In the field of antitumor therapy, small-molecule
drug therapy is a targeted form of treatment with high specificity
and few adverse reactions. Small-molecule drugs are called
‘biological missiles’ and are gradually becoming a hot spot in
research, development and clinical application. Repurposing old
small-molecule drugs is an active focus of research. Metformin is a
typical ‘old medicine with new uses’. Metformin is a
1,1-dimethylbiguanide hydrochloride isolated from the legume
Galega officinalis. It was first reported in 1957 as a
hypoglycemic drug (1). Studies
have found that metformin has weight loss, anti-aging, and
anti-cardiovascular effects (2).
In recent years, studies have reported an antitumoral effect of the
drug, which has been confirmed in various malignant tumor cells
such as liver (3), ovarian
(4) and lung cancer cells. The
present study summarizes knowledge on the anti-tumoral effects of
metformin and its mechanism in order to provide evidence for the
repurposing of this drug for tumor treatment.
Biomacromolecular drugs are considered one of the
most promising fields in drug development in this century. Common
biomacromolecular antitumor drugs include monoclonal antibodies,
recombinant protein drugs and vaccines (5). These biomacromolecular drugs have
some shortcomings in clinical use: i) Lack of selectivity for
targeted lesion tissues in contrast to normal tissues, which may
cause serious adverse reactions; ii) most drugs cannot enter cells
to exert an antitumoral effect and iii) their mechanism of action
on tissues, organs, cells and molecules within the body is
frequently uncertain, therefore it is difficult to evaluate their
efficacy (6). These factors lead
to unsatisfactory clinical therapeutic effects of biomacromolecular
drugs. By contrast, small-molecule drugs are more popular in the
clinics because they may have better therapeutic efficacy and fewer
adverse reactions. More importantly, they are relatively
inexpensive (7). However, with the
widespread application of these drugs, their shortcomings have also
became prominent: Ease in development of drug resistance and
multiple adverse reactions, resulting in reduced clinical efficacy.
For example, gefitinib and erlotinib used in the treatment of
non-small cell lung cancer (NSCLC) (8), imatinib in the treatment of chronic
myeloid leukemia (8) and
gastrointestinal stromal tumors (9) are reported to have several side
effects and develop tumor resistance.
The cellular uptake and secretion rate of metformin
largely depends on the expression of organic cation transporters
and of multidrug and toxin extrusion proteins (17). The organic cation transporter,
toxin extrusion protein and plasma membrane monoamine transporter
distribute metformin to several tissues, such as the liver, kidneys
and small intestine, and also mediate metformin metabolism and
secretion. Oral metformin is mainly absorbed through the upper
small intestine. Knockout mice for the toxin extrusion protein
transporter show a significant reduction in the clearance and
distribution of metformin, usually without an effect on tissue
distribution or pharmacological effects (10). The bioavailability of oral
metformin is 40-60% (18).
Metformin uptake is dose-dependent, but saturable (19). In clinical trials, the plasma
elimination half-life is ~5-6 h in patients with normal renal
function under repeated metformin administration (20). Approximately 90% of oral metformin
is secreted through the kidneys within 24 h. However, for patients
with advanced cancer and gastrointestinal tumors with poor
prognosis, the possibility of reaching high blood concentrations is
low (21). In a cell line model,
the half-inhibitory concentration (IC50) of metformin is
between 5 and 20 mM. In breast cancer cell lines, the
IC50 of metformin is increased and apoptosis and cell
cycle arrest induced by metformin are decreased at high glucose
levels compared with low glucose levels (22). The in vitro activation of
cellular AMP-activated protein kinase (AMPK) requires at least 1 mM
metformin in numerous cancer cell lines (23). This is equivalent to an
intracellular concentration of 131 µM, which is close to the plasma
concentration of metformin in mice receiving an intraperitoneal
injection of 145 µM. Compared with oral metformin, the
intraperitoneal injection can increase the bioavailability of
metformin in mice. In particular, the metformin concentration in
the blood, liver and kidneys increases, significantly (18). In mouse tumor models, the
biodistribution of 11C-metformin administered
intravenously shows relatively high tracer uptake in the kidneys
and liver, and relatively low tracer uptake in the blood and tumors
(24).
11C-metformin-positron emission tomography can be
combined with mutation screening of metformin sensitivity-related
genes and can be used clinically to identify metformin-sensitive
tumors. In a rat model of lipopolysaccharide-induced systemic
inflammation, the concentration of metformin varies between
different brain regions, with the highest concentration of
metformin in the pituitary gland and cerebrospinal fluid (~30
µM).
Studies have reported that metformin has an effect
on tumors of non-obese patients. Metformin can inhibit growth and
promote differentiation of ovarian (54), endometrial (55), and breast cancer (56), thereby reducing the risk and
mortality of these tumors. Jayalath et al (44) found that metformin reduced the
level of serum prostate-specific antigen and delayed the
progression of prostate cancer. In addition, metformin increases
the sensitivity of patients with colorectal cancer (especially
those with diabetes) to radiotherapy and improved the
progression-free survival of patients with NSCLC (57). Additionally, metformin inhibits
metastasis of malignant gliomas (58).
In recent years, epidemiological data have shown
that diabetes increased the risk of a number of tumors. The cancer
mortality rate in patients with diabetes is 1.41 times that of
patients without diabetes (59).
Studies have shown that metformin reduced the incidence of cancer
by 30-50%, especially pancreatic cancer (60), hepatocellular carcinoma (61) and colon cancer (62). Febbraro et al (63) conducted a retrospective cohort
study on 341 patients with ovarian cancer and found that, the
5-year progression-free survival rate of diabetic patients taking
metformin was higher than diabetic patients who did not take
metformin and non-diabetic patients., but the 5-year overall
survival rates were not significantly different. Xu et al
(64) reported that the
pathological complete response rate of patients with diabetes and
breast cancer taking metformin was 24%, which was higher than that
of patients not taking metformin (8%) and also higher than that of
patients without diabetes taking metformin (16%), suggesting that
metformin may improve the prognosis of breast cancer in patients
with diabetes. Wang et al (65) proved through randomized controlled
trials that for patients with breast cancer but without diabetes,
metformin could improve the prognosis of breast cancer by reducing
blood insulin and testosterone levels. Tseng (66) showed that metformin significantly
reduced the incidence of prostate cancer in men with type 2
diabetes mellitus. In a follow-up study of patients with newly
developed diabetes between 1998 and 2002, as of the end of 2009,
the incidence of prostate cancer in patients with diabetes taking
metformin was 239.42/100,000 per year, while the incidence of
non-users was 737.10/100,000 person-years, and the adjusted risk
odds ratio was 0.467 (95% CI, 0.446-0.488). A comparison of the
survival of 750 patients with stage 4 NSCLC aged 65-80 years old
found that 61% of the patients were already taking metformin when
they were diagnosed with lung cancer (67). The median survival time of the
metformin group was 5 months, while that of the non-users was 3
months with a statistically significant difference (67). Studies on the risk of thyroid
cancer in patients with type 2 diabetes taking metformin have also
shown that metformin could reduce the incidence of thyroid cancer
(68). The incidence rate of
patients taking metformin was 24.09/100,000 person-years, while the
incidence rate of those not taking metformin was 87.33/100,000
person-years, with an adjusted risk ratio of 0.683 (95% CI,
0.598-0.780) (68). Other studies
have also shown that metformin decreases the risk of esophageal
adenocarcinoma (69) and
endometrial cancer (70). Bowker
et al (71) conducted a
five-year follow-up of 10,309 newly-diagnosed patients with type 2
diabetes, and found that the tumor-related mortality of patients
taking metformin was significantly lower than that of patients
using sulfonylurea-type hypoglycemic drugs or insulin. Another
meta-analysis on 13,008 patients with type 2 diabetes and tumors
showed that the survival rate of tumor patients using metformin was
significantly higher than that of non-users, and cancer-related
mortality was significantly lower than that of non-users (72). Metformin is likely to inhibit tumor
progression in patients with type 2 diabetes and can reduce the
progression risk of patients with tumors and tumor-related
mortality, thereby improving patient survival.
Emerging evidence has shown that metformin can
inhibit the growth of several types of cancer cells in vitro
(73–75). At the same time, its antitumoral
effect in a mouse tumor model has also been confirmed (76). Huang et al (76) reported that PTEN knockout mice fed
with 300 mg/kg/day metformin had delayed tumorigenesis, and that
metformin effectively inhibits growth of colon polyps. In addition
to inhibiting spontaneous and carcinogen-induced tumors, metformin
could inhibit allograft tumors. Phoenix et al (77) implanted tumor cells cultured in a
high-sugar and high-insulin environment into mice, and found that
the administration of metformin at a dose 40 times higher than the
clinical dose effectively reduces the growth rate of tumors.
Anisimov et al (78) found
that metformin significantly inhibited tumor growth in HER2/neu
transgenic mice and increased their average life span. Memmott
et al (79) used the
tobacco carcinogen NNK to generate an A/J mouse lung cancer model
and found that metformin could delay tumorigenesis and reduced
tumor burden in mice. It was hypothesized that the antitumoral
effect of metformin was related to the downregulation of insulin
and IGF-1 receptor. The AKT receptor pathway was also involved.
Metformin could indirectly reduce the expression of mTOR (the
‘master factor’ of protein synthesis in cells) in the mouse lung
tissue through the AKT pathway, leading to the inhibition of tumor
cell growth.
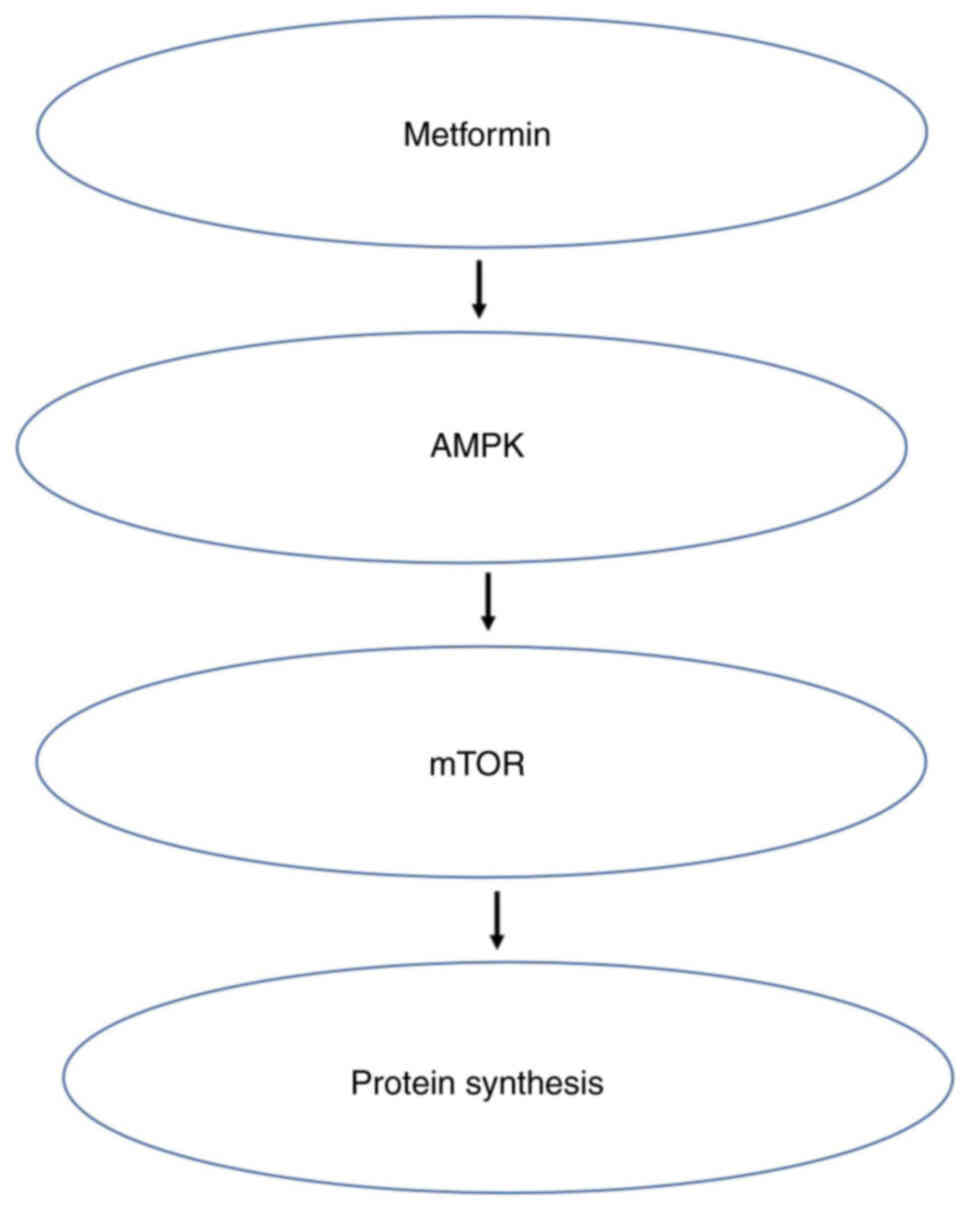
AMPK is one of the main regulators of cell energy
status and key cellular processes such as lipid and glucose
metabolism, cell growth, autophagy and apoptosis. This enzyme
maintains mitochondrial homeostasis and is activated when the ratio
of AMP or ADP to ATP increases, and compensates for energy loss by
upregulating glycolysis (80).
Tumors such as breast (81),
pancreatic (82) and prostate
cancer (83) are related to
obesity, which in turn is associated with the AMPK/mTOR metabolic
pathway (84). AMPK is the cell's
‘energy receptor’ being activated when energy is lacking and
inhibited when energy is excessive. After AMPK is activated, it can
regulate multiple pathways, including the mTOR pathway, thus
dominating intracellular protein synthesis (84). Most studies have shown that
metformin inhibits obesity-related tumors through the AMPK/mTOR
pathway (85,86). In this pathway, AMPK activation by
metformin requires an interaction between the regulatory factor
liver kinase B-1 and inositol polyphosphate multikinase (87). In addition, the lysosomal pathway
is essential for metformin to activate AMPK (88). Metformin activates AMPK through
phosphorylation, which in turn downregulates the expression of key
adipogenic transcription factor sterol regulatory elements combined
with transcription factor 1, leading to the downregulation of
lipogenic enzymes, such as Fas cell surface death receptors
(89). Metformin directly and
indirectly activates AMPK. AMPK can block proliferation and
metastasis of tumor cells by compromising mTOR. Tumor cells cannot
survive because they cannot synthesize proteins normally (90) (Fig.
3).
Metformin does not act only on AMPK to function.
Compared with normal cells, cancer cells have a higher ATP demand.
Metformin can reversibly inhibit NADH dehydrogenase (mitochondrial
complex I) activity of the respiratory chain and exert its role as
an inhibitor of oxidative phosphorylation at the molecular level,
thereby inhibiting ATP production. Metformin accumulates in the
mitochondrial matrix in the presence of a polarized mitochondrial
membrane potential. By inhibiting the effective coupling of redox
and proton transfer domains, it reversibly inhibits the NADH
dehydrogenase (mitochondrial complex I) of the respiratory chain
(91), thereby inhibiting ATP
production.
Metformin also has antitumoral effects on tumors of
non-obese patients and can cause tumor cell death. Cell death can
be divided into programmed and unprogrammed cell death, the latter
of which is also known as necrosis. According to the Clarke
morphological classification, programmed cell death can be divided
into: i) Apoptosis); ii) autophagic cell death and iii)
necrosis-like programmed cell death. Feng et al (92) found that metformin inhibited tumors
by inducing tumor cell apoptosis and autophagy, in the treatment of
esophageal cancer. Metformin-mediated apoptosis of esophageal
cancer cells was subtle, and the main mechanism of cell death was
autophagy. Additionally, it was found that AMPK was not the only
pathway involved in metformin-mediated esophageal cancer cell
apoptosis and autophagy.
Several metformin effects do not rely on AMPK
mechanisms, these are known as the pleiotropic effect of metformin
in tumor treatment (93–95). Biochemical studies have shown that
the redox shuttle enzyme mitochondrial glycerol-3-phosphate
dehydrogenase (GPDH) was another major target (96). The drug reduced the redox state of
mitochondria (in the plasma and liver) and limited the conversion
of lactic acid to glycerol and glucose, thereby reducing liver
gluconeogenesis. Mitochondrial GPDH was overexpressed in the
tumoral thyroid compared with that of normal thyroid tissues.
Thyroid cancer cells with high mitochondrial GPDH expression were
more susceptible to the inhibitory effects of metformin during
proliferation (97). Hexokinase II
is attached to the outer mitochondrial membrane and is highly
expressed in cancer cells. Cell experiments and molecular
simulation studies have shown that metformin inhibited the activity
of hexokinase II by occupying the binding site of
glucose-6-phosphate, and directly impaired glucose metabolism
(98). This induced the
dissociation of hexokinase II from mitochondria, leading to the
activation of apoptotic signals.
Other metformin antitumoral effects were presented
in a recent study that reported that metformin had the potential to
block or delay all kinds of malignant biological behaviors, such as
cell cycle arrest, sensitivity to radiotherapy and chemotherapy,
and inhibition of proliferation and differentiation of cancer stem
cells (101).
Our research group adopted a short-term preoperative
metformin neoadjuvant treatment for esophageal squamous cell
carcinoma and analyzed the changes in the proliferation and
apoptosis indices of cancer tissues of patients with metformin.
Preliminary results confirmed that cancer proliferation is
inhibited by the short-term administration of metformin before
surgery, indicating that the patient can benefit from short-term
sustained-release of metformin treatment before surgery (105). The main side effect of metformin
in patients is gastrointestinal discomfort. These results provide a
theoretical basis for further application of metformin in the
treatment of esophageal cancer.
The results of a meta-analysis showed that for
patients with type 2 diabetes, the effect on blood sugar control
was not sufficient when using metformin alone and that metformin
combined with sulfonylurea hypoglycemic agents could improve blood
sugar control and reduced cardiovascular disease mortality in
patients with type 2 diabetes (106). Recent research has shown that
metformin combined with chemotherapy drugs can significantly
decrease local recurrence in patients with diabetes and NSCLC
(107). In addition, compared
with VEGF-A inhibitors alone, metformin combined with VEGF-A
inhibitors is more effective in inhibiting tumor growth (108), indicating that the combined
application of metformin can be a promising route to increase its
antitumoral efficacy.
Metformin is a small-molecule drug with multiple
pharmacological functions. Although research on metformin as an
antitumoral drug is still in its preliminary stage, its potential
antitumoral efficacy has made people look forward to studying
metformin. Based on current research, it is hypothesized that
metformin combined with radiotherapy and chemotherapy can enhance
the clinical efficacy against tumors, thereby benefiting patients.
Further research on metformin should be carried out on the two
following aspects: i) Improvement of the molecular structure and
pharmaceutical form of metformin, such as modifying tablets into
other pharmaceutical forms to produce targeted drugs to improve
efficacy and reduce adverse reactions, or coupling certain active
groups to the chemical structure of metformin to increase its
specificity and effective concentration; ii) evaluation of the
combination of metformin with other drugs to improve efficacy. In
both cases, the ultimate goal of researchers is to fully understand
and optimize the antitumoral effect of metformin in clinical
practice and benefit patients.
Not applicable.
This study was supported by the following grants: Xianning
high-level personnel project (grant no. 3) and Hubei University of
Science and Technology developing project (grant no. 2019-21GP04)
for NZF, Hubei University of Science and Technology developing
(grant no. 2019-20X018) and pharmacy project (grant no.
2019-20YZ03) for WZ, Hubei University of Science and Technology
doctor initiation project (grant no. BK1431) and Hubei Department
of Education direction project (grant no. B201771) for WHN,
National Natural Science Foundation of China Youth Science
Foundation project (grant no. 81902937) for SYL.
Not applicable.
HZha, ZN, TS and YC designed the study. HW, DH,
HZho, ZW, FW, LW and QW wrote the manuscript. YS, ZN, XS, YR, QH
and HZha revised the manuscript. Data authentication is not
applicable. All authors have read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Nasri H and Rafieian-Kopaei M: Metformin:
Current knowledge. J Res Med Sci. 19:658–664. 2014.
|
|
2
|
Top WMC, Kooy A and Stehouwer CDA:
Metformin: A narrative review of its potential benefits for
cardiovascular disease, cancer and dementia. Pharmaceuticals
(Basel). 15:3122022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kramer JR, Natarajan Y, Dai J, Yu X, Li L,
El-Serag HB and Kanwal F: Effect of diabetes medications and
glycemic control on risk of hepatocellular cancer in patients with
nonalcoholic fatty liver disease. Hepatology. 15:322442021.
View Article : Google Scholar
|
|
4
|
Gralewska P, Gajek A, Marczak A and
Rogalska A: Metformin affects olaparib sensitivity through
induction of apoptosis in epithelial ovarian cancer cell lines. Int
J Mol Sci. 22:105572021. View Article : Google Scholar
|
|
5
|
Tsogas FK, Majerczyk D and Hart PC:
Possible role of metformin as an immune modulator in the tumor
microenvironment of ovarian cancer. Int J Mol Sci. 22:8672021.
View Article : Google Scholar
|
|
6
|
Adeyemi SA and Choonara YE: Current
advances in cell therapeutics: A biomacromolecules application
perspective. Expert Opin Drug Deliv. 12:1–18. 2022. View Article : Google Scholar
|
|
7
|
Liao M, Qin R, Huang W, Zhu HP, Peng F,
Han B and Liu B: Targeting regulated cell death (RCD) with
small-molecule compounds in triple-negative breast cancer: A
revisited perspective from molecular mechanisms to targeted
therapies. J Hematol Oncol. 15:442022. View Article : Google Scholar
|
|
8
|
Marciano O, Mehazri L, Shpungin S, Varvak
A, Zacksenhaus E and Nir U: Fer and fert govern mitochondrial
susceptibility to metformin and hypoxic stress in colon and lung
carcinoma cells. Cells. 10:972021. View Article : Google Scholar
|
|
9
|
Mohammadi S, Arefnezhad R, Danaii S and
Yousefi M: New insights into the core Hippo signaling and
biological macromolecules interactions in the biology of solid
tumors. Biofactors. 46:514–530. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim YS, Hwang KA, Go RE, Kim CW and Choi
KC: Gene therapy strategies using engineered stem cells for
treating gynecologic and breast cancer patients (Review). Oncol
Rep. 33:2107–2112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hotta K, Kiura K, Ueoka H, Tabata M,
Fujiwara K, Kozuki T, Okada T, Hisamoto A and Tanimoto M: Effect of
gefitinib (‘Iressa’, ZD1839) on brain metastases in patients with
advanced non-small-cell lung cancer. Lung Cancer. 46:255–261. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lankheet NA, Schaake EE, Burgers SA, van
Pel R, Beijnen JH, Huitema ADR and Klomp H; NEL Study Group, :
Concentrations of erlotinib in tumor tissue and plasma in
non-small-cell lung cancer patients after neoadjuvant therapy. Clin
Lung Cancer. 16:320–324. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zitvogel L, Rusakiewicz S, Routy B, Ayyoub
M and Kroemer G: Immunological off-target effects of imatinib. Nat
Rev Clin Oncol. 13:431–446. 2016. View Article : Google Scholar
|
|
14
|
Joensuu H: Treatment of inoperable
gastrointestinal stromal tumor (GIST) with imatinib (Glivec,
Gleevec). Med Klin (Munich). 15 (Suppl 1):S28–S30. 2002.
|
|
15
|
Cohen C, Pop D, Icard P, Berthet JP,
Venissac N and Mouroux J: Is there a place for thoracoscopic
enucleation of esophageal gastrointestinal stromal tumors? Thorac
Cardiovasc Surg. 67:585–588. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen YH, Yang SF, Yang CK, Tsai HD, Chen
TH, Chou MC and Hsiao YH: Metformin induces apoptosis and inhibits
migration by activating the AMPK/p53 axis and suppressing PI3K/AKT
signaling in human cervical cancer cells. Mol Med Rep. 23:882021.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng L, Lv W, Zhou Y, Lin X and Yao J:
Progress on the mechanism for aspirin's anti-tumor effects. Curr
Drug Targets. 22:105–111. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pereira PMR, Mandleywala K, Ragupathi A
and Lewis JS: Acute statin treatment improves antibody accumulation
in EGFR- and PSMA-expressing tumors. Clin Cancer Res. 26:6215–6229.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pereira A, de Souza Lima ML, da
Silva-Junior AA, Silva EDS, de Araújo Júnior RF, Martins AA, Alves
JSF, de Santana Oliveira A, Ferreira LDS, de Araújo Costa ECT, et
al: In vitro-in vivo availability of metformin hydrochloride-PLGA
nanoparticles in diabetic rats in a periodontal disease
experimental model. Pharm Biol. 59:1576–1584. 2021. View Article : Google Scholar
|
|
20
|
Horas K, van Herck U, Maier GS, Maus U,
Harrasser N, Jakob F, Weissenberger M, Arnholdt J, Holzapfel BM and
Rudert M: Does vitamin D deficiency predict tumour malignancy in
patients with bone tumours? Data from a multi-center cohort
analysis. J Bone Oncol. 25:1003292020. View Article : Google Scholar
|
|
21
|
Zeng L, Holly JM and Perks CM: Effects of
physiological levels of the green tea extract
epigallocatechin-3-gallate on breast cancer cells. Front Endocrinol
(Lausanne). 5:612014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Varghese S, Samuel SM, Varghese E, Kubatka
P and Büsselberg D: High glucose represses the anti-proliferative
and pro-apoptotic effect of metformin in triple negative breast
cancer cells. Biomolecules. 9:162019. View Article : Google Scholar
|
|
23
|
Kawakita E, Yang F, Kumagai A, Takagaki Y,
Kitada M, Yoshitomi Y, Ikeda T, Nakamura Y, Ishigaki Y, Kanasaki K
and Koya D: Metformin mitigates DPP-4 inhibitor-induced breast
cancer metastasis via suppression of mTOR signaling. Mol Cancer
Res. 19:61–73. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stage TB, Brøsen K and Christensen MM: A
comprehensive review of drug-drug interactions with metformin. Clin
Pharmacokinet. 54:811–824. 2015. View Article : Google Scholar
|
|
25
|
Bian X, Jiang L, Gan Z, Zhang L, Cai L and
Hu X: A glimepiride-metformin multidrug crystal: Synthesis, crystal
structure analysis, and physicochemical properties. Molecules.
24:37862019. View Article : Google Scholar
|
|
26
|
Scheen AJ: Clinical pharmacokinetics of
metformin. Clin Pharmacokinet. 30:359–371. 1996. View Article : Google Scholar
|
|
27
|
Palumbo PJ: Metformin: Effects on
cardiovascular risk factors in patients with non-insulin-dependent
diabetes mellitus. J Diabetes Complications. 12:110–119. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mbara KC, Mato PE, Driver C, Nzuza S,
Mkhombo NT, Gcwensa SK, Mcobothi EN and Owira PM: Metformin turns
62 in pharmacotherapy: Emergence of non-glycaemic effects and
potential novel therapeutic applications. Eur J Pharmacol.
898:1739342021. View Article : Google Scholar
|
|
29
|
Notaro ALG and Neto FTL: The use of
metformin in women with polycystic ovary syndrome: An updated
review. J Assist Reprod Genet. 14:573–579. 2022. View Article : Google Scholar
|
|
30
|
Yu Y, Yu L, Cheng N, Liu X, Fang C, Liu S
and Zhu L: Exercise alleviates the apolipoprotein A5-toll-like
receptor 4 axis impairment in mice with high-fat diet-induced
non-alcoholic steatohepatitis. Front Physiol. 12:7833412021.
View Article : Google Scholar
|
|
31
|
Kalariya NM, Shoeb M, Ansari NH,
Srivastava SK and Ramana KV: Antidiabetic drug metformin suppresses
endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci.
53:3431–3440. 2012. View Article : Google Scholar
|
|
32
|
Agostini F, Masato A, Bubacco L and
Bisaglia M: Metformin repurposing for parkinson disease therapy:
Opportunities and challenges. Int J Mol Sci. 23:3982021. View Article : Google Scholar
|
|
33
|
Ma X, Xiao W, Li H, Pang P, Xue F, Wan L,
Pei L and Yan H: Metformin restores hippocampal neurogenesis and
learning and memory via regulating gut microbiota in the obese
mouse model. Brain Behav Immun. 95:68–83. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim JW, Choe JY and Park SH: Metformin and
its therapeutic applications in autoimmune inflammatory rheumatic
disease. Korean J Intern Med. 37:13–26. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Siskind D, Friend N, Russell A, McGrath
JJ, Lim C, Patterson S, Flaws D, Stedman T, Moudgil V and Sardinha
S: CoMET: A protocol for a randomised controlled trial of
co-commencement of METformin as an adjunctive treatment to
attenuate weight gain and metabolic syndrome in patients with
schizophrenia newly commenced on clozapine. BMJ Open.
8:e0210002018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Taylor A, Siddiqui MK, Ambery P, Armisen
J, Challis BG, Haefliger C, Pearson ER, Doney ASF, Dillon JF and
Palmer CNA: Metabolic dysfunction-related liver disease as a risk
factor for cancer. BMJ Open Gastroenterol. 9:e0008172022.
View Article : Google Scholar
|
|
37
|
Sousa AP, Costa R, Alves MG, Soares R,
Baylina P and Fernandes R: The impact of metabolic syndrome and
type 2 diabetes mellitus on prostate cancer. Front Cell Dev Biol.
10:8434582022. View Article : Google Scholar
|
|
38
|
Yang J, Nishihara R, Zhang X, Ogino S and
Qian ZR: Energy sensing pathways: Bridging type 2 diabetes and
colorectal cancer? J Diabetes Complications. 31:1228–1236. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kabat GC, Kim M, Chlebowski RT, Khandekar
J, Ko MG, McTiernan A, Neuhouser ML, Parker DR, Shikany JM,
Stefanick ML, et al: A longitudinal study of the metabolic syndrome
and risk of postmenopausal breast cancer. Cancer Epidemiol
Biomarkers Prev. 18:2046–2053. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang X and Ding S: The biological and
pharmacological connections between diabetes and various types of
cancer. Pathol Res Pract. 227:1536412021. View Article : Google Scholar
|
|
41
|
Cook A and Cowan C: Adipose. StemBook.
Harvard Stem Cell Institute; Cambridge, MA: 2008
|
|
42
|
Menendez JA, Vazquez-Martin A, Ortega FJ
and Fernandez-Real JM: Fatty acid synthase: Association with
insulin resistance, type 2 diabetes, and cancer. Clin Chem.
55:425–438. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
LeRoith D, Novosyadlyy R, Gallagher EJ,
Lann D, Vijayakumar A and Yakar S: Obesity and type 2 diabetes are
associated with an increased risk of developing cancer and a worse
prognosis; epidemiological and mechanistic evidence. Exp Clin
Endocrinol Diabetes. 116 (Suppl 1):S4–S6. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jayalath VH, Ireland C, Fleshner NE,
Hamilton RJ and Jenkins DJ: The relationship between metformin and
serum prostate-specific antigen levels. Prostate. 76:1445–1453.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Park JH, Hong JY, Park YS, Kang G, Han K
and Park JO: Association of prediabetes, diabetes, and diabetes
duration with biliary tract cancer risk: A nationwide cohort study.
Metabolism. 123:1548482021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang AMY, Chu KH, Daly BF, Ruiter T, Dou
Y, Yang JCC, de Winter TJJ, Chhuor J, Wang S, Flibotte S, et al:
Effects of hyperinsulinemia on pancreatic cancer development and
the immune microenvironment revealed through single-cell
transcriptomics. Cancer Metab. 10:52022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lu L, Koo S, McPherson S, Hull MA, Rees CJ
and Sharp L: Systematic review and meta-analysis: Associations
between metabolic syndrome and colorectal neoplasia outcomes.
Colorectal Dis. 13:160922022. View Article : Google Scholar
|
|
48
|
Nayak S, Rathore V, Bharati J and Sahu KK:
Extending the ambit of SGLT2 inhibitors beyond diabetes: A review
of clinical and preclinical studies on non-diabetic kidney disease.
Expert Rev Clin Pharmacol. 14:1513–1526. 2021. View Article : Google Scholar
|
|
49
|
Lam BQ, Srivastava R, Morvant J, Shankar S
and Srivastava RK: Association of diabetes mellitus and alcohol
abuse with cancer: Molecular mechanisms and clinical significance.
Cells. 10:30772021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Srinivasan M, Arzoun H, Gk LB and
Thangaraj SR: A systematic review: Does insulin resistance affect
the risk and survival outcome of breast cancer in women? Cureus.
14:e217122022.PubMed/NCBI
|
|
51
|
Lemon LS, Orr B, Modugno F, Buckanovich
RJ, Coffman L, Edwards RP and Taylor S: Metformin and survival: Is
there benefit in a cohort limited to diabetic women with
endometrial, breast, or ovarian cancer? Gynecol Oncol. 165:60–66.
2022. View Article : Google Scholar
|
|
52
|
Choi E, Kikuchi S, Gao H, Brodzik K,
Nassour I, Yopp A, Singal AG, Zhu H and Yu H: Mitotic regulators
and the SHP2-MAPK pathway promote IR endocytosis and feedback
regulation of insulin signaling. Nat Commun. 10:14732019.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang L, Zhang B, Zheng W, Kang M, Chen Q,
Qin W, Li C, Zhang Y, Shao Y and Wu Y: Exosomes derived from
pancreatic cancer cells induce insulin resistance in C2C12 myotube
cells through the PI3K/Akt/FoxO1 pathway. Sci Rep. 7:53842017.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yang X, Huang M, Zhang Q, Chen J, Li J,
Han Q, Zhang L, Li J, Liu S, Ma Y, et al: Metformin antagonizes
ovarian cancer cells malignancy through MSLN mediated IL-6/STAT3
signaling. Cell Transplant. 30:96368972110278192021. View Article : Google Scholar
|
|
55
|
Rho SB, Byun HJ, Kim BR and Lee CH:
Knockdown of LKB1 sensitizes endometrial cancer cells via AMPK
activation. Biomol Ther. 29:650–657. 2021. View Article : Google Scholar
|
|
56
|
Cheng T, Wang C, Lu Q, Cao Y, Yu W, Li W,
Liu B, Gao X, Lü J and Pan X: Metformin inhibits the
tumor-promoting effect of low-dose resveratrol, and enhances the
anti-tumor activity of high-dose resveratrol by increasing its
reducibility in triple negative breast cancer. Free Radic Biol Med.
180:108–120. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Eze C, Belka C and Manapov F: Forging a
path for metformin use in inoperable locally advanced non-small
cell lung cancer. JAMA Oncol. 7:1341–1342. 2021. View Article : Google Scholar
|
|
58
|
Mazurek M, Litak J, Kamieniak P, Kulesza
B, Jonak K, Baj J and Grochowski C: Metformin as potential therapy
for high-grade glioma. Cancers (Basel). 12:2102020. View Article : Google Scholar
|
|
59
|
Zhao A, Xiao H, Zhu Y, Liu S, Zhang S,
Yang Z, Du L, Li X, Niu X, Wang C, et al: Omentin-1: A newly
discovered warrior against metabolic related diseases. Expert Opin
Ther Targets. 10:275–289. 2022. View Article : Google Scholar
|
|
60
|
Eibl G and Rozengurt E: Metformin: Review
of epidemiology and mechanisms of action in pancreatic cancer.
Cancer Metastasis Rev. 40:865–878. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang CP, Kuhn J, Shah DP, Schmidt S, Lam
YWF, MacCarthy D, Tenner L and Ramirez AG: Metformin modifies
disparity in hepatocellular carcinoma incidence in men with type 2
diabetes but without chronic liver diseases. Cancer Med.
8:3206–3215. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Erkinantti S, Marttila M, Sund R, Arffman
M, Urpilainen E, Puistola U, Hautakoski A, Karihtala P, Läärä E and
Jukkola A: Association of metformin, other antidiabetic
medications, and statins with incidence of colon cancer in patients
with type 2 diabetes. Clin Colorectal Cancer. 20:e113–e119. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Febbraro T, Lengyel E and Romero IL: Old
drug, new trick: Repurposing metformin for gynecologic cancers?
Gynecol Oncol. 135:614–621. 2014. View Article : Google Scholar
|
|
64
|
Xu H, Chen K, Jia X, Tian Y, Dai Y, Li D,
Xie J, Tao M and Mao Y: Metformin use is associated with better
survival of breast cancer patients with diabetes: A meta-analysis.
Oncologist. 20:1236–1244. 2015. View Article : Google Scholar
|
|
65
|
Wang Q, Ma X, Long J, Du X, Pan B and Mao
H: Metformin and survival of women with breast cancer: A
meta-analysis of randomized controlled trials. J Clin Pharm Ther.
16:135002021.
|
|
66
|
Tseng CH: Metformin significantly reduces
incident prostate cancer risk in Taiwanese men with type 2 diabetes
mellitus. Eur J Cancer. 50:2831–2837. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Brancher S, Støer NC, Weiderpass E,
Damhuis RAM, Johannesen TB, Botteri E and Strand TE: Metformin use
and lung cancer survival: A population-based study in Norway. Br J
Cancer. 124:1018–1025. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Tseng CH: Metformin reduces thyroid cancer
risk in taiwanese patients with type 2 diabetes. PLoS One.
9:e1098522014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Agrawal S, Patel P, Agrawal A, Makhijani
N, Markert R and Deidrich W: Metformin use and the risk of
esophageal cancer in Barrett esophagus. South Med J. 107:774–779.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Becker C, Jick SS, Meier CR and Bodmer M:
Metformin and the risk of endometrial cancer: A case-control
analysis. Gynecol Oncol. 129:565–569. 2013. View Article : Google Scholar
|
|
71
|
Bowker SL, Majumdar SR, Veugelers P and
Johnson JA: Increased cancer-related mortality for patients with
type 2 diabetes who use sulfonylureas or insulin. Diabetes Care.
29:254–258. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Gandini S, Puntoni M, Heckman-Stoddard BM,
Dunn BK, Ford L, DeCensi A and Szabo E: Metformin and cancer risk
and mortality: A systematic review and meta-analysis taking into
account biases and confounders. Cancer Prev Res (Phila). 7:867–885.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wu Z, Wu L, Zou L, Wang M and Liu X:
Metformin induces myeloma cells necrosis and apoptosis and it is
considered for therapeutic use. J Chemother. 15:1–11. 2022.
View Article : Google Scholar
|
|
74
|
Teixeira SF, Guimarães IDS, Madeira KP,
Daltoé RD, Silva IV and Rangel LB: Metformin synergistically
enhances antiproliferative effects of cisplatin and etoposide in
NCI-H460 human lung cancer cells. J Bras Pneumol. 39:644–649.
2013.(In English, Portuguese). View Article : Google Scholar
|
|
75
|
Lin F, Yan W, Wen T and Wu GY: Metformin
induces apoptosis in hepatocellular carcinoma Huh-7 cells in vitro
and its mechanism. Zhonghua Zhong Liu Za Zhi. 35:742–746. 2013.(In
Chinese).
|
|
76
|
Huang X, Hong X, Wang J, Sun T, Yu T, Yu
Y, Fang J and Xiong H: Metformin elicits antitumour effect by
modulation of the gut microbiota and rescues Fusobacterium
nucleatum-induced colorectal tumourigenesis. EBioMedicine.
61:1030372020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Phoenix KN, Vumbaca F and Claffey KP:
Therapeutic metformin/AMPK activation promotes the angiogenic
phenotype in the ERalpha negative MDA-MB-435 breast cancer model.
Breast Cancer Res Treat. 113:101–111. 2009. View Article : Google Scholar
|
|
78
|
Anisimov VN, Egormin PA, Piskunova TS,
Popovich IG, Tyndyk ML, Yurova MN, Zabezhinski MA, Anikin IV,
Karkach AS and Romanyukha AA: Metformin extends life span of
HER-2/neu transgenic mice and in combination with melatonin
inhibits growth of transplantable tumors in vivo. Cell Cycle.
9:188–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Memmott RM, Mercado JR, Maier CR, Kawabata
S, Fox SD and Dennis PA: Metformin prevents tobacco
carcinogen-induced lung tumorigenesis. Cancer Prev Res (Phila).
3:1066–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhang S, Lachance BB, Mattson MP and Jia
X: Glucose metabolic crosstalk and regulation in brain function and
diseases. Prog Neurobiol. 204:1020892021. View Article : Google Scholar
|
|
81
|
Dai JZ, Wang YJ, Chen CH, Tsai IL, Chao YC
and Lin CW: YAP dictates mitochondrial redox homeostasis to
facilitate obesity-associated breast cancer progression. Adv Sci.
18:e21036872022. View Article : Google Scholar
|
|
82
|
Fleming JB, Gonzalez RJ, Petzel MQ, Lin E,
Morris JS, Gomez H, Lee JE, Crane CH, Pisters PWT and Evans DB:
Influence of obesity on cancer-related outcomes after
pancreatectomy to treat pancreatic adenocarcinoma. Arch Surg.
144:216–221. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Purcell SA, Oliveira CLP, Mackenzie M,
Robson P, Lewis J and Prado CM: Body composition and prostate
cancer risk: A systematic review of observational studies. Adv
Nutr. 16:1532021. View Article : Google Scholar
|
|
84
|
Gong L, Wang Z and Zhang Z: Sestrin2 as a
potential target for regulating metabolic-related diseases. Front
Endocrinol (Lausanne). 12:7510202021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zell JA, McLaren CE, Morgan TR, Lawson MJ,
Rezk S, Albers CG, Chen WP, Carmichael JC, Chung J, Richmond E, et
al: A phase IIa trial of metformin for colorectal cancer risk
reduction among individuals with history of colorectal adenomas and
elevated body mass index. Cancer Prev Res (Phila). 13:203–212.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Saini N and Yang X: Metformin as an
anti-cancer agent: Actions and mechanisms targeting cancer stem
cells. Acta Biochim Biophys Sin (Shanghai). 50:133–143. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Bang S, Chen Y, Ahima RS and Kim SF:
Convergence of IPMK and LKB1-AMPK signaling pathways on metformin
action. Mol Endocrinol. 28:1186–1193. 2014. View Article : Google Scholar
|
|
88
|
Najafov A, Luu HS, Mookhtiar AK, Mifflin
L, Xia HG, Amin PP, Ordureau A, Wang H and Yuan J: RIPK1 promotes
energy sensing by the mTORC1 pathway. Mol Cell. 81:370–385. 2021.
View Article : Google Scholar
|
|
89
|
Kim BH, Han S, Lee H, Park CH, Chung YM,
Shin K, Lee HG and Ye SK: Metformin enhances the anti-adipogenic
effects of atorvastatin via modulation of STAT3 and TGF-β/Smad3
signaling. Biochem Biophys Res Commun. 456:173–178. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Xu S, Lam SK, Cheng PN and Ho JC:
Recombinant human arginase induces apoptosis through oxidative
stress and cell cycle arrest in small cell lung cancer. Cancer Sci.
109:3471–3482. 2018. View Article : Google Scholar
|
|
91
|
Cameron AR, Logie L, Patel K, Erhardt S,
Bacon S, Middleton P, Harthill J, Forteath C, Coats JT, Kerr C, et
al: Metformin selectively targets redox control of complex I energy
transduction. Redox Biol. 14:187–197. 2018. View Article : Google Scholar
|
|
92
|
Feng Y, Ke C, Tang Q, Dong H, Zheng X, Lin
W, Ke J, Huang J, Yeung SCJ and Zhang H: Metformin promotes
autophagy and apoptosis in esophageal squamous cell carcinoma by
downregulating Stat3 signaling. Cell Death Dis. 5:e10882014.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Janjetovic K, Vucicevic L, Misirkic M,
Vilimanovich U, Tovilovic G, Zogovic N, Nikolic Z, Jovanovic S,
Bumbasirevic V, Trajkovic V and Harhaji-Trajkovic L: Metformin
reduces cisplatin-mediated apoptotic death of cancer cells through
AMPK-independent activation of Akt. Eur J Pharmacol. 651:41–50.
2011. View Article : Google Scholar
|
|
94
|
Athreya AP, Kalari KR, Cairns J, Gaglio
AJ, Wills QF, Niu N, Weinshilboum R, Iyer RK and Wang L:
Model-based unsupervised learning informs metformin-induced
cell-migration inhibition through an AMPK-independent mechanism in
breast cancer. Oncotarget. 8:27199–27215. 2017. View Article : Google Scholar
|
|
95
|
Yenmiş G, Beşli N, Saraç EY, Emre FS,
Şenol K and Kanıgür G: Metformin promotes apoptosis in primary
breast cancer cells by downregulation of cyclin D1 and upregulation
of P53 through an AMPK-alpha independent mechanism. Turk J Med Sci.
51:826–834. 2021. View Article : Google Scholar
|
|
96
|
Calza G, Nyberg E, Mäkinen M, Soliymani R,
Cascone A, Lindholm D, Barborini E, Baumann M, Lalowski M and
Eriksson O: Lactate-induced glucose output is unchanged by
metformin at a therapeutic concentration-a mass spectrometry
imaging study of the perfused rat liver. Front Pharmacol.
9:1412018. View Article : Google Scholar
|
|
97
|
Thakur S, Daley B, Gaskins K, Vasko VV,
Boufraqech M, Patel D, Sourbier C, Reece J, Cheng SY, Kebebew E, et
al: Metformin targets mitochondrial glycerophosphate dehydrogenase
to control rate of oxidative phosphorylation and growth of thyroid
cancer in vitro and in vivo. Clin Cancer Res. 24:4030–4043. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Salani B, Marini C, Rio AD, Ravera S,
Massollo M, Orengo AM, Amaro A, Passalacqua M, Maffioli S, Pfeffer
U, et al: Metformin impairs glucose consumption and survival in
Calu-1 cells by direct inhibition of hexokinase-II. Sci Rep.
3:20702013. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Jiang Y, Xu D, Song H, Qiu B, Tian D, Li
Z, Ji Y and Wang J: Inflammation and nutrition-based biomarkers in
the prognosis of oesophageal cancer: A systematic review and
meta-analysis. BMJ Open. 11:e0483242021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
El-Tanani M, Al Khatib AO, Aladwan SM,
Abuelhana A, McCarron PA and Tambuwala MM: Importance of STAT3
signalling in cancer, metastasis and therapeutic interventions.
Cell Signal. 92:1102752022. View Article : Google Scholar
|
|
101
|
Zahra MH, Afify SM, Hassan G, Nawara HM,
Kumon K, Seno A and Seno M: Metformin suppresses self-renewal and
stemness of cancer stem cell models derived from pluripotent stem
cells. Cell Biochem Funct. 39:896–907. 2021. View Article : Google Scholar
|
|
102
|
Higurashi T, Hosono K, Takahashi H, Komiya
Y, Umezawa S, Sakai E, Uchiyama T, Taniguchi L, Hata Y, Uchiyama S,
et al: Metformin for chemoprevention of metachronous colorectal
adenoma or polyps in post-polypectomy patients without diabetes: A
multicentre double-blind, placebo-controlled, randomised phase 3
trial. Lancet Oncol. 17:475–483. 2016. View Article : Google Scholar
|
|
103
|
Arrieta O, Zatarain-Barrón ZL, Turcott JG,
Barrón F, Yendamuri S, Cardona AF and Rosell R: Association of BMI
with benefit of metformin plus epidermal growth factor
receptor-tyrosine kinase inhibitors in patients with advanced lung
adenocarcinoma: A secondary analysis of a phase 2 randomized
clinical trial. JAMA Oncol. 8:477–479. 2022. View Article : Google Scholar
|
|
104
|
Pusceddu S, Vernieri C, Di Maio M, Prinzi
N, Torchio M, Corti F, Coppa J, Buzzoni R, Bartolomeo MD, Milione
M, et al: Impact of diabetes and metformin use on enteropancreatic
neuroendocrine tumors: Post hoc analysis of the CLARINET study.
Cancers (Basel). 14:692021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Wang S, Lin Y, Xiong X, Wang L, Guo Y,
Chen Y, Chen S, Wang G, Lin P, Chen H, et al: Low-dose metformin
reprograms the tumor immune microenvironment in human esophageal
cancer: Results of a phase II clinical trial. Clin Cancer Res.
26:4921–4932. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Maruthur NM, Tseng E, Hutfless S, Wilson
LM, Suarez-Cuervo C, Berger Z, Chu Y, Iyoha E, Segal JB and Bolen
S: Diabetes medications as monotherapy or metformin-based
combination therapy for type 2 diabetes: A systematic review and
meta-analysis. Ann Intern Med. 164:740–751. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Wink KC, Belderbos JS, Dieleman EM, Rossi
M, Rasch CRN, Damhuis RAM, Houben RMA and Troost EGC: Improved
progression free survival for patients with diabetes and locally
advanced non-small cell lung cancer (NSCLC) using metformin during
concurrent chemoradiotherapy. Radiother Oncol. 118:453–459. 2016.
View Article : Google Scholar
|
|
108
|
Martin MJ, Hayward R, Viros A and Marais
R: Metformin accelerates the growth of BRAF V600E-driven melanoma
by upregulating VEGF-A. Cancer Discov. 2:344–355. 2012. View Article : Google Scholar : PubMed/NCBI
|