Introduction
Colorectal cancer (CRC) is the second most common
cause of cancer-related mortality worldwide with an increasing
incidence (1,2). The incidence of CRC worldwide is
predicted to increase to 2.5 million new cases in 2035 (3). Although complete surgical resection
remains an important treatment option, 21% of patients newly
diagnosed with CRC had distant metastases (4); further, multidisciplinary treatments,
including radiotherapy and chemotherapy, are important, especially
in advanced cancer. The overall survival of patients with
metastatic CRC (mCRC) has been improving mainly due to advances in
systemic therapy. The overall survival of patients diagnosed with
unresectable mCRC has increased from ~1 year during the era of
fluoropyrimidine monotherapy to more than 30 months with the
integration of multiple cytotoxic agents and targeted therapies,
such as FOLFOX plus bevacizumab (5). A combination of cytotoxic and
molecularly targeted agents is now the standard treatment for
unresectable mCRC.
Recently, for deficient mismatch repair
(dMMR)/microsatellite instability-high (MSI-H) tumors, which
account for 4–5% of CRCs (6),
immune checkpoint inhibitors (ICIs) that target cytotoxic
T-lymphocyte antigen-4 and programmed cell death protein 1 (PD-1)
and its ligand have changed the standard treatment for patients
with unresectable CRC and mCRC (7). The randomized, phase 3 trial
KEYNOTE-177, which compared first-line pembrolizumab to
standard-of-care chemotherapy with or without bevacizumab for
dMMR/MSI-H mCRC, found that pembrolizumab significantly increases
progression-free survival compared with chemotherapy, with fewer
treatment-related adverse events (8). The phase 2 study CHECKMATE-142 showed
the improved efficacy of combination immunotherapy (nivolumab plus
ipilimumab) compared with anti-PD-1 monotherapy (9). These studies compelled the United
States Food and Drug Administration to approve single and dual ICIs
as acceptable standard treatment options for dMMR/MSI-H mCRC.
However, little has been reported about the use of ICIs in
neoadjuvant or conversion settings. Here, we report a case of a
large dMMR/MSI-H colon cancer, which showed a pathologic complete
response after combination treatment with nivolumab and
ipilimumab.
Case report
A 24-year-old man with no previous medical history
had been diagnosed with right-sided colon cancer and was referred
to our institution. He had recurrent constipation and diarrhea for
1 month. He had never smoked cigarettes and consumed alcohol only
socially. Family history was remarkable for his mother with
colorectal cancer and second-degree relatives with one gastric
cancer, two hepatocellular carcinomas, one pharyngeal cancer, and
one cervical cancer. Laboratory studies showed no elevation in
serum CEA (1.1 ng/ml) and CA19-9 (<2.0 U/ml). Initial dynamic
contrast-enhanced computed tomography (CE-CT) showed a huge mass
(110×70 mm) in the ascending colon (Fig. 1A) with direct invasion of the right
ureter and duodenum (Fig. 1B, C),
which was a major concern for upfront surgical resection. This
patient had double inferior vena cava (IVC), and the tumor had
invaded the right IVC (Fig. 1D).
Several enlarged lymph nodes around the tumor were observed, but no
distant metastases were noted. The patient underwent colonoscopy,
which revealed a circumferential ulcerating tumor in the ascending
colon. Biopsy supported the pathological diagnosis of poorly
differentiated adenocarcinoma (Fig.
2A).
We considered upfront surgical resection as a
feasible option, but the size and circumferential invasion would
make the surgery highly invasive and possibly noncurative.
Therefore, we decided to administer neoadjuvant treatment. Before
the chemotherapy, an ileostomy and biopsy of the mesenteric lymph
node were performed. The resected lymph node confirmed the
pathological and molecular diagnosis of lymph node metastasis
(Fig. 2B), with KRAS (codon
13) mutation, non-BRAF V600E, and MSI-H. Neoadjuvant
chemotherapy with capecitabine and oxaliplatin (CAPEOX) plus
bevacizumab was administered (10). During CAPEOX and bevacizumab
treatment, the patient only developed grade 1 nausea. After three
courses of this treatment, a follow-up CT scan revealed an enlarged
tumor (112 ×72 mm) and an appearance of multiple pulmonary
metastases (Figs. 3A and 4). Since the first treatment was a
failure given the disease progression, the combination therapy of
nivolumab (240 mg) plus ipilimumab (1 mg/kg) every 3 weeks was
performed as second-line systemic therapy, based on the result of
high-MSI status. After three courses of such immunotherapy, the
tumor had remarkably shrunk to 52×49 mm, and all the pulmonary
metastases had almost disappeared. After an additional three
courses of nivolumab (240 mg) monotherapy the tumor decreased to
49×46 mm, and all pulmonary metastases had become radiologically
undetectable (Figs. 3B and
4). During these immunotherapies,
the patient only developed grade-2 dermatitis. Eighteen weeks after
nivolumab and ipilimumab combination therapy and four weeks after
the last nivolumab infusion, surgical resection-including right
hemicolectomy and resection of the right kidney, ureter, and right
IVC-was performed. Because the tumor firmly adhered to the
duodenum, resection and reconstruction of part of the third portion
of the duodenum were also implemented. The operation lasted 10 h
and 21 min, and the blood loss amounted to 9011 ml.
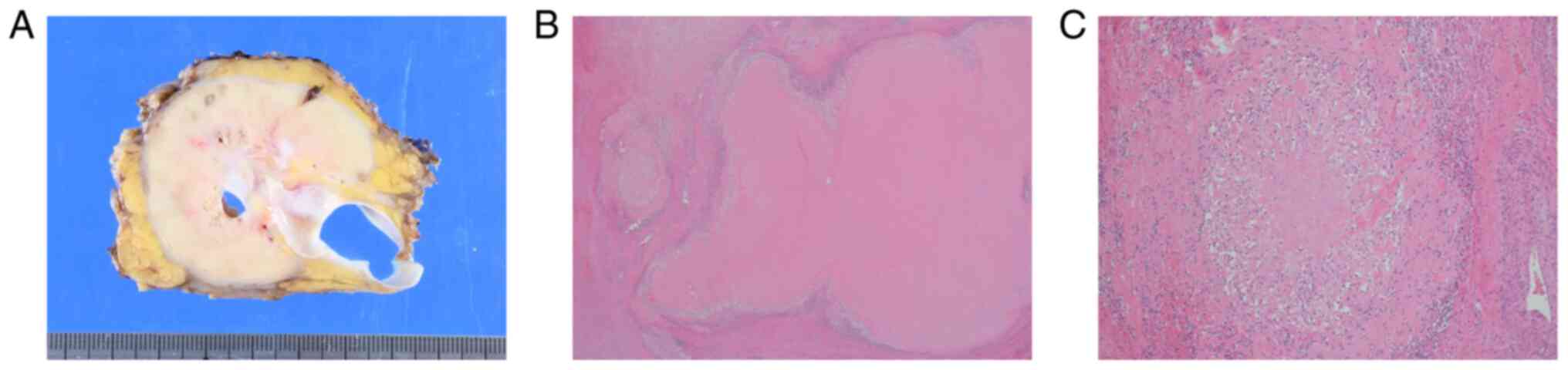
A gross examination of the surgical specimen showed
a cut surface of a creamy yellow tumor (90×65×60 mm3) in
the ascending mesocolon (Fig. 5A).
Pathological examination demonstrated that the tumor was totally
covered with granulation tissue with no viable cells. Thus, the
treatment outcome was pronounced as pathologic complete response
(pCR) (Fig. 5B and C). The patient
presented a grade-B pancreatic fistula (International Study Group
of Pancreatic Surgery pancreatic fistula classification) (11) after surgery but managed to recover
and was discharged on postoperative day 19 with an open drainage
tube, which was removed on postoperative day 93. Because Lynch
syndrome was suspected from personal and familial history, genetic
counseling and testing were performed, and a germline pathogenic
variant was found in MSH2. The patient is alive and well 12 months
after surgery and 22 months after the initial diagnosis.
This study was approved by the Ethics Committee of
Ibaraki Prefectural Central Hospital, Ibaraki Cancer Center.
Written informed consent for publication was obtained from the
patient.
Discussion
Immunotherapy has changed the standard treatment for
metastatic and unresectable dMMR CRC; however, little information
and experience are available on its use before colorectal
surgery.
Neoadjuvant immunotherapy exhibited a high
pathological response rate in trials in melanoma, glioblastoma, and
colon cancer with small numbers of patients (12–15).
As for CRC, one exploratory clinical phase-2 trial and some case
reports provided some information. In the exploratory NICHE trial,
among 20 patients with resectable dMMR/MSI-H tumors who received
nivolumab and ipilimumab, a major pathological response (MPR, ≤10%
residual viable tumor) was observed in 19/20 patients, and pCR was
observed in 12/20 patients (16).
One course of the combination immunotherapy with ipilimumab (1
mg/kg) was administered on day 1 and nivolumab (3 mg/kg) on days 1
and 15 in the NICHE trial. A case report on resected colon cancer
after dual immunotherapy by Kinney and Khalil (17) has described the resection case of a
52-year-old man with right-sided colon cancer who presented pCR
after three courses of combination immunotherapy (ipilimumab 1
mg/kg on day 1 and nivolumab 240 mg on days 1, 15 and 29) every 6
weeks. These studies suggest the potential benefit of immunotherapy
in neoadjuvant settings.
For patients with unresectable metastases, there is
an obvious need for effective conversion therapy. The advancements
in effective chemotherapy yielded higher response and resection
rates (18,19). A study has reported that robust
chemotherapy, such as modified FOLFOXIRI with cetuximab, improved
the objective response rate, and this could be an option for
conversion regimen (20). Case
reports on immunotherapy in the conversion setting are rare, and so
far, no clinical trial of immunotherapy in conversion settings has
been conducted.
To the best of our knowledge, only one case report
has been published on resected colon cancer with distant metastases
achieving pCR after immunotherapy. Yang et al (21) have reported a case of dMMR/MSI-H
ascending colon cancer initially showing multiple mesenteric and
retroperitoneal lymph node metastasis and invasion to the IVC,
ureter, and right kidney vessels, which was successfully converted
to pCR after immunotherapy and radiotherapy (4 cycles of
pembrolizumab 200 mg every 3 weeks and radiotherapy). The present
case is the first report on advanced-stage CRC presenting similar
locoregional extension plus distant pulmonary metastases and
converted to pCR after dual immunotherapy.
Little is known about the choice of therapeutic
agents for mCRC in the conversion setting. The clinical course of
the present case demonstrates that we should have chosen
immunotherapy as a first-line treatment before the surgery. This
idea is supported by the high CR rate reported for pembrolizumab
(8) as a first-line treatment in
patients with dMMR/MIC-H mCRC in KEYNOTE-177 (11% vs. 3.9%). The
final analysis of OS in KEYNOTE-177 was presented at the 2021 ASCO
annual meeting and confirmed pembrolizumab as a new first-line
standard-of-care for patients with MSI-H/dMMR mCRC. The next
question is the best option for immunotherapy. In the
abovementioned studies, immunotherapy was applied in various ways
(16,17,21).
The NICHE and CHECKMATE-142 trials showed the clinical benefit of
combination ICI therapy relative to nivolumab monotherapy.
Nivolumab and ipilimumab act synergistically to promote T-cell
antitumor activity through complementary mechanisms of action
(22,23). Nivolumab plus ipilimumab is
suspected to demonstrate a high response rate in various cancers,
including CRC (9,24). However, in neoadjuvant or
conversion settings, the high toxicity of dual therapy can
potentially delay curative surgery, which needs further
investigation. We provided a longer ICI therapy than that in the
study by Yang et al (21),
which totaled 18 weeks. The optimal duration required for ICI
therapy in conversion settings remains unknown. This patient
underwent surgery 4 weeks after immunotherapy. Determining the
timing of surgical resection is difficult given the scarcity of
information regarding ICI treatment for mCRC metastatic colorectal
cancers in the conversion setting. There was a risk of local
regrowth and distant metastases, and the patient was eager to
undergo complete resection.
Radiological assessment of the tumor response to
immunotherapy is difficult (16).
Pseudoprogression is the initial tumor growth followed by latent or
delayed response (25). Ten
percent of patients with dMMR/MSI-H mCRC exhibited such a
phenomenon during the first 3 months into immunotherapy (26). Such a phenomenon may pose a
quandary on whether immunotherapy should be continued or switched
to another regimen or surgery, especially when surgical resection
is intended. The response assessment by radiology and
histopathology reportedly showed poor correspondence with a
previous study on immunotherapy for colorectal cancer (8). Biomedical imaging of tumor
progression is still mainly designed to determine lesion size
alone, which is reflected in the Response Evaluation Criteria in
Solid Tumors. Conventional assessment of treatment effect using the
tumor size on CT is not appropriate in some cases; the refinement
or innovation of new imaging modalities or new biomarkers that
provide a more accurate assessment of ICI treatment effects is
needed (27). Notably, at the
initial assessment in the present case, the tumor showed a late
enhanced marginal area on CE-CT, which suggests viable tumor cells.
Gadoxetic acid-enhanced magnetic resonance imaging before
preoperative immunotherapy revealed the same enhancement pattern as
CE-CT and showed high-signal intensity in diffusion-weighted
imaging. Such an enhanced area was not observed after
immunotherapy. Positron emission tomography (PET) imaging was found
useful in distinguishing pseudoprogression in melanoma brain
metastases, wherein an enlarged tumor exhibited a low tracer
uptake, from true tumor progression, which presents an intense
tracer uptake (28). In the
present case, we did not perform PET-CT before immunotherapy.
However, PET-CT after immunotherapy showed no elevation of
fluorodeoxyglucose uptake in the tumor. CE-CT and PET-CT may prove
to be useful in detecting viable cells in tumors and in assessing
the treatment effect of ICIs (29).
The present case report demonstrates the successful
resection of an initially unresectable metastatic colon cancer
obtaining pCR after dual ICI therapy. The case findings suggest
methods of using immunotherapy in conversion setting. Initial
immunotherapy could be considered as the standard management
protocol for dMMR/MSI-H mCRC in conversion setting, however,
further research is needed to confirm this suggestion.
Acknowledgements
Not applicable.
Funding
This research was supported by the Japan Agency for Medical
Research and Development (AMED; grant no. JP 18kk0205004), JSPS
KAKENHI (grant no. JP18K07339) and National Cancer Center Research
and Development Fund (grant no. 31-A-2).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SI, TO, HI and JY are responsible for the conception
of the study, development of the study design, and writing of the
paper. SI, TO, HI, MN, MH and HK were involved in the clinical and
therapeutic management of the patient. SI, TO, MN, MH and HK
acquired the data, and MN, MH and HK contributed clinical advice.
JY contributed to the critical revision of the manuscript and
provided important intellectual content. HS, MS and KA contributed
to the interpretation of the pathological findings. SI and TO
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Ibaraki Prefectural Central Hospital, Ibaraki Cancer Center
(approval no. 1040).
Patient consent for publication
Written informed consent for publication of clinical
details and images was obtained from the patient.
Authors' information
Dr Shota Igaue: ORCID ID, 0000-0001-5194-8828.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Colombet M, Soerjomataram I,
Mathers C, Parkin DM, Piñeros M, Znaor A and Bray F: Estimating the
global cancer incidence and mortality in 2018: GLOBOCAN sources and
methods. Int J Cancer. 144:1941–1953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar
|
|
4
|
van der Pool AE, Damhuis RA, Ijzermans JN,
de Wilt JH, Eggermont AM, Kranse R and Verhoef C: Trends in
incidence, treatment and survival of patients with stage IV
colorectal cancer: A population-based series. Colorectal Dis.
14:56–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fakih MG: Metastatic colorectal cancer:
Current state and future directions. J Clin Oncol. 33:1809–1824.
2015. View Article : Google Scholar
|
|
6
|
Akagi K, Oki E, Taniguchi H, Nakatani K,
Aoki D, Kuwata T and Yoshino T: Real-world data on microsatellite
instability status in various unresectable or metastatic solid
tumors. Cancer Sci. 112:1105–1113. 2021. View Article : Google Scholar
|
|
7
|
Lumish MA and Cercek A: Immunotherapy for
the treatment of colorectal cancer. J Surg Oncol. 123:760–774.
2021. View Article : Google Scholar
|
|
8
|
André T, Shiu KK, Kim TW, Jensen BV,
Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs
P, et al: Pembrolizumab in microsatellite-instability-high advanced
colorectal cancer. N Engl J Med. 383:2207–2218. 2020. View Article : Google Scholar
|
|
9
|
Overman MJ, Lonardi S, Wong KYM, Lenz HJ,
Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill
A, et al: Durable clinical benefit with nivolumab plus ipilimumab
in DNA mismatch repair-deficient/microsatellite instability-high
metastatic colorectal cancer. J Clin Oncol. 36:773–779. 2018.
View Article : Google Scholar
|
|
10
|
Seymour MT and Morton D: FOxTROT: An
international randomised controlled trial in 1052 patients (pts)
evaluating neoadjuvant chemotherapy (NAC) for colon cancer. J Clin
Oncol. 37 (Suppl 15):S35042019. View Article : Google Scholar
|
|
11
|
Bassi C, Marchegiani G, Dervenis C, Sarr
M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink
MG, et al: The 2016 update of the International Study Group (ISGPS)
definition and grading of postoperative pancreatic fistula: 11
years after. Surgery. 161:584–591. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Krishnamoorthy M, Lenehan JG and Maleki
Vareki S: Neoadjuvant immunotherapy for high-risk, resectable
malignancies: Scientific rationale and clinical challenges. J Natl
Cancer Inst. 113:823–832. 2021. View Article : Google Scholar
|
|
13
|
Lee AY and Brady MS: Neoadjuvant
immunotherapy for melanoma. J Surg Oncol. 123:782–788. 2021.
View Article : Google Scholar
|
|
14
|
Cloughesy TF, Mochizuki AY, Orpilla JR,
Hugo W, Lee AH, Davidson TB, Wang AC, Ellingson BM, Rytlewski JA,
Sanders CM, et al: Neoadjuvant anti-PD-1 immunotherapy promotes a
survival benefit with intratumoral and systemic immune responses in
recurrent glioblastoma. Nat Med. 25:477–486. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jia XH, Xu H, Geng LY, Jiao M, Wang WJ,
Jiang LL and Guo H: Efficacy and safety of neoadjuvant
immunotherapy in resectable nonsmall cell lung cancer: A
meta-analysis. Lung Cancer. 147:143–153. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chalabi M, Fanchi LF, Dijkstra KK, Van den
Berg JG, Aalbers AG, Sikorska K, Lopez-Yurda M, Grootscholten C,
Beets GL, Snaebjornsson P, et al: Neoadjuvant immunotherapy leads
to pathological responses in MMR-proficient and MMR-deficient
early-stage colon cancers. Nat Med. 26:566–576. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kinney RE and Khalil M: Neoadjuvant
immunotherapy in microsatellite-instability high nonmetastatic
colorectal cancer: A single-institute experience and review of the
literature. Clin Colorectal Cancer. 20:e109–e112. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tomasello G, Petrelli F, Ghidini M, Russo
A, Passalacqua R and Barni S: FOLFOXIRI plus bevacizumab as
conversion therapy for patients with initially unresectable
metastatic colorectal cancer: A systematic review and pooled
analysis. JAMA Oncol. 3:e1702782017. View Article : Google Scholar
|
|
19
|
Gruenberger T, Bridgewater J, Chau I,
García Alfonso P, Rivoire M, Mudan S, Lasserre S, Hermann F,
Waterkamp D and Adam R: Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in
patients with initially unresectable liver metastases from
colorectal cancer: The OLIVIA multinational randomised phase II
trial. Ann Oncol. 26:702–708. 2015. View Article : Google Scholar
|
|
20
|
Hu H, Wang K, Huang M, Kang L, Wang W,
Wang H, Qiu M, Lin R, Zhang H, Lan P, et al: Modified FOLFOXIRI
with or without cetuximab as conversion therapy in patients with
RAS/BRAF wild-type unresectable liver metastases colorectal cancer:
The FOCULM multicenter phase II trial. Oncologist. 26:e90–e98.
2021. View Article : Google Scholar
|
|
21
|
Yang J, Bi F and Gou H: Complete
pathologic response after concurrent treatment with pembrolizumab
and radiotherapy in metastatic colorectal cancer: A case report.
Onco Targets Ther. 14:2555–2561. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Curran MA, Montalvo W, Yagita H and
Allison JP: PD-1 and CTLA-4 combination blockade expands
infiltrating T cells and reduces regulatory T and myeloid cells
within B16 melanoma tumors. Proc Natl Acad Sci USA. 107:4275–4280.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sharma P and Allison JP: The future of
immune checkpoint therapy. Science. 348:56–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Postow MA, Chesney J, Pavlick AC, Robert
C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK,
Agarwala SS, et al: Nivolumab and ipilimumab versus ipilimumab in
untreated melanoma. N Engl J Med. 372:2006–2017. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wolchok JD, Hoos A, O'Day S, Weber JS,
Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, et al:
Guidelines for the evaluation of immune therapy activity in solid
tumors: Immune-related response criteria. Clin Cancer Res.
15:7412–7420. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Colle R, Radzik A, Cohen R, Pellat A,
Lopez-Tabada D, Cachanado M, Duval A, Svrcek M, Menu Y and André T:
Pseudoprogression in patients treated with immune checkpoint
inhibitors for microsatellite instability-high/mismatch
repair-deficient metastatic colorectal cancer. Eur J Cancer.
144:9–16. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gerwing M, Herrmann K, Helfen A,
Schliemann C, Berdel WE, Eisenblätter M and Wildgruber M: The
beginning of the end for conventional RECIST-novel therapies
require novel imaging approaches. Nat Rev Clin Oncol. 16:442–458.
2019. View Article : Google Scholar
|
|
28
|
Kebir S, Rauschenbach L, Galldiks N,
Schlaak M, Hattingen E, Landsberg J, Bundschuh RA, Langen KJ,
Scheffler B, Herrlinger U and Glas M: Dynamic
O-(2-[18F]fluoroethyl)-L-tyrosine PET imaging for the detection of
checkpoint inhibitor-related pseudoprogression in melanoma brain
metastases. Neuro Oncol. 18:1462–1464. 2016. View Article : Google Scholar
|
|
29
|
Ma Y, Wang Q, Dong Q, Zhan L and Zhang J:
How to differentiate pseudoprogression from true progression in
cancer patients treated with immunotherapy. Am J Cancer Res.
9:1546–1553. 2019.PubMed/NCBI
|