | 0.140
(0.052–0.307) | 0.850 | - | - |
| Tumor deposits (yes
vs. no) | −1.367
(−1.183–1.506) | 0.435 | - | - |
| Tumor budding (yes
vs. no) | 0.932
(−1.036–0.613) | 0.049a | 0.895
(−0.926–1.201) | 0.093 |
| IntraTANs (yes vs.
no) | 0.560
(1.212–1.587) | 0.446 | - | - |
| StromaTANs (low vs.
high) | −0.946
(−1.013–1.706) | 0.382 | - | - |
| Necrosis (low vs.
high) | −0.869
(−1.013–0.097) | 0.553 | - | - |
|
IntraTANs/stromaTANs/necrosis (group 1–2
vs. 3–4) | 1.577
(1.372–3.032) | 0.014a | 1.344
(1.235–3.015) | 0.028a |
 | Table VI.Univariate analysis of prognostic
factors for 5-year disease-free survival time in patients with
colorectal cancer. |
Table VI.
Univariate analysis of prognostic
factors for 5-year disease-free survival time in patients with
colorectal cancer.
| Variable | HR (95%CI) | P-value |
|---|
| Age, years (≤60 vs.
≥60) | −1.023
(−0.095–0.259) | 0.714 |
| Sex (female vs.
male) | 0.274
(0.629–3.306) | 0.849 |
| Tumor growth
(expanding vs. infiltrate) | −1.113
(−4.291–4.125) | 0.300 |
| Tumor size, cm
<2.5 vs. 2.5–5 vs. >5 | 1.437
(2.758–3.976) | 0.151 |
| TNM stage (I–II vs.
III–IV) | −0.883
(−0.705–1.215) | 0.562 |
| Adenocarcinoma type
(non-muc vs. partim mucin) | 0.798
(3.094–3.901) | 0.428 |
| Grade of malignancy
(2 vs. 3) | −0.198
(−1.180–6.094) | 0.846 |
| Pre-operative
treatment (yes vs. no) | −0.274
(0.125–0.356) | 0.326 |
| pT stage (1–2 vs.
3–4) | 0.122
(2.669–3.590) | 0.458 |
| Venous invasion
(yes vs. no) | −0.165
(3.718–7.466) | 0.619 |
| Lymphatic invasion
(yes vs. no) | 0.031
(−1.057–8.059) | 0.895 |
| Perineural invasion
(yes vs. no) | 0.807
(−8.567–6.800) | 0.210 |
| Number of removed
lymph nodes (<5 vs. 5–10 vs. <10) | 0.750
(3.401–4.463) | 0.450 |
| Lymph node
metastasis (yes vs. no) | −0.318
(−2.875–4.565) | 0.530 |
| Type of lymph node
metastasis (micro vs. macro) | −1.810
(−1.600–1.817) | 0.383 |
| Number of
metastatic lymph nodes (<5 vs. >5) | 0.204
(2.297–9.057) | 0.801 |
| Lymph node pouch
invasion (yes vs. no) | −0.255
(1.427–6.800) | 0.834 |
| Distant metastasis
(yes vs. no) | −1.399
(5.922–6.379) | 0.355 |
| Distant metastasis
size, mm (<10 vs. >10) | 1.056
(−1.314–1.361) | 0.336 |
| Tumor deposits (yes
vs. no) | −0.652
(−2.044–2.210) | 0.356 |
| IntraTANs (yes vs.
no) | 0.674
(3.282–3.846) | 0.395 |
| StromaTANs (low vs.
high) | −1.061
(−1.558–2.281) | 0.495 |
| Necrosis (low vs.
high) | −0.796
(−2.833–4.140) | 0.489 |
|
IntraTANs/stromaTANs/necrosis (group 1–2
vs. 3–4) | −0.886
(−3.041–3.040) | 0.319 |
Discussion
Neutrophilic cell infiltration and tumor necrosis
often occur in solid tumors (26,27).
Physiologically, immune cells, particularly neutrophils, are able
to protect the host using immune surveillance, eliminating both
microbial pathogens and cancerous cells. Notwithstanding, the
classical function of these cells can be modified, whereby they are
recruited into the tumor mass. They can then be activated via
alternative pathways, and thus exhibit immunosuppressive
activities, including tumor growth and metastasis (28). In the present study, tissues from
patients with CRC exhibited numerous neutrophils localized inside
the tumor tissue in the majority of cases, while a low neutrophilic
inflammation was observed in the center of the tumor stroma. Both
types of neutrophils were associated with pro-tumor factors, such
as the CEA level, the number of resected lymph nodes, lymph node
metastasis and the presence of tumor deposits. Ye et al
(29) indicated that a higher TAN
abundance was found in tissues from patients with CRC with
well-to-moderate tumor differentiation, and fewer numbers of lymph
nodes or metastases, TNM stage I–II disease or rectal cancer.
Subsequent studies have also demonstrated that an increasing TAN
density was associated with a higher stage disease in patients with
CRC (10,30). Furthermore, some studies found an
association between a higher TAN count and the survival time of
patients with cancers of the gastrointestinal tract (30,31).
In the present study, only a slight tendency for a longer DFStime
was observed in patients with a low stromaTAN infiltration. This is
in contrast to the findings of Berry et al (32) who reported that patients with stage
II disease and high TAN scores had a longer overall survival time
as compared with that in patients with stage II disease and a low
TAN score. In addition, Wikberg et al (33) demonstrated that poor infiltration
of CD66b-expressing neutrophils at the invasive front of surgically
resected CRC tumors was associated with a worse prognosis. All the
aforementioned data prove that TANs may have a significant effect
on tumor progression and patient prognosis.
The present study also determined tumor necrosis in
the primary tumor tissues of patients with CRC. It was demonstrated
that the majority of cases had tumors with <30% necrosis across
the entire tumor area. Furthermore, it was revealed that the level
of necrosis was associated with venous, lymphatic and perineural
invasion, lymph node involvement, the number of invaded lymph nodes
and the infiltrate of cancerous cells beyond the lymph node pouch,
as well as with post-operative level of CEA in serum. These
observations are reflected by the hypothesis whereby tumor necrosis
in CRC results from rapid tumor cell invasion, the insufficient
vascular supply of the tumor and the development of intratumoral
hypoxia (34). Schneider et
al (35) indicated that the
extent of necrosis was found to be significantly associated with
histopathological features of disease progression, such as lymph
node metastases, TNM stage, poor tumor differentiation, a large
tumor size and venous invasion. Moreover, Väyrynen et al
(11) demonstrated that more
extensive tumor necrosis in CRC was associated with higher stage
tumors and more frequently with conventional carcinoma
(adenocarcinoma). They also proved the lack of a connection between
tumor necrosis and pre-operative treatment. This finding is in
accordance with the findings of the present study, where no
significant difference was found between the presence of tumor
necrosis and patients who received pre-operative therapy. It has
been demonstrated that short-term neoadjuvant chemo- and
radiotherapies do not markedly modify the morphological appearance
of the tumor tissue (36). The
presence of tumor necrosis revealed in the histological examination
may be reflected in the morphological parameters of whole blood.
With respect to necrosis, the increased recruitment of inflammatory
cells, such as neutrophils was observed. The accumulation of
inflammatory cells in the tissue activates the production of these
erythropoietic cells in the peripheral blood (37). In support of this hypothesis, in
the present study, high levels of necrosis were found to be
associated with morphological parameters measured following
surgical treatment, including WBC, neutrophil count, and NLR-PLR
and PLT-NLR status. It was also demonstrated that tumor necrosis
was associated with MLR calculated from pre-operative whole blood
parameters. Notably, previous studies have highlighted the
potential prognostic value of tumor necrosis in the survival time
of patients with CRC (13,38,39).
In the present study, however, there was a trend for a shorter
5-year DFS time in patients with extensive tumor necrosis.
Previous studies have highlighted the role of immune
cell infiltrates in CRC (23,40).
To determine the role of neutrophils and necrosis, the present
study examined the significance of these parameters in combination.
Patients in groups 3 (high intraTANs, low stromaTANs and low
necrosis) and 4 (high intraTANs, high stromaTANs and high necrosis)
exhibited increased deep primary tumor invasion, and metastases to
venous and lymphatic vessels. The results of the present study also
indicated that the combined parametric value was significantly
associated with serum CEA level prior to surgery. Furthermore, one
of the most notable observations was the prognostic significance of
this novel combined parameter. In the present study, it was
established that patients in groups 1 (low intraTANs, low
stromaTANs and low necrosis), 3 (high intraTANs, low stromaTANs and
low necrosis) and 4 (high intraTANs, high stromaTANs and high
necrosis) had significantly longer 3-year DFS rates compared with
that in patientsin group 2 (low intraTANs, high stromaTANs and high
necrosis). In addition, the analysis of the 3-year DFS rate
revealed that the combined parametric value was an independent
factor for patients with CRC.
The present study has certain limitations, which
should be taken into consideration when interpretating the results.
In the present study, neutrophils were only examined on the basis
of morphological features and counted; however, it is strongly
postulated that the performance of immunohistochemical staining is
also required to reveal the phenotypical characterization of
TANs.
In conclusion, the present study demonstrated that
the examination of neutrophils and necrosis in the tumor tissue
could improve the understanding of CRC pathogenesis. Furthermore,
it was found that the combined value of neutrophil infiltration and
necrosis may be used as an independent factor for patients with
CRC, and may be used in routine pathomorphological diagnostics in
the future.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by a grant from the Medical University
of Bialystok, Poland (grant no. SUB/1/DN/20/001/1194).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KJ collected and analyzed the data, reviewed the
literature and wrote, and revised the manuscript. MK and KL
analyzed and interpreted the pathological results. WF and WK
collected and analyzed data. MG wrote the paper, reviewed the
literature and acquired the data. All authors have read and
approved the final manuscript. KJ and MG confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
This study was reviewed and approved by the Ethics
Committee of the Medical University of Bialystok, Poland.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
World Health Organization (WHO), . Global
Cancer Observatory. WHO; Geneva: 2020, https://gco.iarc.frNovember 10–2020
|
|
2
|
Høydahl Ø, Edna TH, Xanthoulis A, Lydersen
S and Endreseth BH: Long-term trends in colorectal cancer:
Incidence, localization, and presentation. BMC Cancer. 20:10772020.
View Article : Google Scholar
|
|
3
|
Mattiuzzi C, Sanchis-Gomar F and Lippi G:
Concise update on colorectal cancer epidemiology. Ann Transl Med.
7:6092019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
American Cancer Society, . Survival Rates
for Colorectal Cancer. American Cancer Society; Atlanta, GA: 2020,
https://www.cancer.org/camcer/colon-rectal-cancer/detection-diagnosis-staging/survival-rates.htmlNovember
10–2020
|
|
5
|
Hamilton SR, Bosman FT and Boffetta P:
Carcinoma of the colon and rectum. WHO Classification of Tumours of
the Digestive System. Bosman FT, Carneiro F, Hruban RH and Theise
ND: IARC Press; Lyon: pp. 134–46. 2000
|
|
6
|
Cammarota R, Bertolini V, Pennesi G, Bucci
EO, Gottardi O, Garlanda C, Laghi L, Barberis MC, Sessa F, Noonan
DM and Albini A: The tumor microenvironment of colorectal cancer:
Stromal TLR-4 expression as a potential prognostic marker. J Transl
Med. 8:1122010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park JH, McMillan DC, Powell AG, Richards
CH, Horgan PG, Edwards J and Roxburgh CS: Evaluation of a tumor
microenvironment-based prognostic score in primary operable
colorectal cancer. Clin Cancer Res. 21:882–888. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang X, Qiu L, Li Z, Wang XY and Yi H:
Understanding the multifaceted role of neutrophils in cancer and
autoimmune diseases. Front Immunol. 9:24562018. View Article : Google Scholar
|
|
9
|
Ullman TA and Itzkowitz SH: Intestinal
inflammation and cancer. Gastroenterology. 140:1807–1816. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rao HL, Chen JW, Li M, Xiao YB, Fu J, Zeng
YX, Cai MY and Xie D: Increased intratumoral neutrophil in
colorectal carcinomas correlates closely with malignant phenotype
and predicts patients' adverse prognosis. PLoS One. 7:e308062012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Väyrynen SA, Väyrynen JP, Klintrup K,
Mäkelä J, Karttunen TJ, Tuomisto A and Mäkinen MJ: Clinical impact
and network of determinants of tumour necrosis in colorectal
cancer. Br J Cancer. 114:1334–1342. 2016. View Article : Google Scholar
|
|
12
|
Szabó C: Mechanisms of cell necrosis. Crit
Care Med. 33 (Suppl 12):S530–S534. 2005. View Article : Google Scholar
|
|
13
|
Pollheimer MJ, Kornprat P, Lindtner RA,
Harbaum L, Schlemmer A, Rehak P and Langner C: Tumor necrosis is a
new promising prognostic factor in colorectal cancer. Hum Pathol.
41:1749–1757. 2010. View Article : Google Scholar
|
|
14
|
Klarskov L, Holck S, Bernstein I, Okkels
H, Rambech E, Baldetorp B and Nilbert M: Challenges in the
identification of MSH6-associated colorectal cancer: Rectal
location, less typical histology, and a subset with retained
mismatch repair function. Am J Surg Pathol. 35:1391–1399. 2011.
View Article : Google Scholar
|
|
15
|
Zhang X and Chen L: The recent progress of
the mechanism and regulation of tumor necrosis in colorectal
cancer. J Cancer Res Clin Oncol. 142:453–463. 2016. View Article : Google Scholar
|
|
16
|
Benson AB, Venook AP, Al-Hawary MM,
Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D,
Engstrom PF, et al: NCCN guidelines insights: Colon cancer, version
2.2018. J Natl Compr Canc Netw. 16:359–369. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hirahara T, Arigami T, Yanagita S,
Matsushita D, Uchikado Y, Kita Y, Mori S, Sasaki K, Omoto I,
Kurahara H, et al: Combined neutrophil-lymphocyte ratio and
platelet-lymphocyte ratio predicts chemotherapy response and
prognosis in patients with advanced gastric cancer. BMC Cancer.
19:6722019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jakubowska K, Koda M, Grudzińska M,
Kańczuga-Koda K and Famulski W: Monocyte-to-lymphocyte ratio as a
prognostic factor in peripheral whole blood samples of colorectal
cancer patients. World J Gastroenterol. 26:4639–4655. 2020.
View Article : Google Scholar
|
|
20
|
Nagtegaal ID, Odze RD, Klimstra D, Paradis
V, Rugge M, Schirmacher P, Washington KM, Carneiro F and Cree IA;
WHO Classification of Tumours Editorial Board, : The 2019 WHO
classification of tumours of the digestive system. Histopathology.
76:182–188. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin Q, Wei Y, Ren L, Zhong Y, Qin C, Zheng
P, Xu P, Zhu D, Ji M and Xu J: Tumor deposit is a poor prognostic
indicator in patients who underwent simultaneous resection for
synchronous colorectal liver metastases. Onco Targets Ther.
8:233–240. 2015.PubMed/NCBI
|
|
22
|
Mayadas TN, Cullere X and Lowell CA: The
multifaceted functions of neutrophils. Annu Rev Pathol. 9:181–218.
2014. View Article : Google Scholar
|
|
23
|
Harbaum L, Pollheimer MJ, Kornprat P,
Lindtner RA, Bokemeyer C and Langner C: Peritumoral eosinophils
predict recurrence in colorectal cancer. Mod Pathol. 28:403–413.
2015. View Article : Google Scholar
|
|
24
|
Jakubowska K, Koda M, Kisielewski W,
Kańczuga-Koda L and Famulski W: Prognostic significance of
inflammatory cell response in patients with colorectal cancer.
Oncol Lett. 18:783–791. 2019.
|
|
25
|
Gao JF, Arbman G, Wadhra TI, Zhang H and
Sun XF: Relationships of tumor inflammatory infiltration and
necrosis with microsatellite instability in colorectal cancers.
World J Gastroenterol. 11:2179–2183. 2005. View Article : Google Scholar
|
|
26
|
Ling YH, Chen JW, Wen SH, Huang CY, Li P,
Lu LH, Mei J, Li SH, Wei W, Cai MY and Guo RP: Tumor necrosis as a
poor prognostic predictor on postoperative survival of patients
with solitary small hepatocellular carcinoma. BMC Cancer.
20:6072020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu X, Jiang C, Zhang D, Gao M, Peng F,
Huang D, Sun Z, Ni Y, Zhang J and Yin Z: Tumor necrosis targeted
radiotherapy of non-small cell lung cancer using radioiodinated
protohypericin in a mouse model. Oncotarget. 6:26400–26410. 2015.
View Article : Google Scholar
|
|
28
|
Whalen GF: Solid tumours and wounds:
Transformed cells misunderstood as injured tissue? Lancet.
336:1489–1492. 1990. View Article : Google Scholar
|
|
29
|
Ye L, Zhang T, Kang Z, Guo G, Sun Y, Lin
K, Huang Q, Shi X, Ni Z, Ding N, et al: Tumor-infiltrating immune
cells act as a marker for prognosis in colorectal cancer. Front
Immunol. 10:23682019. View Article : Google Scholar
|
|
30
|
Galdiero MR, Bianchi P, Grizzi F, Di Caro
G, Basso G, Ponzetta A, Bonavita E, Barbagallo M, Tartari S,
Polentarutti N, et al: Occurrence and significance of
tumor-associated neutrophils in patients with colorectal cancer.
Int J Cancer. 139:446–456. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kuang DM, Zhao Q, Wu Y, Peng C, Wang J, Xu
Z, Yin XY and Zheng L: Peritumoral neutrophils link inflammatory
response to disease progression by fostering angiogenesis in
hepatocellular carcinoma. J Hepatol. 54:948–955. 2011. View Article : Google Scholar
|
|
32
|
Berry RS, Xiong MJ, Greenbaum A, Mortaji
P, Nofchissey RA, Schultz F, Martinez C, Luo L, Morris KT and
Hanson JA: High levels of tumor-associated neutrophils are
associated with improved overall survival in patients with stage II
colorectal cancer. PLoS One. 12:e01887992017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wikberg ML, Ling A, Li X, Oberg A, Edin S
and Palmqvist R: Neutrophil infiltration is a favorable prognostic
factor in early stages of colon cancer. Hum Pathol. 68:193–202.
2017. View Article : Google Scholar
|
|
34
|
Swinson DE, Jones JL, Richardson D, Cox G,
Edwards JG and O'Byrne KJ: Tumour necrosis is an independent
prognostic marker in non-small cell lung cancer: Correlation with
biological variables. Lung Cancer. 37:235–240. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schneider NI and Langner C: Prognostic
stratification of colorectal cancer patients: Current perspectives.
Cancer Manag Res. 6:291–300. 2014.PubMed/NCBI
|
|
36
|
O'Neil M and Damjanov I: Histopathology of
colorectal cancer after neoadjuvant chemoradiation therapy. Open
Pathol J. 3:91–98. 2009. View Article : Google Scholar
|
|
37
|
Bredholt G, Mannelqvist M, Stefansson IM,
Birkeland E, Bø TH, Øyan AM, Trovik J, Kalland KH, Jonassen I,
Salvesen HB, et al: Tumor necrosis is an important hallmark of
aggressive endometrial cancer and associates with hypoxia,
angiogenesis and inflammation responses. Oncotarget. 6:39676–39691.
2015. View Article : Google Scholar
|
|
38
|
Richards CH, Roxburgh CS, Anderson JH,
McKee RF, Foulis AK, Horgan PG and McMillan DC: Prognostic value of
tumour necrosis and host inflammatory responses in colorectal
cancer. Br J Surg. 99:287–294. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Komori K, Kanemitsu Y, Kimura K, Hattori
N, Sano T, Ito S, Abe T, Senda Y, Misawa K, Ito Y, et al: Tumor
necrosis in patients with TNM stage IV colorectal cancer without
residual disease (R0 Status) is associated with a poor prognosis.
Anticancer Res. 33:1099–1105. 2013.PubMed/NCBI
|
|
40
|
Jakubowska K, Kisielewski W, Kańczuga-Koda
L, Koda M and Famulski W: Diagnostic value of inflammatory cell
infiltrates, tumor stroma percentage and disease-free survival in
patients with colorectal cancer. Oncol Lett. 14:3869–3877. 2017.
View Article : Google Scholar
|