Introduction
Cervical cancer affects millions of women globally,
ranking as the fourth most commonly occurring cancer among women
worldwide (1). The cervical cancer
incidence rate is increasing in China, with 109,700 new cases and
59,000 associated deaths recorded in 2020 (2). A combination of external beam
radiation therapy (EBRT) and brachytherapy (BT) with concurrent
chemotherapy is the standard treatment for locally advanced
cervical cancer. Over the last 20 years, clinical outcomes have
improved and toxicity has been reduced due to the development of
more sophisticated planning and delivery techniques, and the
introduction of computer technology and imaging (3,4).
The delivery of an adequate radiation dose to the
cervical tumor area through the traditional approach is limited by
the normal structures in the pelvic cavity, including the bladder
and rectum, which are sensitive to radiation. Tomotherapy (TOMO) is
a novel radiation therapy modality (5); it is a form of intensity-modulated
radiation therapy (IMRT) that uses a helical 360-degree radiation
delivery system. TOMO delivers image-guided radiation therapy by
comparing daily pretreatment megavoltage computed tomography (CT)
scans with CT scans performed at the time of simulation for
treatment planning. The rapid opening and closing of the leaves in
the collimator rotating around the patient allows TOMO to tailor
the application of radiation doses to tumor regions of complex
shape, while the dose to normal organs is limited (6,7). In
comparison to conventional IMRT techniques, TOMO may provide
sharper dose gradients around the target, leading to more
efficacious sparing of surrounding normal structures and
potentially fewer radiation-related side effects (8–10).
However, the potential benefit of TOMO over IMRT is still unclear
(11,12).
The purpose of the present study was to compare the
therapeutic response, toxicities and dosimetric parameters between
IMRT and TOMO in patients with advanced cervical cancer, in order
to investigate an optimal treatment modality for the disease.
Patients and methods
Clinical materials
A total of 334 patients [Karnofsky Performance
Status (13) ≥70] diagnosed with
International Federation of Gynecology and Obstetrics (FIGO 2009)
(14) stage IIB-IIIB cervical
cancer, who underwent CCRT between August 2015 and March 2018 at
the Department of Gynecological Oncology, Shandong Cancer Hospital
and Institute, Shandong First Medical University and Shandong
Academy of Medical Sciences (Jinan, China), were included in this
study. A total of 2 patients with a history of ischemic heart
disease, 3 patients with mental illness, 5 patients who were
pregnant or lactating, 6 patients with a previous malignancy, 4
patients whose treatment was interrupted or prolonged for >8
weeks due to a serious complication that would affect full
compliance with treatment and 4 patients who were allergic to
chemotherapeutic drugs were excluded. Finally, 310 patients were
selected to be retrospectively studied. The patients were randomly
divided into the IMRT group (n=155) and the TOMO group (n=155)
based on the type of radiotherapy technique used. The choice of
radiotherapy technique was made by the patients and their doctors
following a discussion on the technical differences and the
differences in treatment cost. All patients completed radiotherapy
within 7–8 weeks. Intracavitary BT was performed after the EBRT
course was complete or in the last week of pelvic EBRT. The
treatment planning aim at point A (defined as a reference location
2 cm above the vaginal fornix and 2 cm beside the mid axis of the
uterus) was >85 Gy in an equivalent dose at 2 Gy (EQD2). In the
IMRT group, patient ages ranged from 28 to 70 years, with a median
age of 53 years. Meanwhile, in the TOMO group, patient ages ranged
from 26 to 74 years, with a median age of 51 years. In this study,
17 patients with stage IIB cervical squamous cell carcinoma aged
<40 years were only treated with laparoscopic ovarian
suspension, without removing the cervical tumor, before
radiotherapy. During the operation, the ovary was suspended on the
lateral side of the paracolic sulcus, equivalent to 2–3 cm above
the umbilical level, and fixed to the abdominal wall. The position
of the ovary was marked with a silver clip. Before and after
radiotherapy, ovarian endocrine function was evaluated according to
perimenopausal symptoms and serum hormone levels. All the
procedures were performed in accordance with the Declaration of
Helsinki and relevant policies in China. Ethical approval was
obtained from the Ethics Committee of Shandong Cancer Hospital and
Institute, Shandong First Medical University and Shandong Academy
of Medical Sciences (approval no. SDTHEC2021012058). The
clinicopathological characteristics of all patients are shown in
Table I.
 | Table I.Clinicopathological characteristics of
patients treated with IMRT (n=155) and TOMO (n=155). |
Table I.
Clinicopathological characteristics of
patients treated with IMRT (n=155) and TOMO (n=155).
| Characteristics | IMRT group | TOMO group | P-value |
|---|
| Median age (range),
years | 53 (28–70) | 51 (26–74) | 0.924a |
| FIGO stage, n |
|
| 0.352 |
|
IIB | 46 | 57 |
|
|
IIIA | 3 | 4 |
|
|
IIIB | 106 | 94 |
|
| Histological type,
n |
|
| <0.999 |
|
Squamous carcinoma | 146 | 147 |
|
|
Adenocarcinoma | 6 | 5 |
|
|
Other | 3 | 3 |
|
| Tumor size, n |
|
| 0.36 |
| ≤4
cm | 64 | 72 |
|
| >4
cm | 91 | 83 |
|
| Tumor grade, n |
|
| 0.109 |
| G1 | 48 | 54 |
|
| G2 | 50 | 61 |
|
| G3 | 57 | 40 |
|
| Ovary conserving,
n |
|
| 0.212 |
| No | 149 | 144 |
|
|
Yes | 6 | 11 |
|
| Pathological
morphology type, n |
|
| 0.123 |
|
Exophytic | 135 | 125 |
|
|
Endophytic type | 20 | 30 |
|
| Para-aortic lymph
node metastasis, n |
|
| 0.627 |
|
Positive | 8 | 10 |
|
|
Negative | 147 | 145 |
|
| TNM stage, n |
|
| 0.533 |
|
T2bN0 | 40 | 53 |
|
|
T2bN1 | 6 | 4 |
|
|
T3aN0 | 3 | 4 |
|
|
T3bN0 | 79 | 71 |
|
|
T3bN1 | 27 | 23 |
|
| Pelvic lymph node
metastasis, n |
|
| 0.388 |
|
Positive | 33 | 27 |
|
|
Negative | 122 | 128 |
|
| Therapeutic
response rate, n |
|
|
|
| CR | 147 | 148 | 0.791 |
| PR | 3 | 4 | 0.709 |
| CR +
PR | 150 | 152 | 0.902 |
Chemotherapy
All patients were treated with 4 cycles of
concurrent chemotherapy during RT. The chemotherapy consisted of an
infusion of paclitaxel (135 mg/m2) on day 1 and
cisplatin (75 mg/m2) on day 2 every 4 weeks.
Radiotherapy
For IMRT, a 6-MV photon beam with six to nine
co-planar beams and CT-based treatment planning (Pinnacle version
9.2; Philips Healthcare) was used. The doses were delivered using a
linear-accelerator equipped with multi-leaf collimators (MLCs).
Inverse treatment planning was performed using the ADAC Pinnacle3
Treatment Planning System (Philips Healthcare). All plans used
dynamic MLCs to shape the fields.
The TOMO plans were calculated on the Tomotherapy
Planning Station Hi-Art® Version 4.2.3 workstation
(Tomotherapy Inc.) with a superposition/convolution algorithm. Due
to workstation limitations, CT contouring and organ at risk (OAR)
images were drawn in Version 9.2 of the Pinnacle3 planning system
and transferred to the TOMO planning system.
All patients underwent initial CT simulation in a
supine position with their arms by their sides, using intravenous
contrast agents and free breathing. A customized immobilization
device was fabricated encompassing the lower abdomen, pelvis and
upper thighs to make the daily setup accurate. For the scanning
range, the upper boundary was at the upper edge of the first lumbar
vertebral body, and the lower boundary was 5 cm below the ischium
tuberosity, with a scanning layer thickness of 5 mm.
The therapy plans were delivered with doses of the
planning target volume (PTV). The gross tumor volume (GTV),
clinical target volume (CTV) and PTV were contoured on the
individual CT slices of each patient. The PTV consisted of the CTV
plus a 5-mm margin. The CTV included the whole uterus and cervix,
part of the vagina depending upon the lower extent of the tumor,
the paracervical, parametrial and uterosacral regions, and the
common iliac, external iliac, internal iliac and obturator lymph
nodes. In patients with common iliac and/or para-aortic lymph node
(PALN) involvement, extended-field pelvic and para-aortic
radiotherapy was recommended, up to the level of the renal vessels
(or even more cephalad as directed by involved nodal distribution).
The whole pelvic radiation therapy plan was performed to deliver a
dose of 45–50 Gy in 1.8-Gy daily fractions, with 5 fractions per
week in the center of the PTV. Parametrial boost irradiation of
5–10 Gy was performed in the patients with bulky parametrial/pelvic
sidewall disease after completion of initial whole pelvic radiation
at the discretion of the attending physician. For patients with
common iliac lymph node or PALN metastasis, a para-aortic field
radiotherapy plan was also performed at the same time. The
prescribed dose was 60 Gy.
OARs included the suspended ovary, intestinal pouch,
rectum, bladder and bilateral femoral head, in which the ovary was
considered the whole ovary marked with a silver clip at the lateral
paracolonic sulcus and the intestinal pouch included the intestinal
canal and its surrounding mesenteric tissue as shown by contrast
medium. The upper boundary of the rectum was the junction of the
rectosigmoid colon, the lower boundary was the anus and the bladder
included all the bladders in the filling state. For dose limitation
of OARs, the following parameters were applied: Percentage of
normal tissue receiving at least 5 Gy (V5) in the ovary, <50%;
percentage of normal tissue receiving at least 40 Gy (V40) in the
intestinal pouch, <50%; V40 in the rectum, <40%; V40 in the
bladder, <40%; and V40 in the femoral head, <5%.
To verify the setup accuracy, orthogonal electronic
portal images were captured once a day in the first 3 days of
radiotherapy and then once a week during the whole course of
radiotherapy. After treatment, the physicians informed the patients
whether their bladder and rectum preparation were suitable, in
order to help the patients to prepare their bladder and rectum on
non-imaging days. On non-imaging days, patients were positioned
with skin line marks. The GTV, CTV and PTV were contoured on the
individual axial CT slices of each patient. Normal structures,
including the small bowel, rectum, bladder, kidney and pelvic bone
marrow, were also entered into the planning CT scan. The small
bowel was contoured from the L4-5 interspace to the level of the
sigmoid flexure.
Dose-volume analysis of treatment
plans
Dose-volume histograms (DVHs) of the PTVs and the
critical normal structures were analyzed accordingly. For PTVs, the
volume, the volume covered by 95% of the prescription dose (V95),
and the minimum doses delivered to 5% (D5) and 95% (D95) of the PTV
were evaluated. Critical organs with functional subunits organized
in a series were examined. The conformal index (CI) and homogeneity
index (HI) were used to evaluate the conformity and uniformity of
the plan. The volume received the mean dose for the PTV generated
from the DVH. The CI [International Commission on Radiation Units
and Measurements (ICRU)] for PTV was calculated using the following
formula: CIICRU=VTV/VPTV, where
VTV was the ratio of the treated volume enclosed by the
prescription isodose surface and VPTV was the planning
target volume (15). The HI was
defined as D5/D95, where D5 and D95 were the minimum doses
delivered to 5 and 95% of the PTV reported previously (16).
Intracavitary BT
High-dose-rate source iridium-192 was used with a
vaginal ovoid applicator (Tianjin Rongli Electronics Co. Ltd.;
Hanschke applicator set). Post-implantation dosimetry was performed
with the Rl-hzj192Ir Integrated after loading treatment planning
system (Tianjin Rongli Electronics Co. Ltd.) and included
calculation of the dose to Point A bilaterally, the pelvic sidewall
(point B, defined as the point 3 cm from Point A and 5 cm lateral
to midline) bilaterally, and the rectal point and bladder point as
defined by the ICRU (17). First,
a whole pelvic radiotherapy plan was created to deliver a dose of
45–50 Gy. Intracavitary BT was then administered at doses of 25–30
Gy in 4–5 fractions after the EBRT course was complete or in the
last week of pelvic EBRT. The treatment planning aim at point A was
>85 Gy in EQD2. During the treatment of intracavitary BT,
vaginal packing with gauze pushed the bladder and rectum as far
away as possible to reduce the dose. These treatments were
delivered weekly.
Therapeutic effect evaluation
Therapeutic effects were assessed by clinical
examination, ultrasound, CT scans or/and positron emission
tomography (PET)-CT scans after 2–3 months of treatment. According
to the Response Evaluation Criteria in Solid Tumors (18), therapeutic response was classified
as a complete response (CR), partial response (PR), stable disease
or progressive disease.
Toxicity assessment
The acute and chronic toxicity from radiotherapy was
evaluated using the Radiation Therapy Oncology Group (RTOG)
criteria (19) in the therapy
process, after therapy and during follow-up. In patients with grade
4 hematological or non-hematological toxicity, radiation therapy
was halted until toxicity resolved to at least grade 3.
Follow-up
After treatment completion, the patients were
followed up at 3-month intervals for the first 2 years, at 6-month
intervals for the following 3 years and annually thereafter. At
each visit, a physical and pelvic examination, blood counts,
clinical chemistry and chest radiography were performed. Scans of
the abdomen and pelvic region were conducted using ultrasound.
Imaging as appropriate (MRI, CT and PET-CT) was applied in case of
a suspicion of recurrence. Suspected persistent or recurrent
disease was confirmed using biopsy whenever possible. Overall
survival (OS) was measured from the date of diagnosis to the time
of death, or the time of last follow-up. Progression-free survival
(PFS) was measured from the date of diagnosis to the time of
disease recurrence, or the time of last follow-up. Overall survival
(OS) and progression-free survival (PFS) were calculated from the
date of diagnosis. Surviving patients were censored on the date of
the last follow-up. The cause of death was confirmed by telephone,
correspondence or medical record review.
Statistical analysis
The OS and PFS curves were estimated using the
Kaplan-Meier method and compared using the log-rank test. Clinical
characteristics of patients, toxicities, local control, survival
rates and therapeutic response rate were compared using the
χ2 test or Fisher's exact test. Age was analyzed using
unpaired Student's t-test. Dosimetric parameters were analyzed
using the independent-samples t-test. P<0.05 was used to
indicate a statistically significant difference. All analyses were
performed using SPSS version 17.0 (SPSS Inc.).
Results
The clinicopathological characteristics of all
patients were similar between the two groups (Table I). A total of 310 patients were
included in this study. There were no statistically significant
differences in terms of age, FIGO stage, histologic type, tumor
size, tumor grade, pathologic morphology type, para-aortic lymph
node metastasis, TNM stage, pelvic lymph node metastasis and ovary
conservation between the two groups (P=0.924, P=0.352, P>0.999,
P=0.36, P=0.109, P=0.123, P=0.627, P=0.533, P=0.388 and P=0.212,
respectively). Lymph node metastasis is a risk factor for the
recurrence and metastasis of cervical cancer (20). In the present study, lymph node
metastasis was diagnosed when the minor axis of the lymph node was
≥10 mm, determined using contrast-enhanced CT or MRI. Pelvic lymph
node metastasis (PLNM) occurred in 60 out of 310 patients; 33
patients (21.3%) were treated with IMRT and 27 (17.4%) underwent
TOMO. There was no statistically significant difference between the
two groups (P=0.388). In this study, 18 patients had para-aortic
lymph node metastasis (PALNM); 8 patients underwent IMRT and 10
patients underwent TOMO (Table I).
There was no statistical difference between the two groups
(P=0.627).
DVH statistics for OARs and dosimetric parameters
are described in Table II. In the
comparison of TOMO and IMRT, a better CI (0.82±0.0327 vs.
0.75±0.064, respectively; P=0.006) and HI (1.03±0.006 vs.
1.09±0.076, respectively; P<0.0001) were observed by TOMO
planning. Fig. 1A and B show the
isodose curves of a cross section, sagittal section and coronary
section in two representative patients treated with TOMO and IMRT,
respectively; a 95% isodose curve including the PTV is indicated.
TOMO provided more efficient critical organ sparing than IMRT at
the mean dose, and a lower bladder V40 (P=0.029) and V20 (P=0.001),
femoral head V40 (P=0.014), and lower rectum V40 (P=0.035) and V20
(P=0.005) were observed in the planning using TOMO compared with
that using IMRT. TOMO demonstrated a superior ability to protect
the left ovary (maximum dose (Dmax): 4.61 vs. 5.81 Gy, P=0.026; and
mean dose (Dmean): 2.99 vs. 3.97 Gy, P=0.017, respectively) and the
right ovary (Dmax: 4.53 vs. 5.87 Gy, P=0.013; and Dmean: 2.98 vs.
3.84 Gy, P=0.007, respectively). Femoral head V20 (P=0.066)
exhibited a tendency toward more favorable values in TOMO than
IMRT. There were no statistically significant differences in small
bowel V20 (P=0.251), V40 (P=0.575), and bone marrow protection V20
(P=0.917) and V40 (P=0.53) between the IMRT and TOMO plans.
However, TOMO yielded significantly better values for Dmax
parameters for the bone marrow and small bowel, with a
statistically significant level (P=0.004 and 0.002, respectively).
In this study, the ovarian function was preserved in 2 out of 6
patients (33.3%) in the IMRT group and in 5 patients of 11 patients
(45.5%) in the TOMO group. There was no statistical difference in
terms of ovarian function between the two groups (P=0.627).
 | Table II.Dosimetric results of IMRT and TOMO
for planning dosimetric parameters and organs at risk. |
Table II.
Dosimetric results of IMRT and TOMO
for planning dosimetric parameters and organs at risk.
| Parameter | TOMO group | IMRT group | P-value |
|---|
| PTV |
|
|
|
|
D5% | 52.56±0.28 | 54.82±0.22 | 0.001 |
|
D95% | 50.82±0.31 | 50.27±0.27 | 0.001 |
| HI | 1.03±0.006 | 1.09±0.076 | 0.001 |
| CI | 0.82±0.033 | 0.75±0.064 | 0.006 |
| Bladder |
|
|
|
| V40,
% | 27.31±7.16 | 34.13±7.97 | 0.029 |
| V20,
% | 66.34±8.82 | 80.36±8.16 | 0.001 |
| Dmean,
Gy | 29.28±3.01 | 33.07±3.21 | 0.01 |
| Dmin,
Gy | 10.55±1.43 | 9.93±2.40 | 0.47 |
| Dmax,
Gy | 53.34±0.88 | 56.35±3.22 | 0.007 |
| Femoral head-L |
|
|
|
| V40,
% | 0.57±0.49 | 1.16±0.57 | 0.014 |
| V20,
% | 45.46±4.89 | 38.64±10.57 | 0.066 |
| Dmean,
Gy | 21.46±1.07 | 19.84±2.40 | 0.054 |
| Dmin,
Gy | 15.31±0.93 | 10.39±2.18 | 0.001 |
| Dmax,
Gy | 42.89±2.84 | 42.73±5.88 | 0.935 |
| Femoral head-R |
|
|
|
| V40,
% | 0.50±0.55 | 1.43±1.01 | 0.014 |
| V20,
% | 46.35±5.65 | 41.36±9.08 | 0.138 |
| Dmean,
Gy | 21.60±0.61 | 20.03±2.26 | 0.037 |
| Dmin,
Gy | 15.11±0.81 | 8.10±2.42 | 0.001 |
| Dmax,
Gy | 42.67±2.27 | 44.28±4.94 | 0.337 |
| Rectum |
|
|
|
| V40,
% | 22.82±6.53 | 29.18±6.66 | 0.035 |
| V20,
% | 63.41±11.94 | 79.36±12.01 | 0.005 |
| Dmean,
Gy | 28.00±3.20 | 32.35±2.88 | 0.003 |
| Dmin,
Gy | 12.08±1.52 | 12.39±2.79 | 0.751 |
| Dmax,
Gy | 52.41±1.03 | 55.24±3.29 | 0.007 |
| Small bowel |
|
|
|
| V40,
% | 21.01±9.09 | 23.18±8.75 | 0.575 |
| V20,
% | 66.25±10.00 | 61.55±8.63 | 0.251 |
| Dmean,
Gy | 26.74±3.57 | 26.48±3.46 | 0.865 |
| Dmin,
Gy | 1.90±0.40 | 1.79±0.66 | 0.627 |
| Dmax,
Gy | 28.00±3.20 | 28.00±3.20 | 0.002 |
| Bone marrow |
|
|
|
| V40,
% | 26.81±6.08 | 24.82±8.33 | 0.53 |
| V20,
% | 74.42±8.05 | 74.82±9.36 | 0.917 |
| Dmean,
Gy | 29.09±2.73 | 32.37±3.47 | 0.619 |
| Dmin,
Gy | 3.60±3.88 | 4.75±7.12 | 0.641 |
| Dmax,
Gy | 53.71±0.98 | 56.89±3.04 | 0.004 |
| Ovary-L |
|
|
|
| Dmean,
Gy | 2.99±0.65 | 3.97±1.05 | 0.017 |
| Dmin,
Gy | 2.04±0.55 | 2.71±0.55 | 0.01 |
| Dmax,
Gy | 4.61±1.26 | 5.81±1.07 | 0.026 |
| Ovary-R |
|
|
|
| Dmean,
Gy | 2.98±0.59 | 3.84±0.73 | 0.007 |
| Dmin,
Gy | 1.83±0.55 | 2.47±0.43 | 0.007 |
| Dmax,
Gy | 4.53±0.88 | 5.87±1.37 | 0.013 |
Acute and chronic radiotherapy toxicity was assessed
using the RTOG criteria. Genitourinary, gastrointestinal and
hematological complications were some of the most frequent
unwelcome side effects after pelvic RT. Acute major toxic effects
included cystitis, proctitis, leukopenia, dermatitis and enteritis
(Table III). In total, 17
patients (11.0%) in the IMRT group and 5 patients (3.2%) in the
TOMO group experienced grade 3/4 acute proctitis. Grade 3/4
leukopenia occurred in 71 patients (45.8%) in the IMRT group and 60
patients (38.7%) in the TOMO group. A total of 5 patients (2.6%) in
the IMRT group and 3 patients (1.9%) in the TOMO group experienced
grade 3/4 acute cystitis. As shown in Table III, the acute radiation toxicity
of proctitis, cystitis and leukopenia was significantly lower in
the TOMO group than that in the IMRT group (P=0.033, P=0.049 and
P=0.025, respectively). There was no statistically significant
difference in the acute radiation toxicity of enteritis and
dermatitis between the two groups (P=0.055 and 0.616,
respectively).
 | Table III.Crude incidence of acute toxicity in
the IMRT group (n=155) and the TOMO group (n=155) according to
Radiation Therapy Oncology Group/European Organisation for Research
and Treatment of Cancer acute radiation morbidity criteria in
patients with cervical cancer. |
Table III.
Crude incidence of acute toxicity in
the IMRT group (n=155) and the TOMO group (n=155) according to
Radiation Therapy Oncology Group/European Organisation for Research
and Treatment of Cancer acute radiation morbidity criteria in
patients with cervical cancer.
| Grade | IMRT group, n | TOMO group, n | P-value |
|---|
| Leukopenia |
|
| 0.025 |
| 0 | 15 | 34 |
|
| 1 | 14 | 16 |
|
| 2 | 55 | 45 |
|
| 3 | 47 | 46 |
|
| 4 | 24 | 14 |
|
| Cystitis |
|
| 0.049 |
| 0 | 57 | 78 |
|
| 1 | 66 | 60 |
|
| 2 | 27 | 14 |
|
| 3 | 4 | 3 |
|
| 4 | 1 | 0 |
|
| Enteritis |
|
| 0.055 |
| 0 | 54 | 62 |
|
| 1 | 67 | 77 |
|
| 2 | 30 | 14 |
|
| 3 | 3 | 2 |
|
| 4 | 1 | 0 |
|
| Proctitis |
|
| 0.033 |
| 0 | 53 | 71 |
|
| 1 | 67 | 64 |
|
| 2 | 18 | 15 |
|
| 3 | 16 | 5 |
|
| 4 | 1 | 0 |
|
| Dermatitis |
|
| 0.616 |
| 0 | 56 | 61 |
|
| 1 | 72 | 73 |
|
| 2 | 27 | 21 |
|
| 3 | 0 | 0 |
|
| 4 | 0 | 0 |
|
The chronic toxicities were mainly cystitis and
enterocolitis (Table IV).
Overall, 11 patients (7.1%) in the IMRT group experienced grade 3/4
late radiation cystitis. Grade 3/4 late radiation enterocolitis
occurred in 10 patients (6.5%) in the IMRT group. The incidence of
chronic radiation cystitis and enterocolitis in the TOMO group was
3.9% (6/155) for each. As shown in Table IV, the chronic radiation toxicity
of cystitis and enterocolitis was significantly lower in the TOMO
group than in the IMRT group (P=0.041 and 0.023, respectively).
 | Table IV.Chronic toxicities in the IMRT group
(n=155) and the TOMO group (n=155) according to Radiation Therapy
Oncology Group/European Organisation for Research and Treatment of
Cancer acute radiation morbidity criteria in patients with cervical
cancer. |
Table IV.
Chronic toxicities in the IMRT group
(n=155) and the TOMO group (n=155) according to Radiation Therapy
Oncology Group/European Organisation for Research and Treatment of
Cancer acute radiation morbidity criteria in patients with cervical
cancer.
| Grade | IMRT group, n | TOMO group, n | P-value |
|---|
| Cystitis |
|
| 0.041 |
| 0 | 46 | 59 |
|
| 1 | 64 | 72 |
|
| 2 | 34 | 18 |
|
| 3 | 9 | 6 |
|
| 4 | 2 | 0 |
|
| Enterocolitis |
|
| 0.023 |
| 0 | 40 | 55 |
|
| 1 | 60 | 70 |
|
| 2 | 45 | 24 |
|
| 3 | 8 | 5 |
|
| 4 | 2 | 1 |
|
At the study end date in November 2019, the median
follow-up time was 32 months (5–53 months) in the IMRT group and 28
months (5–48 months) in the TOMO group. A total of 6 out of 155
patients (3.9%) were lost to follow-up in the IMRT group and 2 out
of 155 patients (1.3%) were lost to follow-up in the TOMO group,
resulting in the follow-up rates of 96.1 and 98.7% (P=0.175),
respectively. No significant difference was observed in terms of
CR, PR and total response rate (CR + PR) between the TOMO and IMRT
groups (94.8 vs. 95.5%, P=0.791; 1.9 vs. 2.5%, P=0.709; and 96.8
vs. 98.1%, P=0.902, respectively) (Table I). A plot of the survival curves is
shown in Fig. 2. There were no
statistically significant differences in the 1- and 3-year OS rates
between the TOMO and IMRT groups (94.7 vs. 94.8%, P=0.544; and 81.5
vs. 84.7%, P=0.413, respectively) (Fig. 2A). No significant differences were
found in the 1- and 3-year PFS rates between the two groups (89.5
vs. 87.0%, P=0.904; and 80.6 vs. 82.0%, P=0.708, respectively)
(Fig. 2B). A plot of the survival
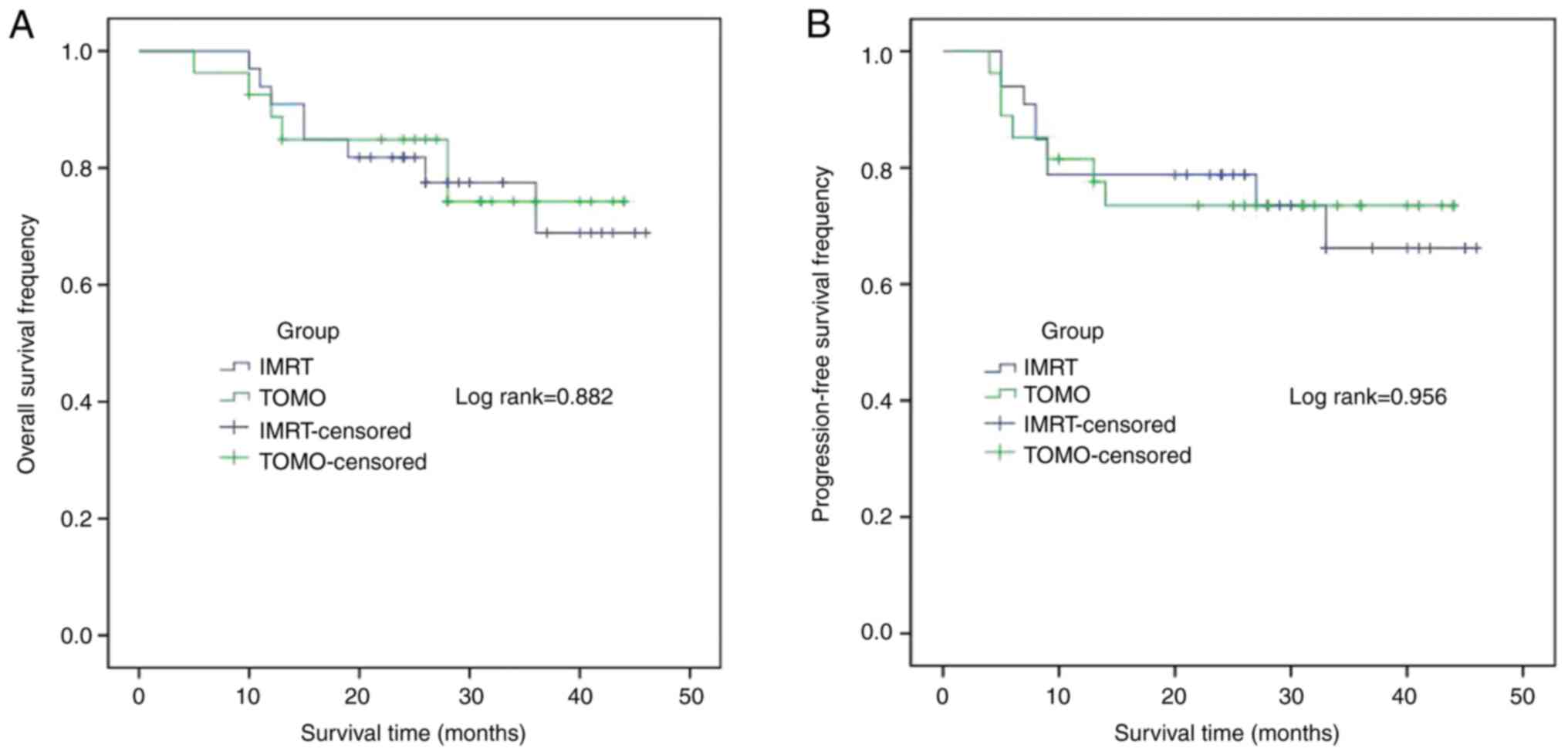
curves for the PLNM patients is shown in Fig. 3. There were no statistical
differences in the 1- and 3-year OS rates between the TOMO and IMRT
groups (88.7 vs. 90.9%, P=0.956; and 74.3 vs. 68.9%, P=0.882,
respectively) (Fig. 3A). In
addition, no significant differences were found in the 1- and
3-year PFS rates between the two groups (81.5 vs. 78.8%, P=0.843;
and 73.5 vs. 66.2%, P=0.956, respectively) (Fig. 3B). A plot of the survival curves
for the patients with PALNM is shown in Fig. 4. For the patients with PALNM, there
were no statistical differences in the 1- and 3-year OS rates
between the TOMO and IMRT groups (90.0 vs. 75.0%, P=0.452; and 80.0
vs. 46.9%, P=0.143, respectively) (Fig. 4A). No significant differences were
found in the 1- and 3-year PFS rates between the two groups with
the PALNM patients (90.0 vs. 50.0%, P=0.078; and 70.0 vs. 25.0%,
P=0.14, respectively) (Fig. 4B) In
the IMRT group, the survival rate was lower than that in the TOMO
group, but there was no statistical difference between the two
groups, which may be related to the small number of cases.
Discussion
The standard treatment for patients affected by
locally advanced cervical cancer is concurrent chemoradiation.
Conformal radiotherapy techniques such as 3D-CRT and IMRT are being
used with increasing frequency with positive results in terms of
decreased toxicity due to the relative sparing of normal tissues.
In the present study, when compared with traditional
intensity-modulated radiotherapy, TOMO was found to can provide
patients with cervical cancer with greater conformal target
coverage, a more homogeneous distribution of the target dose, and
more efficient bladder and rectum sparing.
TOMO is a 360-degree-of-freedom beam projection
radiotherapy, and the number of sub-fields irradiated by a single
dose is >20,000. The addition of further beams would result in
improved conformity without the value of the objective function
being affected (16). The CI value
is typically 0–1, and the closer it is to 1, the better the
conformability of the PTV. A larger HI represents worse PTV
heterogeneity (21,22). The present study results showed
that compared with intensity-modulated radiotherapy, TOMO produced
a significant improvement in dose conformity (0.82±0.0327 vs.
0.75±0.064, respectively; P=0.006) and homogeneity (1.03±0.006 vs.
1.09±0.076, respectively; P<0.001). Several previous studies
have reported that TOMO was superior to IMRT in dose conformity
(0.894±0.006 vs. 0.855±0.008, respectively; P|<0.001) and
homogeneity (1.082±0.006 vs. 1.106±0.006, respectively; P=0.023) in
patients with early cervical cancer and other head and neck cancer
types (23–25).
The ultimate goal of radiotherapy is to improve the
dose control rate of the tumor target and reduce the dose received
by normal tissue as much as possible. Since dose conformity
represents the congruence between iso-dose curves and tumor
contours (26), better conformity
indicates potentially superior tumor target coverage and OAR
protection. In the present study, TOMO provided improved critical
organ sparing compared with IMRT in terms of the average dose.
Compared with IMRT, TOMO had lower bladder V40 (P=0.029), bladder
V20 (P=0.001), and lower rectum V40 (P=0.035) and V20 (P=0.005) in
the planning for patients with advanced cervical cancer. The
femoral head V20 (P=0.066) showed a tendency toward more favorable
values in TOMO than in IMRT, although this was not significant. Guo
et al (23) reported that a
few OARs and dosimetric parameters, including the bladder, rectum
and femoral head, and ovary sparing (P<0.001), exhibited more
favorable values in TOMO than IMRT in patients with early cervical
cancer. This is consistent with several previous studies (26–30),
which have indicated that TOMO outperforms IMRT in terms of dose
conformity in pelvic tumors. The volume of low-dose irradiation of
the intestinal, pelvic and normal tissues is decreased in patients
with TOMO.
It is well known that pelvic radiotherapy can cause
a variety of complications, including small intestinal obstruction,
radiation cystitis, urinary incontinence, fistula and pelvic
fractures (31). TOMO was expected
to reduce the toxicities found when treating pelvic cavity cancer
in practice, in line with these dosimetric data. Retrospective
studies compared the toxicity occurrence in IMRT and TOMO, and
indicated positive results for TOMO (30,32).
In the present study, 17 (11.0%) patients in the IMRT group and 5
(3.2%) in the TOMO group experienced grade 3/4 acute proctitis. In
addition, 4 (2.6%) patients in the IMRT group and 3 patients (1.9%)
in the TOMO group experienced grade 3/4 acute cystitis. The acute
radiation toxicity of proctitis and cystitis was significantly
lower in the TOMO group than that in the IMRT group (P=0.033 and
P=0.049, respectively). These results were in concordance with the
studies by Chang et al (32) and Yao et al (30), which indicated that protection of
the bladder and rectum is a significant advantage of TOMO when
compared with IMRT. Overall, TOMO can decrease the risk of
radiation-induced toxicity in patients undergoing pelvic RT.
Ovarian transposition is mainly suitable for young
patients with cervical cancer who need pelvic RT. In the present
study, before pelvic RT, the arteries and veins of the ovary were
dissected, ovarian blood supply was preserved, and ovaries were
moved outside the irradiation field to avoid the effect of
radiotherapy on ovarian function. The success of ovarian function
preservation after RT is associated with a number of factors, such
as the dose to the ovary during radiotherapy, the age of the
patient, the location of the ovarian displacement and whether
concurrent chemotherapy is administered. The dose to the ovary
during radiotherapy is the most important factor that directly
affects ovarian endocrine function. Therefore, postoperative
radiotherapy planning is required to minimize this dose. TOMO can
produce more complex radiation fields due to the changing
conformation of the multi-leaf collimator. It not only ensures
uniformity and conformity of dose in the target area, but also
avoids OARs that need to be protected in the ray path. At the same
time, its radiation contamination is smaller, so that the OARs can
be accurately excluded (33). For
the displaced ovaries, the target area and ovarian dose can be
better considered, and the possibility of making concessions in the
target range and the conformity is less. Moreover, precise image
guidance can minimize the placement error, ensure the accurate
implementation of the plan, achieve precise treatment and add a
layer of protection for ovarian function. The ovary is a parallel
organ. Damage to ovarian cells during radiotherapy is directly
associated with ovarian function. In the present study, TOMO
demonstrated a superior ability to protect the left ovary (Dmax:
4.61 vs. 5.81 Gy, P=0.026; Dmean: 2.99 vs. 3.97 Gy, P=0.017). Guo
et al (23) reported that
TOMO provided improved ovarian organ sparing compared with IMRT at
the mean dose, and the difference was statistically significant
(P<0.001). Therefore, TOMO radiotherapy is recommended for young
patients who need pelvic radiotherapy. However, ovarian function
was only preserved in 2 out of 6 patients (33.3%) in the IMRT group
and 5 out of 11 patients (45.5%) in the TOMO group. There was no
statistically significant difference in ovarian function between
the two groups (P=0.627). Although there was no significant
difference between the two groups, the ratio of the TOMO group was
higher than that of the IMRT group. This could be associated with
the small sample size of this study. Further prospective randomized
multicenter studies are needed to confirm the benefits of TOMO.
Few published studies (12,34)
have explored tumor control comparing IMRT with TOMO in patients
with locally advanced cervical carcinoma. The present study
demonstrated a similar tumor response rate between the two groups.
There was no significant difference with regard to CR, PR or total
response rate (CR + PR) between the TOMO and IMRT groups (94.8 vs.
95.5%, P=0.791; 1.9 vs. 2.5%, P=0.709; and 96.8 vs. 98.1%, P=0.902,
respectively). There was also no statistically significant
difference in the 1- and 3-year OS rates between the TOMO and IMRT
groups (94.7 vs. 94.8%, P=0.544; and 81.5 vs. 84.7%, P=0.413,
respectively). Furthermore, no significant difference was found in
1- and 3-year PFS rates between the two groups (89.5 vs. 87.0%,
P=0.904; and 80.6 vs. 82.0%, P=0.708, respectively). Wang et
al (34) reported outcomes of
patients with stage IB1-IVA cervical cancer treated with definitive
IMRT. The 3-year OS and PFS rates were 83.0 and 75.0%,
respectively. The study by Chang et al (32) included 15 patients with stage
IB1-IVA cervical cancer, and all patients had received pelvic
irradiation delivered by TOMO. The 3-year OS rate was 93.0% and the
3-year PFS rate was 80.0%. Together, these results indicate that
TOMO is feasible for use in patients with locally advanced cervical
carcinoma. The results also showed that TOMO does not significantly
reduce the recurrence and mortality rates of patients. However,
this is a retrospective analysis that requires further validation
by prospective studies.
In conclusion, the present results showed that TOMO
and IMRT were comparable in terms of mean dose, dose conformity,
dose homogeneity and protection of the ovary. TOMO provided better
critical organ sparing than IMRT in the lower bladder and the lower
rectum, as observed in the planning. The acute and chronic
toxicities were acceptable. Therefore, TOMO is a good option for
treatment of FIGO stage IIB-IIIB cervical cancer, especially in
young patients with ovarian transposition. Further prospective
randomized multicenter studies are required to confirm the benefits
of TOMO.
Acknowledgements
Not applicable.
Funding
This study was supported by the Key Technology Research and
Development Program of Shandong (grant no. 2018GSF118237) and the
National Natural Science Foundation for Young Scientists of China
(grant no. 81602306).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DL recorded and analyzed the experimental raw data,
and was a major contributor in writing the manuscript. DW collected
the clinical data, was involved in the raw data analysis, and
revised the manuscript. QC and JJ contributed to recording the
experimental raw data, and were involved in the raw data analysis
and manuscript draft. SF and XY designed the experiment and revised
the manuscript. JZ designed the experiment and analyzed the dose
volume histogram date. YY designed the experiment, checked the
experimental raw data and was a major contributor to the revised
manuscript. XS supplied study guidance and perfected the experiment
design. DL, DW, JZ and YY confirm the authenticity of all the raw
data. All authors have read and approved the manuscript.
Ethics approval and consent to
participate
Ethics approval was obtained from the Ethics
Committee of Shandong Cancer Hospital and Institute, Shandong First
Medical University and Shandong Academy of Medical Sciences (Jinan,
China; approval no. SDTHEC2021012058).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bhatla N, Aoki D, Sharma DN and
Sankaranarayanan R: Cancer of the cervix uteri. Int J Gynecol
Obstet. 143 (Suppl 2):S22–S36. 2018. View Article : Google Scholar
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dutta S, Nguyen NP, Vock J, Kerr C,
Godinez J, Bose S, Jang S, Chi A, Almeida F, Woods W, et al:
Image-guided radiotherapy and -brachytherapy for cervical cancer.
Front Oncol. 5:642015. View Article : Google Scholar
|
|
4
|
Harkenrider MM, Alite F, Silva SR and
Small W Jr: Image-based brachytherapy for the treatment of cervical
cancer. Int J Radiat Oncol Biol Phys. 92:921–934. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mackie TR, Holmes T, Swerdloff S,
Reckwerdt P, Deasy JO, Yang J, Paliwal B and Kinsella T:
Tomotherapy: A new concept for the delivery of dynamic conformal
radiotherapy. Med Phys. 20:1709–1719. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mackie TR, Kapatoes J, Ruchala K, Lu W, Wu
C, Olivera G, Forrest L, Tome W, Welsh J, Jeraj R, et al: Image
guidance for precision conformal radiotherapy. Int J Radiat Oncol
Biol Phys. 56:89–105. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Balog J, Mackie TR, Pearson D, Hui S,
Paliwal B and Jeraj R: Benchmarking beam alignment for a clinical
helical tomotherapy device. Med Phys. 30:1118–1127. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grigorov G, Kron T, Wong E, Chen J,
Sollazzo J and Rodrigues G: Optimization of helical tomotherapy
treatment plans for prostate cancer. Phys Med Biol. 48:1933–1943.
2003. View Article : Google Scholar
|
|
9
|
van Vulpen M, Field C, Raaijmakers CP,
Parliament MB, Terhaard CH, MacKenzie MA, Scrimger R, Lagendijk JJ
and Fallone BG: Comparing step-and-shoot IMRT with dynamic helical
tomotherapy IMRT plans for head-and-neck cancer. Int J Radiat Oncol
Biol Phys. 62:1535–1539. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen YJ, Liu A, Han C, Tsai PT,
Schultheiss TE, Pezner RD, Vora N, Lim D, Shibata S, Kernstine KH
and Wong JY: Helical Tomotherapy for radiotherapy in esophageal
cancer: A preferred plan with better conformal target coverage and
more homogeneous dose distribution. Med Dosim. 32:166–171. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lian J, Mackenzie M, Joseph K, Pervez N,
Dundas G, Urtasun R and Pearcey R: Assessment of extended-field
radiotherapy for stage IIIC endometrial cancer using
three-dimensional conformal radiotherapy, intensity-modulated
radiotherapy, and helical tomotherapy. Int J Radiat Oncol Biol
Phys. 70:935–943. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsieh CH, Wei MC, Lee HY, Hsiao SM, Chen
CA, Wang LY, Hsieh YP, Tsai TH, Chen YJ and Shueng PW: Whole pelvic
helical tomotherapy for locally advanced cervical cancer: Technical
implementation of IMRT with helical tomotherapy. Radiat Oncol.
4:622009. View Article : Google Scholar
|
|
13
|
Karnofsky DA and Burchenal JH: The
clinical evaluation of chemotherapeutic agents in cancer.
Evaluation of Chemotherapeutic Agents. Macleod CM: Columbia
University Press; New York, NY: pp. p1961949
|
|
14
|
Pecorelli S: Revised FIGO staging for
carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol
Obstet. 105:103–104. 2009. View Article : Google Scholar
|
|
15
|
International Commission on Radiation
Units and Measurements (ICRU), . Prescribing, Recording and
Reporting Photon Beam Therapy (Supplement to ICRU Report 50). ICRU
Report. 62. ICRU; Bethesda, MD: 1999
|
|
16
|
Wang X, Zhang X, Dong L, Liu H, Gillin M,
Ahamad A, Ang K and Mohan R: Effectiveness of noncoplanar IMRT
planning using a parallelized multiresolution beam angle
optimization method for paranasal sinus carcinoma. Int J Radiat
Oncol Biol Phys. 63:594–601. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
International Commission on Radiation
Units and Measurements (ICRU), . Dose and volume specification for
reporting intracavitary therapy in gynecology. ICRU Report. 38.
ICRU; Bethesda, MD: ICRU1985.
|
|
18
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bruner DW: Outcomes research in cancer
symptom management trials: The radiation therapy oncology group
(RTOG) conceptual model. J Natl Cancer Inst Monogr. 37:12–15. 2007.
View Article : Google Scholar
|
|
20
|
Peters WA III, Liu PY, Barrett RJ II,
Stock RJ, Monk BJ, Berek JS, Souhami L, Grigsby P, Gordon W Jr and
Alberts DS: Concurrent chemotherapy and pelvic radiation therapy
compared with pelvic radiation therapy alone as adjuvant therapy
after radical surgery in high-risk early-stage cancer of the
cervix. J Clin Oncol. 18:1606–1613. 2000. View Article : Google Scholar
|
|
21
|
Zhou GX, Xu SP, Dai XK, Ju ZJ, Gong HS,
Xie CB, Yin LM and Yang J: Clinical dosimetric study of three
radiotherapy techniques for postoperative breast cancer: Helical
Tomotherapy, IMRT, and 3D-CRT. Technol Cancer Res Treat. 10:15–23.
2011. View Article : Google Scholar
|
|
22
|
Chitapanarux I, Nobnop W, Tippanya D,
Sripan P, Chakrabandhu S, Klunklin P, Onchan W, Jia-Mahasap B and
Tharavichitkul E: Clinical outcomes and dosimetric study of
hypofractionated Helical TomoTherapy in breast cancer patients.
PLoS One. 14:e02115782019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo MF, Liu XF, Liu L and Wang D;
Department of Gynecologic Oncology, Chongqing University Cancer
Hospital&Chongqing Cancer Institute&Chongqing Cancer
Hospital, : (A dosimetric study of helical tomotherapy in
radiotherapy after ovarian-conserving radical surgery for young
patients with early-stage cervical cancer). J Chongqing Med Univ.
044:39–42. 2019.
|
|
24
|
Wiezorek T, Brachwitz T, Georg D, Blank E,
Fotina I, Habl G, Kretschmer M, Lutters G, Salz H, Schubert K, et
al: Rotational IMRT techniques compared to fixed gantry IMRT and
tomotherapy: Multi-institutional planning study for head-and-neck
cases. Radiat Oncol. 6:202011. View Article : Google Scholar
|
|
25
|
Clemente S, Wu B, Sanguineti G, Fusco V,
Ricchetti F, Wong J and McNutt T: SmartArc-based volumetric
modulated arc therapy for oropharyngeal cancer: A dosimetric
comparison with both intensity-modulated radiation therapy and
helical tomotherapy. Int J Radiat Oncol Biol Phys. 80:1248–1255.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang R, Xu S, Jiang W, Wang J and Xie C:
Dosimetric comparison of postoperative whole pelvic radiotherapy
for endometrial cancer using three-dimensional conformal
radiotherapy, intensity-modulated radiotherapy, and helical
tomotherapy. Acta Oncol. 49:230–236. 2010. View Article : Google Scholar
|
|
27
|
Engels B, De Ridder M, Tournel K, Sermeus
A, De Coninck P, Verellen D and Storme GA: Preoperative helical
tomotherapy and megavoltage computed tomography for rectal cancer:
Impact on the irradiated volume of small bowel. Int J Radiat Oncol
Biol Phys. 74:1476–1480. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tournel K, De Ridder M, Engels B,
Bijdekerke P, Fierens Y, Duchateau M, Linthout N, Reynders T,
Verellen D and Storme G: Assessment of intrafractional movement and
internal motion in radiotherapy of rectal cancer using megavoltage
computed tomography. Int J Radiat Oncol Biol Phys. 71:934–939.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gattaneo GM, Dell'oca I, Broggi S, Fiorino
C, Perna L, Pasetti M, Sangalli G, di Muzio N, Fazio F and
Calandrino R: Treatment planning comparison between conformal
radiotherapy and helical tomotherapy in the case of locally
advanced-stage NSCLC. Radiother Oncol. 88:310–318. 2008. View Article : Google Scholar
|
|
30
|
Yao B, Wang SH, Wang YD, Liu Q and Lu N:
Acute and late toxicities and efficacy of helical tomotherapy and
concurrent chemotherapy in the treatment of locally advanced
cervical cancer. Oncol Prog. 14:544–547. 2016.
|
|
31
|
Rose BS, Aydogan B, Liang Y, Yeginer M,
Hasselle MD, Dandekar V, Bafana R, Yashar CM, Mundt AJ, Roeske JC
and Mell LK: Normal tissue complication probability modeling of
acute hematologic toxicity in cervical cancer patients treated with
chemoradiotherapy. Int J Radiat Oncol Biol Phys. 79:800–807. 2011.
View Article : Google Scholar
|
|
32
|
Chang AJ, Richardson S, Grigsby PW and
Schwarz JK: Split-field helical tomotherapy with or without
chemotherapy for definitive treatment of cervical cancer. Int J
Radiat Oncol Biol Phys. 82:263–269. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin MA: Clinical Application of
Tomotherapy System. China Medical Devices. 29:12–14. 2014.(In
Chinese). View Article : Google Scholar
|
|
34
|
Wang W, Zhang F, Hu K and Hou X:
Image-guided, intensity-modulated radiation therapy in definitive
radiotherapy for 1433 patients with cervical cancer. Gynecol Oncol.
151:444–448. 2018. View Article : Google Scholar
|