Introduction
According to the Global Cancer Observatory,
>2,200,000 new cases of lung cancer were registered in 2020 and
the number of deaths was ~1,800,000, showcasing that lung cancer is
an important disease endangering health worldwide. Lung cancer is
the leading cause of cancer morbidity and mortality in males. Among
females, the incidence of lung cancer is third only to breast
cancer and colorectal cancer, whereas the mortality rate is second
after breast cancer (1). Non-small
cell lung cancer (NSCLC) accounts for ~85% of all cases of lung
cancer. Due to the inconspicuous symptoms of NSCLC, >55% of
patients with NSCLC have already progressed to an advanced stage
when they are diagnosed, with local spread or distant metastasis
and poor prognosis (2,3).
For the treatment of NSCLC, radical surgery is the
preferred option. However, drugs and radiotherapy become
particularly important for patients with advanced NSCLC who cannot
be treated with surgery (4).
Previously, carboplatin or cisplatin combined with gemcitabine,
vinorelbine or paclitaxel were commonly used in the clinic
(5). However, in recent years,
drastic changes have taken place in the treatment of advanced
NSCLC. With the rapidly advancing knowledge of tumor pathogenesis
and the increasing number of studies that have been dedicated to
investigating this phenomenon, molecular targeted therapy has
become a research ‘hotspot’ (6,7). At
present, the drugs that have been indicated to be effective as
first-line therapies in targeted therapy research include erlotinib
(8) and gefitinib (9); certain other drugs, such as
osimertinib (10), have also been
indicated to be efficient as secondline treatments. However, to the
best of our knowledge, limited research has been performed to
investigate drug selection after secondline treatment and docetaxel
combined with cisplatin is frequently used in the clinic at present
(11,12).
Anlotinib is a novel multitarget tyrosine kinase
inhibitor (TKI), which is able to inhibit both tumor angiogenesis
and signal transduction pathways associated with proliferation
(13). The main mechanism may be
inhibition of activation of VEGFR2, PDGFRβ and FGFR1 and downstream
ERK signal transduction. In 2018, Han et al (14) demonstrated the effectiveness of
anlotinib as a third-line treatment for advanced NSCLC through a
multicenter randomized controlled study. However, this study only
compared with a placebo and not with other drugs. After anlotinib
became commercially available in China in May 2018, additional
randomized controlled trials (RCTs) have compared the efficacy of
anlotinib with that of other drugs. In order to explore the most
suitable choice of drugs for the third-line treatment of patients
with advanced NSCLC and to help formulate a better treatment
strategy for NSCLC, the present study comprises a systematic review
and metaanalysis to explore the efficacy and safety of third-line
treatment with anlotinib for patients with advanced NSCLC. The
findings in the present study were reported according to the
‘Preferred Reporting Items for Systematic reviews and
Meta-Analyses’ (15).
Materials and methods
Data sources and searches
Studies were searched from PubMed, Web of Science,
the Cochrane Library (https://www.cochranelibrary.com/) and several Chinese
databases, including Chinese National Knowledge Infrastructure
(CNKI; http://www.cnki.net/), WangFang data
(https://www.wanfangdata.com.cn/) and VIP
(https://www.cqvip.com/), up to February 2022.
Searches were based on combinations of the following index terms:
‘anlotinib’, ‘tyrosine kinase inhibitor’, ‘advanced non-small cell
lung cancer’ and ‘third line therapy’. At the same time, the
references included within each individual study were searched to
supplement the relevant information.
Retrieved articles were included in the present
study as long as the following criteria were satisfied: i) The
study was an RCT; ii) patients with refractory advanced NSCLC that
had been confirmed by pathology or cytology had been treated
previously with two or more chemotherapeutic drugs; iii) the
patient was over 18 years of age; iv) there were no restrictions on
the disclosure of other patient information, such as the sex of the
patient; v) the anlotinib group had been treated with anlotinib;
vi) the control group was treated with either a placebo or other
drugs excluding anlotinib; and vii) the results included at least
one outcome out of progression-free survival (PFS), overall
survival (OS), disease control rate (DCR), objective response rate
(ORR), Karnofsky performance status (KPS) or adverse events
(AEs).
The exclusion criteria were as follows: i) Studies
without original research data; ii) the study was published in a
language other than English or Chinese; and iii) publications
containing the same data as those published elsewhere.
Data extraction and quality
assessment
A total of two researchers (BWZ and YXZ)
independently screened the literature, extracted the data and
cross-checked them. If any differences were identified, these were
settled through discussion or by negotiation with a third party.
When screening articles, the title was read first and after
excluding obviously irrelevant articles, the abstract and full text
were subsequently read to determine whether or not to include them.
If deemed to be necessary, the author of the original research
study was contacted by e-mail or telephone to obtain any
information that was unclear but important for the present study.
The data extraction contents included the following: Basic
information included in the article, i.e., the research topic,
first author, journal wherein the data had been published; baseline
characteristics and intervention measures of research subjects; key
elements of bias risk assessment; and the relevant outcome
indicators and result measurement data.
Two researchers (BWZ and YXZ) also independently
evaluated the bias risk of inclusion in the study and cross-checked
the results. The bias risk assessment that was adopted was the RCT
bias risk assessment tool recommended by the Cochrane Manual 5.1.0
(16).
Data analysis
RevMan 5.3 software (2014; Cochrane Cooperation
Center) was used for the statistical analysis. The survival data
were extracted from Kaplan-Meier curves using Engauge digitizer 4.1
software (17). The hazard ratio
(HR) of PFS and OS, and the risk ratio (RR) of the DCR, ORR and AEs
were calculated by RevMan5.3. The mean differences (MDs) of KPS
were also calculated by RevMan5.3. Q-statistics were used to
evaluate the statistical heterogeneity among experiments. If the
P-value of the Q-statistics was indicated to be <0.1 or
I2 was >50%, this was considered to indicate that the
heterogeneity among studies was statistically significant. If there
was significant heterogeneity, the data were analyzed according to
the random-effects model; otherwise, the fixed-effects model was
adopted (18).
According to the intervention measures of the
control group and the basic information of the patients, subgroup
analysis was conducted to reduce the level of heterogeneity. For
the outcomes of >10 articles, a funnel chart was used to test
the publication bias. P<0.05 was considered to indicate a
statistically significant difference. All P-values were bilateral
and the bilateral coverage rate of the CI was 95%.
Results
Study selection
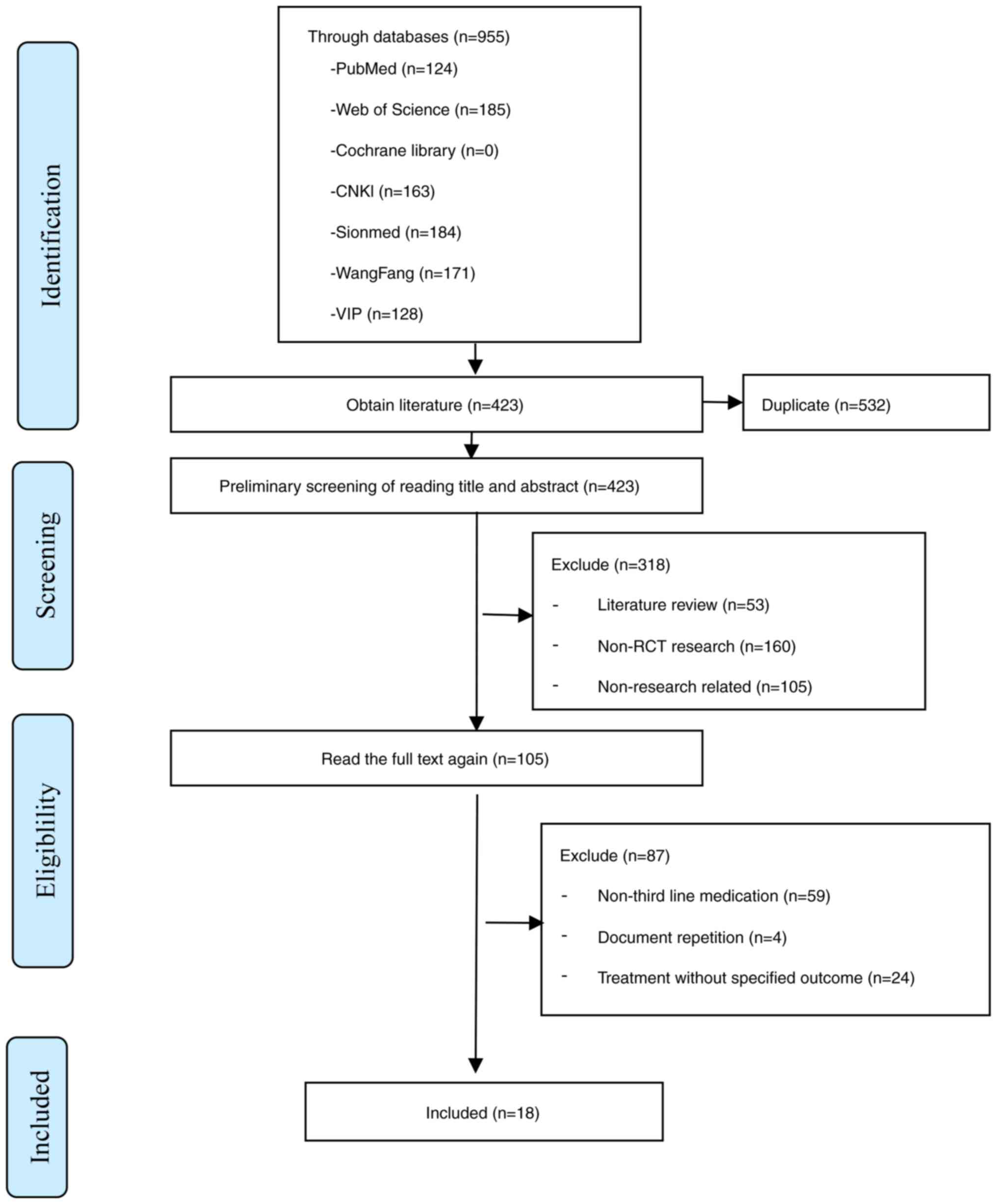
In the present study, a total of 955 articles were
searched in various databases, as detailed above, and after
screening, a total of 18 RCTs were included in the study (14,19–35).
A flow chart of the study selection process is presented in
Fig. 1.
Study characteristics and quality
assessments
A total of 1,658 patients were included in the
present study, comprising 913 patients in the anlotinib group (550
male and 363 female patients) and 745 patients in the control group
(479 male and 266 female patients). The average age of the patients
in each study ranged from 51.30 to 66.50 years. In the present
study, the anlotinib groups of each study were treated with only
anlotinib (12 mg daily for 21 days). Each study control group used
different measures, such as a placebo or supportive treatment. The
details are presented in Table
I.
 | Table I.Basic characteristics of included
studies. |
Table I.
Basic characteristics of included
studies.
|
| Male cases | Age, years | Intervention
measure |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Author and year
(trial number) | T (%) | C (%) | T | C | T | C | Outcomes | (Refs.) |
|---|
| Chen, et al,
2021 | 28 (62.2) | 27 (60.0) | 54.28±5.10 | 54.34±5.06 |
Anlotiniba | Gemcitabine +
cisplatinb | DCR, ORR, KPS,
AEs | (19) |
| Dai, et al,
2019 | 14 (70.0) | 12 (60.0) | - | - |
Anlotiniba | Support
therapyc | PFS, OS, DCR,
ORR | (20) |
| Feng, et al,
2020 | 38 (63.3) | 40 (66.7) | 65.73±5.23 | 64.36±4.84 |
Anlotiniba | Routine
chemotherapyd | DCR, ORR, AEs | (21) |
| Gou, et al,
2020 | 14 (38.3) | 13 (37.1) | 62.72±14.23 | 65.68±16.86 |
Anlotiniba | Pemetrexed +
cisplatine | DCR, ORR, KPS,
AEs | (22) |
| Han, et al,
2021 | 26 (57.8) | 25 (55.6) | 56.93±4.23 | 56.58±4.11 |
Anlotiniba | Gemcitabine +
cisplatinb | DCR, ORR, AEs | (23) |
| Han, et al,
2018 (ALTER0302) | 26 (43.3) | 33 (57.9) | 55.2±10.0 | 55.5±9.1 |
Anlotiniba |
Placebof | PFS, OS, DCR, ORR,
AEs | (24) |
| Han, et al,
2018 (ALTER0303) | 188 (64.0) | 97 (67.8) | 57.9±9.1 | 56.8±9.1 |
Anlotiniba |
Placebof | PFS, OS, DCR, ORR,
AEs | (14) |
| Huang 2020 | 20 (57.1) | 21 (60.0) | 66.7±5.3 | 66.3±5.7 |
Anlotiniba | Routine
chemotherapyd | DCR, ORR, AEs | (25) |
| Kong, et al,
2020 | 15 (68.2) | 14 (63.6) | 57.47±8.13 | 55.38±10.44 |
Anlotiniba |
Placebof | DCR, ORR, KPS | (26) |
| Liu, et al,
2020 | 33 (66.0) | 31 (62.0) | 60.94±10.74 | 60.74±10.98 |
Anlotiniba | Routine
chemotherapyd | DCR, ORR, KPS,
AEs | (27) |
| Luo and Yu,
2021 | 12 (52.2) | 13 (56.5) | 56.43±3.82 | 55.36±4.36 |
Anlotiniba | Gemcitabine+
cisplatinb | DCR, ORR | (28) |
| Si, et al,
2019 | 9 (56.3) | 4 (50.0) | 57.5 | 62.5 |
Anlotiniba |
Placebof | PFS, OS, DCR, ORR,
AEs | (29) |
| Sun, 2021 | 13 (54.2) | 14 (58.3) | 51.48±1.78 | 51.51±1.84 |
Anlotiniba | Gemcitabine+
cisplatinb | ORR, AEs | (30) |
| Tian, 2021 | 30 (61.2) | 28 (62.2) | 50.64±10.36 | 52.00±11.06 |
Anlotiniba | Gemcitabine +
vinorelbineg | DCR, ORR, KPS,
AEs | (31) |
| Wang, 2020 | 15 (68.2) | 16 (72.7) | 66.3±1.7 | 66.5±1.5 |
Anlotiniba |
Placebof | AEs | (32) |
| Yang, 2019 | 23 (63.9) | 9 (50.0) | - | - |
Anlotiniba |
Placebof | PFS, OS, DCR, ORR,
AEs | (33) |
| Yu and Liu,
2021 | 24 (60.0) | 24 (60.0) | 56.6±9.8 | 56.5±9.6 |
Anlotiniba | Support
therapyc | DCR, ORR, AEs | (34) |
| Zhu, 2021 | 22 (64.7) | 23 (67.6) | 65.38±5.81 | 65.47±5.84 |
Anlotiniba | Routine
chemotherapyd | DCR, ORR, AEs | (35) |
A total of 10 studies were based on the method of
random grouping (14,19–24,29,31,34),
whereas two studies had reported the methods of allocation
concealment (14,24). Furthermore, five articles
explicitly used doubleblinding (14,24,26,29,33),
whereas only two articles had missing data (24,29).
None of the studies featured selective reporting. The results are
presented in Fig. 2.
Effectiveness analysis results
PFS
A total of five studies described the PFS (14,20,24,29,33).
The interstudy heterogeneity was low with I2=27%, and
analysis was thus performed using the fixed-effects model. The
results indicated that there was a significant difference in PFS
between the anlotinib group and the control group (HR=0.33; 95% CI:
0.28-0.37; P<0.00001; Fig. 3).
In addition, two studies performed subgroup analyses according to
the basic characteristics of patients (14,24)
and the results indicated that the differences between the
anlotinib group and the control group were not associated with
basic demographic characteristics, such as age and sex. Significant
differences in PFS comparing between the anlotinib group and the
control group were identified when considering patients in the
categories of <60 years or >60 years of age, having a history
or no history of smoking, having a history or no history of
receiving radiotherapy, benefiting or not benefiting from epidermal
growth factor receptor (EGFR)-TKI treatment, and having or lacking
the EGFR gene mutation.
OS
A total of five studies reported on OS (14,20,24,29,33).
The interstudy heterogeneity was low I2=0%, so that
analysis was performed using the fixed-effects model. The results
suggested that there was a significant difference in OS between the
anlotinib group and the control group (HR=0.70; 95% CI, 0.60-0.81;
P<0.00001; Fig. 4). A study
conducted subgroup analysis based on patients’ age, gender, tissue
type, smoking history and other information to determine whether
different disease states have an impact on the efficacy of
anlotinib (14). The results
indicated an improvement of OS after treatment with anlotinib in
all subgroups.
DCR
A total of 16 studies reported on the DCR (14,19–29,31,33–35).
The heterogeneity between studies was I2=82%, so that
analysis was performed using the random-effects model. The results
suggested that the DCR in the anlotinib group was significantly
higher compared with that in the control group (RR=1.51; 95% CI,
1.27-1.79; P<0.00001; Fig. 5).
Subgroup analysis was performed according to the measures used in
the control group. After grouping, the I2 of the
heterogeneity test in each group was 0, indicating that the control
measure may be one of the factors leading to the heterogeneity
among DCR studies. Subgroup analysis indicated that the anlotinib
group was significantly different from the placebo, routine
chemotherapy, gemcitabine + cisplatin, supportive treatment and
gemcitabine + vinorelbine groups. However, no significant
difference was observed between the anlotinib group and the
pemetrexed + cisplatin group. The results are presented in Table II.
 | Table II.Subgroup analysis of the disease
control rate of the third-line treatment of advanced non-small cell
lung cancer in the anlotinib group and control group. |
Table II.
Subgroup analysis of the disease
control rate of the third-line treatment of advanced non-small cell
lung cancer in the anlotinib group and control group.
|
|
|
| Heterogeneity |
| Meta-analysis
results |
|---|
|
|
|
|
|
|
|
|---|
| Control
measure | Number of studies
(Refs.) | Cases | P-value | I2
value | Model | RR (95% CI) | P-value |
|---|
| Placebo | 5 (14,24,26,29,33) | 428/248 | 0.63 | 0 | Fixed | 2.17
(1.83-2.56) | <0.001 |
| Routine
chemotherapy | 4 (21,25,28,35) | 179/179 | 0.78 | 0 | Fixed | 1.23
(1.10-1.36) | <0.001 |
| Gemcitabine +
cisplatin | 3 (19,23,27) | 113/113 | 0.55 | 0 | Fixed | 1.16
(1.04-1.30) | 0.007 |
| Support
therapy | 2 (20,34) | 60/60 | 0.55 | 0 | Fixed | 2.39
(1.57-3.63) | <0.001 |
| Pemetrexed +
cisplatin | 1 (22) | 36/36 | - | - | - | 1.23
(0.97-1.55) | 0.08 |
| Gemcitabine +
vinorelbine | 1 (31) | 49/45 | - | - | - | 2.57
(1.01-6.57) | 0.05 |
ORR
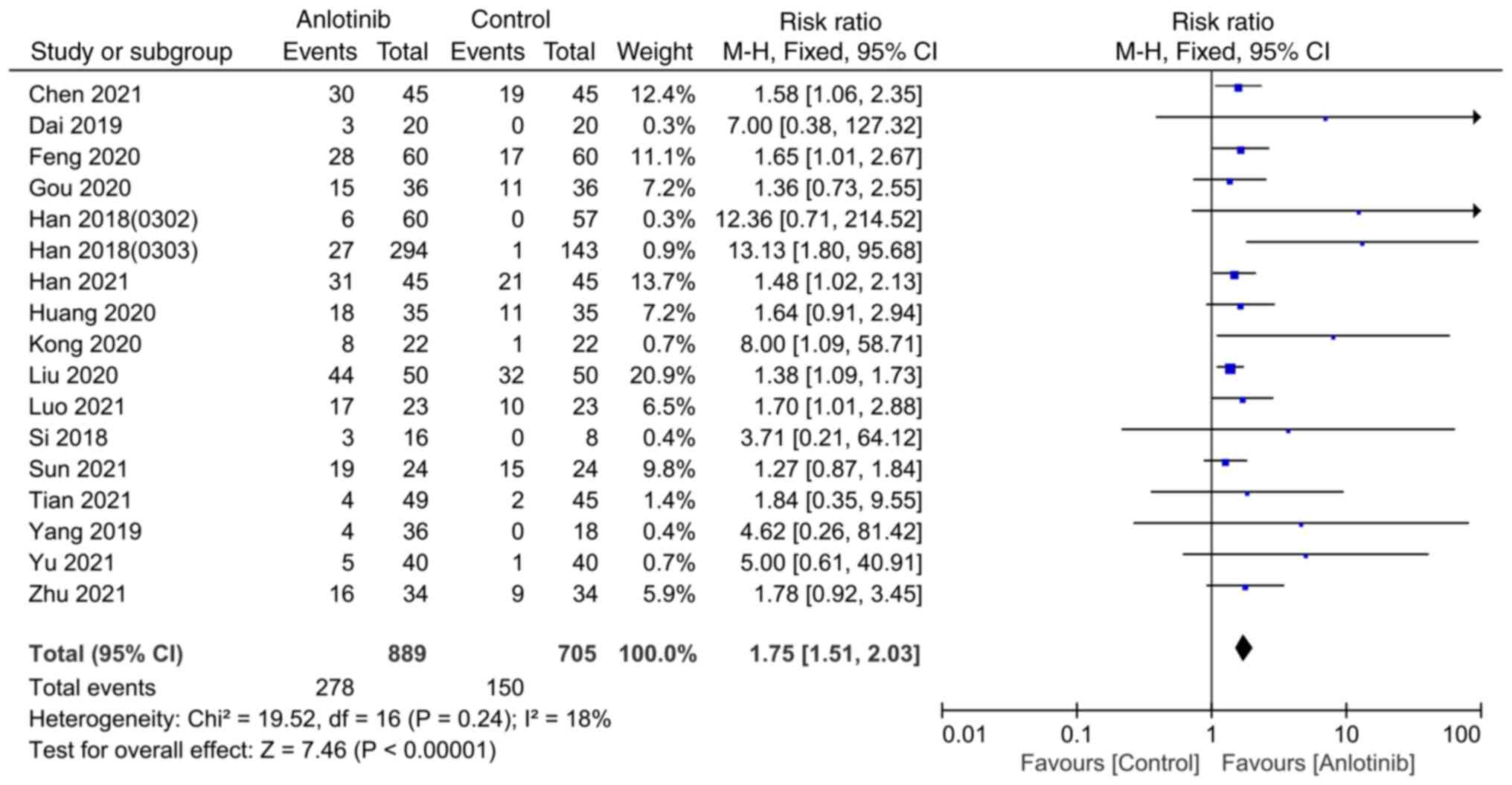
A total of 17 studies reported the ORR (14,19–31,33–35).
The heterogeneity among studies was I2=18%, and the
fixed-effects model was used for analysis. The results indicated
that the ORR of the anlotinib group was significantly higher
compared with that of the control group (RR=1.75; 95% CI,
1.51-2.03; P<0.00001; Fig. 6).
Subgroup analysis was performed according to the measures used by
the control groups of each study. After grouping, the heterogeneity
test results of each group were low (I2=0), indicating
that the control measures may be one of the factors leading to the
heterogeneity of the ORR studies. Subgroup analysis suggested that
there were significant differences between the anlotinib group and
the placebo, conventional chemotherapy, gemcitabine + cisplatin and
supportive treatment groups, although no statistically significant
differences were obtained between the anlotinib group and the
pemetrexed + cisplatin group or the gemcitabine + vinorelbine
group. The results are presented in Table III.
 | Table III.Subgroup analysis of the objective
response rate of the third-line treatment of advanced non-small
cell lung cancer in anlotinib group and control group. |
Table III.
Subgroup analysis of the objective
response rate of the third-line treatment of advanced non-small
cell lung cancer in anlotinib group and control group.
|
|
|
| Heterogeneity |
| Meta analysis
results |
|---|
|
|
|
|
|
|
|
|---|
| Control
measure | Number of studies
(Refs.) | Cases | P-value | I2
value | Model | RR (95% CI) | P-value |
|---|
| Placebo | 5 (14,24,26,29,33) | 428/248 | 0.94 | 0 | Fixed | 8.98
(3.06-26.38) | <0.001 |
| Routine
chemotherapy | 4 (21,25,28,35) | 179/179 | 0.76 | 0 | Fixed | 1.54
(1.25-1.89) | <0.001 |
| Gemcitabine +
cisplatin | 4 (19,23,27,30) | 137/137 | 0.79 | 0 | Fixed | 1.49
(1.22-1.83) | <0.001 |
| Support
therapy | 2 (20,34) | 60/60 | 0.85 | 0 | Fixed | 5.67
(1.04-31.00) | <0.05 |
| Pemetrexed +
cisplatin | 1 (22) | 36/36 | - | - | - | 1.36
(0.73-2.55) | 0.33 |
| Gemcitabine +
vinorelbine | 1 (31) | 49/45 | - | - | - | 1.84
(0.35-9.55) | 0.47 |
KPS
A total of five studies reported the KPS (19,22,26,27,31).
The heterogeneity among studies was I2=87%, and thus,
data were analyzed using the random-effects model. The results
suggested that the KPS of the anlotinib group was significantly
higher compared with that of the control group (MD=9.85; 95% CI,
6.26-13.43; P<0.00001; Fig. 7).
However, due to the small number of studies and the lack of
subgroup information in each study, no further analysis was
possible.
Sensitivity analysis
Sensitivity analysis was performed for PFS, OS, DCR,
ORR and KPS. For this, the results of each of the 18 articles were
excluded at a time and it was observed whether the pooled results
had changed. It was found that exclusion of none of the individual
studies affected the final result (data not shown).
Safety
A total of 13 articles reported the incidence of AEs
in the anlotinib group and the control group in detail (14,19,21–25,27,29–33).
The major AEs were included in the pooled analysis and there were
eight studies on hypertension. The research on hypertension,
hand-foot syndrome, fatigue, oral mucositis and
hypertriglyceridemia had a high level of heterogeneity, so the
random-effects model was used. For the other studies, the
fixed-effects model was used. It was observed the anlotinib group
was significantly higher than the control group in terms of seven
AEs, namely hypertension, hand-foot syndrome, hemoptysis,
proteinuria, cough, diarrhea and hypercholesterolemia, whereas no
statistically significant differences were identified for the other
AEs, as indicated in Table
IV.
 | Table IV.AEs in the anlotinib group and
control group with third-line treatment of advanced non-small cell
lung cancer. |
Table IV.
AEs in the anlotinib group and
control group with third-line treatment of advanced non-small cell
lung cancer.
|
|
|
| Heterogeneity |
| Meta-analysis
results |
|---|
|
|
|
|
|
|
|
|---|
| AEs | Number of studies
(Refs.) | Cases | P-value | I2
value | Model | RR (95% CI) | P-value |
|---|
| Hypertension | 8 | 596/512 | <0.001 | 80 | Random | 3.14
(1.43-6.90) | 0.004 |
| Placebo
or support therapy | 4 (14,24,29,33) | 406/226 | 0.09 | 54 | Random | 5.74
(2.89-11.40) | <0.001 |
| Other
drugs | 4 (19,21,24,31) | 190/186 | 0.11 | 51 | Random | 1.42
(0.60-3.39) | 0.43 |
| Hand-foot
syndrome | 7 | 551/367 | 0.002 | 71 | Random | 3.07
(1.23-7.64) | 0.02 |
| Placebo
or support therapy | 4 (14,24,29,33) | 406/226 | 0.60 | 0 | Fixed | 5.08
(3.19-8.08) | <0.001 |
| Other
drugs | 3 (19,22,31) | 145/141 | 0.02 | 76 | Random | 1.50
(0.21-10.50) | 0.68 |
| Hemoptysis | 7 | 536/370 | 0.41 | 2 | Fixed | 1.81
(1.23-2.66) | 0.003 |
| Placebo
or support therapy | 4 (14,28,33,34) | 392/230 | 0.73 | 0 | Fixed | 2.41
(1.46-3.96) | <0.001 |
| Other
drugs | 3 (21,25,31) | 144/140 | 0.77 | 0 | Fixed | 0.99
(0.52-1.88) | 0.98 |
| Proteinuria | 6 | 502/322 | 0.85 | 0 | Fixed | 2.15
(1.48-3.11) | <0.001 |
| Placebo
or support therapy | 4 (14,24,29,33) | 406/226 | 0.78 | 0 | Fixed | 2.21
(1.47-3.30) | <0.001 |
| Other
drugs | 2 (21,22) | 96/96 | 0.59 | 0 | Fixed | 1.83
(0.71-4.76) | 0.21 |
| Nausea and
vomiting | 6 | 240/229 | 0.56 | 0 | Fixed | 0.76
(0.48-1.18) | 0.22 |
| Placebo
or support therapy | 2 (24,29) | 76/65 | 0.60 | 0 | Fixed | 0.78
(0.42-1.42) | 0.41 |
| Other
drugs | 4 (19,23,27,30) | 164/164 | 0.30 | 18 | Fixed | 0.74
(0.38-1.42) | 0.36 |
| Fatigue | 5 | 466/304 | 0.002 | 76 | Random | 0.87
(0.56-1.37) | 0.55 |
| Placebo
or support therapy | 2 (14,24) | 354/200 | 0.12 | 60 | Random | 1.44
(0.90-2.29) | 0.13 |
| Other
drugs | 3 (21,22,30) | 112/104 | 0.51 | 0 | Fixed | 0.73
(0.52-1.04) | 0.07 |
| Oral Mucositis | 5 | 479/313 | 0.004 | 74 | Random | 3.37
(0.94-12.17) | 0.06 |
| Placebo
or support therapy | 3 (14,24,29) | 370/208 | 0.87 | 0 | Fixed | 8.64
(3.58-20.84) | <0.001 |
| Other
drugs | 2 (21,31) | 109/105 | 0.21 | 37 | Fixed | 1.13
(0.55-2.34) | 0.74 |
| Anaemia | 4 | 150/132 | 0.75 | 0 | Fixed | 0.47
(0.22-1.02) | 0.75 |
| Placebo
or support therapy | 1 (33) | 36/18 | - | - | - | 0.67
(0.27-1.63) | 0.37 |
| Other
drugs | 3 (19,23,30) | 114/114 | 0.87 | 0 | Fixed | 0.29
(0.07-1.17) | 0.08 |
| Cough | 4 | 411/257 | 0.23 | 30 | Fixed | 1.56
(1.19-2.04) | 0.001 |
| Placebo
or support therapy | 3 (14,24,32) | 376/222 | 0.14 | 49 | Fixed | 1.61
(1.21-2.13) | 0.001 |
| Other
drugs | 1 (25) | 35/35 | - | - | - | 1.14
(0.46-2.81) | 0.77 |
| Diarrhea | 3 | 370/208 | 0.48 | 0 | Fixed | 2.52
(1.72-3.69) | <0.001 |
| Placebo
or support therapy | 3 (14,24,29) | 370/208 | 0.48 | 0 | Fixed | 2.52
(1.72-3.69) | <0.001 |
|
Hypercholesterolemia | 3 | 370/208 | 0.75 | 0 | Fixed | 3.14
(2.12-4.66) | <0.001 |
| Placebo
or support therapy | 3 (14,24,29) | 370/208 | 0.75 | 0 | Fixed | 3.14
(2.12-4.66) | <0.001 |
|
Hypertriglyceridemia | 3 | 370/208 | 0.09 | 58 | Random | 1.95
(0.91-4.17) | 0.09 |
| Placebo
or support therapy | 3 (14,24,29) | 370/208 | 0.09 | 58 | Random | 1.95
(0.91-4.17) | 0.09 |
The AEs were analyzed in subgroups and divided into
groups according to the control measures, with placebo or
supportive treatment as one group and other drugs as the other
group. It was determined that the anlotinib group had a
significantly higher incidence of hypertension, hand-foot syndrome,
hemoptysis, proteinuria, oral mucositis, diarrhea and
hypertriglyceridemia than the placebo or supportive treatment
group. However, no significant differences in each outcome index
were identified between the anlotinib group and other drug groups,
as indicated in Table IV.
In addition, four studies reported on the occurrence
of AEs of grade 3 or above (14,24,29,31).
The results indicated that there was no significant difference
between the anlotinib group and control group in terms of
hypertension (RR=4.68, 95% CI, 0.44-49.58; P=0.20), hemoptysis
(RR=1.51, 95% CI, 0.464.91; P=0.49) and oral mucositis (RR=1.26,
95% CI, 0.29-5.41; P=0.76). However, the anlotinib group had a
significantly higher incidence of hand-foot syndrome than the
control group (RR=5.28, 95% CI, 1.18-23.59; P=0.03). Among the four
studies on AEs, three were placebo studies and one used gemcitabine
+ vinorelbine. Subgroup analysis was conducted according to the
control measures. The results indicated that the incidence of grade
3 or above AEs in the anlotinib group was significantly higher than
compared with that in the placebo group with respect to
hypertension and hand-foot syndrome, although no significant
differences were observed when comparing between the anlotinib
group and the gemcitabine + vinorelbine group, as indicated in
Table V.
 | Table V.Grade 3 or above AEs in the anlotinib
group and control group with third-line treatment of advanced
non-small cell lung cancer. |
Table V.
Grade 3 or above AEs in the anlotinib
group and control group with third-line treatment of advanced
non-small cell lung cancer.
|
|
|
| Heterogeneity |
| Meta-analysis
results |
|---|
|
|
|
|
|
|
|
|---|
| AEs | Number of studies
(Refs.) | Cases | P-value | I2
value | Model | RR (95% CI) | P-value |
|---|
| Hypertension | 4 | 419/253 | 0.02 | 68 | Random | 4.68
(0.44-49.58) | 0.20 |
|
Placebo | 3 (14,24,29) | 370/208 | 0.52 | 0 | Fixed | 19.59
(3.76-102.20) | <0.001 |
|
Gemcitabine + Vinorelbine | 1 (31) | 49/45 | - | - | - | 0.31
(0.03-2.84) | 0.30 |
| Hemoptysis | 4 | 419/253 | 0.55 | 0 | Fixed | 1.51
(0.46-4.91) | 0.49 |
|
Placebo | 3 (14,24,29) | 370/208 | 0.86 | 0 | Fixed | 2.17
(0.53-8.10) | 0.30 |
|
Gemcitabine + Vinorelbine | 1 (31) | 49/45 | - | - | - | 0.31
(0.01-7.34) | 0.47 |
| Oral mucositis | 4 | 419/253 | 0.56 | 0 | Fixed | 1.26
(0.29-5.41) | 0.76 |
|
Placebo | 3 (14,24,29) | 370/208 | 0.72 | 0 | Fixed | 2.52
(0.30-21.10) | 0.40 |
|
Gemcitabine + Vinorelbine | 1 (31) | 49/45 | - | - | - | 0.46
(0.04-4.89) | 0.52 |
| Hand-foot
syndrome | 4 | 419/253 | 0.80 | 0 | Fixed | 5.28
(1.18-23.59) | 0.03 |
|
Placebo | 3 (14,24,29) | 370/208 | 0.63 | 0 | Fixed | 5.99
(1.10-32.70) | 0.04 |
|
Gemcitabine + Vinorelbine | 1 (31) | 49/45 | - | - | - | 2.76
(0.12-66.07) | 0.53 |
Publication bias
Drawing funnel graphs based on the DCR and ORR
revealed that the two plots were left- and right-asymmetrical, as
indicated in Figs. 8 and 9. This suggested that there may have been
a certain amount of publication bias.
Discussion
At present, drugs available for the thirdline
treatment of advanced NSCLC are limited. The first-line and
second-line treatment schemes for patients with NSCLC with positive
or negative driver gene mutations have been well-defined, but only
a small number of drugs are in clinical use for the third-line
treatment and the standardized treatment has not been perfected
(36). Previous studies have used
monoclonal antibodies or macromolecular vascular targeting drugs,
such as bevacizumab, to treat advanced NSCLC, although their safety
standards did not reach a satisfactory level (37). At present, pemetrexed or docetaxel
is recommended for patients with the negative driver genes of
non-squamous cell carcinoma. Anlotinib was approved for use in
China in May 2018 (38).
Anlotinib is a novel type of multitarget
small-molecule drug with antiangiogenesis and anti-tumor properties
that is able to inhibit tumor angiogenesis and cell proliferation
by selectively inhibiting vascular endothelial growth factor (VEGF)
receptor, fibroblast growth factor receptor and plateletderived
growth factor receptor (39). In
addition to its usefulness for NSCLC, anlotinib monotherapy has
also achieved good results for a variety of other solid tumors,
including liver cancer (40).
Through systematic evaluation and meta-analysis, the
present study indicated that, compared with the control group, the
third-line treatment of advanced NSCLC with anlotinib was able to
significantly improve the DCR, ORR and KPS, and significantly
prolong PFS and OS. Subgroup analysis according to the treatment
measures of the control group indicated that the DCR and ORR of the
anlotinib group were significantly higher compared with those in
the placebo, supportive treatment, conventional chemotherapy and
gemcitabine + cisplatin groups. However, compared with the
pemetrexed + cisplatin and gemcitabine + vinorelbine groups, the
DCR and ORR values failed to exhibit significant differences,
although, given the relative sparsity of published studies in this
area, it is not possible at present to draw any firm conclusions in
relation to this. Subgroup analysis for PFS indicated that the
difference between the anlotinib group and the control group was
not associated with baseline characteristics, such as the age and
sex of the patients. In conclusion, compared with the placebo or
supportive treatment groups, the curative effect of anlotinib on
advanced NSCLC was appreciable. Compared with treatment with a
combination of gemcitabine + cisplatin and therapy with other drugs
currently used for third-line treatment, anlotinib also had
advantages in terms of effectiveness.
In addition to the abovementioned studies included
in the meta-analysis, several other studies have reported on other
indicators with regard to evaluating their curative effects. Due to
the different measurement methods and the small number of studies,
however, it was not suitable for these studies to be included in
the combined analysis, although several of these studies will now
be briefly described. Two studies reported on the changes in tumor
marker concentration in patients prior to and after intervention
(21,27). The results suggested that, after
treatment, the serum carcinoembryonic antigen and
cytokeratin-19-fragment in the anlotinib group were significantly
lower compared with those in the control group. Another study
reported on the changes in lung function (27), which suggested that the forced
expiratory volume in one second and 6-minute walking distance in
the anlotinib group were significantly higher compared with those
in the control group. A further study discussed the analysis of
blood gas (28) and revealed that
the partial pressure of oxygen and oxygen saturation of the
arterial blood in the anlotinib group were higher compared with
those in the control group, whereas the partial pressure of
CO2 was lower than that in the control group, and this
difference was statistically significant. The above findings also
reflect different aspects of the effectiveness of anlotinib in
improving the physical condition of patients with advanced
NSCLC.
In terms of safety, the present study suggested that
there were significant differences between the anlotinib group and
the control group with respect to hypertension, hand-foot syndrome,
hemoptysis, proteinuria, cough, diarrhea and hypercholesterolemia,
but no statistical differences between the two groups were
identified for the remaining five adverse reactions, including
nausea and vomiting, fatigue, mucositis, anaemia and
hypertriglyceridemia. This finding was not consistent with those of
a previous study by Yu et al (41), presumably because it included three
trials in which the control group was either a placebo or
supportive treatment. However, among the 18 studies included in the
present study, a large proportion of the control groups used other
drugs for treatment, and other therapeutic drugs also caused AEs.
According to the above discussion, the present study suggests that,
compared with other drugs currently used for thirdline treatment,
anlotinib does not exhibit any safety deficiency.
Among the 18 articles included in the present study,
the AEs of interest were hypertension, nausea and vomiting,
hand-foot syndrome and hemoptysis. Nausea is a common
gastrointestinal reaction, which may be caused by drugs directly
stimulating the gastric mucosa or by metabolic factors. It is the
cardiovascular AE that is most commonly associated with
hypertension VEGF pathway inhibitors, a phenomenon that may be
linked to the endothelial dysfunction and the reduction in nitric
oxide release caused by drugs (42). Hemoptysis is an important AE of
anlotinib, which may be associated with its inhibition of the VEGF
receptor, thrombocytopenia and increased bleeding risk. Hand-foot
syndrome is mainly characterized by abnormal sensation, dullness or
numbness of hands and feet, and blisters, ulcers or pain may also
occur in severe cases. This may be associated with anlotinib acting
on the signaling pathway of vascular repair in the compressed parts
of hands and feet (43).
A total of four studies reported on the occurrence
of high-level AEs and the results suggested that there was a
significant difference between the anlotinib group and the control
group in hypertension and handfoot syndrome. Si et al
(29) reported in detail the
treatment of AEs after they occurred; hand-foot syndrome and oral
mucositis were obviously relieved after anlotinib was administered
at a reduced dose of 10 mg, once a day, and other AEs were also
well improved after symptomatic treatment. No treatment-associated
deaths were reported in either study. It may be indicated that AEs
associated with anlotinib are tolerable.
The advantages of the present study are as follows:
i) There were 18 RCTs included in this study, and the results
obtained are reliable; ii) subgroup analysis was carried out
according to the measures of the control group, and the therapeutic
effects and safety of anlotinib were compared with those of
different control measures; and iii) the effectiveness of this
study was evaluated by OS and four other indicators, with special
attention paid to high-level AEs when evaluating safety, and the
evaluation was therefore more comprehensive. The limitations of
this study were as follows: i) The time to market is short and the
patients were all Chinese, so it is impossible to assess the
influence of treatment with anlotinib on other populations; ii)
since certain of the publications did not report on subgroups, the
present study mainly comprised subgroup analysis according to
control measures and sufficient subgroup analysis was not carried
out on other factors; and iii) numerous studies featured
small-sample analysis and there was lack of larger-sample
studies.
According to the results of the present study, it is
considered that for patients with NSCLC who have relapsed after
receiving two types of systemic chemotherapy, it is possible to
consider using anlotinib for treatment. At present, the drug is
administered in a 3-week cycle, at a recommended dose of 12 mg once
a day, with an interval for 1 week (i.e., the third week in the
cycle) after 2 weeks of treatment. However, it is necessary to
detect and prevent the occurrence of AEs. Blood pressure and blood
lipid levels should be monitored regularly and oral mucosa and skin
reactions should also be paid attention to. Anlotinib should also
be used with caution in patients who are at high risk of bleeding
and have hepatic renal insufficiency.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used or analyzed during the study are
available from the corresponding author on reasonable request.
Authors’ contributions
BWZ designed the review and meta-analysis. YXZ and
RZY conceived and wrote the review. BWZ and YXZ acquired and
analyzed the data. BWZ and RZY analyzed and confirmed the integrity
of the data found in the literature. MDK was involved in designing
the research and drafting the manuscript. All authors contributed
to the analysis and reviewed the results and all authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
RCT
|
randomized controlled trial
|
|
HR
|
hazard ratio
|
|
RR
|
risk ratio
|
|
MD
|
mean difference
|
|
AE
|
adverse event
|
|
TKI
|
tyrosine kinase inhibitor
|
|
PFS
|
progression-free survival
|
|
OS
|
overall survival
|
|
DCR
|
disease control rate
|
|
ORR
|
objective response rate
|
|
KPS
|
Karnofsky performance status
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality Worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of nonsmall cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aokage K, Yoshida J, Hishida T, Tsuboi M,
Saji H, Okada M, Suzuki K, Watanabe S and Asamura H: Limited
resection for early-stage non-small cell lung cancer as
function-preserving radical surgery: A review. Jpn J Clin Oncol.
47:7–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Duma N, Santana-Davila R and Molina JR:
Non-small cell lung cancer: Epidemiology, Screening, diagnosis, and
treatment. Mayo Clin Proc. 94:1623–1640. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Broderick SR: Adjuvant and Neoadjuvant
immunotherapy in non-small cell lung cancer. Thorac Surg Clin.
30:215–220. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ruiz-Cordero R and Devine WP: Targeted
therapy and checkpoint immunotherapy in lung cancer. Surg Pathol
Clin. 13:17–33. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al: Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): An open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liang W, Zhong R and He J: Osimertinib in
EGFR-mutated lung cancer. N Engl J Med. 384:6752021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Belani CP and Eckardt J: Development of
docetaxel in advanced non-small-cell lung cancer. Lung Cancer. 46
(Suppl 2):S3–S11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cortot AB, Audigier-Valette C, Molinier O,
Le Moulec S, Barlesi F, Zalcman G, Dumont P, Pouessel D, Poulet C,
Fontaine-Delaruelle C, et al: Weekly paclitaxel plus bevacizumab
versus docetaxel as second- or third-line treatment in advanced
non-squamous non-small-cell lung cancer: Results of the IFCT-1103
ULTIMATE study. Eur J Cancer. 131:27–36. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen G, Zheng F, Ren D, Du F, Dong Q, Wang
Z, Zhao F, Ahmad R and Zhao J: Anlotinib: A novel multi-targeting
tyrosine kinase inhibitor in clinical development. J Hematol Oncol.
11:1202018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han B, Li K, Wang Q, Zhang L, Shi J, Wang
Z, Cheng Y, He J, Shi Y, Zhao Y, et al: Effect of anlotinib as a
third-line or further treatment on overall survival of patients
with advanced non-small cell lung cancer: The ALTER 0303 phase 3
Randomized clinical trial. JAMA Oncol. 4:1569–1575. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The cochrane collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343:d59282011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tierney JF, Stewart LA, Ghersi D, Burdett
S and Sydes MR: Practical methods for incorporating summary
time-to-event data into meta-analysis. Trials. 8:162007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Borenstein M, Hedges LV, Higgins JP and
Rothstein HR: A basic introduction to fixed-effect and
random-effects models for meta-analysis. Res Synth Methods.
1:97–111. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen B, Sun J, Cai W and Chen J: Efficacy
of anlotinib as a third-line therapy for non-small cell lung
cancer. Chin J Clin Oncol Rehabil. 28:558–560. 2021.
|
|
20
|
Dai X, Wang Y, Han L, Liu Y and Li R:
Clinical emcacy of AIllotinib in the treatment of advanced
non-small cell lung cancer. Chin Med Herald. 16:95–98. 2019.
|
|
21
|
Feng Y, Yao F, Wang W, Guo J and Zhu Z:
Short-term curative effect of anlotinib on non-small cell lung
cancer and its influences on CTC and VGEF levels in peripheral
blood and quality of life. J Clin Exp Med. 19:2399–2403. 2020.
|
|
22
|
Gou F, Yu D, Qiao Q and Zhou X: Clinical
observation of anlotinib in the treatment of advanced lung
adenocarcinoma with targeted therapy. Anti-Tumor Pharm. 10:343–348.
2020.
|
|
23
|
Han B, Kan N and Wang S: Explore the
clinical efficacy and short-term prognosis of Anlotinib in the
third-line treatment of advanced non-small cell lung cancer
(NSCLC). Health Guide. 89–90. 2021.(In Chinese).
|
|
24
|
Han B, Li K, Zhao Y, Li B, Cheng Y, Zhou
J, Lu Y, Shi Y, Wang Z, Jiang L, et al: Anlotinib as a third-line
therapy in patients with refractory advanced non-small-cell lung
cancer: A multicentre, randomised phase II trial (ALTER0302). Br J
Cancer. 118:654–661. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang P, Li W and Zhang H: Effect of
anlotinib capsule on the level of VEGF and survival period in
advanced non-small cell lung cancer. J Pract Cancer. 35:360–362.
2020.
|
|
26
|
Kong Q: Clinical effect of antinib in the
thirdline treatment of nonsmall cell lung cancer and its clinical
significance security analysis. Chin J Mod Drug Appl. 14:164–166.
2020.
|
|
27
|
Liu Q, Li J, Wang K and Zhang Y: Clinical
efficacy of antinib in the treatment of patients with advanced lung
cancer after operation. Mod Diagn Treat. 31:2602–2604. 2020.
|
|
28
|
Luo K and Yu H: Clinical observation on
the treatment of advanced non-small cell lung cancer with Anrotinib
III. Healthmust-Readmagazine. 7:452021.(In Chinese).
|
|
29
|
Si XY, Wang HP, Zhang XT, Wang MZ and
Zhang L: Efficacy and safety of anlotinib in 16 patients with
advanced non-small cell lung cancer. Zhonghua Nei Ke Za Zhi.
57:830–834. 2018.(In Chinese). PubMed/NCBI
|
|
30
|
Sun X: Clinical efficacy and safety
analysis of third-line treatment with enrotinib for advanced
non-small cell lung cancer. Oriental Med Diet. 28:1172021.
|
|
31
|
Tian T, He M, Wu F, Lu Y and Liu Y:
Short-term efficacy of anlotinib in the treatment of non-small cell
lung cancer and its influence on serum tumor markers CTC VGEF and
side effects. J Hebei Nat Sci. 27:1908–1912. 2021.
|
|
32
|
Wang C: The efficacy of Anlotinib capsule
in the treatment of advanced non-small cell lung cancer. Chin Heal
Standard Management. 11:75762020.(In Chinese).
|
|
33
|
Yang Y: The Efficacy of Anlotinib as a
thirdor furtherline treatment in Patients with Advanced NSCLC.
Master's Thesis. Zhengzhou University; 2018, (In Chinese).
|
|
34
|
Yu C and Liu L: Observation on the effect
of third-line Anrotinib on advanced non-small cell lung cancer.
Medical Innovation of China. 18:69–72. 2021.(In Chinese).
|
|
35
|
Zhu Y: Short-term efficacy and life of
anlotinib thirdline and above in the treatment of advanced nonsmall
cell lung cancer. Healthmust-Readmagazine. 24:982021.(In
Chinese).
|
|
36
|
Nagano T, Tachihara M and Nishimura Y:
Molecular mechanisms and targeted therapies including immunotherapy
for non-small cell lung cancer. Current Cancer Drug Targets.
19:595–630. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao T, Wang X, Xu T, Xu X and Liu Z:
Bevacizumab significantly increases the risks of hypertension and
proteinuria in cancer patients: A systematic review and
comprehensive meta-analysis. Oncotarget. 8:51492–51506. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Syed YY: Anlotinib: First global approval.
Drugs. 78:1057–1062. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xie C, Wan X, Quan H, Zheng M, Fu L, Li Y
and Lou L: Preclinical characterization of anlotinib, a highly
potent and selective vascular endothelial growth factor receptor-2
inhibitor. Cancer Sci. 109:1207–1219. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
He C, Wu T and Hao Y: Anlotinib induces
hepatocellular carcinoma apoptosis and inhibits proliferation via
Erk and Akt pathway. Biochem Biophys Res Commun. 503:3093–3099.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yu G, Shen Y, Xu X and Zhong F: Anlotinib
for refractory advanced non-small-cell lung cancer: A systematic
review and meta-analysis. PLoS One. 15:e02429822020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Abdel-Qadir H, Ethier JL, Lee DS,
Thavendiranathan P and Amir E: Cardiovascular toxicity of
angiogenesis inhibitors in treatment of malignancy: A systematic
review and meta-analysis. Cancer Treat Rev. 53:120–127. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ogawa C, Morita M, Omura A, Noda T, Kubo
A, Matsunaka T, Tamaki H, Shibatoge M, Tsutsui A, Senoh T, et al:
Hand-foot syndrome and post-progression treatment are the good
predictors of better survival in advanced hepatocellular carcinoma
treated with sorafenib: A multicenter study. Oncology. 93 (Suppl
1):S113–S119. 2017. View Article : Google Scholar
|