Introduction
Pharmacovigilance plays an important role in
evaluating, monitoring and preventing adverse drug reactions (ADRs)
(1). Although randomized clinical
trials are acknowledged as the gold standard to assess the efficacy
and safety of drugs (2), their
design is often based on small and homogeneous populations
monitored for short periods, making it difficult to detect
drug-related reactions (3). Thus,
the identification of suspected ADRs in clinical practice
represents the foundation of postmarketing surveillance (4). Naranjo et al created a tool to
distinguish between true ADRs and suspected ADRs (5). Drugs-induced pleural lesions are
rare. To date, more than 40 drugs have been linked to the
development of pleural lesions, and this number is destined to
increase as new drugs are introduced (6). Like levofloxacin, fluoroquinolones
(FQs), have been identified as being strongly associated with
collagen degradation, aortic dissection, aneurysm and tendinopathy,
but up until now no adverse events have been linked to pleura.
Piperacillin/tazobactam is an injectable
antibacterial product consisting of the semisynthetic antibiotic
piperacillin sodium and the β-lactamase inhibitor tazobactam
sodium. We report a case of recurrent PNX following administration
of levofloxacin and piperacillin/tazobactam in a patient with
chemotherapy-naïve retroperitoneal liposarcoma.
Case report
In January 2020, a 70-year-old Caucasian male was
diagnosed with retroperitoneal dedifferentiated liposarcoma. His
past surgical, medical, and family history were negative in
particular there was no known history of autoimmune conditions,
malignancy and PNX. Furthermore, the patient had stopped smoking a
few years before. An abdominal CT scan performed in December 2019
had revealed a mass of 190×130 mm in the left retroperitoneum,
subsequently confirmed by an MRI. In January 2020, an
F-fluorodeoxyglucose (FDG) PET/CT scan showed intense hyperfixation
of the radiopharmaceutical in the previously described (SUVmax
16.7) (Fig. 1). After a
multidisciplinary evaluation, it was decided to start neoadjuvant
chemotherapy with epirubicin 60 mg/m2 (4-h infusion) in
days 1–2 plus ifosfamide 3,000 mg/m2 (2-h infusion) on
days 1-2-3 (every 21 days). However, the staging investigations
detected signs of lung inflammation in the basal segment of the
right lower lobe (SUVmax 5.8), anterior segment of the right upper
lobe and hilum, bilaterally. The pulmonologist thus recommended a
fibrobronchoscopy study (FBS) and bronchoalveolar lavage (BAL), the
latter revealing the presence of macrophages (44%), lymphocytes
(6%) and neutrophilic granulocytes (50%). Funghi and neoplastic
cells were absent. The quantiferon test was negative.
The patient started levofloxacin 750 mg daily whilst
waiting to start chemotherapy. In February 2020 he was admitted to
the Inpatient Ward of IRCCS Istituto Romagnolo per lo Studio dei
Tumori (IRST) ‘Dino Amadori’ (Meldola, Italy) to start treatment.
Clinical examination was unremarkable and vital signs were: Blood
pressure 120/70, heart rate 82/min, oxygen saturation 98%,
temperature 36.5°C, respiratory rate 18/min. Blood tests revealed
neutrophils 6.9×109/l (range 2.0-8.0), hemoglobin 10.3
g/dl (12.0-17.0), platelets 79×109/l (140–400),
creatinine 0.71 mg/dl (0.70-1.20), e GFR 96 ml/min/1.73
m2, total bilirubin 0.24 mg/dl (<1.20), aspartate
aminotransferase 16 U/l (<40), PCR 106.8 mg/l (<5). Serology
test results were negative for hepatitis B and C. To complete the
search for potential respiratory infections, urinary antigen tests
were performed for Legionella and Pneumococcus, both
negative. The patient was in good clinical conditions but
complained of low back pain that radiated down the left thigh for
which he only took acetaminophen 500 mg + codeine 30 mg, as needed
(usually once a day for nine days). A peripherally inserted central
catheter (PICC) was inserted into the basilic vein of the right arm
(right basilic vein). The chest X-ray after the positioning of the
PICC was negative for lesions and complications (Fig. 2). Given the suspected lung
inflammation and elevated PCR (106.8), it was agreed with the
pulmonologist to add piperacillin 4 g/tazobactam 500 mg three times
a day to levofloxacin. After 5 days of levofloxacin and after three
days of piperacillin/tazobactam, PCR values increased to 141.3
mg/l, without fever, and we decided to perform a chest and
abdominal CT scan to restage the disease and check the situation in
the lungs. After 8 days of antibiotic therapy with levofloxacin and
after six days with piperacillin/tazobactam, the CT scan revealed
an increase in the size of the primary tumour (177×175×241 mm) and,
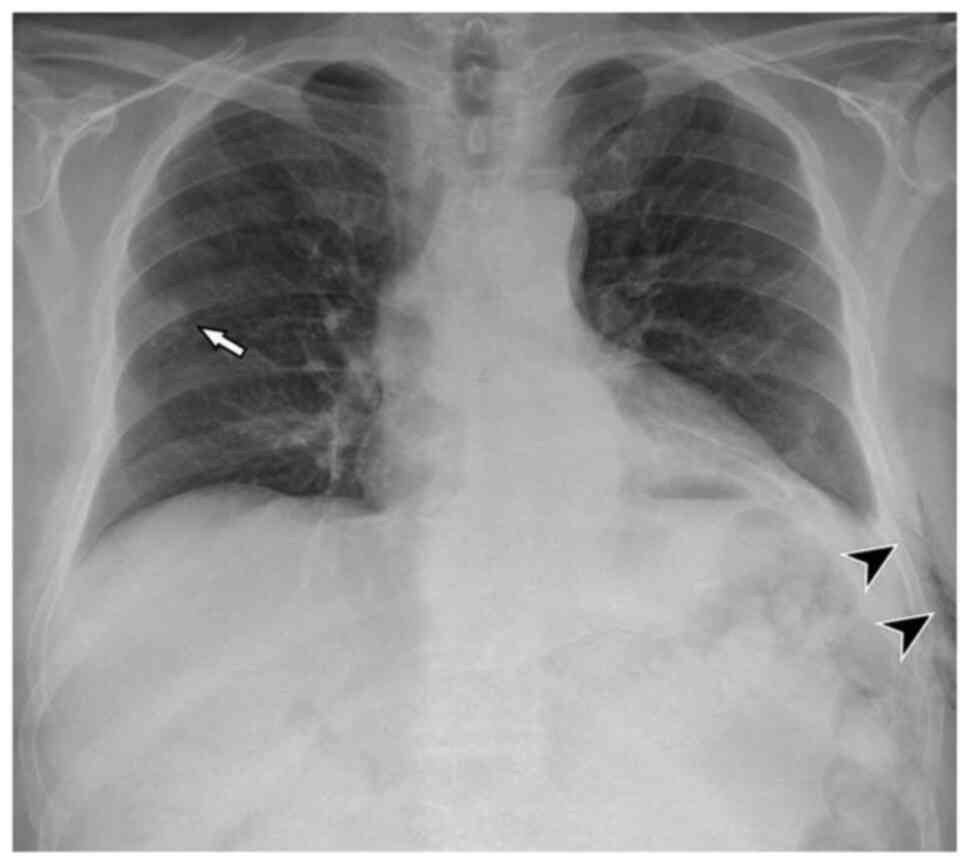
unexpectedly, the presence of an asymptomatic left apical PNX (87
mm) and a slight homolateral pleural effusion (Fig. 3). The pulmonary nodules in the
right upper lobe had increased from 6 to 12 mm and were therefore
strongly suggestive of metastasis (Fig. 4). The PCR had dropped slightly to
128.5 mg/l. PNX was initially treated with oxygen therapy 2 l/min
and rest. A third chest X-ray performed after 6 days, showed an
increase in the PNX (Fig. 5) and
the patient thus underwent left pleural drainage, with complete
resolution of the problem (Fig.
6). Levofloxacin was stopped after ten days, while
piperacillin/tazobactam was continued for a total of 17 days. Given
the presence of metastatic disease, it was decided to change
antiblastic treatment and so the patient started palliative
chemotherapy with doxorubicin 75 mg/m2 (q21). Fourteen
days after the end of the third cycle of doxorubicin (and just over
two months after the first PNX), the patient developed a massive
PNX of the right lung with deviation of cardiomediastinal
structures. We summarize the timeline that correlates the use of
drugs with the two episodes of PNX (Fig. 7). Pleural drainage was performed,
resolving the latter and improving the former. Specific treatment
was also administered for Pneumocystis jirovecii pneumonia.
Best supportive care (BSC) was implemented because of the patient's
poor performance status but death ensued four months later.
Discussion
In the area of pharmacovigilance, the most common
strategies to evaluate causality between reporting the
administration of a drug and a subsequent adverse event are
clinical judgment, probabilistic methods and algorithms. However,
there is still no universally acknowledged method to assess
drug-event causality (7,8). Clinical judgment involves subjective
individual assessments made by expert clinicians based on their
knowledge and experience in the field to assess causality.
Probabilistic methods use specific ‘features’ of drug-related
events within individual case safety reports to transform a
previous probability estimate (calculated from existing
epidemiologic information) into an estimate of drug causation
probability. Algorithms generally use a series of ‘yes/no’
questions regarding specific characteristics of a drug-related
event that have associated scores used to calculate the probability
of a cause-effect relationship.
We believe levofloxacin and/or
piperacillin/tazobactam could give rise to PNX. The
pathophysiologic mechanisms of drug-induced lung injury are still
not fully understood. It is believed that drugs may induce a direct
toxic effect, acting like a hapten or mimicking an immune
cell-activating antigen that is responsible for the damage
(6). Some authors have proposed
the most convincing hypothesis, suggesting that several classes of
antibiotics may trigger oxidative stress in mitochondria,
inhibiting their function (9,10).
PNX can present in one of three ways: primary
spontaneous pneumothorax (PSP), secondary spontaneous pneumothorax
(SSP) and traumatic pneumothorax. PSP is defined as a lack of
manifest lung disease and emphysema-like anomalies (blebs, cysts,
or bullae). Smoking is the most important environmental risk factor
for PSP. SSP is associated with an underlying pulmonary disease,
e.g. chronic obstructive pulmonary disease, asthma, cystic
fibrosis, pneumonia, pulmonary abscess, tuberculosis, malignancy,
interstitial lung disease, connective tissue disease (e.g. Marfan
syndrome, rheumatoid arthritis), pulmonary infarction, foreign body
aspiration, Birt-Hogg-Dube syndrome (11). Traumatic PNX may be a complication
of an invasive procedure such as transthoracic and transbronchial
needle biopsy, insertion of a central venous catheter, or positive
ventilation. Patients may present with a range of symptoms such as
tachycardia and dyspnea. The diagnosis of spontaneous PNX is based
on clinical conjecture subsequently confirmed by imaging.
In our case, the patient was an ex-smoker (20
pack/year up to 5 years before the PNX) and did not have a clinical
history of obstructive bronchopneumopathy. As far as we know, he
was not a user of cocaine, which can also cause PNX (12). We thus hypothesized a correlation
between the onset of spontaneous PNX and the antibiotic therapy
administered. Lung injury induced by levofloxacin, a frequently
used fluoroquinolone (FQ), is rare. FQs kill bacteria by blocking
called class II topoisomerases enzymes. They can also cause severe
collagen-associated adverse events (13). In vivo and animal research
has shown that FQs cause the upregulation of the matrix
metalloproteinase enzyme that degrades collagen, in particular
collagen I and III. Cell biology and animal studies have also
elucidated the pathophysiology by which FQs induce tendon rupture
(14,15). Collagen (90% type I and 10% type
III) makes up 70% of the dry weight of a tendon (16). Types I and III are also the
dominant forms of collagen in the aortic wall, suggesting that a
treatment that contributes to tendon rupture may also induce aortic
aneurysm (17). Collagen is an
essential component of reticular fibres in the interstitial tissue
of the liver, lungs, skin, spleen and blood vessels (18).
As far as we know, only 5 cases of
levofloxacin-induced lung injury have been reported in the
literature (19–23). In particular, Facciolongo et
al published a report (22) of
acute eosinophilic infiltrate after treatment with
piperacillin/tazobactam and levofloxacin for Legionella
pneumophila and subsequent development of PNX. It is plausible
that the PNX in Facciolongo's patient may have been a consequence
of anatomical disruption by the immune system. Only one case of
lung damage has been correlated with the use of either
ciprofloxacin or tosufloxacin (24,25).
Piperacillin is a beta-lactam bactericidal
antibiotic that inhibits the synthesis of specific
penicillin-binding proteins inside the bacterial cell wall.
Tazobactam is an irreversible inhibitor of bacterial
beta-lactamases (e.g. staphylococcal penicillinase and
extended-spectrum beta-lactamases) that increases piperacillin
activity. To the best of our knowledge, the data sheet of only one
of many piperacillin/tazobactam compounds approved by the U.S.
Regulatory Authority (26) reports
a 1.3% incidence of PNX in a nosocomial pneumonia study using a
dose regimen of 3.375 g administered every 4 h together with an
aminoglycoside.
The occurrence of PNX as a complication of the
anticancer effect of chemotherapy has been reported in a small
number of cases and in several tumour types including sarcoma with
multiple lung metastases and osteosarcoma (27). Several hypotheses have been put
forward to explain the mechanism of PNX after chemotherapy: i)
Rupture of a subpleural bulla; ii) rupture of an emphysematous
bulla in a lung partially obstructed by a tumour; and iii)
formation of fistula induced directly by tumour lysis or necrosis
following cytotoxic chemotherapy. Several case reports of PNX after
chemotherapy containing doxorubicin have been published (28,29).
A potential limit of our case report is the fact
that spontaneous PNX is also a rare indication of primary lung
cancer or metastasis. It is estimated that <1% of all cases of
spontaneous PNX are associated with malignancies, in particular
metastatic osteogenic or soft-tissue sarcomas, and documented
mainly in cytotoxic chemotherapy or radiotherapy settings (30). In our case there was a pleural
metastasis in the right lung but no evidence of disease in the left
lung. However, we cannot exclude the possibility of the presence of
micrometastasis in the left lung that was not detectable by CT
scan.
Another possible limit of our study is that
spontaneous PNX can have a genetic or familial origin (31): We did not do genetic testing or a
genetic consultation to verify a genetic cause of PNX on the other
hand, the negative personal and family history for PNX did not
require such tests. However mutations in the FLCN gene have also
been found in sporadic PNX and not only in familial cases.
Our patient had no previous clinical history of such
events and the insertion of the PICC could not have caused the PNX.
If we consider that our patient was an ex-smoker we know that
smoking is associated to bronchiolitis which has a significant
impact on the recurrence rates of PSP (32) but it is not clear in the literature
if an ex-smoker still has the same risk to develop a PNX. Recently
Kim et al (33) reported
that relative humidity, carbon monoxide, and air pressure are
trigger factors for PSP in patients who were younger (<45
years), non- or ex-smokers, and male. A possible theory is the
patient had an idiopathic PSP that then progressed to massive PNX
due to the use of antibiotics. Instead to our knowledge there is no
published literature on PubMed that correlates acetaminophen and/or
codeine to PNX. A correlation between piperacillin/tazobactam and
levofloxacin and the PNX in the left lung seems plausible given the
description of other similar PNX cases in the literature (24–26),
the fact that the patient had not yet started chemotherapy, and the
rapidity with which the PNX occurred during antibiotic therapy. The
ADR Probability Scale (Naranjo) consists of 10 questions that are
answered as either Yes, No, or ‘Do not know’. Different point
values (−1, 0, +1 or +2) are assigned to each answer. Total scores
range from −4 to +13; the reaction is considered definite if the
score is 9 or higher, probable if 5 to 8, possible if 1 to 4, and
doubtful if 0 or less. Our patient scored 3 and 4 on the Naranjo
Algorithm Assessment for adverse drug reactions (a total score
between 1 and 4 indicates a possible adverse drug reaction) in case
of levofloxacin and piperacillin/tazobactam respectively (Table I). The appearance of a second,
massive PNX in the right lung two months after the first PNX in the
contralateral lung (and 14 days after the end of the third cycle of
doxorubicin) could be related to the presence of a right lung
metastasis and to the chemotherapy administered. Furthermore, we
cannot exclude the possibility that the pulmonary inflammation from
Pneumocystis jirovecii may have facilitated the event.
 | Table I.Naranjo algorithm assessment for
adverse drug reactions (5). |
Table I.
Naranjo algorithm assessment for
adverse drug reactions (5).
| Question | Scoring | Levofloxacin |
Piperacillin/tazobactam |
|---|
| Are there previous
conclusive reports on this reaction? | Yes [+1] | 0 | 1 |
| Did the adverse
reaction occur after the suspected drug was given? | Yes [+2] | 2 | 2 |
| Did the adverse
reaction improve when the drug was discontinued, or a specific
antagonist was given? | Yes [+1] | 0 | 0 |
| Did the adverse
reaction appear when the drug was re-administered? | Do not know or not
done [0] | 0 | 0 |
| Are there other
causes that could have triggered the reaction? | No [+2] | 0 | 0 |
| Did the reaction
re-appear when a placebo was given? | Do not know or not
done [0] | 0 | 0 |
| Was the drug detected
in any body fluid in toxic concentrations? | Do not know or not
done [0] | 0 | 0 |
| Was the reaction
more severe when the dose was increased or less severe when the
dose was decreased? | Do not know or not
done [0] | 0 | 0 |
| Did the patient
have a similar reaction to the same or similar drugs in any
previous exposure? | No [0] | 0 | 0 |
| Was the adverse
event confirmed by any objective evidence? | Yes [+1] | 1 | 1 |
| Naranjo score | - | 3 | 4 |
| Adverse drug
reaction | - | Possible | Possible |
We believe that a potential correlation between
levofloxacin and/or piperacillin/tazobactam and PNX may be
underestimated and diagnosed as spontaneous PNX. Although the
reporting of adverse drug reactions via pharmacovigilance is vital
for monitoring drug safety, improvements in the quality and
availability of these documents is urgently needed. The main reason
for the underreporting of adverse drug reactions stems from the
fact that medical professionals often have a limited knowledge
about pharmacovigilance. Training and follow-up activities
organized by regulatory authorities on the basis of the needs of
healthcare operators could represent an important step towards
rectifying this situation.
In conclusion, we invite the scientific community to
take into account that spontaneous PNX could result from the use of
levofloxacin and/or piperacillin/tazobactam but definitely more
retrospective and prospective studies are needed to confirm this
conclusion and that PNX may be a complication of lung metastases
and/or chemotherapy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets generated and/or analysed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
MM drafted the manuscript and examined the patient.
API performed and reviewed the radiological imaging to confirm the
presence/absence of lung metastasis. FGS, CG and GLF assisted in
the preparation of the manuscript and examined the patient. MM, PS
and GB conceptualized and designed the study. LE and DM examined
the patient and conducted the literature research. PS, GB, MV, LE,
DM and SA examined the patient and critically reviewed the
manuscript. MM and GLF confirm the authenticity of all the raw
data. All authors have read and approved the final version of this
manuscript.
Ethics approval and consent to
participate
The study was carried out in accordance with the
principles laid down in the 1964 Declaration of Helsinki. Ethics
approval was not necessary for this work due to its design (case
report). Authors ensured compliance with EQUATOR Guidelines (CARE
Case Report Checklist).
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ADR
|
adverse drug reaction
|
|
BAL
|
bronchoalveolar lavage
|
|
FBS
|
fibrobronchoscopy study
|
|
FDG
|
F-fluorodeoxyglucose
|
|
FQ
|
fluoroquinolone
|
|
PICC
|
peripherally inserted central
catheter
|
|
PNX
|
spontaneous pneumothorax
|
References
|
1
|
Lopes P, Nunes T, Campos D, Furlong LI,
Bauer-Mehren A, Sanz F, Carrascosa MC, Mestres J, Kors J, Singh B,
et al: Gathering and exploring scientific knowledge in
pharmacovigilance. PLoS One. 8:e830162013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Silverman SL: From randomized controlled
trials to observational studies. Am J Med. 122:114–120. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alves C, Macedo AF and Marques FB: Sources
of information used by regulatory agencies on the generation of
drug safety alerts. Eur J Clin Pharmacol. 69:2083–2094. 2013.
View Article : Google Scholar
|
|
4
|
Pal SN, Duncombe C, Falzon D and Olsson S:
WHO strategy for collecting safety data in public health
programmes: Complementing spontaneous reporting systems. Drug Saf.
36:75–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Naranjo CA, Busto U, Sellers EM, Sandor P,
Ruiz I, Roberts EA, Janecek E, Domecq C and Greenblatt DJ: A method
for estimating the probability of adverse drug reactions. Clin
Pharmacol Ther. 30:239–245. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kubo K, Azuma A, Kanazawa M, Kameda H,
Kusumoto M, Genma A, Saijo Y, Sakai F, Sugiyama Y, Tatsumi K, et
al: Consensus statement for the diagnosis and treatment of
drug-induced lung injuries. Respir Investig. 51:260–277. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Agbabiaka TB, Savović J and Ernst E:
Methods for causality assessment of adverse drug reactions: A
systematic review. Drug Saf. 31:21–37. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khan LM, Al-Harthi SE, Osman AM, Sattar MA
and Ali AS: Dilemmas of the causality assessment tools in the
diagnosis of adverse drug reactions. Saudi Pharm J. 24:485–493.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kalghatgi S, Spina CS, Costello JC, Liesa
M, Morones-Ramirez JR, Slomovic S, Molina A, Shirihai OS and
Collins JJ: Bactericidal antibiotics induce mitochondrial
dysfunction and oxidative damage in Mammalian cells. Sci Transl
Med. 5:192ra852013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Zhao X, Malik M and Drlica K:
Contribution of reactive oxygen species to pathways of
quinolone-mediated bacterial cell death. J Antimicrob Chemother.
65:520–524. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Costumbrado J and Ghassemzadeh S:
Spontaneous pneumothorax. StatPearls. StatPearls Publishing;
Treasure Island, FL: 2022
|
|
12
|
Ciriaco P, Rossetti F, Carretta A,
Sant'Angelo M, Arrigoni G and Negri G: Spontaneous pneumothorax in
cocaine users. QJM. 112:519–522. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bennett AC, Bennett CL, Witherspoon BJ and
Knopf KB: An evaluation of reports of ciprofloxacin, levofloxacin,
and moxifloxacin-association neuropsychiatric toxicities, long-term
disability, and aortic aneurysms/dissections disseminated by the
food and drug administration and the European medicines agency.
Expert Opin Drug Saf. 18:1055–1063. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reviglio VE, Hakim MA, Song JK and O'Brien
TP: Effect of topical fluoroquinolones on the expression of matrix
metalloproteinases in the cornea. BMC Ophthalmol. 3:102003.
View Article : Google Scholar
|
|
15
|
Guzzardi DG, Teng G, Kang S, Geeraert PJ,
Pattar SS, Svystonyuk DA, Belke DD and Fedak PWM: Induction of
human aortic myofibroblast-mediated extracellular matrix
dysregulation: A potential mechanism of fluoroquinolone-associated
aortopathy. J Thorac Cardiovasc Surg. 157:109–119.e2. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsai WC, Hsu CC, Chen CP, Chang HN, Wong
AM, Lin MS and Pang JH: Ciprofloxacin up-regulates tendon cells to
express matrix metalloproteinase-2 with degradation of type I
collagen. J Orthop Res. 29:67–73. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Berillis P: The role of collagen in the
aorta structure. Open Circ Vasc J. 6:1–8. 2013. View Article : Google Scholar
|
|
18
|
Gelse K, Pöschl E and Aigner T:
Collagens-structure, function, and biosynthesis. Adv Drug Deliv
Rev. 55:1531–1546. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fujimori K, Shimatsu Y, Suzuki E, Arakawa
M and Gejyo F: Levofloxacin-induced eosinophilic pneumonia
complicated by bronchial asthma. Nihon Kokyuki Gakkai Zasshi.
38:385–390. 2000.(In Japanese).
|
|
20
|
Tohyama M, Arakaki N, Tamaki K and Shimoji
T: A case of drug-induced pneumonitis due to levofloxacin and kampo
medicine. Nihon Kokyuki Gakkai Zasshi. 44:951–956. 2006.(In
Japanese).
|
|
21
|
Shibusa T and Onuma K: A case of possible
drug-induced lung injury caused by levofloxacin. Nihon Kyoubu
Rinsho. 74:691–696. 2015.(In Japanese).
|
|
22
|
Facciolongo N, Menzella F, Castagnetti C,
Cavazza A, Piro R, Carbonelli C and Zucchi L: Eosinophilic
infiltrate in a patient with severe Legionella pneumonia as
a levofloxacin-related complication: A case report. J Med Case Rep.
4:3602010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hosogaya N, Toida K, Ishihara H and
Kugiyama K: A case of drug induced lung injury caused by
levofloxacin eye drops. Respir Med Case Rep. 24:12–15.
2018.PubMed/NCBI
|
|
24
|
Steiger D, Bubendorf L, Oberholzer M, Tamm
M and Leuppi JD: Ciprofloxacin-induced acute interstitial
pneumonitis. Eur Respir J. 23:172–174. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kimura N, Miyazaki E, Matsuno O, Abe Y and
Tsuda T: Drug-induced pneumonitis with eosinophilic infiltration
due to tosufloxacin tosilate. Nihon Kokyuki Gakkai Zasshi.
36:618–622. 1998.(In Japanese).
|
|
26
|
USA Food and Drug Administration,
Department of Health and Human Services, . NDA approval No.
050684/S-055S-061050750/S-016, S-020. Available from. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2012/050684s061,050750s016ltr.pdf
|
|
27
|
Graf-Deuel E and Knoblauch A: Simultaneous
bilateral spontaneous pneumothorax. Chest. 105:1142–1146. 1994.
View Article : Google Scholar
|
|
28
|
Upadya A, Amoateng-Adjepong Y and Haddad
RG: Recurrent bilateral spontaneous pneumothorax complicating
chemotherapy for metastatic sarcoma. South Med J. 96:821–823. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee CH, Park KU, Nah DY and Won KS:
Bilateral spontaneous pneumothorax during cytotoxic chemotherapy
for angiosarcoma of the scalp: A case report. J Korean Med Sci.
18:277–280. 2003. View Article : Google Scholar
|
|
30
|
Matsuura Y, Ninomiya H, Ichinose J, Nakao
M, Ishikawa Y, Okumura S and Mun M: Pathogenesis of secondary
spontaneous pneumothorax complicating osteosarcoma. Ann Thorac
Surg. 110:e81–e83. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Boone PM, Scott RM, Marciniak SJ, Henske
EP and Raby BA: The genetics of pneumothorax. Am J Respir Crit Care
Med. 199:1344–1357. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cheng YL, Huang TW, Lin CK, Lee SC, Tzao
C, Chen JC and Chang H: The impact of smoking in primary
spontaneous pneumothorax. J Thorac Cardiovasc Surg. 138:192–195.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim D, Eom SY, Shin CS, Kim YD, Kim SW and
Hong JM: The clinical effect of smoking and environmental factors
in spontaneous pneumothorax: A case-crossover study in an Inland
province. Ther Adv Respir Dis. 14:17534666209774082020. View Article : Google Scholar : PubMed/NCBI
|