Introduction
Parotid gland tumors consist of 70–80% of salivary
gland tumors, 2–3% of head and neck tumors, and 0.6% of all tumors
(1). Salivary gland tumors vary
widely and are classified into 20 histologic types for malignant
tumors and 11 for benign tumors, according to the 2017
classification of the World Health Organization (WHO) (1). Pleomorphic adenoma is the most common
type and Warthin tumor is the second of benign parotid tumors.
Mucoepidermoid carcinoma is the most common malignancy of the
salivary glands. The etiology and pathogenesis of these tumors have
not been understood. Due to the variety of parotid gland tumors, a
successful outcome of parotid surgery requires careful pre- and
perioperative planning and decision making, as inadequate surgery
may lead to recurrence of not only parotid carcinomas but also
benign parotid tumors such as pleomorphic adenomas. Ultrasound
(US)-guided fine-needle aspiration cytology (FNAC) is the most
reliable preoperative method for the evaluating parotid gland
tumors (2). Recent reports
indicate that liquid-based cytology (LBC) in addition to
conventional smear (CS) in FNAC is useful for the diagnosis of
salivary gland tumors (3). With
regard to the surgery for parotid carcinomas, several factors must
be considered, including the extent of resection (i.e., extended
total, total, or superficial parotidectomy), handling of facial
nerves (i.e., preservation or resection), extent of neck dissection
(i.e., total, selective or prophylactic). Intraoperative facial
nerve monitoring (FNM) was recently reported as helpful in
detecting and preserving the facial nerve (4). Whether postoperative radiotherapy to
parotid carcinoma contributes to improvement of survival has not
been fully evaluated. An independent prognostic factor of parotid
carcinoma except for TNM stage has not been defined yet. We
examined the incidence of different types of parotid gland tumors
and evaluated all parotid gland tumors diagnosed at our
institution, focusing on diagnostic challenges and preoperative
evaluation. This work provides recommendations to better delineate
the management of these tumors.
Patients and methods
Patients
A retrospective study was conducted in 140 patients
(male: 77; female: 63) with parotid gland tumors who underwent
parotidectomy from April 2007 to December 2021 at Hokuto Hospital
Department of Otolaryngology-Head and Neck Surgery. Patient median
age was 60.5 years (range, 18 to 90 years). Data collected included
cigarette smoking status (defined as pack years [packs/day ×
years]), symptoms, tumor location, maximum tumor size measured by
US, preoperative FNAC diagnosis, operation time, histology, and
postoperative complications. With regard to parotid carcinomas,
staging, treatment, and prognosis were also analyzed. Patients with
incomplete clinical and histologic data and malignant lymphoma were
excluded in this study.
Fine-needle aspiration cytology
Both CS and LBC were utilized (5). Briefly, for US-guided FNAC, tumors
were aspirated by 2 experienced otolaryngologists using a 21-gauge
needle attached to a 20-ml disposable plastic syringe and aspirator
developed by Chiba University. Aspirates were immediately processed
on slides and then fixed in 95% ethanol for Papanicolaou staining
and dried for Giemsa staining. The remaining aspirate in the
syringe and needle were rinsed into a vial with 10 ml of CytoRich
Red solution (Becton Dickinson, Franklin Lakes, NJ). Fluid cytology
specimens were processed using the BD SurePath hand method (BD
Biosciences, Franklin Lakes, NJ) and routinely stained with
Papanicolaou solution. Tumors were cytologically classified by 2
experienced cytotechnologists into five categories: non-diagnostic,
benign, indeterminate, suspicious for malignancy, or malignant.
FNAC diagnosis of suspicious for malignancy and malignant and
postoperative histologic diagnosis of parotid carcinoma were
categorized as positive. Other benign results were categorized as
negative. The sensitivity, specificity, and positive and negative
predictive values for detecting malignant lesions by FNAC were
estimated based on the histology results, excluding non-diagnostic
and indeterminate results. Accuracy was estimated based on true
positives and negatives/total number of cases, including
non-diagnostic and indeterminate cases.
Surgical treatments
Surgery for benign parotid tumors was performed by
partial superficial parotidectomy (6). Briefly, after a preauricular to
postauricular (modified Blair incision) S-shaped incision, the
trunk of the facial nerve was identified by intraoperative FNM with
1.0 mA of stimulation (NIM-Response 3.0 system, Medtronic Inc.,
Jacksonville, FL). The tumor was resected with a 1-cm margin with
following the branches of the facial nerve (7,8). For
patients who were diagnosed with malignant tumors by preoperative
FNAC, total parotidectomy was performed. Otherwise, extended total
parotidectomy, defined as resection of adjunct structures such as
either skin, the sternocleidomastoid muscle, the masseter muscle or
the external meatus in addition to total parotidectomy, was
performed (9). For patients with
clinical or imaging evidence of nodal disease (cN+), total neck
dissection (Level I to V) was performed. For patients without lymph
node metastasis (cN0), selective neck dissection of the area from
Level II to I, II and III was performed; otherwise, no neck
dissection was not performed, at the consideration of imaging and
FNAC results. For patients with tumor invasion of the facial nerve
determined intraoperatively, the facial nerve was resected and
transplanted. Skin defects were reconstructed with a free flap.
Histologic diagnosis was assessed by two experienced pathologists
and classified based on the WHO Classification of Head and Neck
Tumours-2017 (1).
Statistical analysis
Associations between groups were determined using
Fisher's exact test for categorical variables, and using the
Kruskal-Wallis test and Steel-Dwass test for continuous variables.
Temporary facial nerve palsy was defined as the complete recovery
of facial palsy within 6 months after surgery. Persistent facial
palsy was defined as any facial palsy lasting more than 6 months.
Time was defined as the period starting from the date of diagnosis
to the date of disease relapse or that of last follow-up visit for
Disease-free survival (DFS) or to the date of death by any cause
for overall survival (OS). DFS and OS rates were calculated using
the Kaplan-Meier method and compared using the log-rank test. For
determination of factors related to DFS and OS, a Cox proportional
hazards model was used. The final results of these analyses are
hazard ratios (HR), their 95% confidence intervals (CI) and
P-value. A p-value less than 0.05 was considered indicative of
statistical significance. BellCurve for Excel (Social Survey
Research Information, Tokyo, Japan) statistical software was used
for all analyses.
Results
Histologic classification
Histologic classification of the 140 patients with
parotid gland tumors included 118 cases (84.3%) of benign tumors,
with 63 (45%) of pleomorphic adenomas, 43 (30.7%) of Warthin
tumors, 6 (4.3%) of myoepitheliomas, and 2 (1.4%) of basal cell
adenomas (Table I). A total of 22
of the 140 patients (15.7%) had parotid carcinoma, of which 10
(7.1%) were of high-grade, 2 (1.4%) of intermediate-grade, and 10
(7.1%) of low-grade in terms of histologic malignancy.
 | Table I.Histologic classification of 140
patients with parotid gland tumors. |
Table I.
Histologic classification of 140
patients with parotid gland tumors.
| Tumor type | No. (%) |
|---|
| Benign tumors | 118 (84.3) |
|
Pleomorphic adenoma | 63 (45) |
| Warthin
tumor | 43 (30.7) |
|
Myoepithelioma | 6 (4.3) |
| Basal
cell adenoma | 2 (1.4) |
|
Others | 4 (2.9) |
| Parotid
carcinomas | 22 (15.7) |
| Low
grade | 10 (7.1) |
|
Mucoepidermoid
ca. | 3 (2.1) |
|
Ca. ex pleomorphic
adenoma | 3 (2.1) |
|
Epithelial
myoepithelial ca. | 2 (1.4) |
|
Mammary analogue
secretory ca. | 1 (0.7) |
|
Intraductal
ca. | 1 (0.7) |
|
Intermediate grade | 2 (1.4) |
|
Adenoid cystic
ca. | 1 (0.7) |
|
Lymphoepithelial
ca. | 1 (0.7) |
| High
grade | 10 (7.1) |
|
Squamous cell
ca. | 3 (2.1) |
|
Adenocarcinoma
NOS | 3 (2.1) |
|
Mucoepidermoid
ca. | 2 (1.4) |
|
Salivary duct
ca. | 2 (1.4) |
Clinicopathologic features
Patients with parotid gland tumors were categorized
into three groups based on clinical features: parotid carcinomas
including all histology, pleomorphic adenoma, and Warthin tumor
(Table II). In terms of age
distribution, patients with pleomorphic adenoma were significantly
younger than those with Warthin tumor (P<0.001). Pleomorphic
adenoma was more frequent among females than parotid carcinoma
(P=0.003) or Warthin tumor (P<0.001). Pack years was
significantly higher in patients with Warthin tumor than patients
with parotid carcinomas (P=0.011) or pleomorphic adenoma
(P<0.001). Pain was significantly more frequent in patients with
parotid carcinoma than in those with pleomorphic adenoma (P=0.001)
or Warthin tumor (P=0.006). Facial nerve palsy was present in 2
patients with parotid carcinomas. There were no significant
differences among the three groups in terms of the location of the
tumor (right or left side; superficial or deep lobe), and maximum
tumor size. Operation time was significantly longer in patients
with parotid carcinoma than in those with pleomorphic adenoma
(P<0.001) or Warthin tumor (P=0.002). Transient facial nerve
palsy occurred in 10 (16%) patients with pleomorphic adenoma and 9
(21%) patients with Warthin tumor. Persistent postoperative facial
nerve palsy was significantly more frequent in patients with
parotid carcinoma than in those with pleomorphic adenoma
(P<0.001) or Warthin tumor (P=0.003).
 | Table II.Clinicopathologic features of parotid
gland tumor patients categorized according to parotid carcinoma,
pleomorphic adenoma, or Warthin tumor. |
Table II.
Clinicopathologic features of parotid
gland tumor patients categorized according to parotid carcinoma,
pleomorphic adenoma, or Warthin tumor.
| Clinicopathologic
factor | Parotid carcinoma
(n=22) | Pleomorphic adenoma
(n=63) | Warthin tumor
(n=43) | P-value |
|---|
| Age,
yearsa | 61 (44–77) | 54 (42–68) | 64 (59–70) | PA vs. WT
<0.001 |
| Gender,
male:femaleb | 16 (73%):6
(27%) | 23 (37%):40
(63%) | 33 (77%):10
(23%) | PC vs. PA 0.003; PA
vs. WT <0.001 |
| Smoking, pack
yearsa,c | 6 (0–25) | 0 (0–20) | 38 (18–50) | PC vs. WT 0.011; PA
vs. WT <0.001 |
|
Symptomsb |
|
|
|
|
|
Pain | 9 (41%) | 5 (8%) | 4 (9.3%) | PC vs. PA 0.001; PC
vs. WT 0.006 |
| Facial
nerve palsy | 2 (9%) | 0 | 0 |
|
|
Locationb |
|
|
|
|
| Side,
right:left | 11 (50%):11
(50%) | 33 (52):30 (48%) | 19 (44%):24
(56%) |
|
| Lobe,
superficial:deep:uncertain | 18 (82%):1 (4%):3
(14%) | 48 (76%):15
(24%) | 37 (86%):6
(14%) |
|
| Maximum tumor size,
mma | 25 (19–32) | 23(18–30) | 32 (23–40) |
|
| Operation time,
mina | 119 (74–160) | 70 (58–85) | 71 (57–97) | PC vs. PA
<0.001; PC vs. WT 0.002 |
| Postoperative
complicationsb |
|
|
|
|
|
Postoperative bleeding | 0 | 2 (3%) | 0 |
|
|
Transient facial nerve
palsy | 3 (14%) | 10 (16%) | 9 (21%) |
|
|
Persistent facial nerve
palsy | 5 (23%) | 0 | 0 | PC vs. PA
<0.001; PC vs. WT <0.003 |
| Frey
syndrome | 1 (2%) | 0 | 0 |
|
|
Recurrence | 4 (18%) | 1 (2%) | 0 |
|
Results of fine-needle aspiration
cytology
FNAC was non-diagnostic for only 6 (4.3%) of 140
patients. The sensitivity, specificity, positive predictive value,
and negative predictive value of FNAC for malignant tumors were 70,
99, 93.3, and 94.4%, respectively (Table III). The accuracy of FNAC for all
parotid gland tumors was 82.9%. Histologic presumption by FNAC
corresponded with the results of histologic analysis of surgical
specimens in 53 (84.1%) of 63 patients with pleomorphic adenoma and
37 (86%) of 43 patients with Warthin tumor.
 | Table III.Correlation between FNAC and
histologic diagnosis among 140 patients with parotid gland
tumors. |
Table III.
Correlation between FNAC and
histologic diagnosis among 140 patients with parotid gland
tumors.
|
| Histologic
diagnosis |
|---|
|
|
|
|---|
|
| Parotid carcinoma
(n=22) | Benign tumors
(n=118) |
|---|
|
|
|
|
|---|
| FNAC | High grade
(n=10) | Intermediate grade
(n=2) | Low grade
(n=10) | Pleomorphic adenoma
(n=63) | Warthin tumor
(n=43) | Myo-epithelioma
(n=6) | Others (n=6) |
|---|
| Non-diagnostic
(n=6) |
|
|
|
| 5 |
| 1 |
| Malignant
(n=10) | 7 | 1 | 2 |
|
|
|
|
| Suspicious for
malignancy (n=5) | 2 |
| 2 | 1 |
|
|
|
| Indeterminate
(n=11) |
| 1 | 1 | 3 | 1 | 4 | 1 |
| Benign (n=108) | 1 |
| 5 | 59 | 37 | 2 | 4 |
|
Pleomorphic adenoma
(n=57) |
|
| 3 | 53 |
| 1 |
|
| Warthin
tumor (n=40) | 1 |
| 2 |
| 37 |
|
|
| Others
(n=11) |
|
|
| 6 |
| 1 | 4 |
Treatments and clinical outcomes of
parotid carcinoma
Staging and treatment methods for the 22 patients
with parotid carcinoma are summarized in Table IV. Extended total/total and
superficial parotidectomy were performed in 10 (45%) and 11 (50%)
patients, respectively. Total and selective neck dissection were
performed in 6 (24%) and 7 (32%) patients, respectively. In 10
patients with malignant diagnosis on FNAC, extended total
parotidectomy with total neck dissection was performed in 3
patients, total parotidectomy with total neck dissection in 2
patients, and total parotidectomy with selective neck dissection in
5 patients. The trunk and part of the facial nerve were resected in
1 (4%) and 4 (18%) patients, respectively, due to tumor invasion of
the facial nerve intraoperatively. All resected facial nerves were
transplanted with the greater auricular nerve. Two (9%) patients
were reconstructed with an ALT flap to repair the defect resulting
from tumor invasion of the skin. Postoperative radiotherapy (50 Gy)
was performed in 15 (68%) of the 22 patients with parotid
carcinoma. A total of 3 of 22 patients (14%) died of parotid
carcinoma, and 4 (18%) patients died of diseases other than parotid
carcinoma. The median of the observation period was 32 months
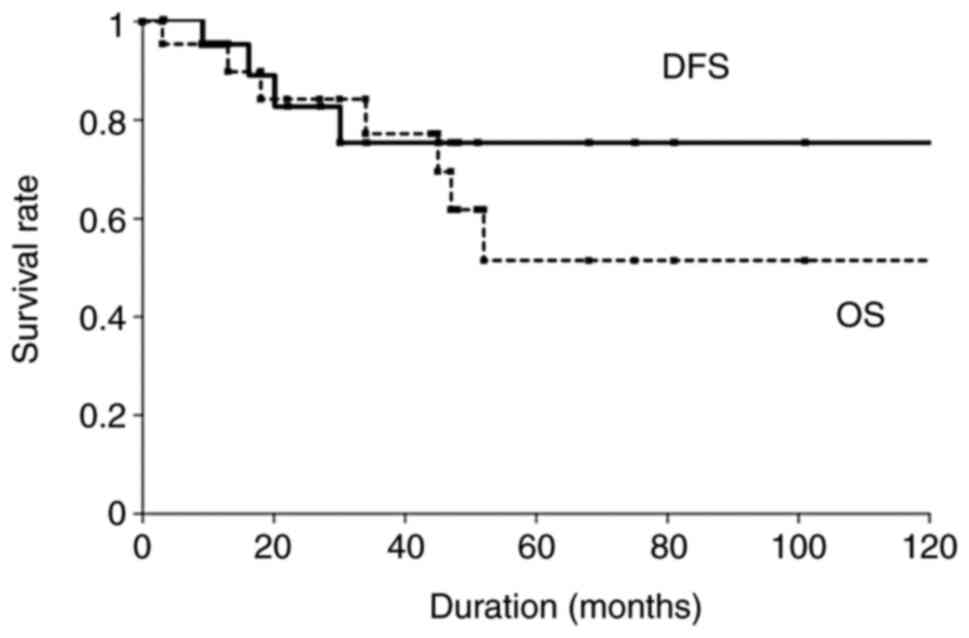
(range, 1–132 months). The 5-year OS and DFS rates among the 22
patients with parotid carcinoma were 51.5 and 76.4%, respectively
(Fig. 1). Univariate analysis
revealed that age >65 years was significantly associated with
poorer 5-year OS (P<0.001) and DFS (P<0.001) (Table V). Male and high-grade histologic
malignancy tended to exhibit worse 5-year OS (P=0.083) and 5-year
DFS (P=0.061), respectively. Multivariate analysis revealed age
>65 years with high-grade histologic malignancy was associated
with worse DFS in this group (P=0.02, hazard ratio: 3.628; 95%
confidence interval: 1.283-9.514).
 | Table IV.Staging and treatment of 22 patients
with parotid carcinoma. |
Table IV.
Staging and treatment of 22 patients
with parotid carcinoma.
| Variable | No. (%) |
|---|
| Clinical
classification |
|
| cT
T1:T2:T3:T4 | 6 (27%):6
(27%):6 |
|
| (27%):4 (18%) |
| cN
N0:N1:N2b | 16 (68%):3 |
|
| (18%):3 (14%) |
| cStage
I:II:III:IVA | 5 (23%):6
(27%):5 |
|
| (23%):6 (27%) |
| Parotidectomy |
|
|
Extended total and total | 10 (45%) |
|
Superficial | 11 (50%) |
| Deep
lobe | 1 (5%) |
| Resection of facial
nerve |
|
|
Trunk | 1 (4%) |
|
Partial | 4 (18%) |
| Not
performed | 17 (82%) |
| Neck
dissection |
|
|
Total | 6 (27%) |
|
Selective | 7 (32%) |
| Not
performed | 9 (41%) |
| Reconstruction with
ALT flap |
|
| + | 2 (9%) |
| - | 20 (91%) |
| Radiotherapy |
|
| + | 15 (68%) |
| - | 7 (32%) |
 | Table V.Univariate analysis of factors
associated with treatment outcome in 22 patients with parotid
carcinoma. |
Table V.
Univariate analysis of factors
associated with treatment outcome in 22 patients with parotid
carcinoma.
|
|
| 5-year OS | 5-year DFS |
|---|
|
|
|
|
|
|---|
| Characteristic | No. (%) | Rate, % | P-value | Rate, % | P-value |
|---|
| Age, years |
|
| 0.001 |
| 0.001 |
|
≤65 | 13 (59) | 88 |
| 100 |
|
|
>65 | 9 (41) | 0 |
| 0 |
|
| Gender |
|
| 0.083 |
| 0.178 |
|
Female | 6 (27) | 100 |
| 100 |
|
|
Male | 16 (73) | 37 |
| 0 |
|
| Pain |
|
| 0.204 |
| 0.549 |
| - | 13 (59) | 69 |
| 80 |
|
| + | 9 (41) | 25 |
| 43 |
|
| Facial nerve
palsy |
|
| 0.797 |
| 0.719 |
| - | 20 (91) | 56 |
| 79 |
|
| + | 2 (9) | 50 |
| 50 |
|
| Facial nerve
invasion |
|
| 0.858 |
| 0.472 |
| - | 17 (77) | 55 |
| 83 |
|
| + | 5 (23) | 53 |
| 53 |
|
| Histologic
grade |
|
| 0.249 |
| 0.061 |
| Low and
intermediate | 12 (55) | 44 |
| 100 |
|
|
High | 10 (45) | 67 |
| 49 |
|
| Pt |
|
| 0.88 |
| 0.285 |
|
pT1-2 | 12 (55) | 51 |
| 89 |
|
|
pT3-4 | 10 (45) | 53 |
| 49 |
|
| pN |
|
| 0.647 |
| 0.566 |
|
pN0 | 15 (68) | 58 |
| 82 |
|
|
pN+ | 7 (32) | 48 |
| 56 |
|
Discussion
This study examined parotid gland tumors treated
with surgery at a single institution. The ratios of parotid
carcinomas and benign parotid tumors were 15.7 and 84.3%,
respectively. The ratio of parotid carcinoma was similar to rates
reported in several other studies of 13.9-31.8% (2,10,11).
With regard to symptoms, mass in the parotid region is the most
common symptom of both benign parotid tumor and parotid carcinoma.
The possibility of malignancy should be considered in the presence
of sudden growing masses, pain, facial nerve palsy, and swelling of
lymph nodes (12). In the present
study, pain in the parotid area was present in 9 (41%) of 22
patients with parotid carcinoma, and the incidence of pain was
significantly higher than among patients with benign parotid
tumors. The frequency of pain in the parotid area is reportedly
31–52% in patients with malignant parotid tumors (13–15).
As the frequency of pain was approximately 10 times higher in
patients with parotid carcinoma than in those with benign tumors,
the presence of pain was considered the first indicator of possible
malignancy (15). In the present
study, facial nerve palsy was present in 2 (9%) of 22 patients with
parotid carcinoma, including a patient with squamous cell carcinoma
and a patient with adenocarcinoma NOS, but facial nerve palsy was
absent in the 118 patients with benign parotid tumors. The
incidence of preoperative partial and complete facial nerve palsy
in patients with parotid carcinoma was reported as 18–35% (16,17),
whereas none of 965 patients with benign tumors presented with
preoperative facial nerve dysfunction (17).
Warthin tumor is the second most common type of
benign parotid tumor, comprising 15–36% of all benign parotid
tumors (18). Studies have
revealed that these tumors predominantly occur in males and those
in the fourth to seventh decades of life. In the present study, 77%
of patients with Warthin tumor were male with a median age at the
surgery of 64 years and ratio of superficial lobe origin of 86%.
Warthin tumor can present bilaterally in 7–10% of cases, either
metachronously (90%) or synchronously (10%) (19,20).
The risk for bilateral Warthin tumors was significantly correlated
with nicotine intake (19). In the
present study, patients with Warthin tumor had higher pack years as
indicator of cigarette smoking status than those with pleomorphic
adenoma and those with parotid carcinoma. Smoking has been
identified as a risk factor for Warthin tumor in several series
(21,22). Evidence for the etiology and
pathogenesis of Warthin tumor remains unclear. To our knowledge,
only a few mechanisms have been proposed to date to explain the
association between Warthin tumor and smoking: 1) direct contact
between inhaled irritants in smoke and the parotid duct lining that
initiate a metaplastic response, which induces the proliferation of
glandular, cystic, and lymphoid elements (21); 2) immune reaction with delayed
hypersensitivity (23); 3) high
level of oxidative damage associated with cigarette smoking that
increases mitochondrial DNA damage in oncocytes (24); and 4) metaplasia of the glandular
tissues entrapped in the parotid lymph nodes triggered by antigens
or chemical irritants in cigarette smoke (25,26).
This may favor the hypothesis of heterotropia (assuming that
Warthin tumor originates in the salivary gland nests entrapped in
intraparotid lymph nodes during encapsulation of the parotid gland)
(27) along with an immunologic
interaction between the epithelial tumor cells and the lymphocytic
infiltration.
Preoperative diagnosis by FNAC (whether benign or
malignant, grade of malignancy, and whether a tumor is a
pleomorphic adenoma or Warthin tumor) is essential for adequate
surgical management (15). In the
present study, the non-diagnostic rate was 4.6%, lower than rates
reported in previous studies of 4.2-12.3% (28). We employed LBC in addition CS to
reduce the non-diagnostic rate and to improve the accuracy of FNAC
results. The methodology of LBC for specimens obtained from thyroid
tumor and lymph node has many advantages, including: 1) decreased
screening area; 2) lack of air-drying artifacts; 3) a more
monolayer cellular surface that is easier to screen; 4)
consistently well-preserved cells, 5) collection of tumor cells
from cystic fluid, 6) possibility of application to immunohistology
and genetic analyses (3,5,29).
However, some disadvantages of LBC for parotid gland tumors include
new artifacts that alter the cellular, architectural and
extracellular matrix appearance, and decreased lymphocytes and
mucinous material in the background (3). The reported sensitivity and
specificity for diagnosing malignant tumors by FNAC with CS ranges
from 56 to 100% and from 57 to 98%, respectively (30–33).
In the present study in which FNAC was combined with CS and LBC,
our data indicated 70% sensitivity and 99% specificity, which was
consistent with these previous results. In general, the relatively
low sensitivity for parotid carcinoma is caused by the high rate of
false-negative results, (i.e., FNAC diagnosis of benign but
histologic diagnosis of malignant). In the present study, 2
patients diagnosed with pleomorphic adenoma by FNAC were
histologically diagnosed with carcinoma ex pleomorphic adenoma.
These results were thought to have been caused by aspiration of
part of the pleomorphic adenoma but not part of the carcinoma.
Other 2 patients diagnosed with Warthin tumor by FNAC were
histologically diagnosed with low-grade mucoepidermoid carcinoma.
To reduce these false-negative results, especially in cystic
lesions, aspiration from several points of the same solid tumor in
the same tumor under US guidance is recommended (34).
The goal of surgical management of benign parotid
tumors is to completely remove the tumor and preserve the facial
nerve (6). Partial superficial
parotidectomy was characterized by the preservation of part of the
unaffected parotid tissue and the dissection of a smaller area of
facial nerve branches (6). Partial
superficial parotidectomy was associated with fewer complications
and lower recurrence rates than superficial parotidectomy (35,36).
In the present study, recurrence after partial superficial
parotidectomy was observed in only 1 patient (2%) with pleomorphic
adenoma. Even if the facial nerve is completely preserved, a
certain rate of postoperative facial nerve palsy is inevitable
(6). The incidence of transient
facial nerve palsy after parotidectomy for benign parotid tumors
ranges from 10–65%, with persistent palsy seen in <5% of cases
(37–39). A report from a single-center study
indicated postoperative facial palsy rate of 20% in pleomorphic
adenoma and 17.9% in Warthin tumor (6). In the present study, the rate of
postoperative transient facial nerve palsy in pleomorphic adenoma
was 16 and 21% in Warthin tumor, and no persistent facial palsy was
observed. These frequencies were consistent with previous reports.
The risk factors for facial nerve palsy reportedly include older
age, tumor size (6,40), tumor in the deep lobe, long
operation time, extensive bleeding, and lack of FNM (41,42).
However, controversy exists, in that some researchers contend that
there is no significant difference in complication rates relative
to tumor size and tumor in the deep lobe (43), the length of the dissected facial
nerve (44), and the extent of
parotidectomy (45). We usually
identify the trunk of the facial nerve using FNM and follow the
branch to confirm nerve integrity. Some studies have reported that
intraoperative FNM decreases the incidence of facial nerve palsy
(4) and the operation time in
parotidectomy (41,46).
Mucoepidermoid carcinoma is the most common
malignancy of the salivary glands, accounting for 10–15% of all
salivary gland neoplasms and 30% of all salivary malignancies
(47,48). In the present study, mucoepidermoid
carcinoma was the most common parotid carcinoma in 5 patients (23%)
including 3 with low-grade and in 2 with high-grade in terms of
histologic malignancy. To date, several histologic grading systems
for mucoepidermoid carcinomas have been used (47,48).
Low-grade tumors tend to be better circumscribed, more cystic,
contain more mucous cells, show minimal cytologic atypia or mitoses
and lack perineural invasion. On the other hand, high-grade lesions
are more infiltrative, more solid, have less mucous cells and more
epidermoid cells, show more cytologic atypia, necrosis and
perineural invasion. High-grade is known to be poor prognostic
factor of mucoepidermoid carcinoma (47,48).
A unique translocation t(11; 19) (q21; p13), the most common
genetic alteration in mucoepidermoid carcinomas, can produce a
fusion oncogene known as CRTC1-MAML2 (49). Accumulating evidence revealed that
the CRTC1-MAML2 expression correlates with a significantly better
prognosis in patients with mucoepidermoid carcinoma (50). CRTC1-MAML2 expression is present in
75–93% of low to intermediate-grade mucoepidermoid carcinoma and
50–89% high-grade mucoepidermoid carcinoma, aiding in histologic
diagnosis (51,52).
Surgical resection is the first choice of the
treatment for parotid carcinoma. For T1-size tumors that are
located in the superficial lobe, have low-grade histology, and are
N0 parotid carcinoma, superficial parotidectomy with safety margins
may suffice (53). Otherwise,
total parotidectomy or extended total parotidectomy with safety
margins is advised according to the extension area. Total neck
dissection (Level I to V) should be carried out in cN+ patients.
However, neck dissection for cN0 patients is still controversial.
In cN0 patients with parotid carcinoma, histologic lymph node
metastasis reportedly ranged from 4.2-30.3% (53–56).
A prophylactic selective neck dissection (Level I to III) should be
performed in cases involving T3 and T4 tumors with high-grade
histology (57), facial nerve
palsy, age >54 years, and tumor invasion of adjacent organs
(58). In the present study, 9
(41%) of 22 patients who did not undergo neck dissection had a
preoperative diagnosis of either cN0, cT1, or benign or
indeterminate result on FNAC. In the present study, the trunk and
part of the facial nerve were resected in 1 (4%) and 4 (18%)
patients, respectively, due to tumor invasion of the facial nerve.
A functioning facial nerve should be preserved unless found to be
infiltrated with the tumor itself at the time of resection
(15). If the nerve is sacrificed
because of invasion, then primary nerve grafting should be
performed. The greater auricular nerve as a donor is an option, but
if that nerve is involved, the sural nerve from the leg may be
preferable. We used ALT flap in 2 patients with skin defects caused
by invasion of the subauricular skin. The ALT flap has been shown
to be effective for covering large defects resulting from the
radical removal of parotid malignancies (59). Postoperative radiotherapy was
associated with improved survival among patients with salivary
gland carcinomas for whom neck dissection was deemed necessary in
an analysis of 4,145 cases (60).
Criteria proposed by the National Comprehensive Cancer Network for
postoperative radiotherapy after the complete resection include
intermediate or high-grade, close or positive margins,
neural/perineural invasion, lymph node metastasis,
lymphatic/vascular invasion, T3 and T4a tumors, and adenoid cystic
carcinoma. Following these guidelines, 15 patients (68%) received
postoperative radiotherapy in the present study. We recommended
postoperative radiotherapy for all patients with parotid carcinoma.
The other 7 patients refused radiotherapy for reasons of advanced
age or difficulty traveling to the hospital due to distant from
home.
In terms of parotid carcinoma prognosis, the 5-year
DFS is 60.2–78% (15,61,62).
The 5-year DFS rate in the present study was 76.4%, which is not
inferior to the rate described in previous reports. Prognostic
factors for parotid carcinoma described in previous studies include
age >60 years (63), pain
(64), facial paralysis (64), skin invasion (64), TNM classification (62,65),
lymph node metastasis (63,66),
high-risk histologic grade (15,62,63,66,67),
perineural invasion (64),
lymphovascular invasion (62,63),
and involved surgical margins (64). We found that age >65 years with
high-grade histologic malignancy was an independent prognostic
factor in determining DFS. Overall, the advantage of this study was
that only 2 otolaryngologists were able to perform FNAC and surgery
using the same surgical methods and techniques. The major
limitations of this study were the small number of the parotid
carcinoma cases and the retrospective study design; thus, the
results should be validated through further prospective comparative
studies.
In conclusion, clinical characteristics and
treatment outcomes of parotid gland tumors at our institution were
consistent with the results of previous reports. Smoking may be
closely related to the pathogenesis of Warthin tumors. LBC
potentially provides better accuracy in FNAC. Considering the
variety of histologic types of parotid gland tumors, it is critical
to obtain the most-accurate preoperative diagnosis and employ the
most-appropriate surgical procedure, including parotidectomy and
neck dissection.
Acknowledgements
The authors would like to thank Professor Mitsuru
Sekido (Department of Plastic and Reconstructive Surgery,
University of Tsukuba, Tsukuba, Japan) and Dr Shujiroh Makino
(Department of Oral Surgery, Hokuto Hospital, Obihiro, Japan) for
performing the reconstruction surgery, and Dr Akihiko Miyamoto
(Department of Radiation Therapy, Hokuto Hospital, Obihiro, Japan)
and Dr Ken-Ichi Matsumoto (Department of Radiation Therapy, Hokuto
Hospital, Obihiro, Japan) for performing the radiotherapy.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SSu, NB and YH conceived and designed the analysis.
TG, AK, AU, MK, RS, RT, SSa, TYI, HN and HT contributed to the
treatments, and collection, analysis and interpretation of the
data. MK, RS, RT and SSa confirm the authenticity of all the raw
data. SSu and NB drafted the manuscript, tables and figures. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Hokuto Hospital (approval no. 1110; Obihiro, Japan).
The requirement for informed consent was waived due to the
retrospective nature of the study using medical records only. The
research content was available publicly on the website of the
Ethics Committee of Hokuto Hospital, which ensured opportunities
for participants to opt out of the research without any
disadvantage.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Seethala RR and Stenman G: Update from the
4th edition of the World Health Organization classification of head
and neck tumours: Tumors of the salivary gland. Head Neck Pathol.
11:55–67. 2017. View Article : Google Scholar
|
|
2
|
Suzuki M, Kawata R, Higashino M, Nishikawa
S, Terada T, Haginomori SI, Kurisu Y and Hirose Y: Values of
fine-needle aspiration cytology of parotid gland tumors: A review
of 996 cases at a single institution. Head Neck. 41:358–365. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rarick JM, Wasman J and Michael CW: The
utility of liquid-based cytology in salivary gland fine-needle
aspirates: Experience of an academic institution. Acta Cytol.
58:552–562. 2014.
|
|
4
|
Sood AJ, Houlton JJ, Nguyen SA and
Gillespie MB: Facial nerve monitoring during parotidectomy: A
systematic review and meta-analysis. Otolaryngol Head Neck Surg.
152:631–637. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamaguchi T, Akahane T, Harada O, Kato Y,
Aimono E, Takei H, Tasaki T, Noguchi H, Nishihara H, Kamata H and
Tanimoto A: Next-generation sequencing in residual liquid-based
cytology specimens for cancer genome analysis. Diagn Cytopathol.
48:965–971. 2020. View
Article : Google Scholar
|
|
6
|
Kawata R, Kinoshita I, Omura S, Higashino
M, Nishikawa S, Terada T, Haginomori SI, Kurisu Y, Hirose Y and
Tochizawa T: Risk factors of postoperative facial palsy for benign
parotid tumors: Outcome of 1,018 patients. Laryngoscope.
131:E2857–E2864. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iizuka K and Ishikawa K: Surgical
techniques for benign parotid tumors: Segmental resection vs
extracapsular lumpectomy. Acta Otolaryngol Suppl. 537:75–81.
1998.
|
|
8
|
Witt RL: Minimally invasive surgery for
parotid pleomorphic adenoma. Ear Nose Throat J. 84(308): 310–1.
2005. View Article : Google Scholar
|
|
9
|
Numano Y, Ogawa T, Ishikawa T, Usubuchi H,
Nakanome A, Ohkoshi A, Ishida E, Rokugo M and Katori Y: Parotid
secretory carcinoma with high-grade transformation. Auris Nasus
Larynx. 47:1043–1048. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li LJ, Li Y, Wen YM, Liu H and Zhao HW:
Clinical analysis of salivary gland tumor cases in West China in
past 50 years. Oral Oncol. 44:187–192. 2008. View Article : Google Scholar
|
|
11
|
Grasl S, Kadletz L, Janik S, Riedl A,
Erlacher B, Formanek M, Grasl MC and Erovic BM: Fine-needle
aspiration cytology and intraoperative frozen section in parotid
gland tumour surgery: A retrospective multicenter analysis of 417
cases. Clin Otolaryngol. 44:461–465. 2019. View Article : Google Scholar
|
|
12
|
Gatta G, Guzzo M, Locati LD, McGurk M and
Prott FJ: Major and minor salivary gland tumours. Crit Rev Oncol
Hematol. 152:1029592020. View Article : Google Scholar
|
|
13
|
Godballe C, Schultz JH, Krogdahl A,
Moller-Grontved A and Johansen J: Parotid carcinoma: Impact of
clinical factors on prognosis in a histologically revised series.
Laryngoscope. 113:1411–1417. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pohar S, Gay H, Rosenbaum P, Klish D,
Bogart J, Sagerman R, Hsu J and Kellman R: Malignant parotid
tumors: Presentation, clinical/pathologic prognostic factors, and
treatment outcomes. Int J Radiat Oncol Biol Phys. 61:112–118. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nishikado A, Kawata R, Haginomori SI,
Terada T, Higashino M, Kurisu Y and Hirose Y: A clinicopathological
study of parotid carcinoma: 18-year review of 171 patients at a
single institution. Int J Clin Oncol. 23:615–624. 2018. View Article : Google Scholar
|
|
16
|
Terhaard C, Lubsen H, Tan B, Merkx T, van
der Laan B, Baatenburg de Jong R, Manni H and Knegt P: Facial nerve
function in carcinoma of the parotid gland. Eur J Cancer.
42:2744–2750. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Inaka Y, Kawata R, Haginomori SI, Terada
T, Higashino M, Omura S and Kikuoka Y: Symptoms and signs of
parotid tumors and their value for diagnosis and prognosis: A
20-year review at a single institution. Int J Clin Oncol.
26:1170–1178. 2021. View Article : Google Scholar
|
|
18
|
de Oliveira FA, Duarte EC, Taveira CT,
Maximo AA, de Aquino EC, Alencar Rde C and Vencio EF: Salivary
gland tumor: A review of 599 cases in a Brazilian population. Head
Neck Pathol. 3:271–275. 2009. View Article : Google Scholar
|
|
19
|
Peter Klussmann J, Wittekindt C, Florian
Preuss S, Al Attab A, Schroeder U and Guntinas-Lichius O: High risk
for bilateral Warthin tumor in heavy smokers-review of 185 cases.
Acta Otolaryngol. 126:1213–1217. 2006. View Article : Google Scholar
|
|
20
|
Ethunandan M, Pratt CA, Morrison A, Anand
R, Macpherson DW and Wilson AW: Multiple synchronous and
metachronous neoplasms of the parotid gland: The Chichester
experience. Br J Oral Maxillofac Surg. 44:397–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kotwall CA: Smoking as an etiologic factor
in the development of Warthin's tumor of the parotid gland. Am J
Surg. 164:646–647. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sadetzki S, Oberman B, Mandelzweig L,
Chetrit A, Ben-Tal T, Jarus-Hakak A, Duvdevani S, Cardis E and Wolf
M: Smoking and risk of parotid gland tumors: A nationwide
case-control study. Cancer. 112:1974–1982. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Allegra SR: Warthin's tumor: A
hypersensitivity disease? Ultrastructural, light, and
immunofluorescent study. Hum Pathol. 2:403–420. 1971. View Article : Google Scholar
|
|
24
|
Lewis PD, Baxter P, Paul Griffiths A,
Parry JM and Skibinski DO: Detection of damage to the mitochondrial
genome in the oncocytic cells of Warthin's tumour. J Pathol.
191:274–281. 2000. View Article : Google Scholar
|
|
25
|
Yu GY, Liu XB, Li ZL and Peng X: Smoking
and the development of Warthin's tumour of the parotid gland. Br J
Oral Maxillofac Surg. 36:183–185. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
de Ru JA, Plantinga RF, Majoor MH, van
Benthem PP, Slootweg PJ, Peeters PH and Hordijk GJ: Warthin's
tumour and smoking. B-ENT. 1:63–66. 2005.PubMed/NCBI
|
|
27
|
Aguirre JM, Echebarria MA, Martinez-Conde
R, Rodriguez C, Burgos JJ and Rivera JM: Warthin tumor. A new
hypothesis concerning its development. Oral Surg Oral Med Oral
Pathol Oral Radiol Endod. 85:60–63. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schmidt RL, Hall BJ, Wilson AR and
Layfield LJ: A systematic review and meta-analysis of the
diagnostic accuracy of fine-needle aspiration cytology for parotid
gland lesions. Am J Clin Pathol. 136:45–59. 2011. View Article : Google Scholar
|
|
29
|
Bandoh N, Goto T, Akahane T, Ohnuki N,
Yamaguchi T, Kamada H, Harabuchi Y, Tanaka S and Nishihara H:
Diagnostic value of liquid-based cytology with fine needle
aspiration specimens for cervical lymphadenopathy. Diagn
Cytopathol. 44:169–176. 2016. View
Article : Google Scholar
|
|
30
|
Que Hee CG and Perry CF: Fine-needle
aspiration cytology of parotid tumours: Is it useful? ANZ J Surg.
71:345–348. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cohen EG, Patel SG, Lin O, Boyle JO, Kraus
DH, Singh B, Wong RJ, Shah JP and Shaha AR: Fine-needle aspiration
biopsy of salivary gland lesions in a selected patient population.
Arch Otolaryngol Head Neck Surg. 130:773–778. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mallon DH, Kostalas M, MacPherson FJ,
Parmar A, Drysdale A, Chisholm E and Sadek S: The diagnostic value
of fine needle aspiration in parotid lumps. Ann R Coll Surg Engl.
95:258–262. 2013. View Article : Google Scholar
|
|
33
|
Singh Nanda KD, Mehta A and Nanda J:
Fine-needle aspiration cytology: A reliable tool in the diagnosis
of salivary gland lesions. J Oral Pathol Med. 41:106–112. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Altin F, Alimoglu Y, Acikalin RM and Yasar
H: Is fine needle aspiration biopsy reliable in the diagnosis of
parotid tumors? Comparison of preoperative and postoperative
results and the factors affecting accuracy. Braz J
Otorhinolaryngol. 85:275–281. 2019. View Article : Google Scholar
|
|
35
|
Stathopoulos P, Igoumenakis D and Smith
WP: Partial superficial, superficial, and total parotidectomy in
the management of benign parotid gland tumors: A 10-year
prospective study of 205 patients. J Oral Maxillofac Surg.
76:455–459. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
O'Brien KF, Shah SD, Pope E, Phillips RJ,
Blei F, Baselga E, Garzon MC, McCuaig C, Haggstrom AN, Hoeger PH,
et al: Late growth of infantile hemangiomas in children >3 years
of age: A retrospective study. J Am Acad Dermatol. 80:493–499.
2019. View Article : Google Scholar
|
|
37
|
Guntinas-Lichius O, Klussmann JP,
Schroeder U, Quante G, Jungehuelsing M and Stennert E: Primary
parotid malignoma surgery in patients with normal preoperative
facial nerve function: Outcome and long-term postoperative facial
nerve function. Laryngoscope. 114:949–956. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lim YC, Lee SY, Kim K, Lee JS, Koo BS,
Shin HA and Choi EC: Conservative parotidectomy for the treatment
of parotid cancers. Oral Oncol. 41:1021–1027. 2005. View Article : Google Scholar
|
|
39
|
Eisele DW, Wang SJ and Orloff LA:
Electrophysiologic facial nerve monitoring during parotidectomy.
Head Neck. 32:399–405. 2010.PubMed/NCBI
|
|
40
|
Bonavolonta P, Dell'Aversana Orabona G,
Maglitto F, Abbate V, Committeri U, Salzano G, Improta G, Iaconetta
G and Califano L: Postoperative complications after removal of
pleomorphic adenoma from the parotid gland: A long-term follow up
of 297 patients from 2002 to 2016 and a review of publications. Br
J Oral Maxillofac Surg. 57:998–1002. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ikoma R, Ishitoya J, Sakuma Y, Hirama M,
Shiono O, Komatsu M and Oridate N: Temporary facial nerve
dysfunction after parotidectomy correlates with tumor location.
Auris Nasus Larynx. 41:479–484. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Patel DK, Ahmad Z and Morton RP: Partial
superficial parotidectomy with retrograde dissection of the facial
nerve for clinically ‘Benign’ parotid tumors. Ann Otol Rhinol
Laryngol. 125:808–814. 2016. View Article : Google Scholar
|
|
43
|
Auger SR, Kramer DE, Hardy B, Jandali D,
Stenson K, Kocak M and Al-Khudari S: Functional outcomes after
extracapsular dissection with partial facial nerve dissection for
small and large parotid neoplasms. Am J Otolaryngol. 42:1027702021.
View Article : Google Scholar
|
|
44
|
Cannon CR, Replogle WH and Schenk MP:
Facial nerve in parotidectomy: A topographical analysis.
Laryngoscope. 114:2034–2037. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wong WK and Shetty S: The extent of
surgery for benign parotid pathology and its influence on
complications: A prospective cohort analysis. Am J Otolaryngol.
39:162–166. 2018. View Article : Google Scholar
|
|
46
|
Savvas E, Hillmann S, Weiss D, Koopmann M,
Rudack C and Alberty J: Association between facial nerve monitoring
with postoperative facial paralysis in parotidectomy. JAMA
Otolaryngol Head Neck Surg. 142:828–833. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Goode RK, Auclair PL and Ellis GL:
Mucoepidermoid carcinoma of the major salivary glands: Clinical and
histopathologic analysis of 234 cases with evaluation of grading
criteria. Cancer. 82:1217–1224. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Brandwein MS, Ivanov K, Wallace DI, Hille
JJ, Wang B, Fahmy A, Bodian C, Urken ML, Gnepp DR, Huvos A, et al:
Mucoepidermoid carcinoma: A clinicopathologic study of 80 patients
with special reference to histological grading. Am J Surg Pathol.
25:835–845. 2001. View Article : Google Scholar
|
|
49
|
Tonon G, Modi S, Wu L, Kubo A, Coxon AB,
Komiya T, O'Neil K, Stover K, El-Naggar A, Griffin JD, et al:
t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates
a novel fusion product that disrupts a Notch signaling pathway. Nat
Genet. 33:208–213. 2003. View Article : Google Scholar
|
|
50
|
Okabe M, Miyabe S, Nagatsuka H, Terada A,
Hanai N, Yokoi M, Shimozato K, Eimoto T, Nakamura S, Nagai N, et
al: MECT1-MAML2 fusion transcript defines a favorable subset of
mucoepidermoid carcinoma. Clin Cancer Res. 12:3902–3907. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Griffith CC, Schmitt AC, Little JL and
Magliocca KR: New developments in salivary gland pathology:
Clinically useful ancillary testing and new potentially targetable
molecular alterations. Arch Pathol Lab Med. 141:381–395. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cipriani NA, Lusardi JJ, McElherne J,
Pearson AT, Olivas AD, Fitzpatrick C, Lingen MW and Blair EA:
Mucoepidermoid carcinoma: A comparison of histologic grading
systems and relationship to MAML2 rearrangement and prognosis. Am J
Surg Pathol. 43:885–897. 2019. View Article : Google Scholar
|
|
53
|
Kawata R, Koutetsu L, Yoshimura K,
Nishikawa S and Takenaka H: Indication for elective neck dissection
for N0 carcinoma of the parotid gland: A single institution's
20-year experience. Acta Otolaryngol. 130:286–292. 2010. View Article : Google Scholar
|
|
54
|
Shinomiya H, Otsuki N, Yamashita D and
Nibu K: Patterns of lymph node metastasis of parotid cancer. Auris
Nasus Larynx. 43:446–450. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lau VH, Aouad R, Farwell DG, Donald PJ and
Chen AM: Patterns of nodal involvement for clinically N0 salivary
gland carcinoma: Refining the role of elective neck irradiation.
Head Neck. 36:1435–1439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Stodulski D, Mikaszewski B, Majewska H,
Wisniewski P and Stankiewicz C: Probability and pattern of occult
cervical lymph node metastases in primary parotid carcinoma. Eur
Arch Otorhinolaryngol. 274:1659–1664. 2017. View Article : Google Scholar
|
|
57
|
Armstrong JG, Harrison LB, Thaler HT,
Friedlander-Klar H, Fass DE, Zelefsky MJ, Shah JP, Strong EW and
Spiro RH: The indications for elective treatment of the neck in
cancer of the major salivary glands. Cancer. 69:615–619. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Medina JE: Neck dissection in the
treatment of cancer of major salivary glands. Otolaryngol Clin
North Am. 31:815–822. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Trojanowski P, Szymanski M, Trojanowska A,
Andrzejczak A, Szczepanek D and Klatka J: Anterolateral thigh free
flap in reconstruction of lateral skull base defects after
oncological resection. Eur Arch Otorhinolaryngol. 276:3487–3494.
2019. View Article : Google Scholar
|
|
60
|
Aro K, Ho AS, Luu M, Kim S, Tighiouart M,
Yoshida EJ, Mallen-St Clair J, Shiao SL, Leivo I and Zumsteg ZS:
Survival impact of adjuvant therapy in salivary gland cancers
following resection and neck dissection. Otolaryngol Head Neck
Surg. 160:1048–1057. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Renehan AG, Gleave EN, Slevin NJ and
McGurk M: Clinico-pathological and treatment-related factors
influencing survival in parotid cancer. Br J Cancer. 80:1296–1300.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kim YH, Chung WK, Jeong JU, Cho IJ, Yoon
MS, Song JY, Nam TK, Ahn SJ, Lee DH, Yoon TM, et al: Evaluation of
prognostic factors for the parotid cancer treated with surgery and
postoperative radiotherapy. Clin Exp Otorhinolaryngol. 13:69–76.
2020. View Article : Google Scholar
|
|
63
|
Erovic BM, Shah MD, Bruch G, Johnston M,
Kim J, O'Sullivan B, Perez-Ordonez B, Weinreb I, Atenafu EG, de
Almeida JR, et al: Outcome analysis of 215 patients with parotid
gland tumors: A retrospective cohort analysis. J Otolaryngol Head
Neck Surg. 44:432015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Vander Poorten VL, Hart AA, van der Laan
BF, Baatenburg de Jong RJ, Manni JJ, Marres HA, Meeuwis CA, Lubsen
H, Terhaard CH and Balm AJ: Prognostic index for patients with
parotid carcinoma: External validation using the nationwide
1985–1994 dutch head and neck oncology cooperative group database.
Cancer. 97:1453–1463. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Mercante G, Marchese C, Giannarelli D,
Pellini R, Cristalli G, Manciocco V, Ruscito P, Pichi B, Marchesi P
and Spriano G: Oncological outcome and prognostic factors in
malignant parotid tumours. J Craniomaxillofac Surg. 42:59–65. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lima RA, Tavares MR, Dias FL, Kligerman J,
Nascimento MF, Barbosa MM, Cernea CR, Soares JR, Santos IC and
Salviano S: Clinical prognostic factors in malignant parotid gland
tumors. Otolaryngol Head Neck Surg. 133:702–708. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Shang J, Wu Y, Wang W, Wang K and Ge M:
Analysis of prognostic risk factors and treatment of parotid
cancer. Oncol Lett. 3:1307–1310. 2012. View Article : Google Scholar
|